Abstract
Although NSAIDs have a well-established place for certain indications in the management of OA and RA, they are associated with significant gastrointestinal (GI) toxicity. The risk of NSAID-related upper GI events, such as dyspepsia or peptic ulcer and complications such as perforation or bleeding, is well characterized. Non-selective NSAIDs increase the risk of peptic ulcer disease ∼5-fold, and that of upper GI bleeding 4-fold, whereas selective cyclo-oxygenase-2 (COX) inhibitors are associated with a significantly lower GI toxicity than non-selective agents. There is evidence that, while the incidence of NSAID-related upper GI complications has decreased in recent years, that of lower GI complications is increasing. Observational studies and analyses from studies, primarily designed to investigate upper GI events, suggest that lower GI complications are relatively common in NSAID users and that COX-2 selective inhibitors are associated with a lower risk of these events. Such events have been poorly characterized, but are associated with significant mortality; indeed, they may have even more serious consequences than the better characterized upper GI events. There is thus a strong case for evaluating the impact of such complications in prospective outcome studies. To facilitate such studies a new endpoint, Clinically Significant Upper or Lower GI Events, has been introduced that captures both upper and lower GI events.
Keywords: Adverse events, Cyclo-oxygenase-2 selective inhibitors, Clinically Significant Upper or Lower GI Events, Gastrointestinal bleeding, Lower gastrointestinal complications, NSAIDs, Peptic ulcer, Upper gastrointestinal complications
Introduction
NSAIDs have consistently been shown to be more effective than acetaminophen (paracetamol) in the management of OA of hip or knee [1, 2] and their use is endorsed in current OA management guidelines, which review the available evidence [3, 4]. However, the use of these agents is associated with gastrointestinal (GI) toxicity [5], including asymptomatic mucosal damage (erosions and ulcers), abdominal pain or dyspepsia with or without mucosal damage, and serious complications such as bleeding ulcers requiring hospitalization [6]. Such problems are common in NSAID-treated patients. For example, in an endoscopic study by Geis et al. [7], gastric or duodenal ulcers were present in 24% of NSAID-treated individuals with OA or RA, whereas the Food and Drug Administration Arthritis Advisory Committee notes that symptomatic ulcers and potentially life-threatening complications have been found in up to 4% of patients per year [5]. The potential impact of these adverse events is highlighted by data from Spain, which show that the mortality rate associated with NSAID or ASA use is ∼5.6%, equivalent to 15.3 deaths per 100 000 users [8]. To put this risk into perspective, data from the USA in 2006 indicate that the risks of dying as a result of a car accident or firearm injury are approximately 15 and 10 per 100 000, respectively [9].
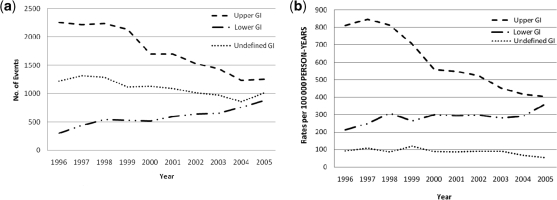
The past decade has seen major advances in the prevention and management of ulcer complications, such as a decrease in the prevalence of Helicobacter pylori infection and improved treatment of acute ulcer bleeding [10], and recent evidence suggests that these developments have been reflected in a change in the pattern of NSAID-related GI complications seen in clinical practice [11]. Thus, while the incidence of complications involving the upper GI tract has decreased steadily during the last decade, perforations and bleeding in the lower GI tract have increased (Fig. 1). Such findings suggest that, whereas attention has traditionally focused on NSAID-related complications in the stomach or duodenum, we need to adopt a broader perspective and consider the potential adverse effects of NSAIDs in the GI tract as a whole. This article reviews the adverse effects of non-selective NSAID and cyclo-oxygenase-2 (COX-2) selective inhibitors in the upper and lower GI tract, and the need for a measurement that incorporates both upper and lower GI complications as an endpoint in outcome studies with NSAIDs.
Fig. 1.
Total number of GI complications per year (a) and estimated incidence of GI complications (per 100 000 person-years) (b) in Spain, 1996–2005 [12]. Reproduced from Lanas et al. [11].
