Abstract
Salivary diagnostics is an emerging field that has progressed through several important developments in the past decade, including the publication of the human salivary proteome and the infusion of federal funds to integrate nanotechnologies and microfluidic engineering concepts into developing compact point-of-care devices for rapid analysis of this secretion. In this article, we discuss some of these developments and their relevance to the prognosis, diagnosis and management of periodontitis, as an oral target, and cardiovascular disease, as a systemic example for the potential of these biodiagnostics. Our findings suggest that several biomarkers are associated with distinct biological stages of these diseases and demonstrate promise as practical biomarkers in identifying and managing periodontal disease, and acute myocardial infarction. The majority of these studies have progressed through biomarker discovery, with the identified molecules requiring more robust clinical studies to enable substantive validation for disease diagnosis. It is predicted that with continued advances in this field the use of a combination of biomarkers in multiplex panels is likely to yield accurate screening tools for these diagnoses in the near future.
Keywords: acute myocardial infarction, lab-on-a-chip, periodontitis, salivary diagnosis
Overview of the field of salivary diagnosis
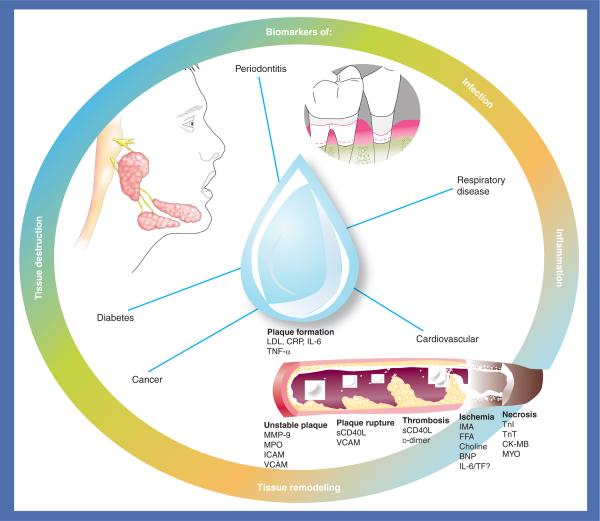
The analysis of blood and its components has been the mainstay for laboratory diagnostic procedures for several decades. However, other biological fluids are also utilized frequently for the diagnosis of disease, for example urine and cerebrospinal fluid, and thus, saliva could offer some distinct advantages in select situations [1–6]. Saliva is a hypotonic fluid composed mostly of water, electrolytes and organic molecules (i.e., amino acids, proteins and lipids). The water component is derived largely from the local capillary bed via intracellular diffusion, aquaporin water channels and extracellular routes [7,8]. Small neutral molecules from the serum enter by passive diffusion from the dense beds of capillaries surrounding and bathing the salivary glands. Electrolytes enter the saliva via osmotic gradients and are regulated by the rate of secretion, nature of the stimulus and level of mineralocorticoids in the circulation. The organic components of glandular saliva are derived largely from protein synthesis and are stored as granules within the acinar cells [4]. Because serum components of saliva are derived primarily from the local vasculature that originates from the carotid arteries [9], saliva has a prodigious fluid source that provides many, if not most, of the same molecules found in the systemic circulation. This makes saliva a potentially valuable fluid for the diagnosis of various systemic diseases (Figure 1).
Figure 1. Potential disease targets for salivary diagnosis.
BNP: B-type natriuretic peptide; CK-MB: Creatine kinase-MB; CRP: C-reactive protein; FFA: Free fatty acid; IMA: Ischemia modified albumin; LDL: Low-density lipoprotein; MMP: Matrix metalloproteinase; MPO: Myeloperoxidase; MYO: Myoglobin; sCD40L: Soluble CD-40 ligand; TF: Tissue factor; TnI: Troponin I; TnT: Troponin T.
The recent cataloguing of the salivary proteome has availed considerable information that is potentially important for diagnostic applications [10–12]. The clinical utility of this information is pertinent to the type of saliva being analyzed, which can be either glandular-specific saliva or whole saliva. There are three major salivary glands (parotid, submandibular and sublingual) that introduce saliva to the oral cavity. Saliva from these glands provides different mixtures of serous- and mucinous-derived fluid, and is primarily useful for the detection of gland-specific pathology. Whole saliva, by contrast, is composed of a mixture of oral fluids from the major salivary (submandibular 65%, parotid 23% and sublingual 4%) and minor salivary glands (8%), and contains constituents of nonsalivary origin, including gingival crevicular fluid (GCF), serum transudate from the mucosa and sites of inflammation, epithelial and immune cells, food debris and many microbes [13,14]. Whole saliva is most frequently studied because its collection is easy, noninvasive and rapid to obtain without the need for specialized equipment. It can also be collected with or without stimulation. Unstimulated whole saliva is commonly collected by the `draining' method where the subject's head is tilted forward so that saliva moves towards the anterior region of the mouth and the pooled saliva is drooled into a wide-bore sterile vessel. Stimulated whole saliva is generally obtained by masticatory action (i.e., from a subject chewing on paraffin) or by gustatory stimulation (i.e., use of citric acid or sour candy drops on the subjects tongue) and is expectorated into a tube. Stimulated whole saliva is less suitable for diagnostic applications because the foreign substances used to stimulate saliva tend to modulate the fluid pH and generally stimulate the water phase of saliva secretion, resulting in a dilution in the concentration of proteins of interest [15,16].
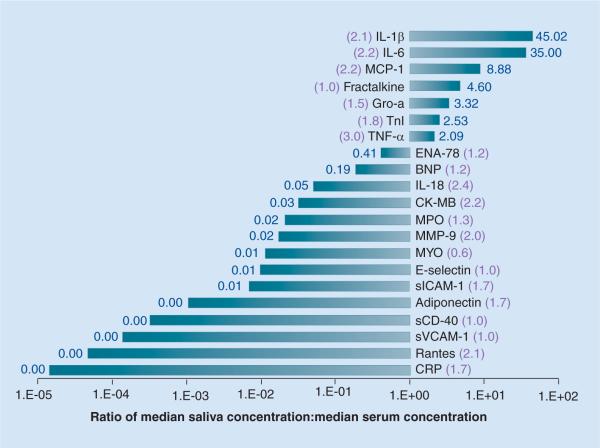
To date, unstimulated whole saliva has been used in the majority of diagnostic studies. Data presented herein from our laboratory are based on the collection of unstimulated whole saliva with a modification in the `draining' method where pooled saliva is expectorated every 30 s instead of `drooled'. This method has provided us with diagnostic information regarding 21 different biomarkers of interest relevant to the assessment of periodontal and cardiovascular disease. As shown in Figure 2, the majority of analytes in unstimulated whole saliva are detected at levels lower than that found in serum in our patient cohort. However, seven markers (IL-1β, IL-6, monocyte chemotactic protein-1, fractalkine, growth-regulated oncogene 1-α, troponin [Tn]I and TNF-α) appear at higher concentrations in saliva than serum. Figure 2 also shows that analyte concentrations are generally higher in unstimulated whole saliva than stimulated whole saliva.
Figure 2. Relative abundance of biomarkers in unstimulated whole saliva shown as median values in saliva compared with median values in serum from 45 healthy adults.
The ratio of median value in unstimulated/stimulated saliva is shown in parenthesis. All samples analyzed by standard enzyme immunoassays.
BNP: B-type natriuretic peptide; CK-MB: Creatine kinase-MB; CRP: C-reactive protein;ENAP: Epithelial neutrophil-activating peptide; Gro-a: Growth-regulated oncogene; MCP: Monocyte chemotactic protein; MMP: Matrix metalloproteinase; MPO: Myeloperoxidase; MYO: myoglobin; sCD-40: soluble CD-40; sICAM: Soluble ICAM; sVCAM: Soluble VCAM; TnI: Troponin I.
New technologies for measuring salivary biomarkers
While medical tests have traditionally been completed in central laboratories filled with specialized equipment and trained technicians, there is currently a trend to complete more tests using portable instrumentation. In this field, tremendous advances have been made that exploit the advantages of miniaturization mediated by the small reagent and sample volumes required. Smaller sample and reagent volumes translate to rapid analysis times and less waste volumes, and result in more cost-effective assays that can be operated with less technological constraints, making them suitable as a high-throughput biomarker validation tool and amenable to point-of-care testing. Most importantly, these characteristics, when fully developed into a functional system, have the potential to lead to significant reductions in the time needed for accurate biomarker testing for the diagnosis and subsequent treatment of a variety of diseases.
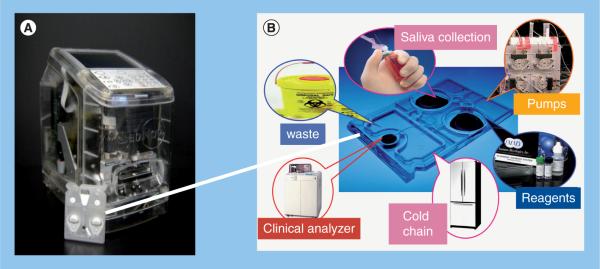
Over the past decade, our research team has sustained efforts that combine and adapt the tools of nanomaterials and microelectronics for the practical implementation of miniaturized sensors, suitable for a variety of important applications. Here, two types of systems have been created. The first is based on a microbead array, wherein micro-pits within a silicon wafer are populated with a variety of chemically sensitized bead `microreactors'. This sensor system is based on a bio-micro-electromechanical systems platform, and may be described as a `chemical processing unit' in analogy to the central processing unit that serves as the brains for a computer chip. Instead of handling electrical signals passing through conductors, as in the case of traditional circuits, the Nano-Biochip technology processes fluids so as to provide a digital fingerprint that can be correlated with the local chemical environment, detecting pH, electrolytes, metal cations, sugars, toxins, proteins and antibodies [17–22]. Building on this technology, our group has pioneered a second class of miniaturized sensor system that contains beads within etchings of stainless steel plates and utilizes a membrane capture element integrated into a fluidics structure [23–25]. These membrane microchip ensembles have been adapted to service cell, spore, and bacteria separation and biomarker identification applications [26]. Importantly, the performance metrics of these miniaturized sensor systems have been shown to closely correlate with established macroscopic gold-standard methods, making them suitable for use as subcomponents of highly functional detection systems for the analysis of complex fluid samples, such as saliva, for a variety of analyte systems [17–25,27–32]. Our efforts have led to the development of a POC device (Figure 3A) that contains a modular and miniaturized sensor system, universal analyzer with functional integrated mechanical/optical interfaces, and flexible microchip architecture that can service the future needs of clinicians and the research communities.
Figure 3. LabNow analyzer and Nano-Biochip.
(A) Analyzer, by contrast to the actual production configuration, is shown here with transparent outer covering to allow inner features to be viewed. (B) A representation of the multiple biochip functions performed within the credit-card-sized Nano-Biochip, which serve to eliminate constraints imposed by traditional laboratory-confined methods.
