Abstract
The central mechanism of fever induction is triggered by an action of prostaglandin E2 (PGE2) on neurons in the preoptic area (POA) through the EP3 subtype of prostaglandin E receptor. EP3 receptor (EP3R)-expressing POA neurons project directly to the dorsomedial hypothalamus (DMH) and to the rostral raphe pallidus nucleus (rRPa), key sites for the control of thermoregulatory effectors. Based on physiological findings, we hypothesize that the febrile responses in brown adipose tissue (BAT) and those in cutaneous vasoconstrictors are controlled independently by separate neuronal pathways: PGE2 pyrogenic signaling is transmitted from EP3R-expressing POA neurons via a projection to the DMH to activate BAT thermogenesis and via another projection to the rRPa to increase cutaneous vasoconstriction. In this case, DMH-projecting and rRPa-projecting neurons would constitute segregated populations within the EP3R-expressing neuronal group in the POA. Here, we sought direct anatomical evidence to test this hypothesis with a double-tracing experiment in which two types of the retrograde tracer, cholera toxin b-subunit (CTb), conjugated with different fluorophores were injected into the DMH and the rRPa of rats and the resulting retrogradely labeled populations of EP3R-immunoreactive neurons in the POA were identified with confocal microscopy. We found substantial numbers of EP3R-immunoreactive neurons in both the DMH-projecting and the rRPa-projecting populations. However, very few EP3R-immunoreactive POA neurons were labeled with both the CTb from the DMH and that from the rRPa, although a substantial number of neurons that were not immunoreactive for EP3R were double-labeled with both CTbs. The paucity of the EP3R-expressing neurons that send collaterals to both the DMH and the rRPa suggests that pyrogenic signals are sent independently to these caudal brain regions from the POA and that such pyrogenic outputs from the POA reflect different control mechanisms for BAT thermogenesis and for cutaneous vasoconstriction by distinct sets of POA neurons.
Keywords: GABA, inflammation, rostral medullary raphe, stress response, sympathetic nervous system, thermoregulation
Fever is a host-defense response that is governed by a brain mechanism triggered by an action of prostaglandin E2 (PGE2), which is mostly produced in the brain vasculature in response to immune signals caused by infections (Elmquist et al., 1997; Matsumura et al., 1998; Yamagata et al., 2001). The EP3 subtype of prostaglandin E receptor is abundantly expressed on neurons in specific subregions of the preoptic area (POA): the median preoptic nucleus (MnPO) and medial preoptic area (MPO) (Nakamura et al., 1999, 2000), and most of these neurons contain the inhibitory neurotransmitter, GABA (Nakamura et al., 2002). The action of PGE2 on these EP3 receptors (EP3Rs), likely an inhibition of EP3R-expressing POA neurons (Narumiya et al., 1999), is responsible for the activation of fever-producing neuronal pathways (Lazarus et al., 2007), although EP3Rs in the POA could also be involved in other physiological functions including thermal hyperalgesia (Hosoi et al., 1997).
The POA contains EP3R-expressing neurons that project directly to the dorsomedial hypothalamus (DMH) and to the rostral raphe pallidus nucleus (rRPa) (Nakamura et al., 2002, 2005b), brain regions that provide excitatory drive for the sympathetically-regulated thermal effectors that are essential for eliciting febrile responses, such as brown adipose tissue (BAT) and cutaneous blood vessels (Nakamura, 2004; Morrison et al., 2008). In particular, the rRPa contains sympathetic premotor neurons that are activated in response to central PGE2 administration and that multi-synaptically control BAT or cutaneous blood vessels such as those in rat tail through their direct innervation of sympathetic preganglionic neurons in the spinal cord (Nakamura et al., 2004, 2005a). Thus, inhibition of neuronal activity in the rRPa blocks heat production in BAT that is evoked by PGE2 action in the POA (Nakamura et al., 2002; Madden et al., 2003; Rathner et al., 2008) and largely attenuates PGE2-evoked cutaneous vasoconstriction in rat tail (Rathner et al., 2008), a sympathetic response to restrict body heat loss and increase body temperature. Intriguingly, inhibition of DMH neurons blocks BAT thermogenesis evoked by PGE2 in the POA (Zaretskaia et al., 2003; Madden et al., 2004; Nakamura et al., 2005b; Rathner et al., 2008), but has no effect on PGE2-evoked cutaneous vasoconstriction (Rathner et al., 2008).
These findings led us to hypothesize (Nakamura, 2004; Nakamura et al., 2005b) that pyrogenic signaling in the POA, i.e. PGE2-mediated inhibition of EP3R-expressing projection POA neurons, increases BAT thermogenesis by removal of direct inhibitory signaling from EP3R-expressing POA neurons to DMH neurons, which would then, in turn, lead to activation of rRPa sympathetic premotor neurons controlling BAT. Similarly, pyrogenic signaling in the POA would stimulate cutaneous vasoconstriction by inhibiting direct inhibitory signaling from EP3R-expressing POA neurons to rRPa sympathetic premotor neurons controlling skin blood vessels (Rathner et al., 2008).
In the present study, we tested the hypothesis that the EP3R-expressing neurons that project to the DMH and to the rRPa constitute segregated populations in POA neurons, potentially mediating differential control of BAT thermogenesis and cutaneous vasoconstriction, respectively, during febrile and cold-defense responses. To this end, we performed a double-tracing experiment in which two types of the retrograde tracer, cholera toxin b-subunit (CTb) conjugated with different fluorophores, were injected into the DMH and the rRPa and the resulting CTb labeling in EP3R-immunoreactive neurons in the POA was analyzed with confocal microscopy.