Upper GI tract complications associated with non-selective NSAIDs and COX-2 selective inhibitors
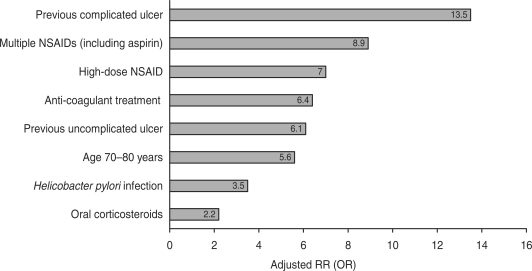
The risks of upper GI toxicity associated with non-selective NSAIDs have been extensively studied. Case-control studies and meta-analyses have shown that the risk of upper GI complications is increased ∼4-fold in NSAID users, compared with non-users [12, 13], and the risk of peptic ulcer disease is increased 5-fold [14]. The risk is highest during the first month of treatment [relative risk (RR) 5.7; 95% CI 4.9, 6.6], and then remains elevated afterwards [12]. Risk factors for NSAID-related bleeding include age ⩾60 years (and especially >70 years) [12, 13], high-dose NSAID treatment, a previous history of peptic ulcer with or without complications, co-therapy with low-dose aspirin, anti-coagulants or steroids and H. pylori infection [14] (Fig. 2). NSAIDs and H. pylori have synergistic effects on risk; in a meta-analysis of 16 studies involving 1625 NSAID users, the odds ratio (OR) for peptic ulcer disease in H. pylori-positive NSAID users was 61.1 (95% CI 9.98, 373), compared with H. pylori-negative non-users [14].
Fig. 2.
Risk factors for upper GI bleeding associated with NSAID use [13–15]. Adapted from Pérez Gutthann et al. [13], Huang et al. [14] and Lanas et al. [15].
The risk of bleeding depends on the individual NSAID. In a case–control study involving 2777 patients with confirmed upper GI bleeding, the highest risk of non-selective NSAIDs was seen with ketorolac (RR, compared with non-use of NSAIDs, 14.4; 95% CI 5.2, 39.9) and the lowest with aceclofenac (RR 2.6; 95% CI 1.5, 4.6) [15], whereas celecoxib was not associated with increased risk of ulcer bleeding (RR 1.0; 95% CI 0.4, 2.1) [15]. The study does not determine why this occurs, but it is quite consistent with other case–control studies. It is important to note that in many cases the first evidence of an NSAID-related ulcer is a life-threatening complication; for example, in a study of 235 patients with life-threatening peptic ulcer complications, 58.2% had previously been asymptomatic [16].
Selective COX-2 inhibitors inhibit the production via COX-2 of PGs mediating pain and inflammation, while preserving COX-1-mediated production of PGs involved in the maintenance of GI mucosal integrity [17, 18]. As a result, these agents might be expected to offer a more favourable safety profile than non-selective NSAIDs with respect to upper GI bleeding. Although COX-1 inhibition is not the only mechanism involved in NSAID-induced GI toxicity, a systematic review of randomized controlled trials has shown that COX-2 selective inhibitors produced significantly fewer gastroduodenal ulcers (RR 0.26; 95% CI 0.23, 0.30) and clinically important ulcer complications (RR 0.39; 95% CI 0.31, 0.50) than non-selective NSAIDs [19]. However, there is evidence, both from this systematic review and from studies such as CLASS [17] and SUCCESS-1 [20] that this safety advantage is reduced in patients receiving concomitant low-dose ASA treatment. For example, in the SUCCESS-1 study, the risk of ulcer complications in patients receiving naproxen or diclofenac was significantly higher than in those receiving the COX-2 selective inhibitor celecoxib (OR 14.1; 95% CI 1.8, 633.5; P = 0.001) in the absence of ASA; in contrast, there was no significant difference in risk between the two groups of ASA users (OR 1.98; 95% CI 0.1, 27.4; P = 0.49) [20]. A recent meta-analysis of all available trials including patients taking low-dose ASA combined with either non-selective NSAIDs or COX-2 selective inhibitors, indicate a 28% risk reduction (RR = 0.72; 95% CI 0.62, 0.95) of the GI risk in patients taking the combination of ASA + COX-2 [21].
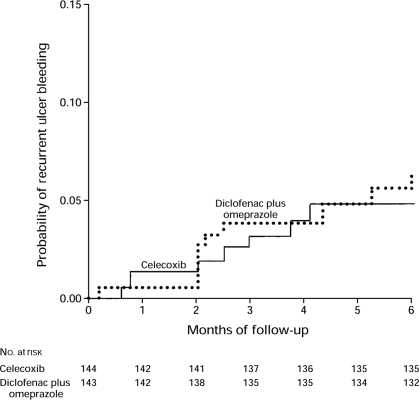
Current OA management guidelines [3, 22] recommend that patients at increased risk of GI complications should receive either a non-selective NSAID with an appropriate gastroprotective agent, such as a proton pump inhibitor (PPI), or a COX-2 selective inhibitor alone. These two strategies were compared in a randomized, double-blind trial involving 287 arthritis patients who received either diclofenac plus omeprazole, or celecoxib, 200 mg twice daily, for 6 months [23]. The risk of recurrent ulcer bleeding did not differ significantly in the two groups (Fig. 3). More recently, the same group have investigated the use of combination therapy with a COX-2 selective inhibitor and a PPI to prevent recurrent ulcer bleeding in high-risk patients. In this randomized, double-blind trial, 441 patients with upper GI bleeding received celecoxib, 200 mg twice daily, alone or in combination with esomeprazole, 20 mg twice daily, for 12 months [24]. The incidence of recurrent bleeding within 12 months was significantly lower with combination therapy than with celecoxib alone (0 vs 8.9%; P = 0.0004), and there were no differences in discontinuation rate or the incidence of adverse events between the two groups. Whereas this study was carried out in H. pylori-negative patients, and therefore their validity in H. pylori-infected patients can be questioned, it must be noted that patients with ulcer history positive for H. pylori should undergo eradication of the infection.