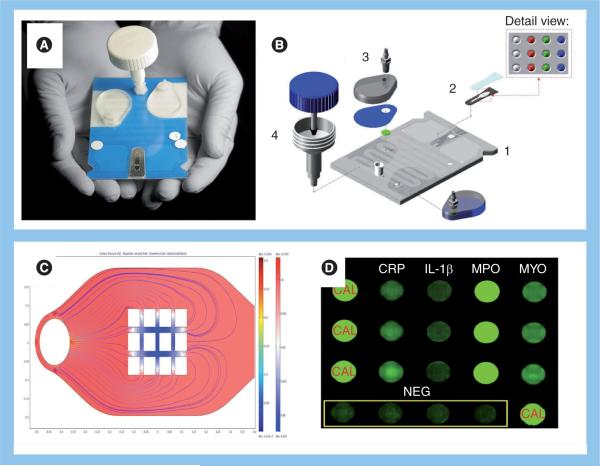
In this POC device, saliva (100–300 μl) is placed into the salivary collection/delivery module, and then delivered into the Nano-Biochip. The injection-molded cartridge is `credit-card' size and encloses the array Nano-Biochip where complex fluorescent immunoas-says are performed (Figure 3B). Here, a network of fluidic components ensure the complete transfer and process of saliva samples to the multiplex bead array to provide quantitative information of target biomarkers of disease. The sample introduction requirements are consistent with the use of saliva or finger-prick quantities of blood that can be directly introduced into the sample introduction port. Detection reagents are stored dry on a conjugate pad embedded within the biochip, and are reconstituted as needed, through the release of a prepacked buffer contained in biochip-integrated pouches. All processing steps are conducted within the microfluidic network of the biochip via actuation inside the analyzer without human intervention. These features eliminate the need for external fluidics, such as pumps, tubing and connectors. Therefore, the integrated system has the potential to reduce cost and reduce the risk for leaks and contamination. The assay is processed entirely through a 5–15 min sequence that is programmed in the main controller board. The flexibility of the control software allows for modifications to be made through an assay-builder interface. Control over the flow rate, incubation time and reagent wash, is achieved by the actuation of stepping motors that direct the fluid flow through the depression of the fluid pouches. The sample is directed to an on-chip waste reservoir, which provides a safe containment of biohazardous fluids. The entire biochip can be discarded as solid waste after the assay, facilitating biohazard waste management. Together, these essential features serve to facilitate the transition from chips-in-a-laboratory to a lab-on-a-chip, and offer significant opportunities for POC technology needs. To date, this system has proven versatile and useful for diagnostic applications involving a variety of bodily fluids in which the analyte concentration may be extremely low [22,25]. Specifically, a multiplex platform designated `cardiac arrest rapid diagnostic information using saliva' (CARDIUS), which uses four matched pairs of highly-specific antibodies (Figure 4), each recognizing target antigens myoglobin (MYO), C-reactive protein (CRP), myeloperoxidase and IL-1β have demonstrated excellent acute myocardial infarction (AMI) screening capabilities [32].
Figure 4. Nano-Biochip elements.
(A) Fully integrated laminate structure. (B) Exploded view for individual modules: (1) the microchip array, (2) microfluidics platform, (3) fluidic interfaces and (4) saliva collection–delivery module. (C) Computational fluid dynamic studies aided our micro-engineers to adjust structures for optimal fluid flow to the beads. (D) Profile of a salivary sample of a heart attack victim from assay performed in the Cardiac Arrest Rapid Diagnostic Information Using Saliva (CARDIUS) study. Here, signals generated on CRP, IL-1β, MPO and MYO-sensitized bead sensors are shown. Also shown are calibrator beads, as well as negative control beads conjugated to an antibody irrelevant to the targets.
CAL: Calibrated; CRP: C-reactive protein; MPO: Myeloperoxidase; MYO: Myoglobin; NEG: Negative.
Salivary biomarkers of periodontal disease
Periodontal disease is a chronic infection involving biofilms of Gram-negative and Gram-positive bacteria characterized by persistent inflammation, breakdown of the connective tissue (i.e., attachment apparatus surrounding teeth) and destruction of alveolar bone [33]. It affects approximately 45% of adults in the USA over 50 years of age, and is a major cause of tooth mobility and tooth loss worldwide [34]. In dentistry, the traditional parameters for diagnosis include probing depths of the gingival crevice, bleeding on probing, clinical attachment levels, plaque index, gingival index and radiographic analysis. These diagnostic parameters are implemented owing to their ease of use, relative noninvasiveness and reliability. However, there are several limitations to this diagnostic approach. First, a highly trained clinician with assistance is needed to record the findings. Second, collection of the diagnostic information includes the use of expensive radiographic equipment that makes the procedure time and labor intensive as well as costly to the consumer. Third, these diagnostic parameters are excellent at determining a past history of disease but provide limited ability to determine ongoing disease unless standardized longitudinal measures are obtained. Fourth, a significant amount of damage must occur before these diagnostic parameters are able to assess the amount or severity of disease [35]. Thus, clinicians have long sought measures with the capacity to detect disease progression more rapidly, at the time of the consultation.
During the past two decades, oral fluids have been investigated as an alternative diagnostic approach. Initially, the focus was on oral fluids emanating from individual teeth. This fluid, known as GCF, is an inflammatory exudate collected by dentists on filter paper strips. GCF is typically in low abundance during health, but increases in quantity and complexity of inflammatory molecules at disease sites. Its use has several diagnostic advantages as contributing inflammatory mediators and tissue-destructive molecules associated with periodontitis appear, and can be detected, in GCF. However, GCF analyses are time consuming by requiring multiple sampling of individual tooth sites onto filter paper strips (i.e., up to 32 teeth, or via clinical decision preselecting the teeth to sample). The procedure is labor intensive and somewhat technically demanding, requiring equipment for calibrating and measuring fluid volumes. Finally, the assessment of analytes is expensive since each sample must be evaluated individually and the required assays are laboratory based and generally cannot be done chairside. In addition, GCF analyses involve miniscule amounts of fluid, often approximately 1 μl, which has an impact on laboratory analysis [36], and can be contaminated with blood, saliva or plaque.
Given some of the problems inherent in sampling GCF, the analysis of salivary biomarkers offers some advantages. Acquisition of saliva is easy, noninvasive, rapid, and requires less manpower and materials than GCF. However, in contrast with GCF, whole saliva clearly provides different diagnostic information. Saliva, for example, represents a pooled sample from all periodontal sites, thereby giving an overall assessment of a particular disease or risk status at the subject level (as opposed to site or tooth-level). Since levels of salivary analytes have the potential to reflect current disease activity and severity, this can be advantageous for providing information used in yes/no decision matrices. Knowledge of levels of specific salivary biomarkers can, in turn, provide patients and healthcare practitioners with the ability to determine whether a disease is present, whether initiation of treatment is needed or if treatment has been successful.
Many analytes associated with periodontitis have been detected in saliva [3,22,37–42]. Cytokines, chemokines, enzymes and immunoglobulins are host-derived factors that can provide potentially important information regarding periodontal status. Our discussion here focuses on markers that hold potential diagnostic significance relevant to three important biological phases of periodontal disease (i.e., inflammatory phase, connective-tissue degradation phase and bone-turnover phase). Specificity demands will likely require use of biomolecules from all three biological phases to `rule in' periodontitis and exclude other inflammatory diseases of the oral cavity. Table 1 provides an overview of these biomarkers.
Table 1.
Salivary biomarkers of periodontal diease.
| Biomarker | Biological role | Concentration linked with periodontal disease compared with healthy controls | Ref. |
|---|---|---|---|
| Inflammatory | |||
| β-glucuronidase | Marker of neutrophil influx | Elevated | [41,43] |
| CRP | Acute-phase reactant | Elevated | [22,44–46] |
| IL-1β | Proinflammatory cytokine | Elevated | [42,49,53–57] |
| IL-6 | Acute-phase protein induction, osteoclast generation and activation | Elevated | [59] |
| MIP1α | CC chemokine stimulates osteoclast progenitors to become active osteoclasts | Elevated in aggressive periodontitis | [56] |
| TNF-α | Proinflammatory cytokine stimulates IL-1, inhibits bone collagen synthesis and induces collagenases | Elevated | [56,57,67,68] |
| Soft-tissue destruction (collagen breakdown) | |||
| α2-macroglobulin | Inactivates a variety of proteinases | Decreased | [46,72–74] |
| MMP-8 | Neutrophil collagenase degrades type I and III collagens | Elevated | [82,89–92,94] |
| MMP-9 | Gelantinase degrades type IV and V collagens | Elevated | [82,89–93] |
| AST | Aminotransferase, marker of cell injury | Elevated | [106–109] |
| ALT | Aminotransferase, marker of cell injury | Elevated, less well characterized than AST | [106–109] |
| TIMPs | Metalloproteinase inhibitors | Decreased | [89,95] |
| Bone remodeling | |||
| Alkaline phosphatase | Hydrolase enzyme involved in bone remodeling | Elevated | [106,122] |
| β C-terminal type I collagen telopeptide | C-terminal degradation products of type I collagen from bone remodeling | Indeterminate | [57,68] |
| C-telopeptide pyridinoline cross-links of type I collagen | C-terminal degradation products of type I collagen from bone remodeling | Indeterminate | [57,68] |
| Osteoprotegerin | Intereferes with RANKL binding | Elevated | [42,129] |
| Osteocalcin | Secreted by osteoblasts that are thought to play a role in mineralization and calcium ion homeostasis | Reduced | [57] |
| SPARC/osteonectin | Secreted by osteoblasts | Reduced | [54] |
| RANKL | Ligand for osteoclast differentiation, induces bone resorption | Indeterminate | [68,129] |
| HGF | Osteoclast-activating factor | Elevated | [54] |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; CC: Cysteine-cysteine; CRP: C-reactive protein; MIP: Macrophage inflammatory protein; MMP: Matrix metalloproteinase; RANKL: Receptor activator of NF-κB ligand; SPARC: Secreted protein, acidic, rich in cysteine; TIMP: Tissue inhibitors of metalloproteinase.
Inflammatory biomarkers
Periodontal disease initiates with inflammation of the gingival apparatus and periodontal tissues in response to bacterial plaque accumulation. The persistent presence of the multispecies bacterial biofilm leads to chronic inflammation and an abundance of inflammatory molecules in oral fluids. To date, several studies have detected one or more inflammatory markers at higher concentrations in patients who have periodontal disease compared with healthy controls. Several of these inflammatory molecules also serve as bone resorptive factors.
β-glucuronidase
Reports of inflammatory markers in saliva, relevant to periodontal disease, were reported more than a decade ago. Initial studies focused on β-glucuronidase, a marker of neutrophil influx, and demonstrated elevated salivary levels in patients with more severe periodontitis compared with healthier states. Elevated levels of β-glucuronidase were significantly correlated with the number of sites with probing depths of at least 5 mm [41]. Clinical and laboratory data, including salivary levels of β-glucuronidase from 380 patients with various levels of periodontitis, were used in logistic regression modeling to identify patients with at least four sites with pocket depths of 5 mm or more [41]. Lamster et al. also reported that high activity levels of salivary β-glucuronidase produced an odds ratio (OR) of 3.77 for periodontal disease. However, levels of β-glucuronidase in saliva did not change in patients with aggressive periodontitis following 2 months of treatment with doxycycline [43].
C-reactive protein
C-reactive protein is an acute-phase reactant that is found at altered levels in the whole saliva of patients who have periodontal disease compared with controls. In 1995, Pedersen et al. first reported that CRP levels were elevated in the saliva of patients with destructive periodontal disease [44]. Subsequently, salivary levels of CRP were demonstrated to be significantly elevated in periodontitis patients compared with edentulous patients (i.e., without teeth) [22]. Consistent with this theme, we demonstrated that CRP levels were 18.2-times higher in whole saliva of patients who had periodontal disease compared with those of healthy dentate patients [45]. By contrast, one study of a limited number of patients reported that CRP levels are lower during chronic periodontitis compared with healthy controls [46]. Although the data from all studies are not in agreement, the majority opinion is that salivary CRP levels appear to be elevated in patients who have periodontitis.
