Abstract
Background
It is unclear whether the disproportionately higher incidence and mortality from colorectal cancer among blacks compared with whites reflect differences in health-care utilization or colorectal cancer susceptibility.
Methods
A total of 60 572 non-Hispanic white and black participants in the ongoing Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial underwent trial-sponsored screening flexible sigmoidoscopy (FSG) without biopsy at baseline in 10 geographically dispersed centers from November 1993 to July 2001. Subjects with polyps or mass lesions detected by FSG were referred to their physicians for diagnostic workup, the cost of which was not covered by PLCO. The records of follow-up evaluations were collected and reviewed. We used log binomial modeling with adjustment for age, education, sex, body mass index, smoking, family history of colorectal cancer, colon examination within previous 3 years, personal history of polyps, and screening center to examine whether utilization of diagnostic colonoscopy and yield of neoplasia differed by race.
Results
Among 57 561 whites and 3011 blacks who underwent FSG, 13 743 (23.9%) and 767 (25.5%) had abnormal examinations, respectively. A total of 9944 (72.4%) whites and 480 (62.6%) blacks had diagnostic colonoscopy within 1 year following the abnormal FSG screening. When compared with whites, blacks were less likely to undergo diagnostic evaluation (adjusted risk ratio = 0.88, 95% confidence interval = 0.83 to 0.93). Overall, among subjects with diagnostic colonoscopy (n = 10 424), there was no statistically significant difference by race in the prevalence of adenoma, advanced adenoma, advanced pathology in small adenomas (high-grade dysplasia or villous histology in adenomas <10 mm), or colorectal cancer.
Conclusions
We observed a lower follow-up for screen-detected abnormalities among blacks when compared with whites but little difference in the yield of colorectal neoplasia. Health-care utilization may be playing more of a role in colorectal cancer racial disparity than biology.
CONTEXT AND CAVEATS
Prior knowledge
In the United States, there is more mortality from colorectal cancer among blacks than among whites, but it was unclear whether this could be attributed differences in cancer susceptibility or health care.
Study design
Data were compared for 57 561 non-Hispanic white and 3.011 non-Hispanic black participants in the ongoing Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) who underwent flexible sigmoidoscopy without biopsy at one of the 10 US screening locations and were referred for diagnostic colonoscopy if abnormalities were detected.
Contribution
Among those with positive screening tests, blacks were only 88% as likely as whites to have undergone diagnostic evaluation. There were no statistically significant differences in tumor characteristics among these races.
Implications
Racial disparities in colorectal cancer rates and outcomes may be due more to differences in health-care utilization than biological differences.
Limitations
Reported colorectal cancer rates may be low because blacks in the PLCO trial were more educated and health conscious than in the general public. Also, only participants with lesions in the distal colon would be detected by this screen.
From the Editors
Colorectal cancer disproportionately affects blacks in the United States, who have higher incidence and mortality compared with whites (1,2). Low socioeconomic status has been shown to be associated with higher incidence of and poorer survival from colorectal cancer, especially among blacks (3,4). It has been suggested that these findings may be related to health-care access. However, data from integrated health-care systems in which colorectal cancer care was a covered benefit demonstrated that advanced stage presentation was more likely to occur among blacks (5). Furthermore, blacks tend to develop colorectal cancer at an earlier age, with a higher prevalence of right-sided neoplasia (6–9). These findings raised a possibility that the biology of the disease may be different in blacks and prompted the American College of Gastroenterology to recommend that blacks be screened for colorectal cancer beginning at 45 years of age (rather than the widely accepted 50 years of age for average-risk persons) and that colonoscopy be used as the preferred screening method (10). Unfortunately, most studies that have evaluated race and/or ethnicity differences in the prevalence of colorectal neoplasia have been limited by retrospective designs, nonscreening populations, single institution experience, small sample sizes, and lack of histopathologic diagnosis (1–9,11,12). No previous study has examined racial disparity in colorectal cancer by health-care utilization and yield of colorectal cancer screening in the same cohort.
Flexible sigmoidoscopy (FSG) is a colorectal cancer screening modality that examines the distal colon. Although FSG is an invasive procedure, it does not require sedation and can be performed by trained nurses, general practitioners, and specialists, thereby making it potentially more available and accessible to the population at large than colonoscopy. One option for colorectal cancer screening would be to screen the population with FSG and refer patients with any abnormal findings (polyps or masses) for diagnostic colonoscopy (13). This approach was used in the ongoing Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. Subjects with abnormal FSG were referred to their personal physicians for diagnostic follow-up. Thus, patients and their physicians determined the plan of care. Therefore, the PLCO trial provided an opportunity to study racial disparity in colorectal cancer as a function of health-care utilization and biological susceptibility within the same cohort.
In this study, we sought to evaluate whether the observed colorectal cancer disparities by race reflect differences in health-care utilization or colorectal cancer susceptibilities. We used adherence to follow-up of abnormal screening as an indicator of health-care utilization and used the yield of colorectal neoplasia to assess colorectal cancer susceptibility. We also investigated racial differences in the prevalence of advanced pathology in small-sized polyps (high-grade dysplasia or villous histology in adenomas <10 mm in size) as another surrogate measure of susceptibility.