Fig. 3.
Kaplan–Meier plot showing the risk of recurrent ulcer bleeding in 287 arthritis patients treated for 6 months with either diclofenac, 75 mg twice daily, plus omeprazole, 20 mg twice daily, or celecoxib, 200 mg twice daily [23]. Reproduced with permission from Chan et al. [23]. Copyright © 2002 Massachusetts Medical Soceity. All rights reserved.
Lower GI tract damage associated with NSAIDs
In contrast to the well-documented risk of upper GI damage associated with NSAIDs, NSAID-related lower GI damage has not been widely studied and remains poorly characterized [25]. This damage includes increased mucosal permeability, mucosal inflammation, overt or occult blood loss, malabsorption, protein loss, ileal dysfunction, diarrhoea, ulceration, strictures, major bleeding and perforation [26]. Data on the incidence of NSAID-related lower GI side effects come from observational or case–control studies, and from analyses of outcome trials in which the primary endpoints were related to upper GI or cardiovascular events.
Observational studies
The association between NSAIDs and injury to the lower intestine has been recognized for almost two decades. In a landmark study, Allison et al. [27] found post-mortem evidence of small intestinal ulceration in 8.4% of NSAID users, compared with 0.6% of non-users (treatment difference 7.8%; 95% CI 5.0, 10.6%; P < 0.001). It must be noted that this study probably underestimated small bowel damage due to post-mortem autolysis [27]. Subsequently, three case–control studies showed the association between NSAID and ASA use with both upper and lower GI complications. One of them was a case–control study involving 566 patients hospitalized for upper or lower GI bleeding, which found that the OR for lower GI bleeding in NSAID users, compared with non-users, was 2.6 (95% CI 1.7, 3.9; P < 0.001) [28]. The other two used similar methodology combining objective testing of ASA use in blood and clinical history for NSAID use. One found that NSAID and ASA use were equally associated with either upper or lower GI bleeding [29] and the other one that both types of drugs were associated with both upper and lower GI perforation [30].
Epidemiological data show that NSAID-related lower GI complications are associated with significant mortality. Data from an observational study in Spain showed that the mortality rate in patients with NSAID-related lower GI bleeding was comparable to that in patients with upper GI bleeding (5.3 and 5.7%, respectively) [8]. Moreover, the mortality rate among patients with either upper or lower GI perforation was markedly higher (30.1%) than in those with GI bleeding. More recent data from Spain show a decrease in mortality in patients hospitalized with upper GI events but not in the lower GI mortality rate [11]. NSAIDS can also aggravate lesions and induce GI complications from pre-existing diseases such as inflammatory bowel disease, diverticulosis or angiodysplasia [31].
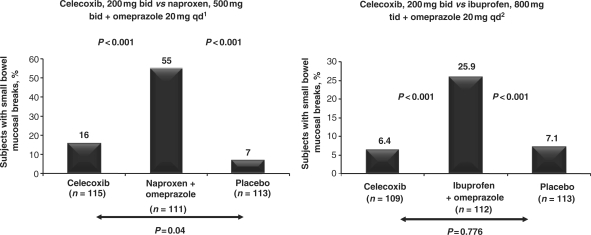
Endoscopy studies. A number of studies have explored the use of new technologies, such as capsule endoscopy or double-balloon enteroscopy, to investigate the occurrence of mucosal lesions or events occurring along the lower bowel. For example, two randomized, placebo-controlled trials have used video capsule endoscopy to assess small bowel injury in healthy volunteers treated with either celecoxib or a combination of a non-selective NSAID and omeprazole [32, 33]. In both studies, the incidence of small bowel mucosal breaks in celecoxib-treated participants was significantly lower than that in those receiving the combination of a non-selective NSAID and omeprazole, and comparable to that in the placebo group (Fig. 4). Double-balloon enteroscopy is an invasive procedure that is associated with a higher risk of surgical complications such as perforation than conventional endoscopy [34]. Visualization of lesions may, however, be better. It has been reported that video capsule endoscopy may have a false positive rate of ∼43% [35], and that this technique misses significant lesions that are detectable by double-balloon enteroscopy [36].
Fig. 4.
Incidence of small bowel mucosal breaks, assessed by video capsule endoscopy, in two randomized, placebo-controlled studies comparing celecoxib, 200 mg twice daily, with the combination of a non-selective NSAID and omeprazole in healthy volunteers [32, 33]. Reproduced from Goldstein et al. [32] with permission from the American Gastroenterological Association and Goldstein et al. [33] with permission from Wiley-Blackwell.
