Abstract
For the family physician, NSAIDs, both traditional and cyclo-oxgenase-2 inhibitors, are a valuable contribution to managing arthritis and other rheumatological conditions in primary care. Yet, many of the patients seen by the family doctor have complex comorbidities and polypharmacy issues. This review looks at the main considerations for primary-care physicians while choosing an anti-inflammatory treatment for a hypothetical patient case study. In addition to looking at the evidence for gastrointestinal and cardiovascular risk, the concomitant use of aspirin with an NSAID is also examined. New evidence for interaction between selective serotonin re-uptake inhibitors is reviewed and the interaction between angiotensin-converting enzyme inhibitors and NSAIDs is considered. Making careful judgements based on individual needs, medical history and comorbidities is recommended based on the evidence reviewed.
Keywords: Gastrointestinal risk, Cardiovascular risk, Cox-2 selective inhibitors, NSAID, Renal risk, Aspirin, Selective serotonin re-uptake inhibitor
Introduction
‘First, do no harm’ is an axiom that has guided practitioners for generations. Lately, the issue of patient safety has been highlighted by data demonstrating the cost associated with ‘medical harm’. It is estimated that 2% of hospital admissions and $37.6 billion of American health care spending can be attributed to medical error or medication side effects [1]. Family physicians who treat a variety of conditions with a large portfolio of therapies have become increasingly sensitized to patient safety and medication side-effect issues.
NSAIDs have been a mainstay in the arsenal of treatments prescribed by family physicians for many years. This class of medication provides an effective, non-sedating, non-addictive and generally well-tolerated treatment for patients with OA and other rheumatological conditions. So effective and well tolerated are these agents that they have also been used to treat other painful conditions that are mainly managed in primary care, such as dysmenorrhoea, migraine and post-partum pain conditions [2]. With the growing range of indications and ageing population, sales of these medications have been rising, tripling between 1975 and 2002 in some cases [3]. With such widespread use, safety concerns are especially important.
The patient
The hypothetical case used in this review illustrates a situation that may be recognized by many family practitioners. OA and other musculoskeletal conditions often occur in a population of patients with co-existing medical conditions, which may be associated with an increased level of gastrointestinal (GI) or cardiovascular risk. Our case study—‘case study A’ like many others, is elderly with a complex treatment regimen and the preservation of his daily activities is an important part of his well-being.
This patient needs a means of controlling his arthritis pain, but his comorbid conditions need to be considered. Notwithstanding the risks of NSAIDs in the elderly patient with cardiac risk factors, they are often ideal medications for those with musculoskeletal pain refractory to acetaminophen (paracetamol). Alternate use of opiate medications is associated with dizziness, falls, constipation, nausea and interaction with psychotropic medications [4, 5]. Clearly, when treating the complex older patient, risks and benefits must be carefully weighed in order to provide patients with an effective and well-tolerated therapy that minimizes risk.
Case study (hypothetical case)
Case study A, a hypothetical case, is an 82-year-old widower who is living independently. With a background medical history of hypertension, Type 2 diabetes, depression, degenerative disc disease in his lower back, mild renal failure (eGFR 55), gastro-oesophageal reflux disease and benign prostatic hypertrophy, his medical management is complex. His current medications include two anti-hypertensive treatments, two oral hypoglycaemic agents, a selective serotonin re-uptake inhibitor (SSRI), proton pump inhibitor (PPI), NSAID, low-dose aspirin (ASA) and selective α-blocker. Case study A enjoyed gardening, daily walks in the neighbourhood and visiting his great grandchildren. His most frequent complaint was that of the arthritis pain that limited his activities and affected his quality of life. After suffering a small myocardial infarction (STEMI) complicated by mild congestive heart failure, he reported back to his family doctor for post-hospital review. His family doctor reviewed his medications and found that he now had three additional agents added to his other drug treatment (β-blocker, diuretic and additional anti-platelet medication). His NSAID had been discontinued and he had been instructed to take acetaminophen and given codeine to be taken ‘as needed for pain’. Case study A was particularly miserable and complained that his new medications were ruining his life. Case study A said that his arthritis pain was ‘killing me worse than the heart attack’. The acetaminophen provided poor pain control—especially at night. When he took the codeine to sleep, he was troubled by constipation. Furthermore, it made him lightheaded, to the point that he suffered a fall one night during his frequent visits to the toilet as a result of his nocturia. Exasperated he asks, ‘Can’t I just have my old arthritis pill back?’.