Experimental Procedures
Animals
All experimental animal protocols were reviewed and approved by Animal Care and Use Committee of Oregon Health and Science University and conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering. Male Sprague-Dawley rats (200–250 g, Charles River, Indianapolis, IN, USA) were housed two per cage with ad libitum access to feed and water in a room air-conditioned at 24±2 °C with a standard 12-h light/dark cycle.
CTb injection
Rats were deeply anesthetized with chloral hydrate (280 mg/kg i.p.) following introductory gas anesthesia with 3% isoflurane and placed in a stereotaxic apparatus; the incisor bar level was adjusted so that bregma and lambda were at the same dorsal level. Unilateral pressure-injections (Picospritzer II, General Valve, Fairfield, NJ, USA) of Alexa488-conjugated CTb and Alexa594-conjugated CTb (1 mg/ml, 240–280 nl; Molecular Probes, Eugene, OR, USA) were made via glass micropipettes (tip inner diameter: 10–20 μm) stereotaxically positioned in the DMH and in the rRPa, respectively. The coordinates for the DMH injections, which targeted the region combining the dorsal hypothalamic area and the dorsomedial hypothalamic nucleus (see Fig. 1A), were 3.3 mm caudal to bregma, 0.3–0.7 mm lateral to the midline and 8.0–8.5 mm ventral to the brain surface, and those for the injections targeting the rRPa were 11.6 mm caudal to bregma, on the midline and 9.5–9.7 mm ventral to the brain surface. Three to six days later, the animals were re-anesthetized and perfused transcardially with 200 ml of a 0.9% sodium chloride solution followed by 250–300 ml of 4% formaldehyde in 0.1 M sodium phosphate buffer (pH 7.4). Subsequently, the brain was post-fixed at 4 °C for 2 h and then cryoprotected with 30% sucrose in 10 mM sodium phosphate buffer (pH 7.4) overnight. The tissues were cut into 25 μm-thick frontal sections on a freezing microtome. The sites of CTb injections were identified with an epifluorescence microscope.
Fig. 1.
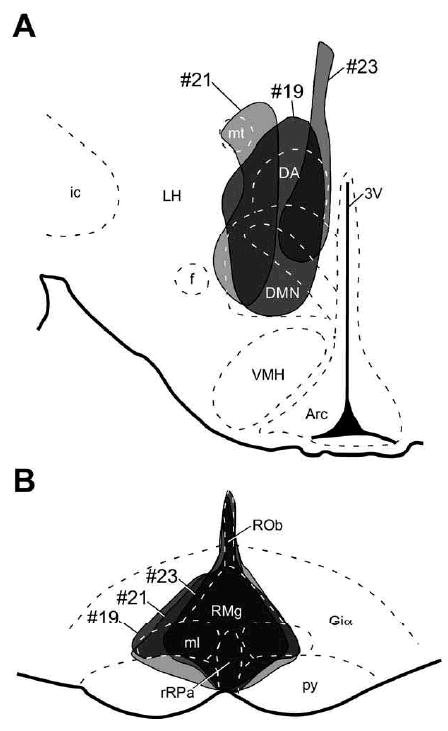
Sites of CTb injections. (A) Alexa488-conjugated CTb and Alexa594-conjugated CTb were injected into the DMH (A; bregma, −3.30 mm) and into the rRPa (B; bregma, −11.80 mm), respectively. Areas where injected CTb spread are shown on the brain maps adopted from an atlas of Paxinos and Watson (1998). Injections in the three animals (#19, #21 and #23) that were used for histochemical analyses are shown. 3V, Third ventricle; Arc, arcuate nucleus; DA, dorsal hypothalamic area; DMN, dorsomedial hypothalamic nucleus; f, fornix; Giα, alpha part of the gigantocellular reticular nucleus; ic, internal capsule; LH, lateral hypothalamic area; ml, medial lemniscus; mt, mammillothalamic tract; py, pyramidal tract; ROb, raphe obscurus nucleus; RMg, raphe magnus nucleus; VMH, ventromedial hypothalamic nucleus.
The post-injection survival time of longer than 3 days was adopted from previous retrograde tracing with CTb in rats (Li et al., 1995; Nakamura et al., 2005b; Nakamura and Morrison, 2008a) and we found no obvious difference in the numbers of CTb-labeled neurons in the POA between the survival times of 3 and 6 days. Out of 23 rats that received the double CTb injections in the present study, three rats had CTb injections centered at both the DMH and the rRPa sites that have been reported to mediate febrile signals from the POA: inactivation of neurons in these regions blocks febrile thermogenic responses to PGE2 into the POA (Nakamura et al., 2002, 2005b). Brain sections of these three rats were subjected to subsequent histological analyses. Prior to confocal microscopy, the other 20 animals were excluded from the present immunohistochemical analyses, since their CTb injections missed or were not centered on either or both of the DMH and rRPa sites.
