Abstract
CCL2 is a chemokine known to recruit monocytes and macrophages to sites of inflammation. A growing body of research suggests CCL2 is progressively overexpressed in tumor beds and may play a role in the clinical progression of solid tumors. Cancer cells derived from several solid tumor types demonstrate functional receptors for CCL2, suggesting this chemokine may achieve tumorigenicity through direct effects on malignant cells; however, a variety of normal host cells that co-exist with cancer in the tumor microenvironment also respond to CCL2. These cells include macrophages, osteoclasts, endothelial cells, T-lymphocytes, and myeloid-derived immune suppressor cells (MDSCs). CCL2 mediated interactions between normal and malignant cells in the tumor microenvironment and plays a multi-faceted role in tumor progression.
Keywords: CCL2, CCR2, tumorigenesis, metastasis, prostate cancer
1. Introduction
Cancer cells coexist in the tumor microenvironment with normal host cells, including hematopoietic precursors. While some host cells inhibit cancer growth, many produce a multitude of soluble factors and nutrients that promote tumor progression and growth. In turn, cancer cells provide growth factors, cytokines, and chemokines that promote host cell proliferation and survival. Understanding the factors that contribute to this vicious cycle of cooperation is of primary importance to understanding malignant progression and has revealed new therapeutic targets for cancer treatment.
Altered regulation of chemokines and their receptors has been demonstrated to promote tumor development and progression through multiple mechanisms including enhanced proliferation, increased invasiveness, increased angiogenesis, and recruitment of immune cells [as reviewed in [1-3]]. Among the many chemokines and their receptor pairs, CC chemokine ligand 2 (CCL2) and its receptor CCR2 have recently been shown to play key roles in promoting tumorigenesis and metastasis [as reviewed in [4]].
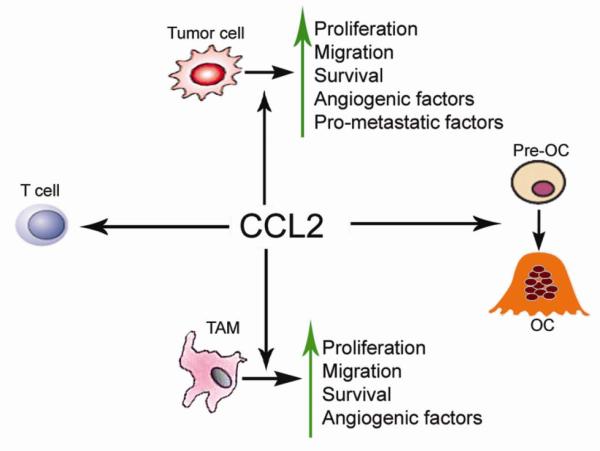
CCL2 is a member of the CC chemokine family that regulates the recruitment of monocytes, macrophages, and other inflammatory cells to sites of inflammation via activation of CCR2 [5]. In addition to its role in inflammatory responses, CCL2 has been demonstrated to directly stimulate prostate cancer cell chemoattraction, proliferation, and survival [6-8]. CCL2 has also been shown to play a central role in the maturation of osteoclasts in the microenvironment of skeletal metastases [9]. The multiple roles of CCL2 in the promotion of tumorigenesis make the CCL2/CCR2 axis an attractive therapeutic target for cancer treatment (Figure 1 and Table 1).
Figure 1.
CCL2 is tumorigenic through its direct effects on tumor cells and its influence on normal host cells.
Table 1.
CCL2 promotes prostate cancer tumorigenesis and metastasis
| Source of CCL2 |
CCL2 functions | Potential mechanisms | References |
|---|---|---|---|
| LNCaP, PC3, VCaP |
Promotes cancer cell proliferation; Protects autophagic cell death |
PI3K/AKT-dependent; autocrine and paracrine manners; PI3K/Akt- dependent survivin up- regulation |
[6-8, 26] |
| LNCaP, PC3, VCaP |
Induces cancer cell migration and invasion |
PAR-1 mediates Rho activation; Differential regulation of PKC and MMP-9 activation |
[6-8, 69] |
| HBME | Promotes PC3 cells transendothelial cell migration |
Small GTPase Rac activation | [28] |
| HBME, VCaP, PC3, LNCaP |
Indirectly induces tumor angiogenesis |
VEGF-A induction from TAMs; FGF-8b production |
[32, 33, 70] |
| Osteoblast | PTHrP induces CCL2 production |
NFκB and C/EBPβ activation |
[9, 32] |
| PC3, C4-2B, Osteoclast, hFOB, HBME |
Mediates osteoclastogenesis |
RANKL-dependent and - independent pathway |
[9, 19, 25, 47] |
| VCaP, PC3 | Promotes tumor growth in vivo |
Regulation of TAMs infiltration |
[33] |
2. CCL2 and its receptor CCR2
CCL2, also known as monocyte chemoattractant protein-1 (MCP-1), was first identified by its ability to attract monocytes in vitro [10-12]. CCL2 is a 76-amino acid polypeptide and is a member of the C-C chemokine family characterized by adjacent cysteine residues. It binds with high affinity to the seven transmembrane G–protein coupled receptor CCR2. The CCL2 gene is one of several cytokine genes clustered on the q-arm of chromosome 17 [13].