Methods
The Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial
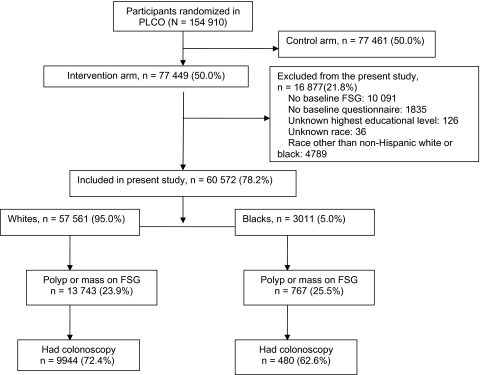
The rationale and design of the PLCO trial have been published (14–16). In brief, PLCO is an ongoing, multicenter, randomized, controlled screening trial designed to evaluate the effect of screening on cancer mortality. It includes 1) FSG (60-cm sigmoidoscope) for colorectal cancer, 2) chest X-ray for lung cancer, 3) digital rectal examination plus serum prostate-specific antigen for prostate cancer, and 4) CA125 measurements plus transvaginal ultrasound for ovarian cancer. The participants were recruited by mailing letters of invitation and the trial’s informational brochures to age-eligible persons who were identified on public, commercial, or screening center-specific mailing lists. Extra efforts were made to recruit minorities so that the study population could closely reflect the general US population (17,18). A total of 154 910 participants who were between 55 and 74 years old (77 449 in the intervention arm and 77 461 control subjects) were recruited from November 1993 to July 2001. The exclusion criteria included any history of prostate, lung, colorectal, or ovarian cancer, a history of treatment for a non-PLCO cancer within a year before recruitment, prior total colectomy, and participation in another cancer screening or primary prevention study. Beginning in April 15, 1995, the PLCO trial did not enroll any new subjects reporting a proctoscopy, sigmoidoscopy, barium enema, or colonoscopy within the previous 3 years. Of the 77 449 participants in the intervention arm, 64 973 (83.9%) were randomly assigned after April 15, 1995. The participants in the intervention arm were offered trial-sponsored screening FSG at baseline at 10 screening centers across the United States (Birmingham, AL; Denver, CO; Detroit, MI; Honolulu, HI; Marshfield, WI; Minneapolis, MN; Pittsburgh, PA; Salt Lake City, UT; St Louis, MO; and Washington, DC). The study was approved by the National Cancer Institute and the institutional review boards at each of the screening centers. All participants gave written informed consent. We excluded 16 877 PLCO participants from this analysis because of missing information or ethnicity other than non-Hispanic white or black race. Thus, our analytic cohort included 60 572 (57 561 non-Hispanic white and 3011 non-Hispanic black) participants who had baseline screening FSG and for whom we had complete information on the highest education achieved (Figure 1).
Figure 1.
Diagram of flow of participants through the study. FSG = flexible sigmoidoscopy.
Exposure and Outcome Assessment
Information on each subject's demographic characteristics, health-related lifestyle, and personal and family medical history was obtained by means of a questionnaire administered at enrollment. Race classification was based on each participant's self-identification. The sigmoidoscopy examiners were recruited from various clinical backgrounds (physicians, registered nurses, nurse practitioners, and physician assistants) and met quality standards. The PLCO protocol required that all examiners, except board-certified gastroenterologists or physicians with hospital privileges to perform FSG or colonoscopy, undergo training and certification by PLCO staff. The examiners watched a videotape and observed 10 procedures. Then, they performed 10 practice procedures followed by a minimum of 25 successful procedures under the guidance of a training gastroenterologist. It is noteworthy that many nurse endoscopists performed thousands of procedures in the PLCO and had adenoma detection rates that were comparable to physician endoscopists (19). The PLCO screening protocol did not include biopsy; rather, subjects with screen-detected polyp or mass were referred to their physicians for diagnostic evaluation, and the cost was not covered by PLCO. This screening approach closely reflects what might happen in a universal screening program targeting the entire US population in which nonphysician health-care providers would also perform screening FSG to increase the capacity for screening. After an abnormal screen in PLCO, the participant was notified and encouraged to seek follow-up with his or her primary care physician. Diagnostic evaluation was managed by community-based practitioners, although subjects and their physicians were able to contact physicians at the screening center if they had questions or needed advice regarding diagnostic evaluation. To obtain details of diagnostic evaluation, centers recontacted participants 3–6 months after the initial report of a positive screen to find out what diagnostic evaluation had been obtained and by which physician. The centers then contacted the physician and requested medical records. If diagnostic evaluation at that time was not definitive, the centers recontacted participants a few months later to obtain information about additional follow-up. Information was collected until a definitive diagnosis was made or until a year had passed, whichever came first. In the instance of colorectal cancer screenings, records documenting diagnostic colonoscopies were obtained and reviewed. The colonoscopy reports provided information on size, number, and location of polyps. Polyp histology, villous content, and high-grade or severe dysplasia were determined by community pathologists and abstracted from the medical records. The location of polyps removed from the rectum to the splenic flexure was defined as distal, whereas polyps from the transverse colon to cecum were defined as proximal. We defined advanced adenomas as those adenomas that were 10 mm or more in diameter or with villous or tubulovillous histology or high-grade or severe dysplasia or invasive cancer.