Immunohistochemistry
The sections containing the POA were incubated overnight in our conventional primary antibody incubation buffer (Nakamura et al., 2000, 2001) containing anti-rat EP3R rabbit polyclonal antibody (0.5 μg/ml; Nakamura et al., 1999, 2000). After rinses in 50 mM phosphate-buffered saline (PBS; pH 7.3) containing 0.3% Triton X-100 (PBS-T), the sections were incubated for 1 h with 10 μg/ml horseradish peroxidase–conjugated anti-rabbit IgG donkey antibody (Chemicon, Temecula, CA, USA) in azide-free PBS-T containing 10% normal goat serum. After rinses in PBS, the sections were incubated for 1 min with biotin-labeled tyramide (Tyramide Signal Amplification Biotin Systems; 1:50; PerkinElmer, Boston, MA, USA) according to the manufacturer's manual and then for 1 h with 5 μg/ml Alexa647-conjugated streptavidin (Molecular Probes) in PBS-T. The sections were then thoroughly washed in PBS-T and mounted onto glass slides.
Confocal microscopy and data analysis
The fluorescence signals for CTb and EP3R immunoreactivity in the sections were detected under a confocal laser-scanning microscope (LSM 510; Zeiss, Oberkochen, Germany). Using the z-stacking function for serial confocal sectioning at 1.7-μm intervals, the images of all CTb-labeled neuronal cell bodies were captured in every sixth section throughout EP3R-immunoreactive regions in the POA and the existence of EP3R immunoreactivity in each CTb-labeled cell was assessed. The identified CTb-labeled cells, with or without EP3R immunoreactivity, were counted and localized on brain maps. Within CTb-labeled cell bodies, the fluorescence signal for CTb accumulated in perinuclear structures, but not in cell nuclei. Therefore, only fluorescence-labeled cell bodies with unlabeled nuclei were counted and mapped and cellular fragments not associated with cell nuclei were not identified as cell bodies. All data are presented as means±SEM and statistical significance was evaluated as needed with a two-tailed paired t-test. The cytoarchitecture and nomenclature of Paxinos and Watson (1998) were adopted in most brain regions, except for the definitions of the MnPO and MPO, which were adopted from a rat brain atlas of Swanson (1992).
Results
Following injections of CTbs conjugated with different fluorophores into the DMH and the rRPa (Fig. 1), many neuronal cell bodies in the POA were retrogradely labeled with CTb (Figs. 2 and 3). Neurons labeled with cholera toxin b-subunit derived from the dorsomedial hypothalamus (DMH-CTb) and those labeled with cholera toxin b-subunit derived from the rostral raphe pallidus nucleus (rRPa-CTb) showed similar distributions in the MnPO and MPO, both of which overlapped EP3R-immunoreactive regions in the POA (Fig. 3). The distributions of these CTb-labeled neuronal populations were consistent with the previously reported distributions of POA neurons projecting to the DMH and those projecting to the rRPa (Hermann et al., 1997; Thompson and Swanson, 1998).
Fig. 2.
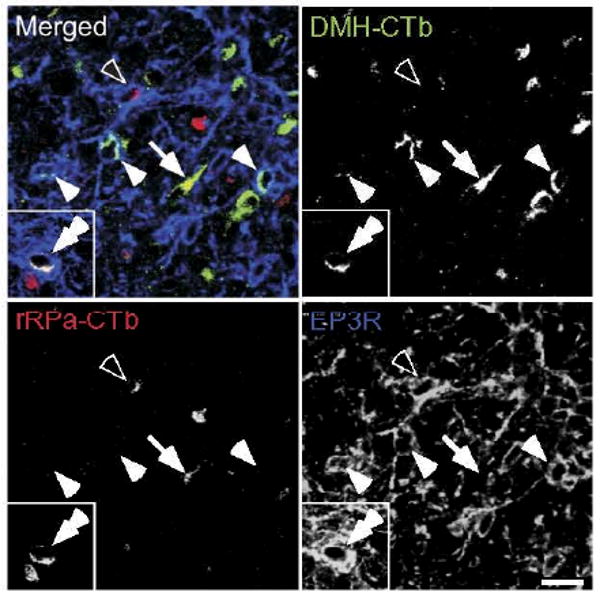
Confocal images of CTb-labeled POA neurons with or without EP3R immunoreactivity. Fluorescence signals of Alexa488-conjugated CTb (transported from the DMH; green), fluorescence signals of Alexa594-conjugated CTb (transported from the rRPa; red) and immunoreactivity for EP3Rs (blue) are pseudocolored and merged. EP3R-immunoreactive neurons that were labeled with DMH-CTb (filled single arrowhead), EP3R-immunoreactive neurons that were labeled with rRPa-CTb (open arrowhead) and neurons that were labeled with both DMH-CTb and rRPa-CTb but lack EP3R immunoreactivity (arrow) were densely distributed in the MnPO and MPO. However, EP3R-immunoreactive neurons that were labeled with both DMH-CTb and rRPa-CTb (inset, double arrowhead) were hardly found. Scale bar=20 μm.
Fig. 3.