IL-1β
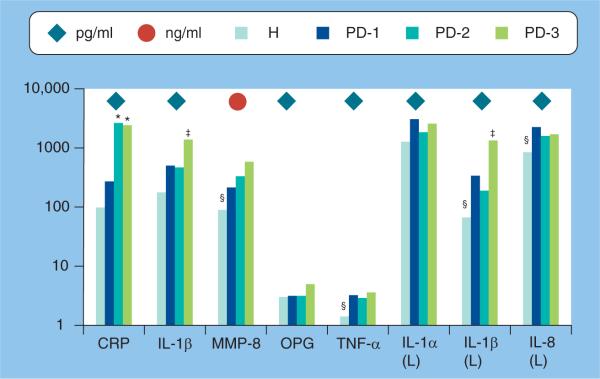
In recent studies, we and others have examined IL-1β as a target molecule of the inflammatory phase of periodontal disease. IL-1β is a pro-inflammatory cytokine that induces widespread gene expression, including cyclo-oxygenase-2, inducible nitric oxide synthetase, and metal-loproteinases that can contribute to activation of osteoclasts and result in bone resorption [47–49]. Of the two isoforms of IL-1 (i.e., IL-1α and IL-1β), IL-1β is more potent in stimulating bone resorption and is the more frequently occurring form in periodontitis [50,51]. In the periodontium, IL-1β may be synthesized and secreted by the local connective tissue cells (fibroblasts and endothelial cells), or by the infiltrating leukocytes [52]. In clinical studies, increased levels of IL-1β have been detected in GCF and have been associated with gingival inflammation, periodontal disease severity and an absence of therapeutic effectiveness [49,53]. In a recent study of whole expectorated saliva, we found that levels of IL-1β were significantly higher in the saliva of patients with periodontitis than in healthy controls [42]. Levels of IL-1β also positively correlated with several periodontal indices including: bleeding on probing, clinical attachment level, percentage of sites with pocket depths of at least 4 mm and overall periodontal disease severity. By establishing diagnostic thresholds at two or more standard deviations above the mean of the controls, we observed that salivary levels of IL-1β above this threshold were significantly associated with increased risk for clinical parameters of periodontal disease (OR = 15.4). Similar findings have been observed by others [54–57] with elevated levels shown to correlate with disease severity (Figure 5).
Figure 5. Mediator levels of six putative biomarkers of periodontal disease in whole expectorated saliva analyzed by enzyme immunosorbent assays and Luminex© technology.
Bars denote mean levels in 35 healthy and 18 periodontitis patients. Severity of periodontitis was categorized based on increasing clinical disease severity (i.e., H < PD-1 < PD-2 < PD-3) using frequency of sites with bleeding on probing, pocket depths of at least 5 mm and clinical attachment levels of at least 2 mm.
*Significantly greater than H and PD-1.
‡Significantly greater than other categories at least at p < 0.01.
§Significantly less than other categories at least at p < 0.01 using Kruskal–Wallis ANOVA on ranks with post-hoc Dunn's test for pairwise comparisons.
CRP: C-reactive protein; H: Healthy; L: Luminex; MMP: Matrix metalloproteinase; OPG: Osteoprotegerin; PD: Periodontitis.
IL-6
IL-6 is produced by T and B cells, macrophages, endothelial cells, epithelial cells and fibroblasts in response to infection, stress and neoplasia. It is also released in response to IL-1 and TNF stimulation of many of these cell types [58]. IL-6 demonstrates a range of functions, including acute-phase protein induction, B- and T-cell growth and differentiation, and plays a crucial role in osteoclast generation and activation [59]. Salivary levels were not elevated with respect to alveolar bone loss in adult periodontitis [54] or aggressive periodontitis in adolescents [56]. However, in one study, IL-6 levels were directly proportional to bone loss scores of adult patients with chronic periodontitis [57].
Macrophage inflammatory protein-1α
Macrophage inflammatory protein (MIP)-1α is a member of the cysteine-cysteine chemokine family, which is secreted by inflammatory cells and is primarily associated with cell adhesion and migration. It stimulates monocytes and/or osteoclast progenitor cells to become active osteoclasts in a dose-dependent manner [60]. MIP-1α has been detected at higher levels in saliva in a longitudinal study of adolescents who had aggressive periodontitis compared with controls [56]. However, we have found that MIP-1α is not elevated in the saliva of chronic adult-periodontitis patients [MILLER CS, UNPUBLISHED DATA].
TNF-α
TNF-α is a proinflammatory and immuno-regulatory cytokine central to the pathogenesis of various inflammatory conditions [61,62]. It plays a role in the recruitment of inflammatory cells and bone resorption through its ability to stimulate IL-1 and granulocyte macrophage colony-stimulating factor (GM-CSF), inhibit bone collagen synthesis, induce collagenases and stimulate osteoclast differentiation in the presence of GM-CSF [63–66]. Although one report suggested that TNF-α was difficult to detect in saliva [46], others found low levels of TNF-α in saliva [56,57,67]. We recently reported that TNF-α levels were detectable in all salivary samples from 35 patients, who had chronic adult periodontal disease and 39 healthy controls [68]. In addition, we found that TNF-α levels in saliva were significantly elevated (by two times) in periodontitis subjects compared with healthy controls. Similar to IL-1β, elevated salivary TNF-α levels correlated with an increased number of sites with bleeding on probing, pocket depth sites of at least 4 mm and clinical attachment levels of at least 2 mm [68]. Similar findings have been reported by Ng et al. [57]. These data suggest that salivary TNF-α levels may have utility for the screening diagnosis of chronic periodontitis in adults.
Molecules of connective tissue destruction
Destruction of connective tissue matrix is responsible for the pathogenesis of chronic inflammatory states, such as periodontitis. Degradation of the matrix is initiated extra- and pericellularly by proteinases produced locally at the inflammatory site and balanced by inhibitors of proteinases. The level of balance/imbalance is thought to determine the progression rate of chronic periodontitis.
α2-macroglobulin
α2-macroglobulin is a large plasma protein found in blood, GCF and saliva [69]. It is produced by the liver and macrophages, and functions to inactivate a variety of proteinases, including serserine-, cysteine-, aspartic- and metallo proteinases produced by granulocytes and other cells [70]. While it also has roles as an inhibitor of coagulation and fibrinolysis, its role in periodontitis is consistent with its ability to regulate proteinases (i.e., gingival collagenase) and tissue destruction within the periodontal complex [71]. Several studies have investigated levels of α2-macroglobulin in saliva [72]. Reduced levels of α2-macroglobulin have been identified in adults with gingivitis and chronic periodontal disease compared with controls [46,73,74]. This suggests an imbalance exists between proteinases and their inhibitors during these conditions.
Matrix metalloproteinases
Matrix metalloproteinases (MMPs) are zinc-dependent proteolytic enzymes that degrade the extracellular collagen matrix and are involved in the healing of injured tissue [75–77]. They are predominantly derived from polymorphonuclear leukocytes. Thus, their expression levels are low in noninflamed periodontium but are significantly higher at sites of periodontal inflammation related to the emigration of polymorpho-nuclear leukocytes into diseased sites [78]. Of the more than 25 members of the family, at least two MMPs are present at elevated levels in inflamed human gingival [79] and GCF [80–85] accompanying adult periodontitis. The unique properties of MMP-8 and MMP-9 have been the focus of the majority of attention related to periodontitis. MMP-8 has the unique ability to break down type I and III collagens, which are the major collagen species within the periodontium. MMP-8 also acts in a protective/anti-inflammatory manner, inhibiting alveolar bone loss in a murine model of bacterium-induced periodontal disease [86]. MMP-9, a gelantinase, degrades type IV and V collagens, which are lesser components of the periodontium [87,88]. Both have been readily detected in the saliva of patients experiencing periodontal disease [42,82,89–94].
Salivary MMP-8 levels are elevated in patients with aggressive periodontitis compared with healthy controls [95]. Mean levels of MMP-8 have also been demonstrated to be more than four times that of healthy controls in the saliva of patients with periodontal disease [42,94]. Elevated salivary MMP-8 levels correlated highly with clinical measures consistent with the features of periodontal disease, and elevated levels of MMP-8 were significantly associated with an increased risk for clinical parameters of periodontal disease (OR = 11) [42]. In another study, patients with periodontitis had significantly higher levels of MMP-9 than healthy subjects in oral rinses [89]. It was also demonstrated that both MMP-8 and MMP-9 levels were detectable in the saliva of patients with chronic periodontitis and levels of MMP-8, but not MMP-9, decreased after conventional therapy (i.e., scaling and root planning) as well as after doxy cycline therapy [96]. Together, these findings confirm earlier reports that salivary concentrations of active collagenases and gelatinases decrease following periodontal scaling and root planning [91,93,97]. Furthermore, these data indicate that MMP-8 may have a greater clinical value in identifying patients with existing periodontal disease and response to therapy than MMP-9.
Tissue inhibitors of metalloproteinases
The activities of MMPs in body tissues, such as the periodontium, are regulated at one level by tissue inhibitors of metalloproteinases (TIMPs). This well-studied family of inhibitors consists of four members (TIMPs 1–4). TIMP-1, -2 and -4 are secreted extracellular proteins and TIMP-3 is bound to the extracellular matrix [98]. All can inhibit MMPs. In addition to proteinase inhibition, TIMPs can exert other functions including, but not limited to, MMP transportation and stabilization, MMP focalization to the cell surface, inhibition of angiogenesis and promotion of bone-resorbing activity [88,98,99]. The most common inhibitor, TIMP-1, is secreted by the regional cells of the periodontium (fibro-blasts, keratinocytes and endothelial cells) and by the migratory cells of the inflammatory infiltrate (monocytes/macrophages). Under natural conditions, inhibitors of MMPs are required for the normal physiological remodeling of connective tissue. An imbalance between the levels of active MMPs and their tissue inhibitors can lead to excessive degradation of extra-cellular matrix proteins [100]. Levels of TIMPs have been demonstrated in the saliva of patients with chronic periodontitis [89,95], and doxycycline treatment, in conjunction with conventional periodontal treatment, increases salivary TIMP-1 concentration in these patients [89].
Aminotransferases
Aminotransferases (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]) are enzymes relevant to periodontal disease diagnosis. Both are cytoplasmic enzymes important for the production of various amino acids and serve as diagnostic analytes of cellular injury in clinical chemistry [101–103]. AST and ALT catalyze amino-transfer or transamination. They are ubiquitous components of saliva and are detected in periodontal tissue, GCF [104], the enamel pellicle [105] and saliva [106,107]. Interestingly, substantially higher levels of AST and ALT were measured in the saliva of healthy subjects as compared with serum [108,109].
Aspartate aminotransferase and ALT are enzymes that are normally confined to the cell, and are released into the GCF and saliva after cell injury and cell death. Their levels in periodontitis are related to the type of tissue affected by necrosis [110,111]. Fibroblasts from the periodontal ligament produce significantly lower levels of aminotransferases than gingival epithelial cells [112]. In one study of patients with periodontal disease, salivary AST activity was significantly increased (by five times) compared with controls, whereas salivary ALT activity was not significantly altered in patients with periodontal disease [106]. Salivary AST levels were significantly higher in a group of patients who had more severe periodontal disease than controls [113,114]. In addition, gingival bleeding and suppuration were observed in 20% of individuals with salivary AST concentrations three-times higher than the median of the controls [113]. Similarly, in a larger patient cohort, AST levels significantly increased with increasing severity of periodontitis, whereas ALT levels increased, but not to a significant level, above the healthy controls [107]. It has also been demonstrated that salivary levels of AST and ALT in patients with periodontitis decrease significantly after scaling [115]. These findings suggest that periodontal destruction, gingival bleeding and suppuration are related to higher AST levels and possibly ALT levels in saliva, and confirm earlier studies demonstrating similar increased levels of AST in crevicular fluid at sites of periodontitis [104,116,117]. Thus, markers of cell injury, such as AST, appear to be useful for assessing periodontal disease in saliva.
Other enzymes
Additional salivary enzymes associated with inflammation and connective tissue destruction of the periodontium include cathepsin G and neutrophil elastase. In one study, salivary levels of cathepsin G and elastase were higher in patients with periodontal disease than those without teeth and those with healthy periodontium [44]. Elevated levels of salivary elastase have also been found in adults with periodontal disease compared with those with a healthy periodontium [118–120]. In longitudinal studies of patients with advanced periodontitis, elastase levels have dropped dramatically as a result of clinically successful therapy [119,120].
Bone remodeling biomarkers
Salivary biomarkers of alveolar bone remodeling are less well defined than the other two biological phases. This may be because alveolar bone remodeling is thought to be an episodic occurrence during periodontal disease progression. This episodic nature requires longitudinal study designs, which are more expensive than cross-sectional designs. A few investigators have addressed this issue by focusing on juvenile patients with aggressive periodontitis, as these patients have a greater number of days with active disease. Unfortunately, the majority of studies to date that have analyzed bone resorption markers have employed cross-sectional study designs utilizing adults with chronic periodontitis, and the readers should understand the limitations of these studies.