Statistical Analyses
Statistical Analysis Systems (SAS) software version 9.1.3 (SAS Institute, Inc, Cary, NC) was used for all analyses. We compared the baseline characteristics of participants by race. Because socioeconomic status may affect colonoscopy use and colon neoplasia detection (20–22), we performed stratified analyses using the highest educational level attained as a proxy for socioeconomic status (4) to mitigate confounding of race by socioeconomic status. We classified subjects into three categories: those with a high school education or less (including those who attended noncollege trade schools), those with some college or college graduates, and those with postgraduate education. Among participants with abnormal baseline FSG, we compared subjects who underwent diagnostic colonoscopy with those who did not using χ2 tests. We used log binomial regression modeling to evaluate the association of race with diagnostic colonoscopy use and also examined the association of race with risk of any adenoma, advanced adenoma, and advanced pathology in small adenoma (size <10 mm) vs no adenoma. We chose log binomial modeling because the prevalence of adenoma (our outcome of interest) is not a rare event in the age group studied, and it has been suggested that odds ratios (ORs) do not approximate risk ratios when the outcome of interest is greater than 10% in the study population (23). We used the PROC GENMOD statistical procedure in SAS and resolved any convergence problems with the COPY method (24,25). Our full models included race (white or black), educational status (high school or less, some college or college graduates, or postgraduate education), age (55–59, 60–64, 65–69, and 70–74 years), sex (male or female), body mass index (<18.5, 18.5–24, 25–29, ≥30 kg/m², or unknown), smoking status (never, former, and current), family history of colorectal cancer (yes, no, and unknown), history of colorectal cancer screening within 3 years of enrollment (yes, no, and unknown), history of colon polyps (yes, no, and unknown), and screening center (10 categories). We calculated risk ratios (RR) with 95% confidence intervals (CIs).
Results
Characteristics of the Participants
A total of 60 572 participants underwent baseline FSG: 57 561 (95%) whites and 3011 (5%) blacks. Overall, there were 30 744 (50.8%) men; 20 414 (33.7%) were 55–59 years old; 25 352 (41.9%) had a high school education or less; and 6249 (10.7%) had a family history of colorectal cancer (Table 1). White participants had more formal education and were more likely to have a personal history of colon polyps or family history of colorectal cancer but were less likely to be obese compared with blacks.
Table 1.
Baseline characteristics by race
| No. (%) |
||||
| Baseline characteristic | Total (n = 60 572) | Whites (n = 57 561) | Blacks (n = 3011) | P* |
| Sex | ||||
| Male | 30 744 (50.8) | 29 386 (51.1) | 1358 (45.1) | <.001 |
| Female | 29 828 (49.2) | 28 175 (48.9) | 1653 (54.9) | |
| Age, y | ||||
| 55–59 | 20 414 (33.7) | 19 337 (33.6) | 1077 (35.8) | |
| 60–64 | 18 809 (31.1) | 17 882 (31.1) | 927 (30.8) | .072 |
| 65–69 | 13 575 (22.4) | 12 934 (22.5) | 641 (21.3) | |
| 70–74 | 7774 (12.8) | 7408 (12.9) | 366 (12.2) | |
| Highest education attained | ||||
| High school or less | 25 352 (41.9) | 23 883 (41.5) | 1469 (48.8) | |
| College | 23 803 (39.3) | 22 709 (39.5) | 1094 (36.3) | <.001 |
| Postgraduate | 11 417 (18.8) | 10 969 (19.1) | 448 (14.9) | |
| Body mass index, kg/m2† | ||||
| <18.5 | 454 (0.8) | 430 (0.8) | 24 (0.8) | |
| 18.5–24 | 19 079 (31.8) | 18 457 (32.3) | 622 (21.0) | <.001 |
| 25–29 | 25 711 (42.8) | 24 540 (43.0) | 1171 (39.6) | |
| ≥30 | 14 833 (24.7) | 13 695 (24.0) | 1138 (38.5) | |
| Smoking status‡ | ||||
| Never | 28 221 (46.6) | 26 994 (46.9) | 1227 (40.8) | |
| Former | 6142 (10.1) | 5587 (9.7) | 555 (18.4) | <.001 |
| Current | 26 200 (43.3) | 24 972 (43.4) | 1228 (40.8) | |
| Has family history of colon cancer§ | 6249 (10.7) | 6023 (10.8) | 226 (7.9) | <.001 |
| Had previous colon examination within 3 y‖ | 7295 (12.2) | 6931 (12.2) | 364 (12.4) | .806 |
| Has history of colon polyps¶ | 4199 (7.2) | 4064 (7.3) | 135 (5.0) | <.001 |
Comparison between blacks and whites using χ2 tests. All comparisons were two-sided.
There were 495 participants with missing body mass index information.
There were nine participants with missing smoking status information.
There were 2060 participants with missing family history of colon cancer.
Some 912 subjects reported no information on colon examinations within 3 years of enrollment.
For 2046 subjects, there was missing information on history of colon polyps.
Baseline Screening FSG and Follow-up Diagnostic Evaluations
The rate of abnormal screening was comparable by race. Among participants who underwent baseline FSG screening, 13 743 of the 57 561 (23.9%) whites and 767 of the 3011 (25.5%) blacks had abnormal screening FSG (P = .257) (Figure 1). Diagnostic colonoscopy was completed in 9944 (72.4%) whites and 480 (62.6%) blacks. Among these, 91% of the colonoscopies were complete to the cecum, with adequate bowel preparation. Baseline characteristics of subjects who did not undergo colonoscopy were comparable to those who did, except that a slightly higher percentage of women (74.4% vs 70.3%, P < .001) and those with family history of colorectal cancer (76.0% vs 71.3%, P < .001) underwent colonoscopy. There were no differences in receipt of diagnostic colonoscopy by age, educational status, body mass index, or history of polyps.
Among white participants, women and those with a family history of colorectal cancer were more likely than men or those without a family history to undergo diagnostic colonoscopy (data not shown). In contrast, among black subjects, only those with a history of colon polyps were more likely to undergo diagnostic colonoscopy, but those with high school education or less were less likely to undergo follow-up evaluations (data not shown).