Investigations of CCL2 and CCR2 genotypic variants through single-nucleotide polymorphism (SNP) analysis suggest that this chemokine may play a role in host susceptibility to the development of cancer and/or cancer metastasis. For example, CCL2 SNPs have been shown to associate with chronic myelogenous leukemia risk [14]. The −2518 G/A SNP occurs in the promoter region of the CCL2 gene. Carriers of the G-allele demonstrate elevated CCL2 transcription [15]. A study of 83 woman from northern Italy undergoing surgery for breast cancer found G-allele carrier status strongly correlates with the presence of metastases at the time of operation [16]. Carriers of the A-allele were prone to developing distant metastasis following radiation therapy when compared to G/G heterozygotes in a study of 411 nasopharyngeal carcinoma patients [17]. Although no association was observed between prostate cancer risk and the CCL2 2518 G/A polymorphism [18], the role of this SNP in prostate cancer progression and metastases remains an open question.
The CCR2 gene, residing in the chemokine receptor gene cluster region, encodes two isoforms. CCR2 mRNA and protein are differentially expressed by various cancer cell lines including prostate, lung, breast cancer, and myeloma [as reviewed in [4]]. Certain prostate cancer cell lines were responsive to CCL2 in proliferation and in vitro cell invasion assays [6, 8], indicating the presence of functional CCR2 receptors. Furthermore, CCR2 antagonist only partially inhibited CCL2-induced proliferation and invasion, suggesting the presence of other functional CCL2 receptors with functional redundancy [8]. Although all prostate cancer cells express CCR2 mRNA and protein, more aggressive cancer cells express higher levels of CCR2 when compared to less aggressive or benign prostatic cells. Diminished CCL2-induced prostate cancer cell invasion and proliferation demonstrated by short-hairpin RNA (shRNA) knockdowns of CCR2 in the prostate cancer cell lines C4-2B and PC3 [19] reiterates the connection between activation of the CCL2/CCR2 axis and the phenotype of aggressive prostate cancer. Interestingly, the I-allele of the CCR2-V64I polymorphism studied extensively as a source of genetic variation in clinical HIV progression [20] may protect against sporadic breast cancer [21] and invasive cervical carcinoma development [22].
3. CCL2 is present in the tumor microenvironment
CCL2 expression has been demonstrated in many cancer types including prostate, breast, lung, melanoma, ovary, gastric, renal, colorectal, esophageal and multiple myeloma [as reviewed in [4]]. Prostate cancer in particular demonstrated elevated CCL2 expression in malignancies when compared to benign prostatic tissues [8]. One study identified 4-fold upregulation of CCL2 as determined by cytokine antibody arrays comparing the tumor-bone microenvironment to non-neoplastic bone [6]. CCL2 is expressed not only in tumor cells, but also in osteoblasts and bone marrow endothelial cells [23, 24]. It was also reported that parathyroid hormone-related peptide (PTHrP) can upregulate CCL2 in both osteoblasts and bone marrow endothelial cells [9]. Although the primary source of CCL2 in bones still needs to be identified, it has been demonstrated that CCL2 contributes to prostate cancer sketetal metastasis through both direct effects on prostate cancer cells and indirect effects on osteoclast activity in tumor-induced bone lesions [25].
4. The effect of CCL2 on cancer cells
4.1. CCL2 and prostate cancer cell proliferation and survival
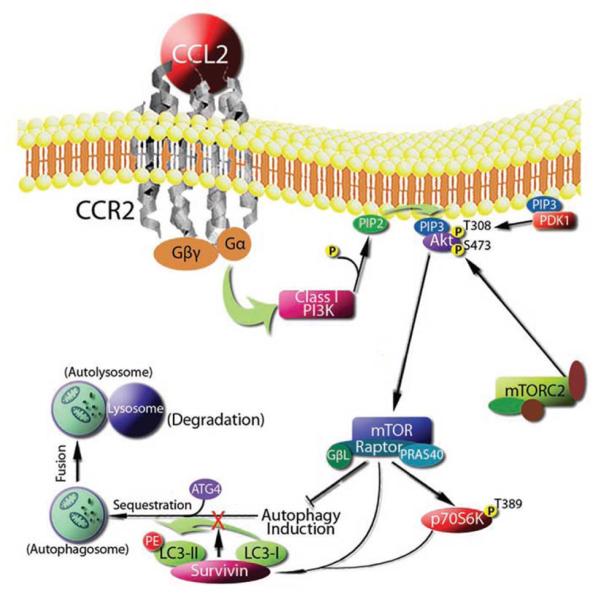
Recombinant human CCL2 induces dose-dependent prostate cancer cell proliferation in vitro through the activation of the phosphatidylinositol 3-kinase (PI3K)/AKT pathway [6, 8]. In addition, this activation of the AKT pathway also provides cancer cells with a survival advantage through up-regulation of survivin [26]. Specifically, PI3K/Akt activation by CCL2 mediates mTORC1 activation, survivin up-regulation, and subsequent down-regulation of autophagosome formation. Autophagy protects cells from nutrient depletion stress; however, excessive autophagy will result in cell death [27]. As survivin may be a key molecule protecting prostate cancer cells from autophagic death (Figure 2), combination therapy targeting both CCL2 and the PI3K/Akt/mTOR/survivin pathways may become a clinical strategy to overcome therapeutic resistance.
Figure 2.
Proposed mechanism by which CCL2 controls autophagic death in prostate cancer cells. Roca H et al., Autophagy.2008, 4(7):969-71.