Alkaline phosphatase
Alkaline phosphatase (ALP) is a nonspecific hydrolase enzyme present in all bodily tissues, but is particularly concentrated in the liver, kidney and bone [121]. It is associated with the calcification process, and elevated ALP levels are commensurate with active bone remodeling. Activity levels of ALP were significantly higher in pregnant women with periodontitis than those with gingivitis or a healthy periodontium [122]. Salivary ALP activity was also significantly higher (five times) in saliva from patients with periodontal disease than controls [106].
C-terminal type I collagen degradation products
There are two C-terminal degradation products of type I collagen: C-terminal cross-linking telo-peptide of type I collagen (βCTX) and pyridinoline crosslinked carboxyterminal telopeptide domains of type I collagen (ICTP). Each is selective in its sensitivity as a marker of bone resorption based on their physiological targets. βCTX is generated by lysosomal cathepsins (cathepsin K) that attack multiple sites of the collagen triple helix, and ICTP is generated subsequently by MMPs such as MMP-9 and MMP-12 [123,124]. ICTP is also known as CTX-MMP [125]. These two end products are released into the circulation under different physiological and pathological conditions. In limited studies of these biomarkers in saliva, degradation products of type I collagen were below the limit of detection in most patients [57,68], and in one report were detected only in patients with periodontal disease [68].
Receptor activator of NF-kB ligand & osteoprotegerin
The balance between receptor activator of NF-κB ligand (RANKL) and osteoprotegerin is critical to bone remodeling [126]. RANKL is essential for the induction of osteoclast differentiation and formation. It is also known as osteoprotegerin (OPG) ligand because it can be bound by the glycoprotein OPG. Binding of these two molecules prevents RANKL from binding to RANK on osteoclast precursors, thus, competitively inhibiting osteoclast differentiation and activity [127,128]. Salivary RANKL levels are significantly higher in untreated non-smoking, periodontitis patients than those who received maintenance therapy [129]. However, in one study, levels of soluble RANKL were barely detectable in the saliva of periodontitis patients [68]. It has been suggested that soluble RANKL levels may be difficult to detect in saliva because this fraction may be bound to OPG or is degraded within saliva [68]. Salivary OPG levels were elevated during periodontitis and correlated positively with probing depth, clinical attachment level and bleeding on probing [42]. However, salivary OPG levels were lower in untreated periodontitis patients who do not smoke than in maintenance-therapy nonsmokers [129]. Larger cohort and longitudinal studies may bring new data on these two markers.
Other bone remodeling markers
Hepatocyte GFs, osteocalcin and osteonectin have potential as salivary biomarkers of periodontal disease with respect to bone remodeling. HGF is secreted by mesenchymal cells, and acts as a multifunctional cytokine on cells of epithelial origin and bone. It stimulates matrix invasion and has central roles in angiogenesis, tissue regeneration and osteoclast activation [130]. Osteocalcin is a noncollagenous protein found in bone-matrix secreted by osteo-blasts; it is thought to function as a localization site for hydroxyapatite crystals during bone matrix synthesis and mineralization [131]. It is released into the serum during osteolysis and osteo genesis. Secreted protein, acidic, rich in cysteine (SPARC)/osteonectin is a non-structural matricellular glycoprotein secreted by osteoblasts, which binds calcium in bone and has an affinity for collagen. It is a normal component of bone matrix and is involved in cell-matrix interaction during tissue remodeling [132]. Salivary levels of HGF and osteonectin have been reported to be elevated and reduced in periodontitis patients, respectively [54]. Salivary levels of osteocalcin and osteonectin have been demonstrated to be inversely correlated with bone-loss scores in patients with periodontal disease [57].
Diagnostic panels
It is generally considered that the combined use of biomarkers increases the sensitivity and specificity for obtaining accurate diagnostic information. An example of this comes from studies where diagnostic thresholds were established using elevated salivary levels of MMP-8 and IL-1β. Individually, these biomarkers are significantly associated with increased risk for periodontal disease (OR = 11–15.4). However, their use in combination demonstrates that the risk for periodontal disease is much greater (OR = 45) when elevated salivary levels of MMP-8 and IL-1β are greater than two standard deviations above the mean of healthy control values [68].
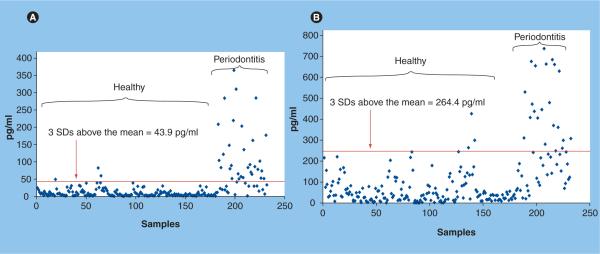
In another example, diagnostic thresholds were established for salivary levels of MMP-8 and IL-1β to discriminate between periodontal health and disease. Here, we analyzed the saliva of 65 periodontitis patients and 30 healthy controls. Figure 6 depicts that salivary levels of IL-1β 43.9 pg/ml or more and levels of MMP-8 of 264.4 ng/ml or more individually provide a positive predictive value of more than 90%, and a negative predictive value of more than 85% for the diagnosis of periodontitis. When used together in rate-of-change analyses, these two biomarkers yield an area under the curve of 0.98, a positive predictive value of 96% and negative predictive value of 82%.
Figure 6. Diagnostic thresholds used in combination predict a screening diagnosis of periodontal disease.
(A) Levels of IL-1β above the threshold (red line) demonstrate a sensitivity of 66%, specificity of 98.3%, positive predictive value of 91.7% and negative predictive value of 91.2% for the diagnosis of periodontal disease. (B) Levels of matrix metalloproteinase-8 above the threshold (red line) demonstrate a sensitivity of 40%, specificity of 98.3%, positive predictive value of 90% and negative predictive value of 85.5%. Samples elevated above both thresholds have a positive predictive value of 96% or higher. All samples analyzed by standard enzyme immunoassays. SD: Standard deviation.
Salivary biomarkers & cardiovascular disease
Approximately 13.2 million Americans have coronary artery disease and nearly 8 million have suffered an AMI [133]. The gravity of this healthcare problem is evident in that 770,000 Americans will have a new myocar-dial infarction and approximately 430,000 will have a recurrent myocardial infarction this year. Furthermore, one of every five deaths in the USA is caused by coronary artery disease and approximately one American dies from a coronary event every minute. Acute coronary syndromes (ACS) collectively refer to a group of clinical syndromes including ST-elevation myocardial infarctions, non-ST-elevation myocardial infarctions and unstable angina. ACS is characterized by atherosclerotic cholesterol plaques that are prone to rupture, causing a spectrum of clinical symptoms ranging from chest pain to AMI to cardiogenic shock.
Currently, the diagnosis of AMI is based on clinical findings, electrocardiogram findings and serum biomarkers. Based on these results, a patient can be classified as having a ST-elevation myocardial infarction, non-ST-elevation myocardial infarction or unstable angina. The biochemical markers of myocardial ischemia and necrosis include MYO, creatine kinase-MB (CK-MB), total CK and cardiac TnT and TnI [134]. The rate of release of these serum biomarkers differs depending on their intracellular location, protein size and local blood-flow characteristics. The temporal pattern of appearance of these markers is of great diagnostic importance. Unfortunately, delays in sample procurement, processing and analysis may be inadequate for early diagnosis and effective intervention [135]. Therefore, methodologies that utilize saliva for diagnosis of an AMI are being sought and could potentially provide a faster screening diagnosis, possibly in an ambulance, a clinic or the emergency department. At present, there are a limited number of published studies on this topic. We have demonstrated that a variety of analytes related to the ischemic cascade, which result from acute coronary syndromes, can be detected in saliva (Figure 2) and salivary multiplexed tests combined with electrocardiology demonstrate sensitivity values in the range of 90–100% for the detection of an AMI [32].
Cardiac enzymes
CK-MB, MYO & TnI
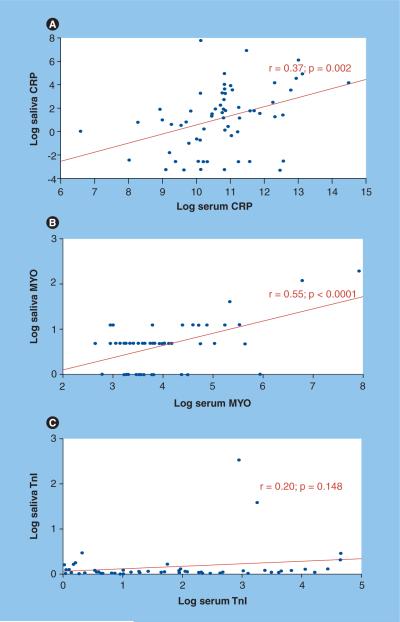
The presence of cardiac enzymes in saliva allowed us to determine if levels of these enzymes are elevated in AMI patients. Using standard immunoassays, we found that salivary MYO levels are significantly higher within 48 h of chest-pain onset in AMI patients compared with concentrations in non-AMI patients (Figure 7). Furthermore, salivary MYO levels correlated positively with serum concentrations (Figure 8). The discriminatory capacity of MYO is greater at time points at or before 24 h from the onset of chest pain [MILLER CS,UNPUBLISHED DATA]. Although CK-MB and TnI are excellent serum biomarkers of AMI, they have not yet been shown to be useful salivary biomarkers for diagnosing AMI.
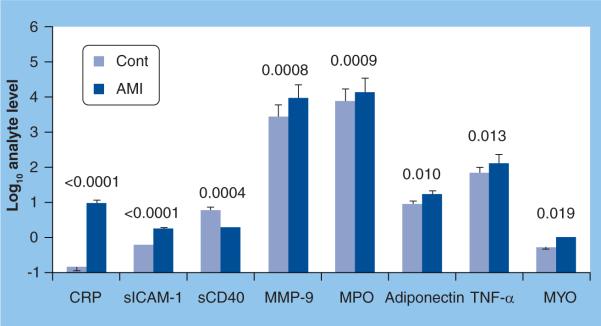
Figure 7. Median analyte levels expressed as log values in unstimulated whole saliva from 45 acute myocardial infarction patients and 40 non-acute myocardial infarction adult controls.
Samples were obtained within 48 h of chest-pain onset and analyzed in duplicate by Luminex© and Beckman Access instruments. P values shown above the bars indicate significant differences between the groups.
AMI: Acute myocardial infarction; Cont: Control; CRP: C-reactive protein; MMP: Matrix metalloproteinase; MPO: Myeloperoxidase; MYO: myoglobin; s: Soluble; sICAM: Soluble ICAM.
Figure 8. Correlations between serum and salivary biomarkers of acute myocardial infarction demonstrate C-reactive protein and myoglobin potential utility.
Log concentrations in the serum (X-axis) and saliva (Y-axis) of: (A) CRP from 64 acute myocardial infarction patients, (B) MYO from 55 acute myocardial infarction patients, and (C) TnI from 53 acute myocardial infarction patients. Samples were analyzed using Luminex and Beckman Access.
CRP: C-reactive protein; MYO: Myoglobin; TnI: Troponin I.
Inflammatory markers
CRP, TNF-α, MMP-9 & myeloperoxidase
C-reactive protein is a marker of systemic inflammation, and plays a central role in atherosclerosis and its complications [136]. We have found that salivary levels of CRP are significantly higher in patients who have suffered an AMI (Figure 7) [32] and correlate positively with serum concentrations (Figure 8). TNF-α is also elevated in the saliva of patients who have suffered an AMI [MILLER CS, UNPUBLISHED DATA].
The atherosclerotic lesions that are present in acute coronary syndromes are rich in macrophages that release lytic enzymes like metalloproteinases [137]. In our studies, salivary levels of MMP-9 are significantly higher in patients who have suffered an AMI (Figure 7) [32] and are elevated within 24 h after the onset of chest pain [MILLER CS, UNPUBLISHED DATA].