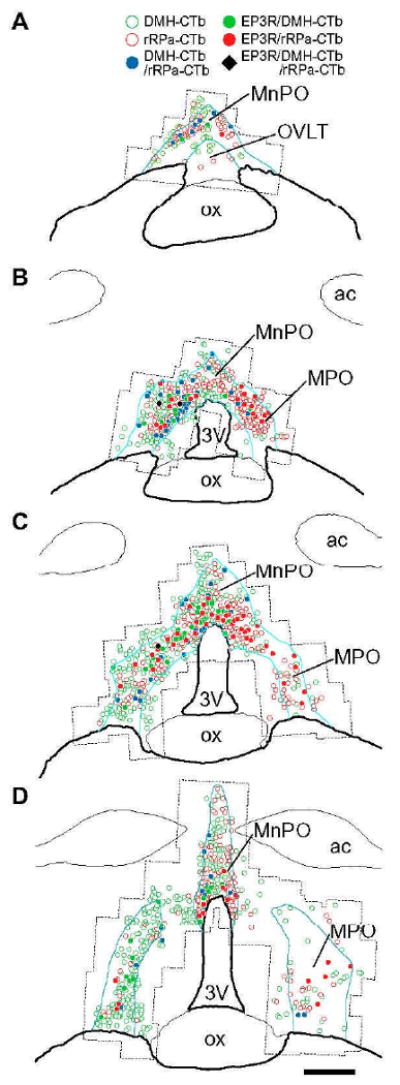
Distribution of CTb-labeled neuronal cell bodies with or without EP3R immunoreactivity in the POA. Drawings of POA sections from the animal #21 are shown in a rostrocaudal order (A–D). Blue lines delineate EP3R-immunoreactive regions. Dotted lines outline the areas that were scanned with a confocal microscope. The left side is ipsilateral to the CTb injection into the DMH. ac, Anterior commissure; ox, optic chiasm. Scale bar=0.5 mm.
Confocal microscopy revealed EP3R immunoreactivity within both the POA neuronal population labeled with DMH-CTb and that labeled with rRPa-CTb (Fig. 2), consistent with our previous results (Nakamura et al., 2002, 2005b). In 808±12 cells (mean±SEM; n=3) that were labeled with DMH-CTb, 59±7 cells (7.3%±0.9%) exhibited EP3R immunoreactivity and in 541±110 cells that were labeled with rRPa-CTb, 67±9 cells (12.8%±0.9%) exhibited EP3R immunoreactivity. We also found a population (78±6 cells) of POA neurons that were labeled with both DMH-CTb and rRPa-CTb (Fig. 2), which constituted 9.7%±0.8% of DMH-CTb-labeled POA neurons and 15.3%±2.0% of rRPa-CTb-labeled POA neurons. In the neuronal population that was labeled with both CTbs, however, we found only 4±1 cells that were immunopositive for EP3R (Fig. 2, inset), which constituted less than 1% of either the DMH-CTb-labeled or rRPa-CTb-labeled POA neurons.
We analyzed the distribution of EP3R-immunoreactive, CTb-labeled neurons within the POA. EP3R-immunoreactive neurons that were labeled with either DMH-CTb or rRPa-CTb were densely localized dorsal and dorsolateral to the third ventricle at the rostrocaudal levels at which the EP3R-immunoreactive MnPO and MPO regions are fused together (Fig. 3B, C). Although a small region surrounding the organum vasculosum of the lamina terminalis (OVLT), located at the rostral end of the EP3R-immunoreactive POA region, has been described as a PGE2-sensitive site for eliciting fever (Matsuda et al., 1992; Osaka, 2008), this region contained only a few EP3R-immunoreactive neurons that were labeled with CTb from either injection site (Fig. 3A).
Unilateral CTb injection into the DMH retrogradely labeled more neurons in the ipsilateral MPO (333±48 cells) than those in the contralateral MPO (53±4 cells), indicating a predominance of ipsilateral projections from the POA to the DMH, The MnPO, which is centered on the midline, contained 422±41 cells that were labeled with DMH-CTb. The DMH-CTb-labeled neuronal populations in the MnPO and the ipsilateral MPO contained comparable numbers of EP3R-immunoreactive neurons (MnPO: 30±6 cells, 7.2%±1.4%; ipsilateral MPO: 27±6 cells, 7.8%±0.7%; P>0.05, paired t-test; Fig. 4). Since CTb injection into the midline rRPa resulted in retrograde labeling of a comparable number of neurons in either side of the MPO (MPO ipsilateral to CTb injection into the DMH: 139±35 cells; contralateral MPO: 120±18 cells), the numbers of these CTb-labeled neurons in both sides of the MPO were combined (259±52 cells). Although a comparable number of MnPO neurons were also labeled with rRPa-CTb (281±64 cells), more rRPa-CTb-labeled neurons in the MPO exhibited EP3R immunoreactivity (43±6 cells) than those in the MnPO (24±6 cells), resulting in the significantly higher percentage of EP3R-immunoreactive population in the rRPa-CTb-labeled MPO neurons than that in the rRPa-CTb-labeled MnPO neurons (MPO: 17.0%±1.4%; MnPO: 8.6%±0.7%; P<0.05, paired t-test; Fig. 4).
Fig. 4.