4.2. CCL2 and prostate cancer cell migration and metastasis
CCL2 is chemotatic for prostate cancer cells in both autocrine and paracrine manners [6, 8]. CCL2 induces actin rearrangement through phosphorylation of p70-S6 kinase (a downstream target of Akt) and activates the small GTPase Rac through the actin-associated protein PCNT1 [28]. This activation of Rac allows the cells to undergo diapedesis through the bone marrow endothelium. This migration is mediated in part by thrombin/thrombin receptor facilitated positioning of cancer cells in a “ready state” for migration in response to a chemotactic signal (23). Taken together, these data suggest that CCL2 has a significant role in promoting prostate cancer cell extravasation into the bone microenvironment.
5. CCL2 and tumor angiogenesis
Angiogenesis correlates with cancer progression and/or poor prognosis in a variety of solid tumors [29]. The process can be activated at different stages of tumor development, depending on the cancer types and the tumor microenvironment. Identifying critical angiogenic factors and mediators in the tumor microenvironment remains an important challenge.
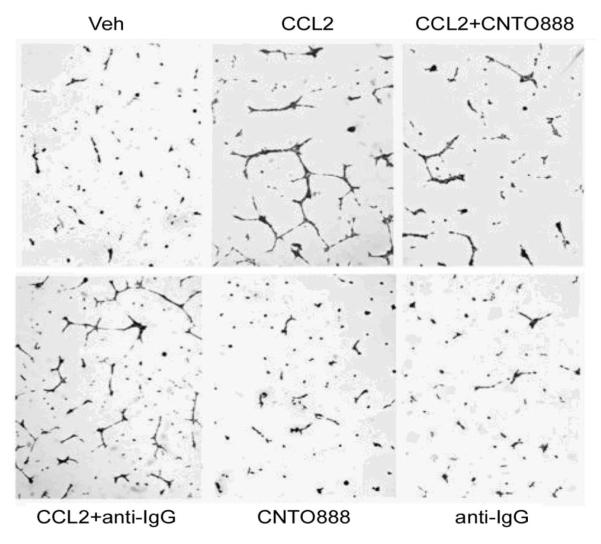
Previous findings suggest that CCL2 can directly mediate angiogenesis in endothelial cells, which express CCR2 [30]. Human brain endothelial cells, human umbilical cord vein endothelial cells (HUVEC), and human dermal microvascular endothelial cells (HDMVEC) were reported to express CCR2 and respond to CCL2 by forming more vessel spouts in vitro [30, 31]. It was reported in a study of prostate cancer angiogenesis that CCL2 stimulated tumor cells to produce proangiogenic factor(s) instead of directly stimulating bone marrow endothelial cells (HBMEs) [32]. Conditioned media from CCL2-treated PC-3 cells significantly increased sprout formation in HBMEs, whereas pretreating PC-3 cells with anti-CCL2 antibody abolished this effect. CCL2 itself failed to directly stimulate capillary tube formation by HBMEs. The antibody against human CCL2 CNTO 888 also had no effect as a monotherapy on capillary tube formation; however, CCL2 increased VEGF-A mRNA expression levels in PC-3 cells after 4-6 hours of treatment. The induction of VEGF-A mRNA expression in PC-3 cells was blocked by pretreatment with a neutralizing antibody, indicating this induction was mediated by CCL2 (Figure 3).
Figure 3.
CCL2 has pro-angiogenic effects on endothelial cells through elevated tumor-derived VEGF-A. Li X et al., Cancer Res. 2009, 69(4):1685-92.
In addition, it has been shown that systemic administration of CCL2 neutralizing antibodies significantly decrease PC3 tumor burden in vivo and dramatically reduce tumor blood vessel density [32]. Similar findings were reported in a subcutaneous VCaP xenograft model [33]. In that study, VCaP cells were implanted in SCID mice and monitored for tumor growth and microvascular density. Systemic administration of CCL2 neutralizing antibodies significantly reduced VCaP tumor growth and decreased tumor microvascular density. Therefore, these results support that CCL2 promotes the tumor angiogenesis both in vitro and in vivo.
In other tumor types such as breast cancer and multiple myeloma, CCL2 expression has been reported to associate with tumor progression and angiogenesis [as reviewed in [34, 35]]. CCL2 was suggested to induce angiogenesis through multiple mechanisms including direct induction of VEGF-A [36] and hypoxia inducible factor-1 (HIF-1) [37]. CCL2 can also stimulate angiogenesis through the attraction of tumor associated macrophages (TAMs) which are known to secrete VEGF [38]. TAMs are a major component in the inflammatory response and produce a variety of growth factors for epithelial and endothelial cells, as well as inflammatory cytokines and chemokines that contribute to the tumor cell survival, proliferation, and invasion.
6. CCL2 and TAMs in the tumor progression
Many solid tumors contain an inflammatory infiltrate that often comprises a majority of the cells in the tumor’s mass [39]. In many tumors, the majority of the infiltrating cells are TAMs derived from myeloid progenitor cells in the bone marrow compartment [40]. Myeloid progenitor cells develop into pro-monocytes in the bone marrow prior to being released into the circulation where they undergo differentiation into monocytes. Monocytes then migrate into tissues where they differentiate into resident macrophages, protect against infection, and respond to tissue injury. In cancer these cells differentiate into TAMs; however, it is not clear whether TAMs are derived from peripheral blood monocytes recruited into tumors from the circulation or from resident macrophages present in the otherwise healthy tissue where a tumor develops [41].
Macrophages demonstrate functional “polarization” toward one of two phenotypically distinct macrophage subsets: TH1 (also known as M1 macrophages) or TH2 (also known as M2 macrophages). M1 macrophages are known to produce pro-inflammatory cytokines and play an active role in cell destruction, whereas M2 macrophages primarily scavenge debris, promote angiogenesis, and facilitate wound repair [42]. The M2 macrophage population is thought to be phenotypically similar to the TAM population [40].