Patients at increased GI risk
Uppermost in the minds of clinicians both in secondary and primary care has been the upper GI toxicity associated with traditional NSAIDs (tNSAIDs) [6]. The risk of upper GI bleeding is associated with risk factors, such as age, concomitant ASA or aspirin and prednisone use, previous GI bleeding, anti-coagulation and other chronic medical conditions [7, 8] . In Canada, deaths associated with GI bleeds related to NSAIDs have been reported as being more frequent than deaths due to motor vehicle accidents [9]. The more widespread use of ASA for cardioprotection has resulted in more patients being put at risk of GI haemorrhage.
A safety issue often overlooked is the risk of lower GI bleeding associated with tNSAID use. Lower GI bleeding may come from diverticular disease, colonic ulcer or ectasia [10] and represents ∼40% of total GI bleeding [11]. Small intestinal bleeding occurring below the duodenum is often very difficult to identify due to the lack of availability of capsule endoscopy and for family practitioners can involve referral to a large, possibly distant specialized centre. It is important for practitioners to consider that the use of PPIs is unlikely to protect the lower GI tract from bleeding. Thus, the concomitant use of a PPI with a tNSAID still leaves a significant amount of the GI tract at risk of bleeding. Cyclo-oxgenase-2 (Cox-2) selective inhibitors, with or without additional gastroprotection in very high-risk patients, may be the best strategy for reducing the risk of GI complications in those individuals, with evidence indicating that in healthy individuals Cox-2s result in a lower incidence of small bowel lesions than tNSAIDs. [12, 13] More evidence in a high-risk population would be valuable in this assessment.
GI haemorrhage related to NSAID use is often occult [14]. Thus, treating physicians and patients cannot be reassured by lack of abdominal symptoms. Although bleeding may be acute and catastrophic, it is more likely to be indolent and subtle, often resulting in a picture of iron deficiency anaemia. Furthermore, the onset of bleeding from NSAID-related GI damage can occur with even short duration therapy [15]. Both of these factors are clinically relevant—patients are still at risk despite short course NSAID therapy, with no overt GI symptoms. GI risk assessment should be independent of duration of therapy or assumed tolerance.
The introduction of the selective Cox-2 inhibitors marked an important breakthrough in patient safety. This class of medications was found to be associated with less GI toxicity but similar efficacy to tNSAIDs [16]. The use of this new class of medications became widespread with early guidelines urging their use as first line for patients who were at high risk of GI complications from tNSAIDs [4, 17].
Patients at increased cardiovascular risk
Initial enthusiasm for the Cox-2 inhibitors was tempered by emerging data, which reflected increased cardiac events in patients who were on long-term Cox-2 inhibitor therapy [18]. Rofecoxib was the first Cox-2 reported as causing increases in blood pressure and also cardiac events—particularly at high dosages [19]. A study with high-dose celecoxib also demonstrated increased cardiovascular incidents [20]. Later, a primarily retrospective review suggested variable-increased cardiovascular risk attributable to all NSAIDs—both Cox-2 selective and non-selective [21]. This effect seemed to be dose dependent and widespread throughout Cox-2 selective and tNSAIDs.