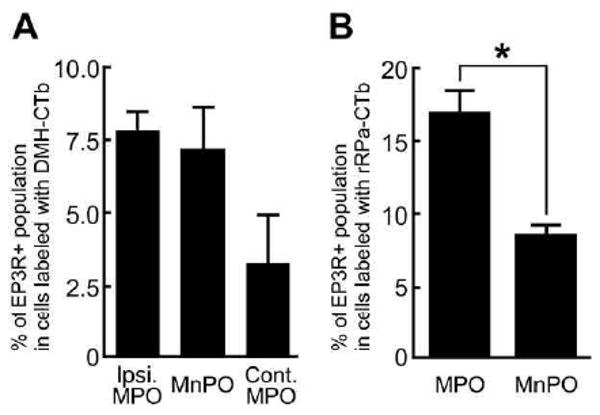
Percentages of EP3R-immunoreactive neurons in DMH-CTb-labeled (A) and rRPa-CTb-labeled (B) populations in POA subregions. CTb-labeled cells in the EP3R-immunoreactive regions were counted in every sixth 25-mm-thick frontal section throughout the POA. The POA was divided into three subregions in (A): the MnPO and the MPO ipsilateral (Ipsi. MPO) and contralateral (Cont. MPO) to the unilateral CTb injection into the DMH, and into two subregions in (B): the MnPO and the MPO. The data represent the mean±SEM (n=3). * P<0.05, paired t-test.
Discussion
The major finding in the present study is that although the DMH and the rRPa receive projections from many POA neurons that express prostaglandin EP3Rs, only a very small number of EP3R-expressing POA neurons project to both the DMH and the rRPa. In light of the critical role of EP3Rs in POA neurons as a sensor of the pyrogenic mediator, PGE2 (Lazarus et al., 2007), the present result indicates that fever signaling to the DMH and to the rRPa from the POA is mediated by separate populations of EP3R-expressing neurons.
In the present study, EP3R immunoreactivity was used as a marker to judge whether the CTb-labeled cell bodies of DMH- and/or rRPa-projecting POA neurons would have been receptive to the pyrogenic mediator, PGE2. However, due to the dense localization of EP3R-immunoreactive cell bodies and dendritic processes in the POA, especially in the MnPO, we could not use the EP3R immunoreactivity itself to convincingly identify each separate EP3R-expressing cell body and thus, we could not provide a count of the total number of EP3R-immunoreactive POA neurons. A substantial population of POA neurons that were negative for EP3R immunoreactivity was labeled with both DMH-CTb and rRPa-CTb. This result minimizes the likelihood that the paucity of EP3R-immunoreactive POA neurons that were labeled with both DMH-CTb and rRPa-CTb was due to an intracellular or transport mechanism in POA neurons that specifically prevented the simultaneous uptake or transport of both tracers, although we cannot eliminate the possibility that such a mechanism exists only in EP3R-expressing POA neurons. Although the physiological function of the EP3R-immunonegative POA neurons that project to both the DMH and the rRPa is currently unknown, these neurons could provide a substrate for synchronization of DMH and rRPa neuronal activity.
The present mapping with confocal microscopy provides more detailed information on the distribution of EP3R-expressing POA neurons projecting to either DMH or rRPa than did our previous single-tracing studies with epifluorescence microscopy (Nakamura et al., 2002, 2005b) and revealed that these neurons are densely distributed in the MnPO/MPO region dorsal and dorsolateral to the third ventricle especially at the rostrocaudal levels where the EP3R-immunoreactive MnPO and MPO regions are fused together (Fig. 3B, C). A similar distribution is shown by POA neurons multi-synaptically innervating the interscapular BAT, about 40% of which express EP3Rs (Yoshida et al., 2003). Physiologically, this is the same POA region that is highly sensitive to the pyrogenic action of PGE2 (Scammell et al., 1996) and inhibition of neurons in the MPO subregion dorsolateral to the third ventricle causes thermogenesis (Osaka, 2004), although its effect on cutaneous vasoconstrictors is unknown.
EP3R-expressing, DMH-projecting neurons appeared to be distributed more evenly between the MnPO and MPO regions than did those projecting to the rRPa, which were more concentrated in the MPO than in the MnPO. This difference in the distributions of EP3R-expressing neurons may reflect differential topographies of the POA regions controlling thermoregulatory effectors in fever development. The dorsal cap of the OVLT at the rostral end of the POA (Fig. 3A) is also sensitive to the pyrogenic actions of PGE2 (Scammell et al., 1996; Osaka, 2008). Although we observed many EP3R-immunoreactive dendritic processes and some cell bodies localized in this region, only a few of these EP3R-expressing neurons projected to either DMH or rRPa. Thus, the pyrogenic action of PGE2 in this region might be mediated by EP3Rs on the dendrites of DMH- or rRPa-projecting, EP3R-expressing neurons that are distributed more caudally in the MnPO/MPO region dorsal and dorsolateral to the third ventricle.
Inhibition of neuronal activity either in the DMH or in the rRPa blocks BAT thermogenesis evoked by an action of PGE2 in the POA (Nakamura et al., 2002, 2005b; Madden et al., 2003, 2004; Zaretskaia et al., 2003; Rathner et al., 2008). In contrast, the cutaneous vasoconstriction stimulated by PGE2 in the POA is largely attenuated by inhibiting rRPa neurons, but is unaffected by inhibition of neurons in the DMH (Rathner et al., 2008). Together, these results have led us to propose that pyrogenic signaling in the POA, specifically the PGE2-mediated inhibition of warm-sensitive POA neurons (Schoener and Wang, 1976; Ranels and Griffin, 2003), leads to activation of BAT thermogenesis by disinhibition of sympathoexcitatory neurons in the DMH that drive BAT sympathetic premotor neurons in the rRPa (Nakamura, 2004; Morrison et al., 2008) and to a parallel increase in cutaneous vasoconstrictor sympathetic outflow through disinhibition of cutaneous vasoconstrictor sympathetic premotor neurons in the rRPa, i.e. without involvement of neurons in the DMH (Rathner et al., 2008). The present results identify potential anatomical substrates for the POA–DMH and POA–rRPa pathways, the reduction of whose inhibitory control of sympathoexcitatory neurons in the DMH and the rRPa is key to the development of the febrile response to PGE2 in the POA.