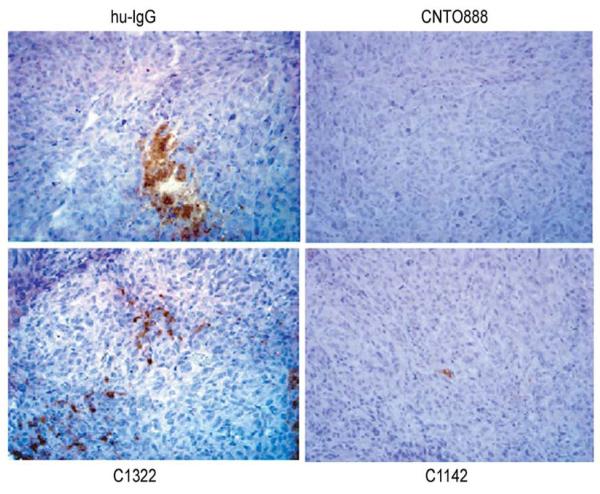
TAMs have been demonstrated to play a direct role in the regulation of tumor growth and angiogenesis in prostatic malignancies [33]. Elevated expression by both cancer and stromal cells of a number of monocyte chemoattractants including CCL2, CCL3, CCL4, CCL5 (RANTES), and CCL8 has been shown to positively correlate with increased TAM numbers in several human tumors [43, 44]. Specifically, CCL2 has been demonstrated to regulate monoctye/macrophage trafficking and has been reported to be present in prostatic tumor beds (Figure 4), suggesting a role for this chemokine in TAM recruitment [45].
Figure 4.
Inhibition of CCL2 reduced the number of infiltrating macrophages in VCaP xenografts. Loberg RD et al., Neoplasia. 2007, 9(7):556-62.
7. The effect of CCL2 on osteoclasts
Prostate cancer preferentially metastasizes to bone, resulting in high mortality. Histopathological examination of tumor-induced bone lesions reveals a mixture of pre-requisite osteolytic bone resorption and a predominantly bone forming osteoblastic reaction. The tumor-induced osteoclast activity is essential for prostate cancer establishment in the bone microenvironment.
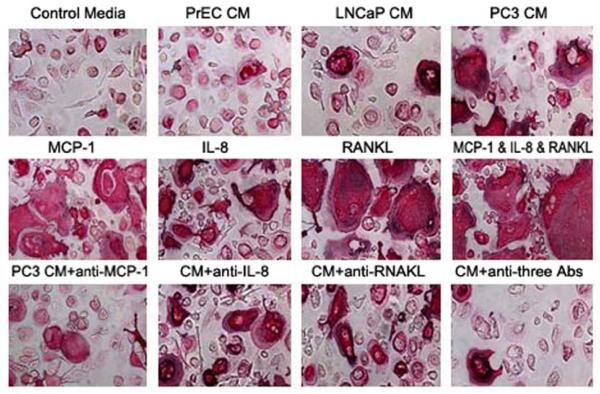
It has been reported that blocking Receptor Activator of Nuclear Factor KappaB Ligand (RANKL) in murine models partially inhibits the establishment and progression of prostatic tumors in bone [46]; however, the cell line used in these studies produced very low levels of RANKL, suggesting other soluble factors may mediate prostate cancer-induced osteoclast activity. Recently, it was reported that CCL2 significantly induces osteoclast fusion both in vitro [25] and in vivo [32]. Human bone marrow mononuclear cells (HBMC) cultured in vitro using conditioned media collected from prostate cancer PC3 cell cultures differentiated into bone resorbing osteoclast-like cells (Figure 5). Both recombinant human CCL2 and IL-8 directly stimulated HBMC differentiation into osteoclast-like cells, but only IL-8 induced bone resorption on dentin slices following 21 days of culture in the absence of RANKL. Nevertheless, conditioned media-induced bone resorption was inhibited by a CCL2 neutralizing antibody. The inhibition was enhanced by combining CCL2 neutralizing antibody with an IL-8 neutralizing antibody, indicating that CCL2 and IL-8 cooperatively mediate tumor-induced osteoclastogenesis and bone resorption. In support of this findings, another report showed CD11b+ cells isolated from human peripheral blood differentiate into functional osteoclasts when grown in PC3 conditioned media [47].
Figure 5.
CCL2 neutralizing antibodies diminish osteoclast formation induced by prostate cancer conditioned media. Lu Yet al., Cancer Res. 2007, 67(8):3646-53.
The effects of CCL2 on osteoclastogenesis were further demonstrated in vivo using intratibial-injections of prostate cancer cells in mice as a preclinical mouse model of prostate cancer bone metastasis [32]. Systemic treatment with CCL2 antibody not only deceased tumor burden, but also decreased osteoclast numbers at the tumor–bone interface in this model. These observations were accompanied by a decrease in the serum marker of bone resorption TRAP5b [32]. These results demonstrate an active role for CCL2 in tumor-induced osteoclastogenesis.
8. The effects of CCL2 on T lymphocytes in the tumor microenvironment
Several studies have demonstrated CCL2 overexpression at sites of inflammation and the contribution of CCL2 to the inflammatory components of such diseases as atherosclerosis, multiple sclerosis, and rheumatoid arthritis [5, 48]. In addition, growing evidence has implicated chemokines as a key component of cancer-related inflammation due to the role of chemokines in inflammatory cell recruitment and function in the tumor microenvironment [36]. Although, CCL2 is chemoattractant not only for inflammatory monocytes, but also for memory T-lymphocytes, natural killer cells, and perhaps dendritic cells [49]. Several studies indicate that CCL2 may also influence T-cell differentiation and function.