In multivariate analyses, when compared with whites, blacks were less likely to undergo diagnostic evaluation (RR = 0.88, 95% CI = 0.83 to 0.93) (Table 2). When the analyses were stratified by educational level, the black–white differences in lack of follow-up were only statistically significant among participants with high school education or less (RR = 0.83, 95% CI = 0.75 to 0.91) (Table 2).
Table 2.
Follow-up colonoscopy after abnormal flexible sigmoidoscopy (FSG) by race and highest education attained
| Characteristic | No. of patients with abnormal FSG | No. of patients without follow-up colonoscopy | No. of patients who had follow-up colonoscopy | Percentage with follow-up colonoscopy (95% CI) | Relative risk of undergoing follow-up colonoscopy (95% CI)* |
| White (all) | 13 743 | 3799 | 9944 | 72 (72% to 73%) | 1.0 (reference) |
| Black (all) | 767 | 287 | 480 | 63 (59% to 66%) | 0.88 (0.83 to 0.93) |
| High school or less (all) | 6260 | 1730 | 4530 | 72 (71% to 74%) | 1.0 (reference) |
| College (all) | 5760 | 1659 | 4101 | 71 (70% to 72%) | 1.01 (0.99 to 1.03) |
| Postgraduate (all) | 2490 | 697 | 1793 | 72 (70% to 74%) | 1.00 (0.98 to 1.03) |
| White, high school or less | 5888 | 1570 | 4318 | 73 (72% to 75%) | 1.0 (reference) |
| Black, high school or less | 372 | 160 | 212 | 57 (52% to 62%) | 0.83 (0.75 to 0.91) |
| White, college | 5470 | 1567 | 3903 | 71 (70% to 73%) | 1.0 (reference) |
| Black, college | 290 | 92 | 198 | 68 (63% to 74%) | 0.93 (0.86 to 1.01) |
| White, postgraduate | 2385 | 662 | 1723 | 72 (70% to 74%) | 1.0 (reference) |
| Black, postgraduate | 105 | 35 | 70 | 67 (57% to 76%) | 0.92 (0.80 to 1.05) |
Multivariable models included race (white or black), educational status (high school or less, some college or college graduates, or postgraduate education), age (55–59, 60–64, 65–69, or 70–74 years), sex (male or female), body mass index (<18.5, 18.5–24, 25–29, ≥30, or unknown), smoking status (never, former, or current), family history of colorectal cancer (yes, no, or unknown), history of colorectal cancer screening within 3 years of enrollment (yes, no, or unknown), history of colon polyps (yes, no, or unknown), and screening center (10 categories). CI = confidence interval.
Yield of Colorectal Neoplasia at Diagnostic Evaluations
Among subjects with diagnostic colonoscopies (n = 10 424), the percentage of subjects with adenoma was comparable by race: 54% (95% CI = 53% to 55%) for whites and 51% (95% CI = 46% to 55%) for blacks (Table 3). Overall, there were no statistically significant differences in the prevalence of adenoma, advanced adenoma, or advanced pathology (high-grade dysplasia or villous component) in small (<10 mm) adenomas by race (Table 3). However, in stratified analysis by educational level, black participants with postgraduate education had increased risk of adenoma compared with whites (RR = 1.29, 95% CI = 1.09 to 1.54).
Table 3.
Yield of colorectal neoplasia among subjects who had follow-up colonoscopy after abnormal flexible sigmoidoscopy
| Findings at colonoscopy, No. of patients |
Relative risk (95% CI)* |
|||||||
| Characteristic | No adenoma | Any adenoma | Advanced adenoma | <10 mm advanced adenoma | Percentage with any adenoma (95% CI) | Any adenoma vs no adenoma | Advanced adenoma vs no adenoma | <10 mm advanced adenoma vs no adenoma |
| White, all (n = 9944) | 4556 | 5388 | 2222 | 385 | 54 (53% to 55%) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| Black, all (n = 480) | 236 | 244 | 111 | 15 | 51 (46% to 55%) | 1.01 (0.92 to 1.11) | 1.11 (0.94 to 1.30) | 1.01 (0.60 to 1.71) |
| High school or less, all (n = 4530) | 1980 | 2550 | 1109 | 178 | 56 (55% to 58%) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| College, all (n = 4101) | 1961 | 2140 | 850 | 153 | 52 (51% to 54%) | 0.94 (0.90 to 0.97) | 0.87 (0.81 to 0.94) | 0.90 (0.73 to 1.11) |
| Postgraduate, all (n = 1793) | 851 | 942 | 374 | 69 | 53 (50% to 55%) | 0.94 (0.89 to 0.99) | 0.88 (0.80 to 0.97) | 0.91 (0.69 to 1.20) |
| White, high school or less (n = 4318) | 1881 | 2437 | 1052 | 172 | 56 (55% to 58%) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| Black, high school or less (n = 212) | 99 | 113 | 57 | 6 | 53 (46% to 60%) | 1.01 (0.88 to 1.15) | 1.16 (0.93 to 1.44) | 1.01 (0.44 to 2.30) |
| White, college (n = 3903) | 1854 | 2049 | 813 | 147 | 53 (51% to 54%) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| Black, college (n = 198) | 107 | 91 | 37 | 6 | 46 (39% to 53%) | 0.94 (0.80 to 1.10) | 0.95 (0.71 to 1.27) | 0.88 (0.38 to 2.04) |
| White, postgraduate (n = 1723) | 821 | 902 | 357 | 66 | 52 (50% to 55%) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| Black, postgraduate (n = 70) | 30 | 40 | 17 | 3 | 57 (45% to 69%) | 1.29 (1.09 to 1.54) | 1.48 (0.98 to 2.23) | 1.83 (0.58 to 5.80) |
Multivariable models included race (white or black), educational status (high school or less, some college or college graduates, or postgraduate education), age (55–59, 60–4, 65–9, or 70–74 years), sex (male or female), body mass index (<18.5, 18.5–24, 25–29, ≥30, or unknown), smoking status (never, former, or current), family history of colorectal cancer (yes, no, or unknown), history of colorectal cancer screening within 3 years of enrollment (yes, no, or unknown), history of colon polyps (yes, no, or unknown), and screening center (10 categories). CI = confidence interval.