Outcome clinical trials
Despite the high incidence of NSAID-related lower GI complications in observational studies, and the mortality associated with such complications, no published outcome studies have specifically addressed the clinical impact of these adverse effects on NSAID users. Indirect evidence comes from outcome trials investigating NSAID-related upper GI or cardiovascular complications.
The Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) study compared diclofenac and etoricoxib in 34 701 patients with OA or RA, who were treated for up to 3.5 years, with a primary endpoint of confirmed thrombotic cardiovascular events [37, 38]. The overall incidence of upper and lower GI events in this study was 0.47 and 0.56%, respectively. Upper GI events (symptomatic ulcers plus upper GI complications) were significantly less common with etoricoxib than with diclofenac [hazard ratio (HR) 0ċ69; 95% CI 0ċ57, 0ċ83; P = 0ċ0001] [37]. In this study, 35% of patients were receiving low-dose ASA and 39% were receiving a PPI. These two factors may have affected the results of the study. First, low-dose ASA combined with either etoricoxib or diclofenac may have increased the incidence of both upper and lower GI events. Secondly, the use of a PPI may result in a relative increase in lower GI events when compared with those of the upper GI tract. In any case, gastroprotective therapy did not completely abolish the risk of upper GI events. However, although this therapy was supposed to be taken throughout the duration of the trial in all patients at risk, it was not taken by the majority of patients, and the authors suggest that it did decrease the risk in patients who were at the greatest GI risk and were taking PPIs.
The finding that the incidence of lower GI events in this study was higher than that of upper GI events is not surprising because, while PPIs can reduce the risk of upper GI events they would not be expected to have a protective effect in the mid- and large bowel. There was no significant difference between rates of lower GI clinical events for etoricoxib and diclofenac: rates being 0.32 and 0.38 per 100 patient-years (HR = 0.84; 95% CI 0.63, 1.13), respectively. The analysis of lower GI events did indicate that significant risk factors for a lower GI event are a prior lower GI event (HR = 4.06; 95% CI 2.93, 5.62) and age ⩾65 years (HR = 1.98; 95% CI 1.45, 2.71) [38].
In the Misoprostol Ulcer Complications Outcomes Safety Assessment (MUCOSA) trial with misoprostol, serious lower GI events were found by the investigators to be more common than those from the upper GI tract and occurred in 147 patients, whereas only 95 individuals experienced upper GI complications [39]. Similarly, in the Vioxx GI Outcomes Research (VIGOR) trial, which compared naproxen and rofecoxib in 8076 patients with RA, serious lower GI events, such as perforation, obstruction or major bleeding, accounted for 39.4% of all serious GI adverse events among naproxen-treated patients and 42.7% of such events among rofecoxib-treated patients [40].
The incidence of lower GI events
Together, the available evidence from epidemiological and outcome studies indicates that NSAID-related lower GI damage is relatively common. Only one recent study from Canada reported lower rates of hospitalization due to lower GI complications than upper [41]. This was a population-based retrospective cohort study that included 644 183 elderly patients, aged ⩾65 years, who received 1 778 541 prescriptions for non-selective NSAIDs (315 222, 17.7% with a PPI). The study examined ulceration, perforation or bleeding in the GI tract in study individuals taking non-selective NSAIDs or acetaminophen with or without a PPI. Among users of non-selective NSAIDs without a PPI, the crude rates of hospitalization were 0.7 cases per 1000 patient-years for lower GI complications compared with 4.4 cases per 1000 patient-years for complications in the upper GI tract [41]. However, when the non-selective NSAID was taken with a PPI, the rates of hospitalization were 1.4 cases per 1000 patient-years for the lower GI tract compared with 2.0 cases per 1000 patient-years for the upper GI tract [40]. It might be suggested that hospitalizations for upper but not lower GI complications associated with non-selective NSAIDs were reduced by the addition of a PPI.
A systematic literature review reported that mucosal breaks or small intestinal injuries were present in up to 71% of NSAID users, and that up to 88% of patients with lower GI bleeding were NSAID users [25]; the ORs for bleeding or perforation associated with NSAID treatment ranged from 1.9 to 18.4 or from 2.5 to 8.1, respectively. The risk of such problems was lower in patients receiving COX-2 selective inhibitors than in those receiving non-selective NSAIDs; the RRs for small mucosal breaks, haematochezia (maroon-coloured blood in the stool) and lower GI clinical events were 0.3 (95% CI 0.2, 0.5), 0.4 (95% CI 0.2, 0.8) and 0.5 (95% CI 0.2, 0.9; P = 0.03), respectively, compared with non-selective agents [25].