Myeloperoxidase is a protein abundantly expressed by neutrophils that is secreted during cell activation [138]. It can contribute to tissue injury, and elevated serum levels predict an increased risk for subsequent cardiovascular events in patients with ACS [139]. We have found that salivary levels are elevated in AMI patients (Figure 7) [32].
Adhesion markers
Soluble CD40 ligand & sICAM-1
Adhesion molecules are expressed on the endothelium, and plaque destabilization/rupture is associated with release of soluble CD40 ligand and specific adhesion molecules [140,141]. In our studies, salivary soluble ICAM-1 is significantly elevated in AMI patients (Figure 7), whereas salivary soluble CD40 ligand is significantly lower in AMI patients [32].
As mentioned earlier, the significance of these putative salivary cardiac biomarkers requires a better understanding of the potential confounders, and will most likely be gained through their use in multiplex panels as we have reported [32].
Potential confounders
Salivary diagnostics face many challenges before entry into mainstream clinical care is achieved. Proteins, inhibitors and enzymes, known to be present in saliva, may obscure or destroy antigenic determinants needed for immunoassays, and many inflammatory markers and proteases are at elevated levels in patients who experience gingivitis and periodontitis [95,142]. For example, elevated levels of salivary CRP, MMP-8, MMP-9 and IL-1β during periodontitis [22,42,44–46,82,89–94] could confound the utility of these markers for AMI diagnoses and studies are needed to identify their discriminatory capacity. By contrast, systemic diseases can also influence molecules appearing in saliva. Post-traumatic stress disorder has been shown to modulate salivary CRP and α2-macroglobulin levels [74], and nephrotic syndrome is known to alter the serum concentration of α2-macroglobulin, thus potentially impacting salivary concentrations. Infectious disease states, such as HIV infection, are known to be associated with altered salivary MMP and TIMP levels [143] and serum MIP-1α levels are elevated in multiple myeloma and other lytic bone disorders potentially confounding its utility in salivary diagnosis of periodontal disease [60,144]. Cyclic changes in salivary ALP levels have been associated with the menstrual cycle [145] and oral lesions [146], and periodontal therapy has resulted in altered levels of inflammatory cytokines and TIMPs in saliva [89,147]. Additional confounders include low flow rates due to dehydration, drug administration or systemic diseases that can affect/limit saliva collection, and day-to-day variations. All these factors must be rigorously considered before saliva gains real-world application.
Future perspective
This article presents information on biomarkers found in oral fluids relevant to oral and systemic disease, with emphasis on salivary molecules and their potential to provide screening diagnoses for periodontal disease and AMI. The field of salivary diagnostics is rather new, but a growing number of reports have been published on the topic. Its emerging status is evident, in that many analytes have been investigated by a limited number of scientists; many only in cross-sectional study designs. Accordingly, a few promising analytes have been identified. Before salivary diagnostics becomes established in clinical practice, biomarker discovery needs greater development and validation, especially with respect to which salivary biomarkers best correlate with periodontal disease and AMI. Targeted approaches that identify key biomarkers linked to distinct biological phases of disease are needed to generate the panels required to provide the sensitivity and specificity needed for accurate and reproducible disease diagnosis. For example, identifying key molecules that appear in saliva during the processes of inflammation, connective tissue destruction and bone remodeling are critical to the field of salivary diagnosis and periodontal disease. Similarly, key markers of processes associated with inflammation, plaque formation, plaque rupture, coronary ischemia, necrosis and cardiac remodeling are critical for diagnosing different phases of acute coronary syndromes.
In the near future, biomarker panels are likely to gain the specificity needed for the utility of saliva as a true diagnostic fluid. However, this will only be achieved once the proper combination of markers are validated in longitudinal studies and their reliability confirmed with respect to cyclical day-to-day variations and potential confounders [148]. Regarding periodontal disease, studies are needed that sample patients at regular intervals in order to capture analyte profiles seen during different phases of active disease. This information can then be used to identify key salivary biomarkers associated with bone remodeling that are useful in diagnostic panels. We expect that in a few years, salivary diagnostics will be perfected such that a panel of six or fewer diagnostic biomarkers can be assessed and results obtained within 15 min. This application will allow healthcare providers to rapidly rule in or rule out diseases that need immediate therapy (i.e., patients experiencing chest pain from an AMI) versus less urgent therapy (i.e., patients experiencing noncardiac chest pain). Future developments in the field of salivary diagnostics are likely to lead to POC devices that can revolutionize our approach to screening, risk assessment and therapeutic management for a range of health conditions. This approach will hopefully allow more individualized treatment to be provided before significant tissue destruction occurs.
Executive summary.
Salivary diagnostics is an emerging field
-
■
Saliva contains biomarkers derived from serum, gingival crevicular fluid and mucosal transudate.
-
■
Systemic and oral diseases produce markers that appear in saliva.
-
■
Unstimulated saliva contains higher concentrations of diagnostic biomarkers than stimulated saliva.
Relevant clinical markers associated with specific biological phases of periodontal disease & cardiovascular disease appear in saliva
-
■
Markers of inflammation, connective tissue destruction and bone remodeling associated with periodontitis appear in saliva.
-
■
Markers of tissue necrosis (cardiac enzymes), inflammation and cell adhesion appear in saliva.
Promising biomarkers of periodontitis
-
■
Several markers related to inflammation, connective tissue destruction and bone remodeling are elevated in chronic periodontitis.
-
■
Inhibitors of proteinases are reduced in saliva in chronic periodontitis.
-
■
Specific markers (macrophage inflammatory protein-1a) are associated with aggressive forms of periodontitis.
Promising biomarkers of cardiovascular disease
-
■
Several markers related to cardiac tissue necrosis, inflammation and plaque adhesion/rupture are at altered levels in saliva during the first 48 h after an acute myocardial infarction.
Technological advances are resulting in the development of point-of-care diagnostic devices
-
■
Several biomarkers relevant to the diagnosis of periodontitis and cardiovascular disease can already be detected using lab-on-a-chip technology.
-
■
Multiplex formats allow for a greater sensitivity and specificity relevant to disease diagnosis.
-
■
Lab-on-a-chip technology will allow for portable, point-of-care diagnoses.
Conclusion
-
■
Detection of analytes in saliva shows great promise for enhancing the ability to diagnose periodontal disease and acute myocardial infarction at the chair/bedside
Acknowledgments
Financial & competing interests disclosure Funding was provided by the NIH through grant U01DE017793. B Bhagwandin, JW Jacobson and JT McDevitt have financial interests in LabNow, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Mandel ID. The diagnostic uses of saliva. J. Oral Pathol. Med. 1990;19(3):119–125. doi: 10.1111/j.1600-0714.1990.tb00809.x. [DOI] [PubMed] [Google Scholar]
- 2.Haeckel R, Hanecke P. The application of saliva, sweat and tear fluid for diagnostic purposes. Ann. Biol. Clin. (Paris) 1993;51(10–11):903–910. [PubMed] [Google Scholar]
- 3.Kaufman E, Lamster IB. The diagnostic applications of saliva — a review. Crit. Rev. Oral Biol. Med. 2002;13(2):197–212. doi: 10.1177/154411130201300209. [DOI] [PubMed] [Google Scholar]
- 4.Malamud D. Saliva as a diagnostic fluid. BMJ. 1992;305(6847):207–208. doi: 10.1136/bmj.305.6847.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malamud D, Niedbala RS, editors. Oral Based Diagnostics. Vol. 1098. Annals of the New York Academy of Sciences; Wiley-Blackwell, NY, USA: 2007. pp. 1–515. [DOI] [PubMed] [Google Scholar]
- 6.Wong DT, editor. Salivary Diagnostics. John Wiley & Sons; Philadelpha, PA, USA: 2008. pp. 1–320. [Google Scholar]
- 7.Verkman AS, Yang B, Song Y, Manley GT, Ma T. Role of water channels in fluid transport studied by phenotype ana lysis of aquaporin knockout mice. Exp. Physiol. 2000;85:233S–241S. doi: 10.1111/j.1469-445x.2000.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 8.Haeckel R, Hanecke P. Application of saliva for drug monitoring. An in vivo model for transmembrane transport. Eur. J. Clin. Chem. Clin. Biochem. 1996;34(3):171–191. [PubMed] [Google Scholar]
- 9.Johnson LR. Salivary secretion. In: Johnson LR, editor. Gastrointestinal Physiology. 6th Edition Mosby; St. Louis, MO, USA: 2001. pp. 65–74. [Google Scholar]
- 10.Denny P, Hagen FK, Hardt M, et al. The proteomes of human parotid and submandibular/sublingual gland salivas collected as the ductal secretions. J. Proteome. Res. 2008;7(5):1994–2006. doi: 10.1021/pr700764j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu S, Xie Y, Ramachandran P, et al. Large-scale identification of proteins in human salivary proteome by liquid chromatography/mass spectrometry and two-dimensional gel electrophoresis-mass spectrometry. Proteomics. 2005;5(6):1714–1728. doi: 10.1002/pmic.200401037. [DOI] [PubMed] [Google Scholar]
- 12.Helmerhorst EJ, Oppenheim FG. Saliva: a dynamic proteome. J. Dent. Res. 2007;86(8):680–693. doi: 10.1177/154405910708600802. [DOI] [PubMed] [Google Scholar]
- 13.Mandel ID, Wotman S. The salivary secretions in health and disease. Oral Sci. Rev. 1976;8:25–47. [PubMed] [Google Scholar]
- 14.Fox PC. Saliva composition and its importance in dental health. Compend. Suppl. 1989;13:S457–S460. [PubMed] [Google Scholar]
- 15.Oberg SG, Izutsu KT, Truelove EL. Human parotid saliva protein composition: dependence on physiological factors. Am. J. Physiol. 1982;242(3):G231–G236. doi: 10.1152/ajpgi.1982.242.3.G231. [DOI] [PubMed] [Google Scholar]
- 16.Rudney JD, Kajander KC, Smith QT. Correlations between human salivary levels of lysozyme, lactoferrin, salivary peroxidase and secretory immunoglobulin A with different stimulatory states and over time. Arch. Oral Biol. 1985;30(11–12):765–771. doi: 10.1016/0003-9969(85)90129-3. [DOI] [PubMed] [Google Scholar]
- 17.Christodoulides N, Tran M, Floriano PN, et al. A microchip-based multianalyte assay system for the assessment of cardiac risk. Anal. Chem. 2002;74(13):3030–3036. doi: 10.1021/ac011150a. [DOI] [PubMed] [Google Scholar]
- 18.Curey TE, Goodey A, Tsao A, et al. Characterization of multicomponent monosaccharide solutions using an enzyme-based sensor array. Anal. Biochem. 2001;293(2):178–184. doi: 10.1006/abio.2001.5114. [DOI] [PubMed] [Google Scholar]
- 19.Goodey A, Lavigne JJ, Savoy SM, et al. Development of multianalyte sensor arrays composed of chemically derivatized polymeric microspheres localized in micromachined cavities. J. Am. Chem. Soc. 2001;123(11):2559–2570. doi: 10.1021/ja003341l. [DOI] [PubMed] [Google Scholar]
- 20.McCleskey SC, Griffin MJ, Schneider SE, McDevitt JT, Anslyn EV. Differential receptors create patterns diagnostic for ATP and GTP. J. Am. Chem. Soc. 2003;125(5):1114–1115. doi: 10.1021/ja021230b. [DOI] [PubMed] [Google Scholar]
- 21.Ali MF, Kirby R, Goodey AP, et al. DNA hybridization and discrimination of single-nucleotide mismatches using chip-based microbead arrays. Anal. Chem. 2003;75(18):4732–4739. doi: 10.1021/ac034106z. [DOI] [PubMed] [Google Scholar]
- 22.Christodoulides N, Mohanty S, Miller CS, et al. Application of microchip assay system for the measurement of C-reactive protein in human saliva. Lab Chip. 2005;5(3):261–269. doi: 10.1039/b414194f. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez WR, Christodoulides N, Floriano PN, et al. A microchip CD4 counting method for HIV monitoring in resource-poor settings. PLoS Med. 2005;2(7):e182. doi: 10.1371/journal.pmed.0020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christodoulides N, Floriano PN, Acosta SA, et al. Toward the development of a lab-on-a-chip dual-function leukocyte and C-reactive protein ana lysis method for the assessment of inflammation and cardiac risk. Clin. Chem. 2005;51(12):2391–2395. doi: 10.1373/clinchem.2005.054882. [DOI] [PubMed] [Google Scholar]
- 25.Floriano PN, Christodoulides N, Romanovicz D, et al. Membrane-based on-line optical ana lysis system for rapid detection of bacteria and spores. Biosens. Bioelectron. 2005;20(10):2079–2088. doi: 10.1016/j.bios.2004.08.046. [DOI] [PubMed] [Google Scholar]
- 26.Cohen J. Monitoring treatment: at what cost? Science. 2004;304(5679):1936. doi: 10.1126/science.304.5679.1936. [DOI] [PubMed] [Google Scholar]
- 27.Goodey AP, McDevitt JT. Multishell microspheres with integrated chromatographic and detection layers for use in array sensors. J. Am. Chem. Soc. 2003;125(10):2870–2871. doi: 10.1021/ja029696h. [DOI] [PubMed] [Google Scholar]
- 28.McCleskey SC, Floriano PN, Wiskur SL, Anslyn EV, McDevitt JT. Citrate and calcium determination in flavored vodkas using artificial neural networks. Tetrahedron. 2003;59(50):10089–10092. [Google Scholar]
- 29.Wiskur SL, Floriano PN, Anslyn EV, McDevitt JT. A multicomponent sensing ensemble in solution: differentiation between structurally similar analytes. Angew. Chem. Int. Ed. Engl. 2003;42(18):2070–2072. doi: 10.1002/anie.200351058. [DOI] [PubMed] [Google Scholar]
- 30.Li S, Floriano PN, Christodoulides N, et al. Disposable polydimethylsiloxane/silicon hybrid chips for protein detection. Biosens. Bioelectron. 2005;21(4):574–580. doi: 10.1016/j.bios.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Li S, Fozdar D, Ali MF, et al. A continuous flow polymerase chain reaction microchip with regional velocity control. J. Microelectromech. Syst. 2005;14(6):223–236. doi: 10.1109/JMEMS.2005.859083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Floriano PN, Christodoulides N, Miller CS, et al. Use of saliva-based nano-biochip tests for acute myocardial infarction at the point of care: a feasibility study. Clin. Chem. 2009;55(8):1530–1538. doi: 10.1373/clinchem.2008.117713. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ Key paper related to salivary biomarkers important for detection of acute myocardial infarction.