It is clear from the model that we have proposed for the central pathways underlying the febrile response and cold-defense responses (Nakamura, 2004; Morrison et al., 2008) that the POA–DMH and the POA–rRPa projection neurons are expected to exert a tonic GABAergic inhibition of sympathoexcitatory target neurons in the DMH and in the rRPa. Evidence for such tonic, GABAergic inhibitory pathways controlling BAT and cutaneous vasoconstrictors includes the findings (a) that transections of descending outputs from the POA induce BAT thermogenesis and cutaneous vasoconstriction (Chen et al., 1998; Rathner et al., 2008), (b) that the majority of EP3R-expressing POA neurons contain GABA (Nakamura et al., 2002) and (c) that GABAergic POA neurons innervate DMH neurons projecting to the rRPa (Nakamura et al., 2005b). Furthermore, blockade of tonic GABAergic inputs to the DMH evokes BAT thermogenesis (Zaretskaia et al., 2002; Cao et al., 2004) and blockade of GABAergic inputs to the rRPa reverses cutaneous vasodilation evoked by warming the POA (Tanaka et al., 2002). However, the GABAergic phenotype of the distinct populations of POA neurons described in the present study that express the EP3R and project to the DMH and/or the rRPa remains to be demonstrated directly.
Similarly to their role in febrile responses, BAT thermogenesis and cutaneous vasoconstriction also occur as cold-defense responses when animals are exposed to environments of reduced temperatures and here as well, neurons in the POA play a pivotal role in controlling the activation of these thermal effectors. Similar to the effect of PGE2 in the POA, the discharge of projection POA neurons controlling BAT thermogenesis and cutaneous vasoconstriction is probably altered by the integrated cool sensory signals from the skin and from other thermoreceptive sites (Boulant and Hardy, 1974; Romanovsky, 2007; Morrison et al., 2008). Indeed, the finding that cool sensory signals delivered from the skin to the POA via neurons in the lateral parabrachial nucleus (Nakamura and Morrison, 2008a) likely inhibit warm-sensitive POA neurons through GABAergic interneurons in the MnPO (Nakamura and Morrison, 2008b) suggests a strong similarity to the inhibition of POA neuronal activity expected from the binding of PGE2 to EP3Rs (Narumiya et al., 1999) on POA neurons. The findings that inhibitors of cyclooxygenase, an enzyme producing prostaglandins, do not affect cold-induced cutaneous vasoconstriction in humans (Hechtman and Jageneau, 1985) and that EP3R-knockout mice can maintain their basal body temperature with normal circadian changes (Oka et al., 2003) indicate that the PGE2-EP3R mechanism is not required for the cold-defense responses that contribute to the maintenance of basal body temperature, but do not mean that EP3R-expressing POA neurons themselves are not involved in these non-febrile thermoregulatory functions. Thus, PGE2- or cooling-mediated attenuation of the tonic GABAergic signaling from POA neurons, including those that express EP3R, that project to the sympathoexcitatory neurons in the DMH and in the rRPa seems to be a key event in eliciting febrile and cold-defense responses including increases in BAT thermogenesis and cutaneous vasoconstriction.
As with the febrile responses to PGE2 in the POA, the cooling-evoked increase in sympathetic outflow to BAT and the resulting BAT thermogenesis are blocked by inhibiting neurons in either the DMH or the rRPa (Nakamura and Morrison, 2007) and body temperature-dependent outflow to cutaneous vasoconstrictors is eliminated by inhibiting rRPa neurons, but is unaffected by inhibiting DMH neurons (Rathner et al., 2008). These findings support the view that the cold-defensive responses in BAT and in cutaneous vasoconstrictors, as with their febrile responses, are differentially controlled by separate sets of inhibitory projection POA neurons, potentially those target-specific projection POA neurons expressing EP3Rs revealed in the present study. The finding that during cooling, the sympathetic nerves to BAT and those to the rat tail skin vasculature are activated at different threshold temperatures (Ootsuka and McAllen, 2006) is also consistent with a model including effector-specific populations of warm-sensitive projection POA neurons. To date, however, there is no direct evidence to indicate whether a single population of EP3R-expressing, warm-sensitive POA neurons mediates both the cold-defense and the febrile responses in each thermal effector. It may be that for each thermal effector, the populations of POA neurons controlling its febrile and cold-defense responses are different, the latter perhaps represented by the EP3R-immunonegative neurons projecting to the DMH or to the rRPa which were found abundantly in the present study.
While our previous studies and our models for central febrile and cold-defense pathways have focused on the control of BAT thermogenesis and cutaneous vasoconstriction, other thermoregulatory effectors, such as skeletal muscles for shivering, arrector pili muscles for heat conservation and sweat and salivary glands and the respiratory system for evaporative heat dissipation, make important contributions to febrile responses and are likely to be differentially activated in the course of thermoregulatory responses, including cold defense. Although little is known about the central efferent pathways controlling the thermal responses of these effectors, it is reasonable to assume that projection POA neurons play a key role in controlling their activation during fever and during thermoregulatory responses. Thus, if neurons in the DMH or those in the rRPa are involved in determining the activation of the neural outflows to these organs, some of the neurons within the EP3R-expressing projection pathways from the POA revealed in the present study would also be expected to contribute to their febrile responses.