The significance of lower GI complications to the patient
Although serious lower GI complications such as perforation or overt bleeding are of major clinical concern because of their life-threatening nature, events that are less severe or even asymptomatic can still have a marked impact on the patient. Symptomatic lesions can lead to the patient discontinuing potentially beneficial NSAID treatment, whereas occult blood loss or anaemia can result in impaired physical performance and diminished quality of life. For example, in the InChianti (Invecchiare in Chianti [Ageing in the Chianti Area]) study, the presence of anaemia (defined according to World Health Organization criteria as a haemoglobin concentration <13 g/dl in men or <12 g/dl in women) was associated with poorer performance (mean short physical performance battery scores 8.8 vs 9.6; P = 0.003) and more disabilities (P < 0.001), compared with non-anaemic individuals [42]. People with anaemia also had significantly lower knee extensor strength (14.1 vs 15.2 kg; P = 0.02) and handgrip strength (25.3 vs 27.1 kg; P = 0.04) than those without anaemia.
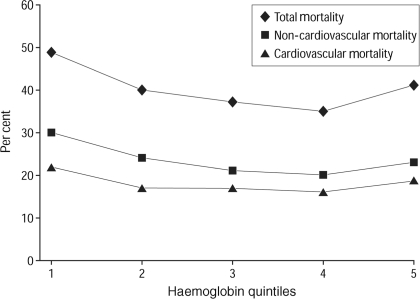
In addition to impairing performance and quality of life, anaemia can also increase the risk of other adverse events. Evidence for this comes from the Cardiovascular Health Study, a prospective cohort study involving 5888 individuals aged ⩾65 years [43]. Overall, 1205 participants were in the lowest quintile of haemoglobin concentration (<13.6 g/dl for men and <12.6 g/dl for women), and 498 (8.5%) were anaemic. Individuals in both the lowest and highest quintiles of haemoglobin concentrations were at significantly higher risk of both cardiovascular and overall mortality (Fig. 5), with adjusted HRs of 1.57 (95% CI 1.38, 1.78) and 1.38 (95% CI 1.19, 1.54), respectively. These associations persisted after adjustment for causes and consequences of anaemia, such as renal dysfunction, inflammation or frailty.
Fig. 5.
Unadjusted mortality over 11.2 years, according to haemoglobin concentration quintiles, in the Cardiovascular Health Study [43]. Reproduced with permission from Zakai et al. [43]. Copyright © 2005 American Medical Association. All rights reserved.
The need for a new endpoint in NSAID outcome studies
Previously, outcome studies with NSAIDs have focused on adverse events affecting the upper GI tract, in view of the well-established association between NSAIDs and such complications. However, given the strong evidence that NSAIDs also damage the lower GI tract, and that this can have important consequences for the patient, there is a strong case for evaluating the impact of such complications in prospective outcome studies. This will require the use of an endpoint that captures both upper and lower GI events.
Studies that have reported lower GI complications in NSAID-treated patients have used a variety of endpoints, ranging from changes in intestinal permeability resulting from mucosal damage to overt clinical complications such as perforation, ulceration or bleeding [25]. In general, however, the clinical endpoints of NSAID-induced damage to the lower GI tract are not well defined, and the impact of those that have already been described is unclear. Thus, reliable endpoints that evaluate the entire GI tract are needed for NSAID outcome studies.
Development of a new endpoint: clinically significant upper or lower GI events
It is well known that bleeding, perforation or obstruction can occur in both the upper and the lower GI tract and can all be associated with NSAID use [5, 6, 29]. Therefore, evaluation of NSAID-related events in the GI tract should include all these endpoints. Furthermore, identification of the responsible lesion associated with a major complication is sometimes a difficult task, especially when the responsible lesion is not identified in the upper GI endoscopy performed soon after hospital admission. Exploration of the small and large bowel may be difficult, complex and require additional technology and trained staff, which are not always available in all hospitals. Also, delay in performing the appropriate test reduces the probability of finding mucosal lesions induced by NSAIDs. Consequently, major GI events not linked to a specific lesion should not prevent the inclusion of those events as clinical endpoints. In addition, patients using NSAIDs may develop a significant haemoglobin drop [25], which is not always macroscopically visualized as GI bleeding, but that has a clear GI origin, and as noted above, may have a relevant clinical impact. In view of these considerations, a new endpoint has been introduced that comprises both upper and lower GI events, with or without endoscopic lesions (Table 1). This endpoint has been named Clinically Significant Upper or Lower GI Events (CSULGIEs) [44].
Table 1.
CSULGIEs
| With lesion | Without lesion |
|---|---|
| Gastroduodenal haemorrhage | Acute GI haemorrhage of unknown origin, including presumed |
| Gastric outlet obstruction | small bowel haemorrhage |
| Gastroduodenal, small bowel or large bowel perforation | Clinically significant anaemia of presumed occult GI origin |
| Small bowel haemorrhage | including possible small bowel blood loss |
| Large bowel haemorrhage | |
| Clinically significant anaemia of defined GI origin |
This endpoint has been used in the recent Celecoxib vs Omeprazole aNd Diclofenac for at-risk OA and RA patients (CONDOR) study (NCT00141102), which compared celecoxib, 200 mg twice daily, with diclofenac slow release (SR), 75 mg twice daily, plus omeprazole, 20 mg once daily [44]. The primary aim of this study was to determine whether celecoxib is superior to combined therapy with diclofenac SR plus omeprazole in preventing CSULGIEs in high-risk patients with OA or RA. This study is the first major outcome study in which lower GI events were included in a prospectively designated endpoint. It is expected that CONDOR will provide additional data that may be useful for the clinician who now has two options (COX-2 alone vs non-selective NSAIDs plus a PPI), which are equally effective in the prevention of GI damage in the upper GI tract. It remains to be seen whether, when considering the entire GI tract, one of the options (COX-2 alone) will be superior to the other one (ns-NSAIDs plus a PPI) since PPIs are not expected to have any effect beyond the duodenum.