In analyses of colorectal neoplasia by location, the percentage of subjects with proximal adenoma was similar between whites (19%, 95% CI = 18% to 20%) and blacks (21%, 95% CI = 18% to 25%). The overall risk of proximal adenoma was comparable between blacks and whites (RR = 1.09, 95% CI = 0.91 to 1.29). However, the risk of proximal advanced adenoma was higher in blacks (RR = 1.56, 95% CI = 1.13 to 2.14). There were no differences in risk in the distal colon (Table 4).
Table 4.
Yield of advanced adenoma by location among subjects who had follow-up colonoscopy after abnormal flexible sigmoidoscopy
| Findings at colonoscopy, No. of patients |
Relative risk (95% CI)* |
||||
| Characteristic | No adenoma | Proximal advanced adenoma | Distal advanced adenoma | Proximal advanced adenoma vs no adenoma | Distal advanced adenoma vs no adenoma |
| White, all (n = 9944) | 4556 | 550 | 1830 | 1.0 (reference) | 1.0 (reference) |
| Black, all (n = 480) | 236 | 41 | 82 | 1.56 (1.13 to 2.14) | 1.03 (0.85 to 1.26) |
| High school or less, all (n = 4530) | 1980 | 265 | 931 | 1.0 (reference) | 1.0 (reference) |
| College, all (n = 4101) | 1961 | 224 | 691 | 0.92 (0.78 to 1.09) | 0.84 (0.78 to 0.91) |
| Postgraduate, all (n = 1793) | 851 | 102 | 290 | 0.96 (0.77 to 1.20) | 0.83 (0.74 to 0.93) |
| White, high school or less (n = 4318) | 1881 | 243 | 891 | 1.0 (reference) | 1.0 (reference) |
| Black, high school or less (n = 212) | 99 | 22 | 40 | 1.90 (1.21 to 3.00) | 1.04 (0.79 to 1.37) |
| White, college (n = 3903) | 1854 | 215 | 661 | 1.0 (reference) | 1.0 (reference) |
| Black, college (n = 198) | 107 | 9 | 30 | 0.85 (0.44 to 1.65) | 0.95 (0.68 to 1.33) |
| White, postgraduate (n = 1723) | 821 | 92 | 278 | 1.0 (reference) | 1.0 (reference) |
| Black, postgraduate (n = 70) | 30 | 10 | 12 | 2.96 (1.55 to 5.66) | 1.34 (0.81 to 2.24) |
Multivariable models included race (white or black), educational status (high school or less, some college or college graduates, or postgraduate education), age (55–59, 60–64, 65–69, or 70–74 years), sex (male or female), body mass index (<18.5, 18.5–24, 25–29, ≥30, or unknown), smoking status (never, former, or current), family history of colorectal cancer (yes, no, or unknown), history of colorectal cancer screening within 3 years of enrollment (yes, no, or unknown), history of colon polyps (yes, no, or unknown), and screening center (10 categories). CI= confidence interval.
A total of 156 colorectal cancers were diagnosed among all subjects who had diagnostic colonoscopy. Of these, 118 (75.6%) were diagnosed in stage I or stage II. A total of 146 colorectal cancers were diagnosed among whites (146/9944; prevalence = 1.5%, 95% CI = 1.2% to 1.7%), and 10 colorectal cancers were diagnosed among blacks (10/480; prevalence = 2.1%, 95% CI = 1.0% to 3.8%). There was no statistically significant difference in the risk of colorectal cancer in blacks (RR = 1.58, 95% CI = 0.80 to 3.12) compared with whites.
Discussion
We observed a lower rate of diagnostic evaluation following an abnormal screening FSG among blacks as compared with whites. This lower rate of follow-up evaluation for screen-detected abnormalities implies that the reported racial disparity in colorectal cancer may not be eliminated by a universal screening program if a separate follow-up diagnostic testing is required. Overall, we did not observe any meaningful difference in the yield of colorectal neoplasia by race. This suggests that the biology of colorectal cancer may not be materially different by race, at least in the early stages of carcinogenesis, but instead that health-care utilization differences among the races may play a more important role in the observed disparities in colorectal cancer.
Although we did not observe any difference in the overall prevalence of adenoma in the proximal colon by race (RR = 1.09, 95% CI = 0.91 to 1.29), we found a higher prevalence of proximal advanced adenomas among blacks (RR = 1.56, 95% CI = 1.13 to 2.14). However, this finding was inconsistent across education groups. We observed a higher risk of proximal advanced adenoma among blacks with high school education or less (RR = 1.90, 95% CI = 1.21 to 3.00) and postgraduate education (RR = 2.96, 95% CI = 1.55 to 5.66) compared with their white counterparts, but not in the group with college education (RR = 0.85, 95% CI = 0.44 to 1.65). Although this may suggest biological differences, it could also be related to the small sample sizes in these categories or residual confounding. Regardless, our study does not provide any strong evidence to support a different colorectal cancer screening recommendation for blacks.