NSAIDs and aspirin
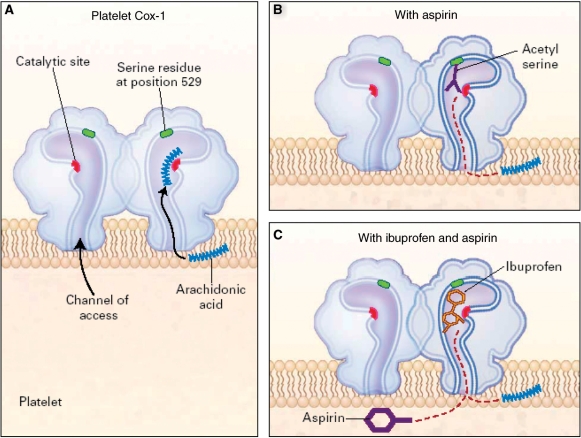
The use of NSAIDs in patients who have risk factors for coronary artery disease is complex. Some tNSAIDs adversely impact the cardioprotective, anti-platelet properties of low-dose ASA by competition for Cox-1 binding sites (Fig. 1). In a study by Catella-Lawson et al. [22], patients with arthritis and vascular disease were given aspirin (81 mg) with either ibuprofen (400 mg), paracetamol (1000 mg) or rofecoxib (25 mg). In the first 6-day period, the aspirin was given 2 h before the ibuprofen, paracetamol or rofecoxib and then after a wash-out period of at least 14 days, it was given in reverse order. When aspirin preceded ibuprofen, Cox-1 activity and platelet aggregation were inhibited nearly 100% during the ensuing 24 h. However, when ibuprofen was taken with aspirin, Cox-1 activity was inhibited by only 53% and platelet aggregation by only 2% after 24 h. Ensuring ASA is taken prior to taking an NSAID can thus overcome the adverse effects of these NSAIDs on cardioprotective capability associated with low-dose ASA.
Fig. 1.
The effect of aspirin alone and ibuprofen plus aspirin on platelet Cox-1. (A) Demonstrates ‘Normal functioning’ of Cox-1 receptor. (B) demonstrates how ASA irreversibly binds with the Cox 1 receptor, providing long term platelet inhibition. (C) shows how ibuprofen similarly but temporarily blocks the receptor preventing ASA from binding and thereby permanently inhibiting the Cox 1 receptor. Thus the cardioprotective effect of ASA may be minimized when ibuprofen is administered prior to the ASA because the ibuprofen ‘protects’ the Cox 1 receptor from irreversible inhibition. Reproduced with permission from Catella-Lawson et al. [22]. Copyright © 2001 Massachusetts Medical Society. All rights reserved.
However, this may not be entirely straightforward. In the same study there were also participants who were given multiple-dose NSAIDs: enteric-coated aspirin 2 h before ibuprofen (400 mg three times a day) or 2 h before a delayed-release diclofenac 75 mg two times a day). In the ibuprofen group, platelet aggregation was inhibited by only ∼10% the following morning. In contrast, in the diclofenac group, platelet aggregation remained nearly 100% inhibited the next morning [22]. This may be explained by the delayed absorption of the ASA due to its enteric coating. This could have delayed absorption and thus effect until after the ibuprofen was absorbed. In the case of the delayed-release diclofenac, the mechanism of slower drug absorption allowed the ASA to be active prior to it coming on board. Cox-2 inhibitors do not appear to have the same effect. In the two groups taking acetaminophen or rofecoxib in the same study, neither drug inhibited aspirin’s anti-platelet effect when given 2 h before aspirin [22].
NSAIDs and other conditions
All NSAIDs can also raise blood pressure and interfere with the blood pressure lowering effects of certain medication classes, such as angiotensin-converting enzyme (ACE) inhibitors [23]. The concomitant use of NSAIDs with diuretics increases the likelihood of nephrotoxicity as well as interfering with their blood pressure lowering effects [24]. Electrolyte issues—particularly hyperkalaemia, are also associated with NSAID use [24]. NSAIDs, both traditional and Cox-2 selective inhibitors must, therefore, be used with caution in all patients with renal failure. Congestive heart failure can also be exacerbated by traditional and Cox-2 selective NSAIDs [25].
NSAIDs and selective serotonin uptake inhibitors
A further emerging area of interest to family doctors is the potential increase in GI bleeding for patients who are taking the SSRIs. Although the data are preliminary and retrospective, there appears to be an increase in GI bleeding in patients who are on SSRIs alone, a risk which is increased when patients are also taking NSAIDs [26, 27]. Over 26 000 users of anti-depressants in North Jutland, Denmark, were included in the study by Dalton and coworkers [26, 27]. In individuals taking SSRIs without other medication, the rate of upper GI bleeding was 3.6 times more than expected (95% CI 2.7, 4.7). When combined with either an NSAID or low-dose aspirin (GI), the risk increased to 12.2 (95% CI 7.1, 19.5) and 5.2 (95% CI 3.2, 8.0), respectively (Table 1).
Table 1.