Conclusions
The available evidence shows that the incidence of lower GI adverse events associated with NSAID use is increasing. Such events have been poorly characterized, but are associated with significant mortality; indeed, they may have even more serious consequences than the widely recognized and well-characterized upper GI events.
There is a clear need for new outcome measurements that reflect the risk of lower GI events in clinical trials with non-selective NSAIDs and COX-2 selective inhibitors. This need has been met by the introduction of CSULGIEs as an endpoint, which captures events occurring in the entire GI tract. The CONDOR study, in which CSULGIEs were a primary endpoint, is the first major outcome NSAID trial in which lower GI adverse events were prospectively included in primary outcome measurement. The results of this study should demonstrate whether celecoxib is superior to the combination of diclofenac SR and omeprazole in preventing CSULGIEs in high-risk patients with OA or RA.
Acknowledgements
Medical writing assistance was provided by Just:: Health PR Ltd, with financial support from Pfizer Inc. A medical writer assisted with searches of the literature and collation of data and supported the author in the drafting of the text. The author was fully involved in all stages of the preparation of the manuscript.
Supplement: This paper forms part of the supplement entitled ‘Balancing gastrointestinal benefit–risk in individuals who are prescribed NSAIDs for arthritis’. This supplement was supported by an unrestricted grant from Pfizer Inc.
Disclosure statement: A.L. is an advisor to Pfizer and AstraZeneca. He has received research support from AstraZeneca and Pfizer and is involved in multicentre randomized clinical trials sponsored by Bayer and Pfizer.
References
- 1.Pincus T, Koch G, Lei H, et al. Patient preference for placebo, acetaminophen (paracetamol) or celecoxib efficacy studies (PACES): two randomised, double blind, placebo controlled, crossover clinical trials in patients with knee or hip osteoarthritis. Ann Rheum Dis. 2004;63:931–9. doi: 10.1136/ard.2003.020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geba GP, Weaver AL, Polis AB, Dixon ME, Schnitzer TJ for the VACT Group. Efficacy of rofecoxib, celecoxib, and acetaminophen in osteoarthritis of the knee. JAMA. 2002;287:64–71. doi: 10.1001/jama.287.1.64. [DOI] [PubMed] [Google Scholar]
- 3.Jordan KM, Arden NK, Doherty M, et al. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT) Ann Rheum Dis. 2003;62:1145–55. doi: 10.1136/ard.2003.011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43:1905–15. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 5.Paulus HE. FDA Arthritis Advisory Committee meeting: serious gastrointestinal toxicity of nonsteroidal anti-inflammatory drugs; drug-containing renal and biliary stones; diclofenac and carprofen approved. Arthritis Rheum. 1988;31:1450–1. [Google Scholar]
- 6.Singh G, Rosen Ramey D. NSAID induced gastrointestinal complications: the ARAMIS perspective–1997: arthritis, rheumatism and aging medical information system. J Rheumatol. 1998;51(Suppl.):8–16. [PubMed] [Google Scholar]
- 7.Geis GS, Stead H, Wallemark CB, Nicholson PA. Prevalence of mucosal lesions in the stomach and duodenum due to chronic use of NSAID in patients with rheumatoid arthritis or osteoarthritis, and interim report on prevention by misoprostol of diclofenac associated lesions. J Rheumatol. 1991;18(Suppl. 28):11–4. [PubMed] [Google Scholar]
- 8.Lanas A, Perez-Aisa MA, Feu F, et al. A nationwide study of mortality associated with hospital admission due to severe gastrointestinal events and those associated with nonsteroidal anti-inflammatory drug use. Am J Gastroenterol. 2005;100:1685–93. doi: 10.1111/j.1572-0241.2005.41833.x. [DOI] [PubMed] [Google Scholar]
- 9.Henry J. [(28 July 2009, date last accessed)]. Kaiser Family Foundation. State Health Facts 2006. http://www.statehealthfacts.org/comparemaptable.jsp?ind=113&cat=2 and http://www.statehealthfacts.org/comparemaptable.jsp?ind=118&cat=2.