Our results are comparable to the report of similar colorectal cancer survival between blacks and whites in the US Veterans Affairs Health Care System (26), where access to health-care resources is equivalent. A similar finding was also reported among participants in a randomized clinical trial of adjuvant chemotherapy (27), which reported no difference in 5-year disease-free survival between whites and blacks (58% vs 57%) nor in overall survival (66% vs 65%). These findings suggest that differences in access and utilization of health-care resources may in part explain the lower survival in blacks with colorectal cancer. Studies have shown that minority populations are less likely to use health-care resources even in an equal access environment (28–30).
It is uncertain what factors are directly responsible for the lower rates of follow-up for abnormal testing among blacks, but a number of factors could be considered. Low socioeconomic status may affect health-care utilization because of the direct cost of care such as co-pay or indirect cost such as lost wages while accessing health-care resources. It is possible that the need for multiple tests following abnormal PLCO screening may adversely affect the ability of some subjects to undergo follow-up testing. Lack of knowledge of cancer prevention may contribute to low uptake of diagnostic testing, and lack of cultural competence on the part of care providers may also constitute a barrier to health-care utilization (31–33).
In general, our study demonstrates that blacks were less likely to undergo diagnostic evaluations following an abnormal screening FSG compared with their white counterparts at every level of education, but this reached statistical significance only among participants with high school education or less. It is quite possible that our findings of lower health-care utilization among blacks may actually be an underestimation. Black participants in the PLCO were more educated and health conscious than comparable members of the general population (34) and were encouraged to undergo follow-up evaluations by virtue of being enrolled in an ongoing clinical trial. Therefore, the fact that even these study participants who had abnormal screening tests did not undergo appropriate diagnostic evaluations, would suggest that utilization of health-care resources in the general population may be lower than what we captured in our study.
We are not aware of any study that has examined the use of diagnostic evaluation after screening and yield of colorectal neoplasia by race for direct comparison. However, three large studies recently compared the prevalence of colorectal neoplasia during screening colonoscopy using the Clinical Outcomes Research Initiative database, an endoscopic data repository of geographically diverse gastroenterology practices in the United States. Two of these studies did not use histopathologic diagnosis. Thornton et al. (11) reported an increased odds of proximal polyps in blacks (OR = 1.30, 95% CI = 1.11 to 1.52) compared with whites, whereas Lieberman et al. (12) reported an increased odds of large (>9 mm) polyps among blacks but similar odds of proximal large polyps (OR = 1.13, 95% CI = 0.93 to 1.38). The third study (35) used the histopathologic diagnosis of detected polyps from the Clinical Outcomes Research Initiative database and reported an increased odds of advanced adenoma among blacks (OR = 1.35, 95% CI = 1.04 to 1.75), which is at variance with our findings. However, those authors also reported no difference by race and/or ethnicity in the prevalence of advanced histology in adenoma, either with size less than 10 mm in diameter or with size more than 10 mm in diameter (35). The difference in the findings between that study and our own may be because serrated histology was included as advanced pathology in the other study. Another difference is that our study used a multilevel screening strategy in which only subjects with abnormal FSG results had colonoscopies rather than the entire screened population.
The strengths of our study are that we prospectively evaluated both utilization of health-care resources and the yield of colorectal neoplasia by race. Our study population was from an ongoing, large, community-based screening trial recruited from 10 geographically dispersed centers. Also, the diagnostic colonoscopies were arranged by participants’ physicians and performed by the community gastroenterologists whom their physicians chose. This enables our study to closely reflect what might happen in a real-world scenario.
Our study also has limitations. Blacks were underrepresented in the PLCO trial despite intense efforts during recruitment. The enrollment was 5% for blacks in PLCO as compared with an age-eligible US population that was 9.5% black. Participants in the PLCO are also more educated, more likely to exercise regularly, and less likely to be current smokers than comparable members of the general public (18,34). Hence, the yield of colorectal neoplasia may be lower compared with the general population. However, we do not believe that this should differentially affect the blacks as compared with whites in our study. The racial difference in the rates of follow-up for abnormal screening (an endpoint of our study) may mask differences in the yield of colorectal neoplasia because of incomplete ascertainment, and we used the highest education attained as a proxy for socioeconomic status. Furthermore, participants with proximal colon neoplasia but without any distal lesions would not be detected because the participants who were referred for colonoscopy had distal colon lesions. Therefore, if blacks were more likely to have isolated proximal advanced adenoma compared with whites, this would not be captured in our study. This should be a focus of future studies. Proximal advanced adenoma has been estimated to occur in 1.5%–2.7% of subjects without any distal colon polyps in colonoscopy-based screening studies (36,37). Of note, Thornton et al. (11) suggested that blacks have a higher rate of isolated proximal polyps, but the study lacked histopathologic diagnosis. Also, in an epidemiological necropsy study of 3558 persons (aged 20–89 years) in a single referral center of whom 26% died from malignancy, Pendergrass et al. (38) reported a higher prevalence of adenoma among young whites (aged 20–49 years) compared with young blacks (ie, 2.52 vs 1.97 per hundred; RR = 1.28, 95% CI = 1.26 to 1.31) but noted a higher right-to-left ratio in adenoma prevalence among young blacks.
It is noteworthy that our study was not designed to address the recommendation by the American College of Gastroenterology that blacks be screened for colorectal cancer starting at 45 years of age. We evaluated the differences in health-care utilization and biological outcomes (in terms of colorectal neoplasia) by race. Our study suggests that among whites and blacks aged 55–74 years, biological differences may not be as important as health-care utilization as a factor for racial disparity in colorectal cancer. We have no data on racial disparity between the ages of 45 and 54 years, but it is unlikely that the entire population of blacks would have increased risk compared with whites at age 45–54 and then not at age 55–74. However, there may be a subpopulation of blacks who are at increased risk at a younger age.