T-lymphocytes express the CCR2 and may react to CCL2 to differentiate into Th2 “helper” T-cells [50]. Th2 cells are a subset of CD4+ T-helper cells that stimulate B-lymphocytes to produce antibody and suppress the action of cytotoxic CD8+ T-cells [51]. The Karpus group showed that naïve T-lymphocytes from TCR transgenic mice differentiate toward a Th1 phenotype in vitro when exposed to antigen in the presence of macrophage inflammatory protein-1a [52]. In contrast, the same cells became Th2-like in the presence of CCL2. CCL2-induced Th2 differentiation was blocked by a neutralizing antibody against IL-4, suggesting CCL2 induced Th2 differentiation by enhancing IL-4 expression. It has also been reported that IL-4 induces CCL2 expression in endothelial cells [53], suggesting a self-amplifying loop whereby CCL2 and IL-4 mutually enhance their expression, resulting in T-cell differentiation toward a Th2 phenotype in the tumor microenvironment [54].
9. The effects of CCL2 on immune suppressor cells
Failure of host anti-tumor immunity may be caused by exaggerated suppression of tumor-associated antigen-reactive lymphocytes. Suppression of reactive lymphocytes is mediated by regulatory T-cells (Tregs). Tumor derived TGFβ can convert CD4+ CD25− T cells into CD4+ CD25+ T cells, which is one immune evasion mechanism available to tumor cells [55]. Other mechanisms include production of the immunosuppressive cytokine IL-10 [56], generation of immunosuppressive DCs [57], and induction of T-cell anergy [58]. Abrogating immune evasion by eliminating Treg cells appears to be an effective immunotherapy approach against both murine and human melanoma [59, 60]. Thus, identifying and targeting chemokines facilitating Tregs-mediated immune evasion remains a noteworthy goal.
An analysis of malignant human glioma cell lines and tumor cells from patients with Glioblastoma multiforme (GBM) detected intracellular expression of chemokines CCL22 and CCL2; however, only CCL2 was detected by ELISA. Supernatant collected from the glioma cell lines in this study induced Treg cell migration, as did recombinant human CCL2 and CCL22. The induced migration was successfully inhibited by neutralizing antibodies [61]. Taken together, these findings suggest targeting CCL2 as a strategy to inhibit Treg recruitment by tumors.
In addition to Treg cells, recent reports demonstrate the role of another cell population in immune suppression - immature myeloid-derived suppressor cells (MDSCs) [62, 63]. These cells are identified by a GR-1+CD11b+ F4/80- surface antigen profile [62]. It has been reported that MDSC accumulation associates with the inflammatory milieu of the tumor microenvironment. In addition to suppressing T cells, MDSCs also demonstrate a role in repressing the cytotoxicity of natural killer (NK) cells in tumor-bearing hosts [64]. More recently, Sinha reported that immature myeloid cells may also induce macrophage differentiation from an M1 type to an M2 phenotype [65]. Several tumor-derived cytokines and chemokines have been shown to influence MDSC function [66, 67]. In particular, the CCL2/CCR2 pathway has been demonstrated to play a critical role in the migration of MDSCs to tumors [68]. This suggests CCL2 facilitates cancer’s evasion of an anti-tumor immune response by recruiting immune suppressor cells into the tumor microenvironment.
10. Conclusion
CCL2 influences cancer tumorigenesis and metastasis via distinct mechanisms:
A direct promotional effect on tumor cell growth, survival and function
A modulatory effect on the tumor microenvironment by promoting macrophage mobilization and infiltration into the tumor bed
Promotion of osteoclast maturation in the bone tumor microenvironment
Suppression of cytotoxic lymphocytes.
Direct CCL2 stimulation regulates the growth, survival, invasiveness, and migration of tumor cells. This ability to stimulate cancer epithelial cells directly has implicated CCL2 in the progression and metastasis of several human carcinomas including breast, pancreas, and prostate. An equally influential role of CCL2 in the development and progression of tumors is its interaction with a variety of normal host cells in the tumor microenvironment. Through its interaction with vascular endothelial cells, CCL2 promotes the development of tumor vasculature critical to sustained neoplastic growth. As a chemokine for monocytes and macrophages, CCL2 recruits inflammatory infiltrates that often constitute a significant portion of the tumor volume, contribute to tumor growth, and appear to promote tumor angiogenesis. In the bone microenvironment CCL2 induces osteoclast formation and activation, a critical step in the seeding of bone tumors. Finally, CCL2 acts as a chemokine attracting immunosuppressive Tregs and MDSCs into the tumor microenvironment, which contributes to cancers’ evasion of an anti-tumor immune response. Taken together, these findings reveal CCL2 as an attractive, multifaceted target for cancer therapy.