- 33.Armitage GC. Diagnosis of periodontal diseases. J. Periodontol. 2003;74(8):1237–1247. doi: 10.1902/jop.2003.74.8.1237. [DOI] [PubMed] [Google Scholar]
- 34.Petersen PE, Ogawa H. Strengthening the prevention of periodontal disease: the WHO approach. J. Periodontol. 2005;76(12):2187–2193. doi: 10.1902/jop.2005.76.12.2187. [DOI] [PubMed] [Google Scholar]
- 35.Greenstein G. Current interpretations of periodontal probing evaluations: diagnostic and therapeutic implications. Compend. Contin. Educ. Dent. 2005;26(6):381–382. 384, 387–390. quiz 398–389. [PubMed] [Google Scholar]
- 36.Griffiths GS. Formation, collection and significance of gingival crevice fluid. Periodontol. 2000. 2003;31:32–42. doi: 10.1034/j.1600-0757.2003.03103.x. [DOI] [PubMed] [Google Scholar]
- 37.Fox PC. Salivary monitoring in oral diseases. Ann. NY Acad. Sci. 1993;694:234–237. doi: 10.1111/j.1749-6632.1993.tb18356.x. [DOI] [PubMed] [Google Scholar]
- 38.Kaufman E, Lamster IB. Analysis of saliva for periodontal diagnosis - a review. J. Clin. Periodontol. 2000;27(7):453–465. doi: 10.1034/j.1600-051x.2000.027007453.x. [DOI] [PubMed] [Google Scholar]
- 39.Lamster IB, Grbic JT. Diagnosis of periodontal disease based on ana lysis of the host response. Periodontol. 2000. 1995;7:83–99. doi: 10.1111/j.1600-0757.1995.tb00038.x. [DOI] [PubMed] [Google Scholar]
- 40.Seymour GJ, Gemmell E. Cytokines in periodontal disease: where to from here? Acta Odontol. Scand. 2001;59(3):167–173. doi: 10.1080/000163501750266765. [DOI] [PubMed] [Google Scholar]
- 41.Lamster IB, Kaufman E, Grbic JT, Winston LJ, Singer RE. β-glucuronidase activity in saliva: relationship to clinical periodontal parameters. J. Periodontol. 2003;74(3):353–359. doi: 10.1902/jop.2003.74.3.353. [DOI] [PubMed] [Google Scholar]
- 42.Miller CS, King CP, Jr, Langub MC, Kryscio RJ, Thomas MV. Salivary biomarkers of existing periodontal disease: a cross-sectional study. J. Am. Dent. Assoc. 2006;137(3):322–329. doi: 10.14219/jada.archive.2006.0181. [DOI] [PubMed] [Google Scholar]; ■■ Original paper on salivary biomarkers of three biological phases associated with periodontal disease.
- 43.Pietruska M, Bernaczyk A, Knas M, Pietruski J, Zwierz K. Assessment of salivary levels of the chosen exoglycosidases in patients with aggressive periodontitis after treatment with doxycycline. Adv. Med. Sci. 2006;51(Suppl. 1):158–161. [PubMed] [Google Scholar]
- 44.Pederson ED, Stanke SR, Whitener SJ, Sebastiani PT, Lamberts BL, Turner DW. Salivary levels of α2-macroglobulin, α1-antitrypsin, C-reactive protein, cathepsin G and elastase in humans with or without destructive periodontal disease. Arch. Oral Biol. 1995;40(12):1151–1155. doi: 10.1016/0003-9969(95)00089-5. [DOI] [PubMed] [Google Scholar]
- 45.Christodoulides N, Floriano PN, Miller CS, et al. Lab-on-a-chip methods for point-of-care measurements of salivary biomarkers of periodontitis. Ann. NY Acad. Sci. 2007;1098:411–428. doi: 10.1196/annals.1384.035. [DOI] [PubMed] [Google Scholar]; ■ Original paper on the utility of salivary biomarkers and a point-of-care device for diagnostic purposes related to periodontal disease.
- 46.Aurer A, Jorgic-Srdjak K, Plancak D, Stavljenic-Rukavina A, Aurer-Kozelj J. Proinflammatory factors in saliva as possible markers for periodontal disease. Coll. Antropol. 2005;29(2):435–439. [PubMed] [Google Scholar]
- 47.Kupper TS. Mechanisms of cutaneous inflammation. Interactions between epidermal cytokines, adhesion molecules, and leukocytes. Arch. Dermatol. 1989;125(10):1406–1412. doi: 10.1001/archderm.125.10.1406. [DOI] [PubMed] [Google Scholar]
- 48.Larsen CG, Anderson AO, Oppenheim JJ, Matsushima K. Production of interleukin-8 by human dermal fibroblasts and keratinocytes in response to interleukin-1 or tumour necrosis factor. Immunology. 1989;68(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- 49.Meikle MC, Atkinson SJ, Ward RV, Murphy G, Reynolds JJ. Gingival fibroblasts degrade type I collagen films when stimulated with tumor necrosis factor and interleukin 1: evidence that breakdown is mediated by metalloproteinases. J. Periodont. Res. 1989;24(3):207–213. doi: 10.1111/j.1600-0765.1989.tb02007.x. [DOI] [PubMed] [Google Scholar]
- 50.Stashenko P, Dewhirst FE, Peros WJ, Kent RL, Ago JM. Synergistic interactions between interleukin 1, tumor necrosis factor, and lymphotoxin in bone resorption. J. Immunol. 1987;138(5):1464–1468. [PubMed] [Google Scholar]
- 51.Masada MP, Persson R, Kenney JS, Lee SW, Page RC, Allison AC. Measurement of interleukin-1 αand -1 β in gingival crevicular fluid: implications for the pathogenesis of periodontal disease. J. Periodontal Res. 1990;25(3):156–163. doi: 10.1111/j.1600-0765.1990.tb01038.x. [DOI] [PubMed] [Google Scholar]
- 52.Tsai CC, Ho YP, Chen CC. Levels of interleukin-1 β and interleukin-8 in gingival crevicular fluids in adult periodontitis. J. Periodontol. 1995;66(10):852–859. doi: 10.1902/jop.1995.66.10.852. [DOI] [PubMed] [Google Scholar]
- 53.Alexander DC, Martin JC, King PJ, Powell JR, Caves J, Cohen ME. Interleukin-1 β, prostaglandin E2, and immunoglobulin G subclasses in gingival crevicular fluid in patients undergoing periodontal therapy. J. Periodontol. 1996;67(8):755–762. doi: 10.1902/jop.1996.67.8.755. [DOI] [PubMed] [Google Scholar]
- 54.Scannapieco FA, Ng P, Hovey K, Hausmann E, Hutson A, Wactawski-Wende J. Salivary biomarkers associated with alveolar bone loss. Ann. NY Acad. Sci. 2007;1098:496–497. doi: 10.1196/annals.1384.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tobon-Arroyave SI, Jaramillo-Gonzalez PE, Isaza-Guzman DM. Correlation between salivary IL-1β levels and periodontal clinical status. Arch. Oral Biol. 2008;53(4):346–352. doi: 10.1016/j.archoralbio.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Fine DH, Markowitz K, Furgang D, et al. Macrophage inflammatory protein-1a: a salivary biomarker of bone loss in a longitudinal cohort study of children at risk for aggressive periodontal disease? J. Periodontol. 2009;80(1):106–113. doi: 10.1902/jop.2009.080296. [DOI] [PubMed] [Google Scholar]; ■Original paper on a salivary biomarkers associated with risk of bone loss in aggressive periodontitis.