Conclusion
In conclusion, the results from the present anatomical study using a molecular marker for pyrogen-receptive neurons indicate that separate sets of projection neurons mediate pyrogenic signaling from the POA to neurons in two different caudal brain sites: the DMH and the rRPa. These separate outflow pathways from the POA potentially mediate differential inhibitory control of the sympathoexcitatory drive determining BAT thermogenesis and cutaneous vasoconstriction during fever development, and possibly during cold defense. Such differential control of multiple effectors by distinct populations of projection neurons in the POA is important for eliciting appropriate thermoregulatory responses contributing to survival from infection and to compensation for a wide variety of thermal environments.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants NS40987 and DK57838 to S.F.M. K.N. was a fellow for research abroad supported by the Japan Society for the Promotion of Science. Acquisition of confocal images was supported by NIH instrumentation grant RR016858.
Abbreviations
- BAT
brown adipose tissue
- CTb
cholera toxin b-subunit
- DMH
dorsomedial hypothalamus
- DMH-CTb
cholera toxin b-subunit derived from the dorsomedial hypothalamus
- EP3R
EP3 receptor
- MnPO
median preoptic nucleus
- MPO
medial preoptic area
- OVLT
organum vasculosum of the lamina terminalis
- PBS
phosphate-buffered saline
- PBS-T
phosphate-buffered saline containing Triton X-100
- PGE2
prostaglandin E2
- POA
preoptic area
- rRPa
rostral raphe pallidus nucleus
- rRPa-CTb
cholera toxin b-subunit derived from the rostral raphe pallidus nucleus
References
- Boulant JA, Hardy JD. The effect of spinal and skin temperatures on the firing rate and thermosensitivity of preoptic neurones. J Physiol. 1974;240:639–660. doi: 10.1113/jphysiol.1974.sp010627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience. 2004;126:229–240. doi: 10.1016/j.neuroscience.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Chen XM, Hosono T, Yoda T, Fukuda Y, Kanosue K. Efferent projection from the preoptic area for the control of non-shivering thermogenesis in rats. J Physiol. 1998;512:883–892. doi: 10.1111/j.1469-7793.1998.883bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, Scammell TE, Saper CB. Mechanisms of CNS response to systemic immune challenge: the febrile response. Trends Neurosci. 1997;20:565–570. doi: 10.1016/s0166-2236(97)01138-7. [DOI] [PubMed] [Google Scholar]
- Hechtman DH, Jageneau A. Inhibition of cold-induced vasoconstriction with ketanserin. Microvasc Res. 1985;30:56–62. doi: 10.1016/0026-2862(85)90037-8. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars α demonstrated by iontophoretic application of choleratoxin (subunit b) J Chem Neuroanat. 1997;13:1–21. doi: 10.1016/s0891-0618(97)00019-7. [DOI] [PubMed] [Google Scholar]
- Hosoi M, Oka T, Hori T. Prostaglandin E receptor EP3 subtype is involved in thermal hyperalgesia through its actions in the preoptic hypothalamus and the diagonal band of Broca in rats. Pain. 1997;71:303–311. doi: 10.1016/s0304-3959(97)03380-0. [DOI] [PubMed] [Google Scholar]
- Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci. 2007;10:1131–1133. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- Li YQ, Takada M, Kaneko T, Mizuno N. Premotor neurons for trigeminal motor nucleus neurons innervating the jaw-closing and jaw-opening muscles: differential distribution in the lower brain-stem of the rat. J Comp Neurol. 1995;356:563–579. doi: 10.1002/cne.903560407. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Excitatory amino acid receptor activation in the raphe pallidus area mediates prostaglandin-evoked thermogenesis. Neuroscience. 2003;122:5–15. doi: 10.1016/s0306-4522(03)00527-x. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Excitatory amino acid receptors in the dorsomedial hypothalamus mediate prostaglandin-evoked thermogenesis in brown adipose tissue. Am J Physiol. 2004;286:R320–R325. doi: 10.1152/ajpregu.00515.2003. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Hori T, Nakashima T. Thermal and PGE2 sensitivity of the organum vasculosum lamina terminalis region and preoptic area in rat brain slices. J Physiol. 1992;454:197–212. doi: 10.1113/jphysiol.1992.sp019260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K, Cao C, Ozaki M, Morii H, Nakadate K, Watanabe Y. Brain endothelial cells express cyclooxygenase-2 during lipopolysaccharide-induced fever: light and electron microscopic immunocytochemical studies. J Neurosci. 1998;18:6279–6289. doi: 10.1523/JNEUROSCI.18-16-06279.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K. Fever-inducing sympathetic neural pathways. J Therm Biol. 2004;29:339–344. [Google Scholar]