In conclusion, we did not observe meaningful differences in the yield of colorectal neoplasia by race among participants with diagnostic colonoscopy, but blacks were less likely to undergo diagnostic evaluation following abnormal screening when compared with whites. Targeted interventions aimed at increasing the uptake of screening among minority populations, and provision of an adequate diagnostic component for screening programs is essential if colorectal cancer disparities were to be eliminated.
Funding
The study was funded by the National Cancer Institute through contracts to the participating centers associated with the PLCO trial: University of Colorado Denver (N01-CN-25514); Georgetown University (N01-CN-25522); Pacific Health Research Institute (N01-CN-25515); Henry Ford Health System (N01-CN-25512); University of Minnesota (N01-CN-25513); Washington University (N01-CN-25516); University of Pittsburgh Medical Center (N01-CN-25511); University of Utah (N01-CN-25524); Marshfield Clinic Research Foundation (N01-CN-25518); University of Alabama at Birmingham (N01-CN-75022); Westat, Inc. (N01-CN-25476); UCLA Immunogenetics Ctr. (N01-CN-25404). The clinical trials number for PLCO trial: NCT (00002540).
Footnotes
The authors report no conflicts of interest. The funding agency had a role in the design and reporting of the study and in the decision to submit the manuscript for publication and approved the final version of the manuscript.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2005. Bethesda, MD: National Cancer Institute; 2008. Based on November 2007 SEER data submission, posted to the SEER Web site. http://seer.cancer.gov/csr/1975_2005/. Accessed March 24, 2009. [Google Scholar]
- 3.Siegel RL, Jemal A, Thun MJ, Hao Y, Ward EM. Trends in the incidence of colorectal cancer in relation to county-level poverty among blacks and whites. J Natl Med Assoc. 2008;100(12):1441–1444. doi: 10.1016/s0027-9684(15)31544-3. [DOI] [PubMed] [Google Scholar]
- 4.Kinsey T, Jemal A, Liff J, Ward E, Thun M. Secular trends in mortality from common cancers in the United States by educational attainment, 1993-2001. J Natl Cancer Inst. 2008;100(14):1003–1012. doi: 10.1093/jnci/djn207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doubeni CA, Field TS, Buist DS, et al. Racial differences in tumor stage and survival for colorectal cancer in an insured population. Cancer. 2007;109(3):612–620. doi: 10.1002/cncr.22437. [DOI] [PubMed] [Google Scholar]
- 6.Fairley TL, Cardinez CJ, Martin J, et al. Colorectal cancer in U.S. adults younger than 50 years of age, 1998-2001. Cancer. 2006;107(5 suppl):1153–1161. doi: 10.1002/cncr.22012. [DOI] [PubMed] [Google Scholar]
- 7.Shavers VL. Racial/ethnic variation in the anatomic subsite location of in situ and invasive cancers of the colon. J Natl Med Assoc. 2007;99(7):733–748. [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson H, Jr, Carstens R. Anatomical distribution of colonic carcinomas. Interracial differences in a community hospital population. Cancer. 1986;58(4):997–1000. doi: 10.1002/1097-0142(19860815)58:4<997::aid-cncr2820580435>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Francois F, Park J, Bini EJ. Colon pathology detected after a positive screening flexible sigmoidoscopy: a prospective study in an ethnically diverse cohort. Am J Gastroenterol. 2006;101(4):823–830. doi: 10.1111/j.1572-0241.2006.00433.x. [DOI] [PubMed] [Google Scholar]
- 10.Agrawal S, Bhupinderjit A, Bhutani MS, et al. Committee of Minority Affairs and Cultural Diversity, American College of Gastroenterology. Colorectal cancer in African Americans. Am J Gastroenterol. 2005;100(3):515–523. doi: 10.1111/j.1572-0241.2005.41829.x. [DOI] [PubMed] [Google Scholar]
- 11.Thornton JG, Morris AM, Thornton JD, Flowers CR, McCashland TM. Racial variation in colorectal polyp and tumor location. J Natl Med Assoc. 2007;99(7):723–728. [PMC free article] [PubMed] [Google Scholar]
- 12.Lieberman DA, Holub JL, Moravec MD, Eisen GM, Peters D, Morris CD. Prevalence of colon polyps detected by colonoscopy screening in asymptomatic black and white patients. JAMA. 2008;300(12):1417–1422. doi: 10.1001/jama.300.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramsoekh D, van Leerdam ME, van Ballegooijen M, Habbema JD, Kuipers EJ. Population screening for colorectal cancer: faeces, endoscopes or X-rays? Cell Oncol. 2007;29(3):185–194. doi: 10.1155/2007/610496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21(suppl):S309. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 15.Gohagan JK, Prorok PC, Hayes RB, Kramer BS Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial Project Team. The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: history, organization, and status. Control Clin Trials. 2000;21(6 suppl):251S–272S. doi: 10.1016/s0197-2456(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 16.Weissfeld JL, Schoen RE, Pinsky PF, et al. Flexible sigmoidoscopy in the PLCO Cancer Screening Trial: results from the baseline screening examination of a randomized trial. J Natl Cancer Inst. 2005;97(13):989–997. doi: 10.1093/jnci/dji175. [DOI] [PubMed] [Google Scholar]