Acknowledgements
This work was supported by the DOD PC061231 (JZ); NIH PO1 CA093900 (KJP), an American Cancer Society Clinical Research Professorship (KJP), NIH SPORE P50 CA69568 (KJP), Cancer Center P30 CA46592 (KJP), Southwest Oncology Group CA32102 (KJP), Prostate Cancer Foundation (KJP)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004 Jul;4(7):540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 2.Taichman RS, Loberg RD, Mehra R, Pienta KJ. The evolving biology and treatment of prostate cancer. J Clin Invest. 2007 Sep;117(9):2351–61. doi: 10.1172/JCI31791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balkwill F. Chemokine biology in cancer. Semin Immunol. 2003 Feb;15(1):49–55. doi: 10.1016/s1044-5323(02)00127-6. [DOI] [PubMed] [Google Scholar]
- 4.Craig MJ, Loberg RD. CCL2 (Monocyte Chemoattractant Protein-1) in cancer bone metastases. Cancer Metastasis Rev. 2006 Dec;25(4):611–9. doi: 10.1007/s10555-006-9027-x. [DOI] [PubMed] [Google Scholar]
- 5.Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004 Oct 29;95(9):858–66. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 6.Loberg RD, Day LL, Harwood J, Ying C, St John LN, Giles R, et al. CCL2 is a potent regulator of prostate cancer cell migration and proliferation. Neoplasia. 2006 Jul;8(7):578–86. doi: 10.1593/neo.06280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loberg RD, Tantivejkul K, Craig M, Neeley CK, Pienta KJ. PAR1-mediated RhoA activation facilitates CCL2-induced chemotaxis in PC-3 cells. J Cell Biochem. 2007 Aug 1;101(5):1292–300. doi: 10.1002/jcb.21252. [DOI] [PubMed] [Google Scholar]
- 8.Lu Y, Cai Z, Galson DL, Xiao G, Liu Y, George DE, et al. Monocyte chemotactic protein-1 (MCP-1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate. 2006 Sep 1;66(12):1311–8. doi: 10.1002/pros.20464. [DOI] [PubMed] [Google Scholar]
- 9.Lu Y, Xiao G, Galson DL, Nishio Y, Mizokami A, Keller ET, et al. PTHrP-induced MCP-1 production by human bone marrow endothelial cells and osteoblasts promotes osteoclast differentiation and prostate cancer cell proliferation and invasion in vitro. Int J Cancer. 2007 Aug 15;121(4):724–33. doi: 10.1002/ijc.22704. [DOI] [PubMed] [Google Scholar]
- 10.Valente AJ, Graves DT, Vialle-Valentin CE, Delgado R, Schwartz CJ. Purification of a monocyte chemotactic factor secreted by nonhuman primate vascular cells in culture. Biochemistry. 1988 May 31;27(11):4162–8. doi: 10.1021/bi00411a039. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimura T, Robinson EA, Tanaka S, Appella E, Kuratsu J, Leonard EJ. Purification and amino acid analysis of two human glioma-derived monocyte chemoattractants. J Exp Med. 1989 Apr 1;169(4):1449–59. doi: 10.1084/jem.169.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989 Apr 1;169(4):1485–90. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehrabian M, Sparkes RS, Mohandas T, Fogelman AM, Lusis AJ. Localization of monocyte chemotactic protein-1 gene (SCYA2) to human chromosome 17q11.2-q21.1. Genomics. 1991 Jan;9(1):200–3. doi: 10.1016/0888-7543(91)90239-b. [DOI] [PubMed] [Google Scholar]
- 14.Enjuanes A, Benavente Y, Bosch F, Martin-Guerrero I, Colomer D, Perez-Alvarez S, et al. Genetic variants in apoptosis and immunoregulation-related genes are associated with risk of chronic lymphocytic leukemia. Cancer Res. 2008 Dec 15;68(24):10178–86. doi: 10.1158/0008-5472.CAN-08-2221. [DOI] [PubMed] [Google Scholar]
- 15.Rovin BH, Lu L, Saxena R. A novel polymorphism in the MCP-1 gene regulatory region that influences MCP-1 expression. Biochem Biophys Res Commun. 1999 Jun 7;259(2):344–8. doi: 10.1006/bbrc.1999.0796. [DOI] [PubMed] [Google Scholar]
- 16.Ghilardi G, Biondi ML, La Torre A, Battaglioli L, Scorza R. Breast cancer progression and host polymorphisms in the chemokine system: role of the macrophage chemoattractant protein-1 (MCP-1) -2518 G allele. Clin Chem. 2005 Feb;51(2):452–5. doi: 10.1373/clinchem.2004.041657. [DOI] [PubMed] [Google Scholar]
- 17.Tse KP, Tsang NM, Chen KD, Li HP, Liang Y, Hsueh C, et al. MCP-1 Promoter Polymorphism at 2518 is associated with metastasis of nasopharyngeal carcinoma after treatment. Clin Cancer Res. 2007 Nov 1;13(21):6320–6. doi: 10.1158/1078-0432.CCR-07-1029. [DOI] [PubMed] [Google Scholar]
- 18.Smith MW, Dean M, Carrington M, Winkler C, Huttley GA, Lomb DA, et al. Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC), ALIVE Study. Science. 1997 Aug 15;277(5328):959–65. doi: 10.1126/science.277.5328.959. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y, Chen Q, Corey E, Xie W, Fan J, Mizokami A, et al. Activation of MCP-1/CCR2 axis promotes prostate cancer growth in bone. Clin Exp Metastasis. 2009;26(2):161–9. doi: 10.1007/s10585-008-9226-7. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien SJ, Moore JP. The effect of genetic variation in chemokines and their receptors on HIV transmission and progression to AIDS. Immunol Rev. 2000 Oct;177:99–111. doi: 10.1034/j.1600-065x.2000.17710.x. [DOI] [PubMed] [Google Scholar]