- 57.Ng PY, Donley M, Hausmann E, Hutson AD, Rossomando EF, Scannapieco FA. Candidate salivary biomarkers associated with alveolar bone loss: cross-sectional and in vitro studies. FEMS Immunol. Med. Microbiol. 2007;49(2):252–260. doi: 10.1111/j.1574-695X.2006.00187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakajima K, Matsuda T, Fujitani Y, et al. Signal transduction through IL-6 receptor: involvement of multiple protein kinases, stat factors, and a novel H7-sensitive pathway. Ann. NY Acad. Sci. 1995;762:55–70. doi: 10.1111/j.1749-6632.1995.tb32314.x. [DOI] [PubMed] [Google Scholar]
- 59.Janeway CA, Jr, Yagi J, Rojo J, et al. Immune recognition and effector function in subsets of CD4 T cells. Princess Takamatsu Symp. 1988;19:193–208. [PubMed] [Google Scholar]
- 60.Terpos E, Politou M, Viniou N, Rahemtulla A. Significance of macrophage inflammatory protein-1 α (MIP-1α) in multiple myeloma. Leuk. Lymphoma. 2005;46(12):1699–1707. doi: 10.1080/10428190500175049. [DOI] [PubMed] [Google Scholar]
- 61.Bartold PM, Marshall RI, Haynes DR. Periodontitis and rheumatoid arthritis: a review. J. Periodontol. 2005;76(Suppl. 11):2066–2074. doi: 10.1902/jop.2005.76.11-S.2066. [DOI] [PubMed] [Google Scholar]
- 62.Haynes DR. Bone lysis and inflammation. Inflamm. Res. 2004;53(11):596–600. doi: 10.1007/s00011-004-1303-z. [DOI] [PubMed] [Google Scholar]
- 63.Bertolini DR, Nedwin GE, Bringman TS, Smith DD, Mundy GR. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986;319(6053):516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- 64.Katagiri T, Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002;8(3):147–159. doi: 10.1034/j.1601-0825.2002.01829.x. [DOI] [PubMed] [Google Scholar]
- 65.Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-α induces differentiation of and bone resorption by osteoclasts. J. Biol. Chem. 2000;275(7):4858–4864. doi: 10.1074/jbc.275.7.4858. [DOI] [PubMed] [Google Scholar]
- 66.Kobayashi K, Takahashi N, Jimi E, et al. Tumor necrosis factor α stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J. Exp. Med. 2000;191(2):275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wozniak KL, Arribas A, Leigh JE, Fidel PL., Jr Inhibitory effects of whole and parotid saliva on immunomodulators. Oral Microbiol. Immunol. 2002;17(2):100–107. doi: 10.1046/j.0902-0055.2001.00101.x. [DOI] [PubMed] [Google Scholar]
- 68.Frodge BD, Ebersole JL, Kryscio RJ, Thomas MV, Miller CS. Bone remodeling biomarkers of periodontal disease in saliva. J. Periodontol. 2008;79(10):1913–1919. doi: 10.1902/jop.2008.080070. [DOI] [PubMed] [Google Scholar]
- 69.Grimoud AM, Pontet F, Rousselet F, Lodter JP, Duffaut D, Seguela JP. Comparison of protein concentrations in saliva and serum. Ann. Biol. Clin. (Paris) 1988;46(6):361–370. [PubMed] [Google Scholar]
- 70.Chu CT, Howard GC, Misra UK, et al. A 2-macroglobulin: a sensor for proteolysis. Ann. NY Acad. Sci. 1994;737:291–307. doi: 10.1111/j.1749-6632.1994.tb44319.x. [DOI] [PubMed] [Google Scholar]
- 71.Birkedal-Hansen H, Cobb CM, Taylor RE, Fullmer HM. Serum inhibition of gingival collagenase. J. Oral Pathol. 1974;3(6):284–290. doi: 10.1111/j.1600-0714.1974.tb01723.x. [DOI] [PubMed] [Google Scholar]
- 72.Ohlsson M, Rosengren M, Tegner H, Ohlsson K. Quantification of granulocyte elastase inhibitors in human mixed saliva and in pure parotid secretion. Hoppe Seylers Z. Physiol. Chem. 1983;364(9):1323–1328. doi: 10.1515/bchm2.1983.364.2.1323. [DOI] [PubMed] [Google Scholar]
- 73.Rao RN, Balamuralikrishnan K, Vasantkumar A, Karanth KS, Bhat MK, Aroor AR. A study of antitrypsin and macroglobulin levels in serum and saliva of patients with gingivitis. Indian J. Dent. Res. 1995;6(2):41–46. [PubMed] [Google Scholar]
- 74.Aurer A, Aurer-Kozelj J, Stavljenic-Rukavina A, Kalenic S, Ivic-Kardum M, Haban V. Inflammatory mediators in saliva of patients with rapidly progressive periodontitis during war stress induced incidence increase. Coll. Antropol. 1999;23(1):117–124. [PubMed] [Google Scholar]
- 75.Delaisse JM, Engsig MT, Everts V, et al. Proteinases in bone resorption: obvious and less obvious roles. Clin. Chim. Acta. 2000;291(2):223–234. doi: 10.1016/s0009-8981(99)00230-2. [DOI] [PubMed] [Google Scholar]
- 76.Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5(8):2145–2154. [PubMed] [Google Scholar]
- 77.Birkedal-Hansen H. Role of matrix metalloproteinases in human periodontal diseases. J. Periodontol. 1993;64:474–484. doi: 10.1902/jop.1993.64.5s.474. [DOI] [PubMed] [Google Scholar]
- 78.Sodek J, Overall CM. Matrix metalloproteinases in periodontal tissue remodelling. Matrix Suppl. 1992;1:352–362. [PubMed] [Google Scholar]
- 79.Tonetti MS, Freiburghaus K, Lang NP, Bickel M. Detection of interleukin-8 and matrix metalloproteinases transcripts in healthy and diseased gingival biopsies by RNA/PCR. J. Periodont. Res. 1993;28:511–513. doi: 10.1111/j.1600-0765.1993.tb02114.x. [DOI] [PubMed] [Google Scholar]
- 80.Villela B, Cogen RB, Bartolucci AA, Birkedal-Hansen H. Collagenolytic activity in crevicular fluid from patients with chronic adult periodontitis, localized juvenile periodontitis and gingivitis, and from healthy control subjects. J. Periodontal Res. 1987;22(5):381–389. doi: 10.1111/j.1600-0765.1987.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 81.Ingman T, Sorsa T, Suomalainen K, et al. Tetracycline inhibition and the cellular source of collagenase in gingival crevicular fluid in different periodontal diseases. A review article. J. Periodontol. 1993;64(2):82–88. doi: 10.1902/jop.1993.64.2.82. [DOI] [PubMed] [Google Scholar]
- 82.Sorsa T, Suomalainen K, Uitto VJ. The role of gingival crevicular fluid and salivary interstitial collagenases in human periodontal diseases. Arch. Oral Biol. 1990;35(Suppl.):193S–196S. doi: 10.1016/0003-9969(90)90156-5. [DOI] [PubMed] [Google Scholar]
- 83.Mäntylä P, Stenman M, Kinane DF, et al. Gingival crevicular fluid collagenase-2 (MMP-8) test stick for chair-side monitoring of periodontitis. J. Periodontal Res. 2003;38(4):436–439. doi: 10.1034/j.1600-0765.2003.00677.x. [DOI] [PubMed] [Google Scholar]
- 84.Mäntylä P, Stenman M, Kinane D, et al. Monitoring periodontal disease status in smokers and nonsmokers using a gingival crevicular fluid matrix metalloproteinase-8-specific chair-side test. J. Periodontal Res. 2006;41(6):503–512. doi: 10.1111/j.1600-0765.2006.00897.x. [DOI] [PubMed] [Google Scholar]
- 85.Sorsa T, Hernández M, Leppilahti J, et al. Detection of gingival crevicular fluid MMP-8 levels with different laboratory and chair-side methods. Oral Dis. 2009 doi: 10.1111/j.1601-0825.2009.01603.x. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 86.Kuula H, Salo T, Pirilä E, Tuomainen AM, et al. Local and systemic responses in matrix metalloproteinase 8-deficient mice during Porphyromonas gingivalis-induced periodontitis. Infect. Immun. 2009;77(2):850–859. doi: 10.1128/IAI.00873-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bartold PM, Narayanan AS. The biochemistry and physiology of periodontal connective tissues. In: Wilson TG, Kornman KS, editors. Fundamentals of Periodontics. Quintessence Publishing Co; IL, USA: 2003. pp. 67–93. [Google Scholar]
- 88.Sorsa T, Tjaderhane L, Konttinen YT, et al. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann. Med. 2006;38(5):306–321. doi: 10.1080/07853890600800103. [DOI] [PubMed] [Google Scholar]
- 89.Gorska R, Nedzi-Gora M. The effects of the initial treatment phase and of adjunctive low-dose doxycycline therapy on clinical parameters and MMP-8, MMP-9, and TIMP-1 levels in the saliva and peripheral blood of patients with chronic periodontitis. Arch. Immunol. Ther. Exp. (Warsz.) 2006;54(6):419–426. doi: 10.1007/s00005-006-0047-6. [DOI] [PubMed] [Google Scholar]
- 90.Halinen S, Sorsa T, Ding Y, et al. Characterization of matrix metalloproteinase (MMP-8 and -9) activities in the saliva and in gingival crevicular fluid of children with Down's syndrome. J. Periodontol. 1996;67(8):748–754. doi: 10.1902/jop.1996.67.8.748. [DOI] [PubMed] [Google Scholar]
- 91.Gangbar S, Overall CM, McCulloch CA, Sodek J. Identification of polymorphonuclear leukocyte collagenase and gelatinase activities in mouthrinse samples: correlation with periodontal disease activity in adult and juvenile periodontitis. J. Periodontal Res. 1990;25(5):257–267. doi: 10.1111/j.1600-0765.1990.tb00914.x. [DOI] [PubMed] [Google Scholar]
- 92.Ingman T, Sorsa T, Lindy O, Koski H, Konttinen YT. Multiple forms of gelatinases/type IV collagenases in saliva and gingival crevicular fluid of periodontitis patients. J. Clin. Periodontol. 1994;21(1):26–31. doi: 10.1111/j.1600-051x.1994.tb00272.x. [DOI] [PubMed] [Google Scholar]
- 93.Makela M, Salo T, Uitto VJ, Larjava H. Matrix metalloproteinases (MMP-2 and MMP-9) of the oral cavity: cellular origin and relationship to periodontal status. J. Dent. Res. 1994;73(8):1397–1406. doi: 10.1177/00220345940730080201. [DOI] [PubMed] [Google Scholar]
- 94.Herr AE, Hatch AV, Throckmorton DJ, et al. Microfluidic immunoassays as rapid saliva-based clinical diagnostics. Proc. Natl Acad. Sci. USA. 2007;104(13):5268–5273. doi: 10.1073/pnas.0607254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ingman T, Tervahartiala T, Ding Y, et al. Matrix metalloproteinases and their inhibitors in gingival crevicular fluid and saliva of periodontitis patients. J. Clin. Periodontol. 1996;23(12):1127–1132. doi: 10.1111/j.1600-051x.1996.tb01814.x. [DOI] [PubMed] [Google Scholar]
- 96.Sorsa T, Ding Y, Salo T, et al. Effects of tetracyclines on neutrophil, gingival, and salivary collagenases. A functional and western-blot assessment with special reference to their cellular sources in periodontal diseases. Ann. NY Acad. Sci. 1994;732:112–131. doi: 10.1111/j.1749-6632.1994.tb24729.x. [DOI] [PubMed] [Google Scholar]
- 97.Uitto VJ, Suomalainen K, Sorsa T. Salivary collagenase. Origin, characteristics and relationship to periodontal health. J. Periodontal Res. 1990;25(3):135–142. doi: 10.1111/j.1600-0765.1990.tb01035.x. [DOI] [PubMed] [Google Scholar]
- 98.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 2003;92(8):827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 99.Shibutani T, Yamashita K, Aoki T, Iwayama Y, Nishikawa T, Hayakawa T. Tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) stimulate osteoclastic bone resorption. J. Bone Miner. Metab. 1999;17(4):245–251. doi: 10.1007/s007740050091. [DOI] [PubMed] [Google Scholar]
- 100.Reynolds JJ, Meikle MC. Mechanisms of connective tissue matrix destruction in periodontitis. Periodontol. 2000. 1997;14:144–157. doi: 10.1111/j.1600-0757.1997.tb00195.x. [DOI] [PubMed] [Google Scholar]
- 101.Rider NL, Craig TJ. Liver enzyme elevation and normal pulmonary function in an adult with a declining forced expiratory volume in 1 second. Allergy Asthma Proc. 2008;29(3):345–348. doi: 10.2500/aap.2008.29.3120. [DOI] [PubMed] [Google Scholar]
- 102.Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245(3):194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 103.Sherman KE. Alanine aminotransferase in clinical practice. A review. Arch. Intern. Med. 1991;151(2):260–265. [PubMed] [Google Scholar]
- 104.Chambers DA, Imrey PB, Cohen RL, Crawford JM, Alves ME, McSwiggin TA. A longitudinal study of aspartate aminotransferase in human gingival crevicular fluid. J. Periodontal Res. 1991;26(2):65–74. doi: 10.1111/j.1600-0765.1991.tb01628.x. [DOI] [PubMed] [Google Scholar]
- 105.Hannig C, Spitzmuller B, Hannig M. Transaminases in the acquired pellicle. Arch. Oral Biol. 2009;54(5):445–448. doi: 10.1016/j.archoralbio.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 106.Totan A, Greabu M, Totan C, Spinu T. Salivary aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase: possible markers in periodontal diseases? Clin. Chem. Lab. Med. 2006;44(5):612–615. doi: 10.1515/CCLM.2006.096. [DOI] [PubMed] [Google Scholar]
- 107.Nomura Y, Tamaki Y, Tanaka T, et al. Screening of periodontitis with salivary enzyme tests. J. Oral Sci. 2006;48(4):177–183. doi: 10.2334/josnusd.48.177. [DOI] [PubMed] [Google Scholar]
- 108.Welker D. Dental caries susceptibility and transaminases in human saliva. Dtsch Stomatol. 1966;16(12):910–917. [PubMed] [Google Scholar]
- 109.Musumeci V, Cherubini P, Zuppi C, Zappacosta B, Ghirlanda G, Di Salvo S. Aminotransferases and lactate dehydrogenase in saliva of diabetic patients. J. Oral Pathol. Med. 1993;22(2):73–76. doi: 10.1111/j.1600-0714.1993.tb00047.x. [DOI] [PubMed] [Google Scholar]
- 110.Shimada K, Mizuno T, Uchida T, Kato T, Ito K, Murai S. Relationship between levels of aspartate aminotransferase in gingival crevicular fluid and conventional measures of periodontal status assessed using PocketWatch: a cross-sectional study. J. Oral Sci. 1999;41(1):35–40. doi: 10.2334/josnusd.41.35. [DOI] [PubMed] [Google Scholar]
- 111.Yucekal-Tuncer B, Uygur C, Firatli E. Gingival crevicular fluid levels of aspartate amino transferase, sulfide ions and N-benzoyl-dl-arginine-2-naphthylamide in diabetic patients with chronic periodontitis. J. Clin. Periodontol. 2003;30(12):1053–1060. doi: 10.1046/j.0303-6979.2003.00426.x. [DOI] [PubMed] [Google Scholar]
- 112.Shimada K, Mizuno T, Ohshio K, Kamaga M, Murai S, Ito K. Analysis of aspartate aminotransferase in gingival crevicular fluid assessed by using PocketWatch: a longitudinal study with initial therapy. J. Clin. Periodontol. 2000;27(11):819–823. doi: 10.1034/j.1600-051x.2000.027011819.x. [DOI] [PubMed] [Google Scholar]
- 113.Cesco Rde T, Ito IY, de Albuquerque RF., Jr Levels of aspartate aminotransferase (AST) in saliva of patients with different periodontal conditions. J. Clin. Periodontol. 2003;30(8):752–755. doi: 10.1034/j.1600-051x.2003.00373.x. [DOI] [PubMed] [Google Scholar]; ■ Original paper on a salivary biomarker associated with cell injury in periodontitis.