The O/E for upper GI tract bleeding among 26 005 current users of anti-depressant medication in the county of North Jutland, Denmark, 1991–95a
| Variable | No. of personsb | Person-years at risk | Obs. | O/E (95% CI), RDc/1000 | Treatment years |
|---|---|---|---|---|---|
| SSRIs | |||||
| Current use | |||||
| SSRI only | 17 320 | 12 760.2 | 55 | 3.6 (2.7, 4.7) | 3.1 |
| SSRI and NSAIDs only | 4107 | 960.2 | 17 | 12.2 (7.1, 19.5) | 16.3 |
| SSRI and low-dose aspirin only | 2640 | 1532.9 | 20 | 5.2 (3.2, 8.0) | 12.4 |
| SSRI and other drugs only | 4678 | 1566.8 | 27 | 11.6 (7.5, 16.6) | 15.8 |
| Former use | |||||
| No use of any other drug | 13 362 | 14 465.6 | 18 | 1.2 (0.7, 1.9) | 0.2 |
| Non-SSRIs | |||||
| Current use | |||||
| Non-SSRI only | 7716 | 8804.7 | 27 | 2.3 (1.5, 3.4) | 1.8 |
| Non-SSRI and NSAIDs only | 2418 | 827.2 | 9 | 8.2 (3.7, 15.5) | 9.6 |
| Non-SSRI and low-dose aspirin only | 927 | 657.7 | 7 | 4.6 (1.8, 9.4) | 8.3 |
| Non-SSRI and other drugs only | 2932 | 1063.3 | 7 | 4.5 (1.8, 9.2) | 5.1 |
| Former use | |||||
| No use of any other drug | 6604 | 9592.4 | 25 | 2.5 (1.6, 3.6) | 1.6 |
| Other anti-depressants | |||||
| Current use | |||||
| Other anti-depressants only | 4436 | 4153.7 | 9 | 1.7 (0.8, 3.1) | 0.9 |
| Other anti-depressants and NSAIDs only | 1224 | 340.9 | 3 | 6.3 (1.3, 18.4) | 7.4 |
| Other anti-depressants and low-dose aspirin only | 542 | 356.6 | 2 | 2.5 (0.3, 9.2) | 3.4 |
| Other anti-depressants and other drugs only | 1979 | 726.7 | 5 | 5.2 (1.7, 12.2) | 5.6 |
| Former use | |||||
| No use of any other drug | 3927 | 5764.7 | 12 | 1.9 (1.0, 3.3) | 1.0 |
aOther drugs include high-dose aspirin (N02B A01 and N02B A51), vitamin K antagonists (B01A A03 and B01A A04) and oral corticosteroids (H02A B), used alone or in combination with NSAIDs (M01A) or low-dose aspirin (B01A C06 and N02B A01). (Anatomical Therapeutic Chemical classification system codes are in parentheses.); bthe grouping of persons is not mutually exclusive; persons can contribute to more than one category of current use; cthe RD is the difference between the incidence rate of the exposed population and the incidence rate of the unexposed population (where the unexposed population equals the relevant background population incidence). Reproduced with permission from Dalton et al. [26]. Obs: observed number of hospitalizations for upper GI tract bleeding; O/E: observed–expected ratio; RD: rate difference.
Increased risk, but a much lower one, was found in a retrospective study conducted in the UK with 11 261 participants with upper GI bleeding and 53 156 controls. This study examined the risk of GI bleeding associated with NSAID treatments and SSRIs when prescribed separately and together [28]. They found that concurrent prescription of both drugs led to a marginally higher risk than when one drug was prescribed [SSRI: odds ratio (OR) 2.38, 95% CI 2.08, 2.72; NSAIDs: OR 2.15, 95% CI 2.02, 2.28; SSRI + NSAID: OR 2.93, 95% CI 2.25, 3.82]. The study also found that this risk appears to be slightly less when a selective Cox-2 inhibitor is combined with an SSRI. This interaction with anti-inflammatory medications is an important area for further research, as depression is a commonly treated condition by family physicians [28].