- Nakamura K, Kaneko T, Yamashita Y, Hasegawa H, Katoh H, Ichikawa A, Negishi M. Immunocytochemical localization of prostaglandin EP3 receptor in the rat hypothalamus. Neurosci Lett. 1999;260:117–120. doi: 10.1016/s0304-3940(98)00962-8. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kaneko T, Yamashita Y, Hasegawa H, Katoh H, Negishi M. Immunohistochemical localization of prostaglandin EP3 receptor in the rat nervous system. J Comp Neurol. 2000;421:543–569. doi: 10.1002/(sici)1096-9861(20000612)421:4<543::aid-cne6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Li YQ, Kaneko T, Katoh H, Negishi M. Prostaglandin EP3 receptor protein in serotonin and catecholamine cell groups: a double immunofluorescence study in the rat brain. Neuroscience. 2001;103:763–775. doi: 10.1016/s0306-4522(01)00027-6. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Hübschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogköi Z, König M, Thiel HJ, Gerstberger R, Kobayashi S, Kaneko T. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci. 2004;24:5370–5380. doi: 10.1523/JNEUROSCI.1219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci. 2002;22:4600–4610. doi: 10.1523/JNEUROSCI.22-11-04600.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Kobayashi S, Kaneko T. Sympathetic premotor neurons mediating thermoregulatory functions. Neurosci Res. 2005a;51:1–8. doi: 10.1016/j.neures.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Nakamura K, Matsumura K, Kobayashi S, Kaneko T, Morrison SF. Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur J Neurosci. 2005b;22:3137–3146. doi: 10.1111/j.1460-9568.2005.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol. 2007;292:R127–R136. doi: 10.1152/ajpregu.00427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci. 2008a;11:62–71. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. Preoptic mechanism for cold-defensive responses to skin cooling. J Physiol. 2008b;586:2611–2620. doi: 10.1113/jphysiol.2008.152686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Oka T, Oka K, Kobayashi T, Sugimoto Y, Ichikawa A, Ushikubi F, Narumiya S, Saper CB. Characteristics of thermoregulatory and febrile responses in mice deficient in prostaglandin EP1 and EP3 receptors. J Physiol. 2003;551:945–954. doi: 10.1113/jphysiol.2003.048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ootsuka Y, McAllen RM. Comparison between two rat sympathetic pathways activated in cold defense. Am J Physiol. 2006;291:R589–R595. doi: 10.1152/ajpregu.00850.2005. [DOI] [PubMed] [Google Scholar]
- Osaka T. Cold-induced thermogenesis mediated by GABA in the preoptic area of anesthetized rats. Am J Physiol. 2004;287:R306–R313. doi: 10.1152/ajpregu.00003.2004. [DOI] [PubMed] [Google Scholar]
- Osaka T. Prostaglandin E2 fever mediated by inhibition of the GABAergic transmission in the region immediately adjacent to the organum vasculosum of the lamina terminalis. Pflugers Arch. 2008;456:837–846. doi: 10.1007/s00424-007-0443-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Ranels HJ, Griffin JD. The effects of prostaglandin E2 on the firing rate activity of thermosensitive and temperature insensitive neurons in the ventromedial preoptic area of the rat hypothalamus. Brain Res. 2003;964:42–50. doi: 10.1016/s0006-8993(02)04063-5. [DOI] [PubMed] [Google Scholar]
- Rathner JA, Madden CJ, Morrison SF. Central pathway for spontaneous and prostaglandin E2-evoked cutaneous vasoconstriction. Am J Physiol. 2008;295:R343–R354. doi: 10.1152/ajpregu.00115.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol. 2007;292:R37–R46. doi: 10.1152/ajpregu.00668.2006. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Elmquist JK, Griffin JD, Saper CB. Ventromedial preoptic prostaglandin E2 activates fever-producing autonomic pathways. J Neurosci. 1996;16:6246–6254. doi: 10.1523/JNEUROSCI.16-19-06246.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoener EP, Wang SC. Effects of locally administered prostaglandin E1 on anterior hypothalamic neurons. Brain Res. 1976;117:157–162. doi: 10.1016/0006-8993(76)90567-9. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. Amsterdam, The Netherlands: Elsevier; 1992. [Google Scholar]
- Tanaka M, Nagashima K, McAllen RM, Kanosue K. Role of the medullary raphé in thermoregulatory vasomotor control in rats. J Physiol. 2002;540:657–664. doi: 10.1113/jphysiol.2001.012989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with fluorogold and PHAL in the rat. Brain Res Rev. 1998;27:89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Matsumura K, Inoue W, Shiraki T, Suzuki K, Yasuda S, Sugiura H, Cao C, Watanabe Y, Kobayashi S. Coexpression of microsomal-type prostaglandin E synthase with cyclooxygenase-2 in brain endothelial cells of rats during endotoxin-induced fever. J Neurosci. 2001;21:2669–2677. doi: 10.1523/JNEUROSCI.21-08-02669.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Nakamura K, Matsumura K, Kanosue K, König M, Thiel HJ, Boldogköi Z, Toth I, Roth J, Gerstberger R, Hübschle T. Neurons of the rat preoptic area and the raphe pallidus nucleus innervating the brown adipose tissue express the prostaglandin E receptor subtype EP3. Eur J Neurosci. 2003;18:1848–1860. doi: 10.1046/j.1460-9568.2003.02919.x. [DOI] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, DiMicco JA. Role of the dorsomedial hypothalamus in thermogenesis and tachycardia caused by microinjection of prostaglandin E2 into the preoptic area in anesthetized rats. Neurosci Lett. 2003;340:1–4. doi: 10.1016/s0304-3940(03)00047-8. [DOI] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, Shekhar A, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res. 2002;928:113–125. doi: 10.1016/s0006-8993(01)03369-8. [DOI] [PubMed] [Google Scholar]