- 114.Zappacosta B, Manni A, Persichilli S, et al. Salivary thiols and enzyme markers of cell damage in periodontal disease. Clin. Biochem. 2007;40(9–10):661–665. doi: 10.1016/j.clinbiochem.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 115.Yoshie H, Tai H, Kobayashi T, et al. Salivary enzyme levels after scaling and interleukin-1 genotypes in Japanese patients with chronic periodontitis. J. Periodontol. 2007;78(3):498–503. doi: 10.1902/jop.2007.060216. [DOI] [PubMed] [Google Scholar]; ■ Original paper on a salivary biomarker related to response to periodontal therapy.
- 116.Persson GR, DeRouen TA, Page RC. Relationship between gingival crevicular fluid levels of aspartate aminotransferase and active tissue destruction in treated chronic periodontitis patients. J. Periodontal Res. 1990;25(2):81–87. doi: 10.1111/j.1600-0765.1990.tb00896.x. [DOI] [PubMed] [Google Scholar]
- 117.Imrey PB, Crawford JM, Cohen RL, Alves ME, McSwiggin TA, Chambers DA. A cross-sectional ana lysis of aspartate aminotransferase in human gingival crevicular fluid. J. Periodontal Res. 1991;26(2):75–84. doi: 10.1111/j.1600-0765.1991.tb01629.x. [DOI] [PubMed] [Google Scholar]
- 118.Ingman T, Sorsa T, Konttinen YT, et al. Salivary collagenase, elastase- and trypsin-like proteases as biochemical markers of periodontal tissue destruction in adult and localized juvenile periodontitis. Oral Microbiol. Immunol. 1993;8(5):298–305. doi: 10.1111/j.1399-302x.1993.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 119.Uitto VJ, Nieminen A, Coil J, Hurttia H, Larjava H. Oral fluid elastase as an indicator of periodontal health. J. Clin. Periodontol. 1996;23(1):30–37. doi: 10.1111/j.1600-051x.1996.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 120.Nieminen A, Nordlund L, Uitto VJ. The effect of treatment on the activity of salivary proteases and glycosidases in adults with advanced periodontitis. J. Periodontol. 1993;64(4):297–301. doi: 10.1902/jop.1993.64.4.297. [DOI] [PubMed] [Google Scholar]
- 121.Paster BJ, Russell MK, Alpagot T, et al. Bacterial diversity in necrotizing ulcerative periodontitis in HIV-positive subjects. Ann. Periodontol. 2002;7(1):8–16. doi: 10.1902/annals.2002.7.1.8. [DOI] [PubMed] [Google Scholar]
- 122.Kugahara T, Shosenji Y, Ohashi K. Screening for periodontitis in pregnant women with salivary enzymes. J. Obstet. Gynaecol. Res. 2008;34(1):40–46. doi: 10.1111/j.1447-0756.2007.00681.x. [DOI] [PubMed] [Google Scholar]
- 123.Garnero P, Ferreras M, Karsdal MA, et al. The type I collagen fragments ICTP and CTX reveal distinct enzymatic pathways of bone collagen degradation. J. Bone Miner. Res. 2003;18(5):859–867. doi: 10.1359/jbmr.2003.18.5.859. [DOI] [PubMed] [Google Scholar]
- 124.Garnero P. Biomarkers for osteoporosis management: utility in diagnosis, fracture risk prediction and therapy monitoring. Mol. Diagn. Ther. 2008;12(3):157–170. doi: 10.1007/BF03256280. [DOI] [PubMed] [Google Scholar]
- 125.Delmas PD. Bone marker nomenclature. Bone. 2001;28(6):575–576. doi: 10.1016/s8756-3282(01)00480-x. [DOI] [PubMed] [Google Scholar]
- 126.Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res. Ther. 2007;9(Suppl. 1):S1. doi: 10.1186/ar2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. J. Am. Med. Assoc. 2004;292(4):490–495. doi: 10.1001/jama.292.4.490. [DOI] [PubMed] [Google Scholar]
- 128.Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 129.Buduneli N, Biyikoglu B, Sherrabeh S, Lappin DF. Saliva concentrations of RANKL and osteoprotegerin in smoker versus non-smoker chronic periodontitis patients. J. Clin. Periodontol. 2008;35(10):846–852. doi: 10.1111/j.1600-051X.2008.01310.x. [DOI] [PubMed] [Google Scholar]
- 130.Callander NS, Roodman GD. Myeloma bone disease. Semin. Hematol. 2001;38(3):276–285. doi: 10.1016/s0037-1963(01)90020-4. [DOI] [PubMed] [Google Scholar]
- 131.Lieben L, Callewaert F, Bouillon R. Bone and metabolism: a complex crosstalk. Horm. Res. 2009;71(Suppl. 1):134–138. doi: 10.1159/000178056. [DOI] [PubMed] [Google Scholar]
- 132.Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol. 2001;19(8):816–827. doi: 10.1016/s0945-053x(00)00133-5. [DOI] [PubMed] [Google Scholar]
- 133.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics - 2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 134.The National Academy of Clinical Biochemistry . Biomarkers of acute coronary syndromes and heart failure. In: Christenson RH, editor. Laboratory Medicine Practice Guidelines. The American Association for Clinical Chemistry; Washington, DC, USA: 2007. pp. 1–26. [Google Scholar]
- 135.Wilper AP, Woolhandler S, Lasser KE, et al. Waits to see an emergency department physician: U.S. trends and predictors, 1997–2004. Health Aff. (Millwood) 2008;27(2):84–95. doi: 10.1377/hlthaff.27.2.w84. [DOI] [PubMed] [Google Scholar]
- 136.Ross R. Atherosclerosis - an inflammatory disease. N. Engl. J. Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 137.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113(19):2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]; ■ Up-to-date paper on tissue and serum biomarkers of cardiovascular disease.
- 138.Wu T, Trevisan M, Genco RJ, Dorn JP, Falkner KL, Sempos CT. Periodontal disease and risk of cerebrovascular disease: the first national health and nutrition examination survey and its follow-up study. Arch. Intern. Med. 2000;160(18):2749–2755. doi: 10.1001/archinte.160.18.2749. [DOI] [PubMed] [Google Scholar]
- 139.Baldus S, Heeschen C, Meinertz T, et al. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108(12):1440–1445. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 140.Malik I, Danesh J, Whincup P, et al. Soluble adhesion molecules and prediction of coronary heart disease: a prospective study and meta-analysis. Lancet. 2001;358(9286):971–976. doi: 10.1016/S0140-6736(01)06104-9. [DOI] [PubMed] [Google Scholar]
- 141.Apple FS, Wu AH, Mair J, et al. Future biomarkers for detection of ischemia and risk stratification in acute coronary syndrome. Clin. Chem. 2005;51(5):810–824. doi: 10.1373/clinchem.2004.046292. [DOI] [PubMed] [Google Scholar]
- 142.Nakamura M, Slots J. Salivary enzymes. Origin and relationship to periodontal disease. J. Periodontal Res. 1983;18(6):559–569. doi: 10.1111/j.1600-0765.1983.tb00393.x. [DOI] [PubMed] [Google Scholar]
- 143.Mellanen L, Lahdevirta J, Tervahartiala T, Meurman JH, Sorsa T. Matrix metalloproteinase-7, -8, -9, -25, and -26 and CD43, -45, and -68 cell-markers in HIV-infected patients' saliva and gingival tissue. J. Oral Pathol. Med. 2006;35(9):530–539. doi: 10.1111/j.1600-0714.2006.00449.x. [DOI] [PubMed] [Google Scholar]
- 144.Terpos E, Anagnostopoulos A, Kastritis E, Bamias A, Tsionos K, Dimopoulos MA. Abnormal bone remodelling and increased levels of macrophage inflammatory protein-1α (MIP-1a) in Waldenstrom macroglobulinaemia. Br. J. Haematol. 2006;133(3):301–304. doi: 10.1111/j.1365-2141.2006.06017.x. [DOI] [PubMed] [Google Scholar]
- 145.Boyer KG, France JT. Alkaline phosphatase, arylsulfatase and β-glucuronidase in saliva of cyclic women. Int. J. Fertil. 1976;21(1):43–48. [PubMed] [Google Scholar]
- 146.Rhodus NL, Ho V, Miller CS, Myers S, Ondrey F. NF-κB dependent cytokine levels in saliva of patients with oral preneoplastic lesions and oral squamous cell carcinoma. Cancer Detect. Prev. 2005;29(1):42–45. doi: 10.1016/j.cdp.2004.10.003. [DOI] [PubMed] [Google Scholar]; ■ Original paper demonstrating that premalignant and malignant oral disease can modulate salivary biomarkers.
- 147.Rhodus NL, Cheng B, Bowles W, Myers S, Miller L, Ondrey F. Proinflammatory cytokine levels in saliva before and after treatment of (erosive) oral lichen planus with dexamethasone. Oral Dis. 2006;12(2):112–116. doi: 10.1111/j.1601-0825.2005.01165.x. [DOI] [PubMed] [Google Scholar]
- 148.Thomas MV, Branscum A, Miller CS, Ebersole J, Al-Sabbagh M, Schuster JL. Within-subject variability in repeated measures of salivary analytes in healthy adults. J. Periodontol. 2009;80(7):1146–1153. doi: 10.1902/jop.2009.080654. [DOI] [PMC free article] [PubMed] [Google Scholar]