- 21.Zafiropoulos A, Crikas N, Passam AM, Spandidos DA. Significant involvement of CCR2-64I and CXCL12-3a in the development of sporadic breast cancer. J Med Genet. 2004 May;41(5):e59. doi: 10.1136/jmg.2003.013649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng B, Wiklund F, Gharizadeh B, Sadat M, Gambelunghe G, Hallmans G, et al. Genetic polymorphism of chemokine receptors CCR2 and CCR5 in Swedish cervical cancer patients. Anticancer Res. 2006 Sep-Oct;26(5B):3669–74. [PubMed] [Google Scholar]
- 23.Bost KL, Bento JL, Petty CC, Schrum LW, Hudson MC, Marriott I. Monocyte chemoattractant protein-1 expression by osteoblasts following infection with Staphylococcus aureus or Salmonella. J Interferon Cytokine Res. 2001 May;21(5):297–304. doi: 10.1089/107999001300177484. [DOI] [PubMed] [Google Scholar]
- 24.Vanderkerken K, Vande Broek I, Eizirik DL, Van Valckenborgh E, Asosingh K, Van Riet I, et al. Monocyte chemoattractant protein-1 (MCP-1), secreted by bone marrow endothelial cells, induces chemoattraction of 5T multiple myeloma cells. Clin Exp Metastasis. 2002;19(1):87–90. doi: 10.1023/a:1013891205989. [DOI] [PubMed] [Google Scholar]
- 25.Lu Y, Cai Z, Xiao G, Keller ET, Mizokami A, Yao Z, et al. Monocyte chemotactic protein-1 mediates prostate cancer-induced bone resorption. Cancer Res. 2007 Apr 15;67(8):3646–53. doi: 10.1158/0008-5472.CAN-06-1210. [DOI] [PubMed] [Google Scholar]
- 26.Roca H, Varsos Z, Pienta KJ. CCL2 protects prostate cancer PC3 cells from autophagic death via phosphatidylinositol 3-kinase/AKT-dependent survivin up-regulation. J Biol Chem. 2008 Sep 5;283(36):25057–73. doi: 10.1074/jbc.M801073200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roca H, Varsos ZS, Mizutani K, Pienta KJ. CCL2, survivin and autophagy: new links with implications in human cancer. Autophagy. 2008 Oct 1;4(7):969–71. doi: 10.4161/auto.6822. [DOI] [PubMed] [Google Scholar]
- 28.van Golen KL, Ying C, Sequeira L, Dubyk CW, Reisenberger T, Chinnaiyan AM, et al. CCL2 induces prostate cancer transendothelial cell migration via activation of the small GTPase Rac. J Cell Biochem. 2008 Aug 1;104(5):1587–97. doi: 10.1002/jcb.21652. [DOI] [PubMed] [Google Scholar]
- 29.Fox SB, Harris AL. Markers of tumor angiogenesis: clinical applications in prognosis and anti-angiogenic therapy. Invest New Drugs. 1997;15(1):15–28. doi: 10.1023/a:1005714527315. [DOI] [PubMed] [Google Scholar]
- 30.Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, et al. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000 Jul 1;96(1):34–40. [PubMed] [Google Scholar]
- 31.Goede V, Brogelli L, Ziche M, Augustin HG. Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int J Cancer. 1999 Aug 27;82(5):765–70. doi: 10.1002/(sici)1097-0215(19990827)82:5<765::aid-ijc23>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Loberg R, Liao J, Ying C, Snyder LA, Pienta KJ, et al. A destructive cascade mediated by CCL2 facilitates prostate cancer growth in bone. Cancer Res. 2009 Feb 15;69(4):1685–92. doi: 10.1158/0008-5472.CAN-08-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loberg RD, Ying C, Craig M, Yan L, Snyder LA, Pienta KJ. CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia. 2007 Jul;9(7):556–62. doi: 10.1593/neo.07307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008 Aug 28;267(2):271–85. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 35.Aggarwal R, Ghobrial IM, Roodman GD. Chemokines in multiple myeloma. Exp Hematol. 2006 Oct;34(10):1289–95. doi: 10.1016/j.exphem.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000 Aug;6(8):3282–9. [PubMed] [Google Scholar]
- 37.Hong KH, Ryu J, Han KH. Monocyte chemoattractant protein-1-induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood. 2005 Feb 15;105(4):1405–7. doi: 10.1182/blood-2004-08-3178. [DOI] [PubMed] [Google Scholar]
- 38.Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000 Mar 23;404(6776):407–11. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- 39.Kelly PM, Davison RS, Bliss E, McGee JO. Macrophages in human breast disease: a quantitative immunohistochemical study. Br J Cancer. 1988 Feb;57(2):174–7. doi: 10.1038/bjc.1988.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006 Apr;42(6):717–27. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Stephens TC, Currie GA, Peacock JH. Repopulation of gamma-irradiated Lewis lung carcinoma by malignant cells and host macrophage progenitors. Br J Cancer. 1978 Nov;38(5):573–82. doi: 10.1038/bjc.1978.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006 Jan 15;66(2):605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 43.Negus RP, Stamp GW, Hadley J, Balkwill FR. Quantitative assessment of the leukocyte infiltrate in ovarian cancer and its relationship to the expression of C-C chemokines. Am J Pathol. 1997 May;150(5):1723–34. [PMC free article] [PubMed] [Google Scholar]
- 44.Azenshtein E, Luboshits G, Shina S, Neumark E, Shahbazian D, Weil M, et al. The CC chemokine RANTES in breast carcinoma progression: regulation of expression and potential mechanisms of promalignant activity. Cancer Res. 2002 Feb 15;62(4):1093–102. [PubMed] [Google Scholar]