- 17.Stallings FL, Ford ME, Simpson NK, et al. Black participation in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21(6 suppl):379S–389S. doi: 10.1016/s0197-2456(00)00093-3. [DOI] [PubMed] [Google Scholar]
- 18.Pinsky PF, Ford M, Gamito E, et al. Enrollment of racial and ethnic minorities in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. J Natl Med Assoc. 2008;100(3):291–298. doi: 10.1016/s0027-9684(15)31241-4. [DOI] [PubMed] [Google Scholar]
- 19.Pinsky PF, Schoen RE, Weissfeld JL, Kramer B, Hayes RB, Yokochi L PLCO Project Team. Variability in flexible sigmoidoscopy performance among examiners in a screening trial. Clin Gastroenterol Hepatol. 2005;3(8):792–797. doi: 10.1016/s1542-3565(05)00286-7. [DOI] [PubMed] [Google Scholar]
- 20.O'Malley AS, Forrest CB, Feng S, Mandelblatt J. Disparities despite coverage: gaps in colorectal cancer screening among Medicare beneficiaries. Arch Intern Med. 2005;165(18):2129–2135. doi: 10.1001/archinte.165.18.2129. [DOI] [PubMed] [Google Scholar]
- 21.Doubeni CA, Laiyemo AO, Reed G, Field TS, Fletcher RH. Socioeconomic and racial patterns of colorectal cancer screening among Medicare enrollees in 2000 to 2005. Cancer Epidemiol Biomarkers Prev. 2009;18(8):2170–2175. doi: 10.1158/1055-9965.EPI-09-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haas JS, Earle CC, Orav JE, Brawarsky P, Neville BA, Williams DR. Racial segregation and disparities in cancer stage for seniors. J Gen Intern Med. 2008;23(5):699–705. doi: 10.1007/s11606-008-0545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 24.Deddens JA, Petersen MR, Lei X. Proceedings of the 28th Annual SAS Users Group International conference. Cary, NC: SAS Institute, Inc; 2003. Estimation of prevalence ratios when PROC GENMOD does not converge (paper 270–28) [Google Scholar]
- 25.Deddens JA, Petersen MR. Re: “Estimating the relative risk in cohort studies and clinical trials of common outcomes”. Am J Epidemiol. 2004;159(2):213–214. doi: 10.1093/aje/kwh022. [DOI] [PubMed] [Google Scholar]
- 26.Dominitz JA, Samsa GP, Landsman P, Provenzale D. Race, treatment, and survival among colorectal carcinoma patients in an equal-access medical system. Cancer. 1998;82(12):2312–2320. doi: 10.1002/(sici)1097-0142(19980615)82:12<2312::aid-cncr3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 27.McCollum AD, Catalano PJ, Haller DG, et al. Outcomes and toxicity in African Americans and Caucasian patients in a randomized adjuvant chemotherapy trial for colon cancer. J Natl Cancer Inst. 2002;94(15):1160–1167. doi: 10.1093/jnci/94.15.1160. [DOI] [PubMed] [Google Scholar]
- 28.Baldwin LM, Dobie SA, Billingsley K, et al. Explaining black-white differences in receipt of recommended colon cancer treatment. J Natl Cancer Inst. 2005;97(16):1211–1220. doi: 10.1093/jnci/dji241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farjah F, Wood DE, Yanez ND, III, et al. Racial disparities among patients with lung cancer who were recommended operative therapy. Arch Surg. 2009;144(1):14–18. doi: 10.1001/archsurg.2008.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garland AF, Lau AS, Yeh M, McCabe KM, Hough RL, Landsverk JA. Racial and ethnic differences in utilization of mental health services among high-risk youths. Am J Psychiatry. 2005;162(7):1336–1343. doi: 10.1176/appi.ajp.162.7.1336. [DOI] [PubMed] [Google Scholar]
- 31.Sharma S, O'Keefe SJ. Environmental influences on the high mortality from colorectal cancer in African Americans. Postgrad Med J. 2007;83(983):583–589. doi: 10.1136/pgmj.2007.058958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powe BD. Fatalism among elderly African Americans. Effects on colorectal cancer screening. Cancer Nurs. 1995;18:385–392. [PubMed] [Google Scholar]
- 33.Jandorf L, Fatone A, Borker PV, et al. Creating alliances to improve cancer prevention and detection among urban medically underserved minority groups. The East Harlem Partnership for Cancer Awareness. Cancer. 2006;107(8 suppl):2043–2051. doi: 10.1002/cncr.22153. [DOI] [PubMed] [Google Scholar]
- 34.Pinsky PF, Miller A, Kramer BS, et al. Evidence of a healthy volunteer effect in the prostate, lung, colorectal, and ovarian cancer screening trial. Am J Epidemiol. 2007;165(8):874–881. doi: 10.1093/aje/kwk075. [DOI] [PubMed] [Google Scholar]
- 35.Lieberman D, Moravec M, Holub J, Michaels L, Eisen G. Polyp size and advanced histology in patients undergoing colonoscopy screening: implications for CT colonography. Gastroenterology. 2008;135(4):1100–1105. doi: 10.1053/j.gastro.2008.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med. 2000;343(3):169–174. doi: 10.1056/NEJM200007203430302. [DOI] [PubMed] [Google Scholar]
- 37.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343(3):162–168. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 38.Pendergrass CJ, Edelstein DL, Hylind LM, et al. Occurrence of colorectal adenomas in younger adults: an epidemiologic necropsy study. Clin Gastroenterol Hepatol. 2008;6(9):1011–1015. doi: 10.1016/j.cgh.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]