- 10.Barkun A, Fallone CA, Chiba N, et al. A Canadian clinical practice algorithm for the management of patients with nonvariceal upper gastrointestinal bleeding. Nonvariceal upper GI bleeding consensus conference group. Can J Gastroenterol. 2004;18:605–9. doi: 10.1155/2004/595470. [DOI] [PubMed] [Google Scholar]
- 11.Lanas A, Garcia-Rodríguez LA, Polo-Tomás M, et al. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol. 2009;104:1633–41. doi: 10.1038/ajg.2009.164. [DOI] [PubMed] [Google Scholar]
- 12.Hernández-Diaz S, Garcia-Rodríguez LA. Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation. An overview of epidemiologic studies published in the 1990s. Arch Intern Med. 2000;160:2093–9. doi: 10.1001/archinte.160.14.2093. [DOI] [PubMed] [Google Scholar]
- 13.Pérez Gutthann S, Garciá Rodríguez LA, Raiford DS. Individual nonsteroidal anti-inflammatory drugs and other risk factors for upper gastrointestinal bleeding and perforation. Epidemiology. 1997;8:18–24. doi: 10.1097/00001648-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Huang J-Q, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic ulcer disease: a meta-analysis. Lancet. 2002;359:14–22. doi: 10.1016/S0140-6736(02)07273-2. [DOI] [PubMed] [Google Scholar]
- 15.Lanas A, García-Rodríguez LA, Arroyo MT, et al. Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo-oxygenase-2 inhibitors, traditional non-aspirin non-steroidal anti-inflammatory drugs, aspirin and combinations. Gut. 2006;55:1731–8. doi: 10.1136/gut.2005.080754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armstrong CP, Blower AL. Non-steroidal anti-inflammatory drugs and life threatening complications of peptic ulceration. Gut. 1987;28:527–32. doi: 10.1136/gut.28.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis. The CLASS study: a randomized controlled trial. JAMA. 2000;284:1247–55. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 18.Crofford LJ, Lipsky PE, Brooks P, Abramson SB, Simon LS, Van de Putte LBA. Basic biology and clinical application of specific cyclooxygenase-2 inhibitors. Arthritis Rheum. 2000;43:4–13. doi: 10.1002/1529-0131(200001)43:1<4::AID-ANR2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 19.Rostom A, Muir K, Dubé C, et al. Gastrointestinal safety of cyclooxygenase-2 inhibitors: a Cochrane Collaboration systematic review. Clin Gastroenterol Hepatol. 2007;5:818–28. doi: 10.1016/j.cgh.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Singh G, Fort JG, Goldstein JL, et al. Celecoxib versus naproxen and diclofenac in osteoarthritis patients: SUCCESS-1 study. Am J Med. 2006;119:255–66. doi: 10.1016/j.amjmed.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 21.Rostom A, Muir K, Dube C, Lanas A, Jolicoeur E, Tugwell P. Prevention of NSAID-related upper gastrointestinal toxicity: a meta-analysis of traditional NSAIDs with gastroprotection and COX-2 inhibitors. Drug Health Patient Safety. 2009;1:1–25. doi: 10.2147/dhps.s4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NICE clinical guideline 59. Osteoarthritis: the care and management of osteoarthritis in adults. 2008. Feb, [(December 2009, date last accessed)]. http://www.nice.org.uk/nicemedia/pdf/CG59NICEguideline.pdf.
- 23.Chan FKL, Hung LCT, Suen BY, et al. Celecoxib versus diclofenac and omeprazole in reducing the risk of recurrent ulcer bleeding in patients with arthritis. N Engl J Med. 2002;347:2104–10. doi: 10.1056/NEJMoa021907. [DOI] [PubMed] [Google Scholar]
- 24.Chan FKL, Wong VWS, Suen BY, et al. Combination of a cyclo-oxygenase-2 inhibitor and a proton-pump inhibitor for prevention of recurrent ulcer bleeding in patients at very high risk: a double-blind, randomised trial. Lancet. 2007;369:1621–6. doi: 10.1016/S0140-6736(07)60749-1. [DOI] [PubMed] [Google Scholar]
- 25.Laine L, Smith R, Min K, Chen C, Dubois RW. Systematic review: the lower gastrointestinal adverse effects of non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2006;24:751–67. doi: 10.1111/j.1365-2036.2006.03043.x. [DOI] [PubMed] [Google Scholar]
- 26.Lanas A, Scarpignato C. Microbial flora in NSAID-induced intestinal damage: a role for antibiotics? Digestion. 2006;73(Suppl. 1):136–50. doi: 10.1159/000089789. [DOI] [PubMed] [Google Scholar]
- 27.Allison MC, Howatson AG, Torrance CJ, Lee FD, Russell RI. Gastrointestinal damage associated with the use of nonsteroidal anti-inflammatory drugs. N Engl J Med. 1992;327:749–54. doi: 10.1056/NEJM199209103271101. [DOI] [PubMed] [Google Scholar]