- 45.Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunol Today. 1992 Jul;13(7):265–70. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- 46.Morrissey C, Kostenuik PL, Brown LG, Vessella RL, Corey E. Host-derived RANKL is responsible for osteolysis in a C4-2 human prostate cancer xenograft model of experimental bone metastases. BMC Cancer. 2007;7:148. doi: 10.1186/1471-2407-7-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mizutani K, Sud S, Pienta KJ. Prostate cancer promotes CD11b positive cells to differentiate into osteoclasts. J Cell Biochem. 2009 Mar 1;106(4):563–9. doi: 10.1002/jcb.22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daly C, Rollins BJ. Monocyte chemoattractant protein-1 (CCL2) in inflammatory disease and adaptive immunity: therapeutic opportunities and controversies. Microcirculation. 2003 Jun;10(3-4):247–57. doi: 10.1038/sj.mn.7800190. [DOI] [PubMed] [Google Scholar]
- 49.Musso T, Cappello P, Stornello S, Ravarino D, Caorsi C, Otero K, et al. IL-10 enhances CCL2 release and chemotaxis induced by CCL16 in human monocytes. Int J Immunopathol Pharmacol. 2005 Apr-Jun;18(2):339–49. doi: 10.1177/039463200501800216. [DOI] [PubMed] [Google Scholar]
- 50.Loetscher P, Seitz M, Baggiolini M, Moser B. Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J Exp Med. 1996 Aug 1;184(2):569–77. doi: 10.1084/jem.184.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Viola A, Contento RL, Molon B. T cells and their partners: The chemokine dating agency. Trends Immunol. 2006 Sep;27(9):421–7. doi: 10.1016/j.it.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 52.Karpus WJ, Lukacs NW, Kennedy KJ, Smith WS, Hurst SD, Barrett TA, Differential CC. chemokine-induced enhancement of T helper cell cytokine production. J Immunol. 1997 May 1;158(9):4129–36. [PubMed] [Google Scholar]
- 53.Rollins BJ, Pober JS. Interleukin-4 induces the synthesis and secretion of MCP-1/JE by human endothelial cells. Am J Pathol. 1991 Jun;138(6):1315–9. [PMC free article] [PubMed] [Google Scholar]
- 54.Gu L, Rutledge B, Fiorillo J, Ernst C, Grewal I, Flavell R, et al. In vivo properties of monocyte chemoattractant protein-1. J Leukoc Biol. 1997 Nov;62(5):577–80. doi: 10.1002/jlb.62.5.577. [DOI] [PubMed] [Google Scholar]
- 55.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001 Sep 3;194(5):629–44. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999 Oct 4;190(7):995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate costimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000 Jun;30(6):1538–43. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 58.Ermann J, Szanya V, Ford GS, Paragas V, Fathman CG, Lejon K. CD4(+)CD25(+) T cells facilitate the induction of T cell anergy. J Immunol. 2001 Oct 15;167(8):4271–5. doi: 10.4049/jimmunol.167.8.4271. [DOI] [PubMed] [Google Scholar]
- 59.Nagai H, Horikawa T, Hara I, Fukunaga A, Oniki S, Oka M, et al. In vivo elimination of CD25+ regulatory T cells leads to tumor rejection of B16F10 melanoma, when combined with interleukin-12 gene transfer. Exp Dermatol. 2004 Oct;13(10):613–20. doi: 10.1111/j.0906-6705.2004.00198.x. [DOI] [PubMed] [Google Scholar]
- 60.Mahnke K, Schonfeld K, Fondel S, Ring S, Karakhanova S, Wiedemeyer K, et al. Depletion of CD4+CD25+ human regulatory T cells in vivo: kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int J Cancer. 2007 Jun 15;120(12):2723–33. doi: 10.1002/ijc.22617. [DOI] [PubMed] [Google Scholar]
- 61.Jordan JT, Sun W, Hussain SF, DeAngulo G, Prabhu SS, Heimberger AB. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother. 2008 Jan;57(1):123–31. doi: 10.1007/s00262-007-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006 Feb;16(1):53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 63.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007 Jun 20;25(18):2546–53. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 64.Liu C, Yu S, Kappes J, Wang J, Grizzle WE, Zinn KR, et al. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood. 2007 May 15;109(10):4336–42. doi: 10.1182/blood-2006-09-046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim JH, Dhanasekaran SM, Mehra R, Tomlins SA, Gu W, Yu J, et al. Integrative analysis of genomic aberrations associated with prostate cancer progression. Cancer Res. 2007 Sep 1;67(17):8229–39. doi: 10.1158/0008-5472.CAN-07-1297. [DOI] [PubMed] [Google Scholar]
- 66.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007 Oct 15;67(20):10019–26. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005 Oct 3;202(7):931–9. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang B, Lei Z, Zhao J, Gong W, Liu J, Chen Z, et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 2007 Jul 8;252(1):86–92. doi: 10.1016/j.canlet.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 69.Herman JG, Stadelman HL, Roselli CE. Curcumin blocks CCL2-induced adhesion, motility and invasion, in part, through down-regulation of CCL2 expression and proteolytic activity. Int J Oncol. 2009 May;34(5):1319–27. [PMC free article] [PubMed] [Google Scholar]
- 70.Valta MP, Tuomela J, Vuorikoski H, Loponen N, Vaananen RM, Pettersson K, et al. FGF-8b induces growth and rich vascularization in an orthotopic PC-3 model of prostate cancer. J Cell Biochem. 2009 May 4; doi: 10.1002/jcb.22175. [DOI] [PubMed] [Google Scholar]