Conclusion
What is the right approach to case study A? Clearly, he is at increased risk for NSAID therapy—his recent cardiac event, renal failure, bleeding risk from medications and underlying risk factors must be balanced by the impact of his musculoskeletal pain on his quality of life. An initial approach of non-pharmaceutical interventions would be appropriate. Involvement with physiotherapy and occupational therapy, home care support and weight loss would be helpful. Despite this, many patients such as case study A would benefit from adjunct medical therapy. Before considering the options, careful discussion with case study A, and his family, of risks and benefits of other therapies is important. Particularly, if NSAID therapy was considered, careful monitoring of blood pressure, renal function, electrolytes, haemoglobin and fluid status would be critical. Choice of a non-tNSAID with the lowest bleeding risk may be appropriate. Narcotic and other alternate pain medications will almost certainly increase risk for falls, cognitive impairment and constipation. Assisting case study A in choosing the right treatment and close monitoring following the initiation therapy will maximize the outcomes while reducing the risks.
No solution is offered in this case because there is no one course of treatment with a guaranteed effective outcome: the case simply illustrates the kind of decisions that primary-care doctors face on a regular basis. Furthermore, any decision would be made not by the physician alone but with the patient (and his family) there to consider the risks and benefits of the options.
With ageing patient populations, who have multiple comorbidities and are taking increasingly more medications, patient safety issues are more important than ever for family physicians and other primary-care providers to consider. In the appropriate patient population, with proper monitoring and assessment, NSAID therapy can significantly benefit our patients’ quality of life. In selected patients, Cox-2 selective inhibitors provide additional safety benefit and may be considered preferentially. However, notwithstanding their significant safety benefit in some patient populations, they must be used in caution in patients with renal failure, hypertension and heart disease.

Acknowledgements
Medical writing assistance was provided by Just:: Health PR Ltd, with financial support from Pfizer Inc. A medical writer assisted with searches of the literature and collation of data and supported the author in the drafting of the text. The author was fully involved in all stages of the preparation of the manuscript.
Supplement: This paper forms part of the supplement entitled ‘Balancing gastrointestinal benefit–risk in individuals who are prescribed NSAIDs for arthritis’. This supplement was supported by an unrestricted grant from Pfizer Inc.
Disclosure statement: R.W. has received compensation for speaking, program development and/or research from Sanofi Aventis, Merck Frosst, Pfizer, Novartis, Bristol Myer Squibb and AstraZeneca.
References
- 1.Kohn LT, Corrigan JM, Donaldson MS. To err is human: building a Safer Health System. Washington, DC: National Academy Press; 1999. [PubMed] [Google Scholar]
- 2.Todd PA, Clissold SP. Naproxen. A reappraisal of its pharmacology, and therapeutic use in rheumatic diseases and pain states. Drugs. 1990;40:91–137. doi: 10.2165/00003495-199040010-00006. [DOI] [PubMed] [Google Scholar]
- 3.Hermansson M, Ekedahl A, Ranstam J, Zilling T. Decreasing incidence of peptic ulcer complications after the introduction of the proton pump inhibitors, a study of the Swedish population from 1974–2002. BMC Gastroenterol. 2009;9:25. doi: 10.1186/1471-230X-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee. 2000 Update. Arthritis Rheum. 2000;43:1905–15. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 5.Maurer PM, Bartkowski RR. Drug interactions of clinical significance with opioid analgesics. Drug Saf. 1993;8:30–48. doi: 10.2165/00002018-199308010-00005. [DOI] [PubMed] [Google Scholar]
- 6.Paulus HE. FDA Arthritis Advisory Committee meeting: serious gastrointestinal toxicity of nonsteroidal anti-inflammatory drugs; drug-containing renal and biliary stones; diclofenac and carprofen approved. Arthritis Rheum. 1988;31:1450–1. [Google Scholar]