- 28.Wilcox CM, Alexander LN, Cotsonis GA, Clark WS. Nonsteroidal anti-inflammatory drugs are associated with both upper and lower gastrointestinal bleeding. Dig Dis Sci. 1997;42:990–7. doi: 10.1023/a:1018832902287. [DOI] [PubMed] [Google Scholar]
- 29.Lanas A, Sekar MC, Hirschowitz BI. Objective evidence of aspirin use in both ulcer and nonulcer upper and lower gastrointestinal bleeding. Gastroenterology. 1992;103:862–9. doi: 10.1016/0016-5085(92)90018-t. [DOI] [PubMed] [Google Scholar]
- 30.Lanas A, Serrano P, Bajador E, Esteva F, Benito R, Sainz R. Evidence of aspirin use in both upper and lower gastrointestinal perforation. Gastroenterology. 1997;112:683–9. doi: 10.1053/gast.1997.v112.pm9041228. [DOI] [PubMed] [Google Scholar]
- 31.Lanas A, Panes J, Pique JM. Clinical implications of COX-1 and/or COX-2 inhibition for the distal gastrointestinal tract. Curr Pharm Des. 2003;9:2253–66. doi: 10.2174/1381612033453992. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Zlotnick S, Fort JG. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3:133–41. doi: 10.1016/s1542-3565(04)00619-6. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein JL, Eisen GM, Lewis B, et al. Small bowel mucosal injury is reduced in healthy subjects treated with celecoxib compared with ibuprofen plus omeprazole, as assessed by video capsule endoscopy. Aliment Pharmacol Ther. 2007;25:1211–22. doi: 10.1111/j.1365-2036.2007.03312.x. [DOI] [PubMed] [Google Scholar]
- 34.Gerson LB, Tokar J, Chiorean M, et al. Complications associated with double balloon enteroscopy at 9 U.S. centers. Clin Gastroenterol Hepatol. 2009;7:828–33. doi: 10.1016/j.cgh.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Signorelli C, Villa F, Rondonotti E, Abbiati C, Beccari G, de Franchis R. Sensitivity and specificity of the suspected blood identification system in video capsule enteroscopy. Endoscopy. 2005;37:1170–3. doi: 10.1055/s-2005-870410. [DOI] [PubMed] [Google Scholar]
- 36.Chong AK, Chin BW, Meredith CG. Clinically significant small-bowel pathology identified by double-balloon enteroscopy but missed by capsule endoscopy. Gastrointest Endosc. 2006;64:445–9. doi: 10.1016/j.gie.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Laine L, Curtis SP, Cryer B, Kaur A, Cannon CP. Assessment of upper gastrointestinal safety of etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomised comparison. Lancet. 2007;369:465–73. doi: 10.1016/S0140-6736(07)60234-7. [DOI] [PubMed] [Google Scholar]
- 38.Laine L, Curtis SP, Langman M, et al. Lower gastrointestinal events in a double-blind trial of the cyclo-oxygenase-2 selective inhibitor etoricoxib and a traditional non-steroidal anti-inflammatory drug diclofenac. Gastroenterology. 2008;135:1517–25. doi: 10.1053/j.gastro.2008.07.067. [DOI] [PubMed] [Google Scholar]
- 39.Silverstein FE, Graham DY, Senior JR, et al. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995;123: 241–9. doi: 10.7326/0003-4819-123-4-199508150-00001. [DOI] [PubMed] [Google Scholar]
- 40.Laine L, Connors LG, Reicin A, et al. Serious lower gastrointestinal clinical events with nonselective NSAID or coxib use. Gastroenterology. 2003;124:288–92. doi: 10.1053/gast.2003.50054. [DOI] [PubMed] [Google Scholar]
- 41.Rahme E, Barkun A, Nedjar H, Gaugris S, Watson D. Hospitalizations for upper and lower GI events associated with traditional NSAIDs and acetaminophen among the elderly in Quebec, Canada. Am J Gastroenterol. 2008;103:872–82. doi: 10.1111/j.1572-0241.2008.01811.x. [DOI] [PubMed] [Google Scholar]
- 42.Penninx BWJH, Pahor M, Cesari M, et al. Anemia is associated with disability and decreased physical performance and muscle strength in the elderly. J Am Geriatr Soc. 2004;52:719–24. doi: 10.1111/j.1532-5415.2004.52208.x. [DOI] [PubMed] [Google Scholar]
- 43.Zakai NA, Katz R, Hirsch C, et al. A prospective study of anemia status, haemoglobin concentration, and mortality in an elderly cohort. Arch Intern Med. 2005;165:2214–20. doi: 10.1001/archinte.165.19.2214. [DOI] [PubMed] [Google Scholar]
- 44.Chan FKL, Cryer B, Goldstein JL, et al. A novel composite endpoint to evaluate the gastrointestinal (GI) effects of non-steroidal anti-inflammatory drugs through the entire GI tract. J Rheumatol. 2009;36:12. doi: 10.3899/jrheum.090168. [DOI] [PubMed] [Google Scholar]