- 7.Lanas A, García-Rodríguez LA, Arroyo MT, et al. Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo-oxygenase-2 inhibitors, traditional non-aspirin non-steroidal anti-inflammatory drugs, aspirin and combinations. Gut. 2006;55:1731–8. doi: 10.1136/gut.2005.080754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pérez Gutthann S, Garciá Rodríguez LA, Raiford DS. Individual nonsteroidal anti-inflammatory drugs and other risk factors for upper gastrointestinal bleeding and perforation. Epidemiology. 1997;8:18–24. doi: 10.1097/00001648-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Hunt RH, Barkun AN, Baron D, et al. Recommendations for the appropriate use of anti-inflammatory drugs in the era of coxibs: defining the role of gastroprotective agents. Can J Gastroenterol. 2002;16:231–40. doi: 10.1155/2002/516092. [DOI] [PubMed] [Google Scholar]
- 10.Wilcox CM, Clarke WS. Association of nonsteroidal anti inflammatory drugs with outcome in upper and lower gastrointestinal bleeding. Dig Dis Sci. 1997;42:985–9. doi: 10.1023/a:1018880818217. [DOI] [PubMed] [Google Scholar]
- 11.Laine L, Connors LG, Reicin A, et al. Serious lower gastrointestinal clinical events with nonselective NSAID or coxib use. Gastroenterology. 2003;124:288–92. doi: 10.1053/gast.2003.50054. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Zlotnick S, Fort JG. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3:133–41. doi: 10.1016/s1542-3565(04)00619-6. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein JL, Eisen GM, Lewis B, et al. Small bowel mucosal injury is reduced in healthy subjects treated with celecoxib compared with ibuprofen plus omeprazole, as assessed by video capsule endoscopy. Aliment Pharmacol Ther. 2007;25:1211–22. doi: 10.1111/j.1365-2036.2007.03312.x. [DOI] [PubMed] [Google Scholar]
- 14.Lanas A, Scarpignato C. Microbial flora in NSAID-induced intestinal damage: a role for antibiotics? Digestion. 2006;73 (Suppl. 1):136–50. doi: 10.1159/000089789. [DOI] [PubMed] [Google Scholar]
- 15.Hernández-Dias S, Garcia-Rodríguez LA. Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation. An overview of epidemiological studies published in the 1990s. Arch Intern Med. 2000;160:2093–9. doi: 10.1001/archinte.160.14.2093. [DOI] [PubMed] [Google Scholar]
- 16.Rostom A, Muir K, Dubé C, et al. Gastrointestinal safety of cyclooxygenase-2 inhibitors: a Cochrane Collaboration systematic review. Clin Gastroenterol Hepatol. 2007;5:818–28. doi: 10.1016/j.cgh.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 17.American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Guidelines for the Management of Rheumatoid Arthritis. 2002 Update. Arthritis Rheum. 2002;46:328–46. doi: 10.1002/art.10148. [DOI] [PubMed] [Google Scholar]
- 18.Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 19.Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med. 2000;343:1520–8. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 20.Solomon SD, McMurray JV, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–80. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 21.McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA. 2006;296:1633–44. doi: 10.1001/jama.296.13.jrv60011. [DOI] [PubMed] [Google Scholar]
- 22.Catella-Lawson F, Reilly MP, Kapoor SC, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Eng J Med. 2001;345:1809–17. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- 23.Stys T, Lawson WE, Smaldone GC, Stys A. Does aspirin attenuate the beneficial effects of angiotensin-converting enzyme inhibition in heart failure? Arch Intern Med. 2000;160:1409–13. doi: 10.1001/archinte.160.10.1409. [DOI] [PubMed] [Google Scholar]
- 24.Whelton A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: physiologic foundations and clinical implications. Am J Med. 1999;106 (Suppl. 5B):13S–24. doi: 10.1016/s0002-9343(99)00113-8. [DOI] [PubMed] [Google Scholar]
- 25.Gislason HG, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169:141–9. doi: 10.1001/archinternmed.2008.525. [DOI] [PubMed] [Google Scholar]
- 26.Dalton SO, Sørensen HT, Johansen C. SSRIs and upper gastrointestinal bleeding: what is known and how should it influence prescribing? CNS Drugs. 2006;20:143–51. doi: 10.2165/00023210-200620020-00005. [DOI] [PubMed] [Google Scholar]
- 27.Dalton SO, Johansson C, Mellemkjaer L, et al. Use of selective serotonin re-uptake inhibitors and risk of upper gastrointestinal tract bleeding. Arch Intern Med. 2003;163:59–64. doi: 10.1001/archinte.163.1.59. [DOI] [PubMed] [Google Scholar]
- 28.Tata LJ, Fortun PJ, Hubbard RB, et al. Does concurrent prescription of selective serotonin reuptake inhibitors and non-steroidal anti-inflammatory drugs substantially increase the risk of upper gastrointestinal bleeding? Aliment Pharmacol Ther. 2005;22:175–81. doi: 10.1111/j.1365-2036.2005.02543.x. [DOI] [PubMed] [Google Scholar]