Abstract
Human leukocyte antigen-G (HLA-G) is a non-classical major histocompatibility complex class Ib molecule that acts as a specific immunosuppressor. Some studies have demonstrated that human papillomavirus (HPV) seems to be involved in lower or absent HLA-G expression, particularly in cervical cancer. In this study, we performed a cross-sectional study, systematically comparing the qualitative expression of the HLA-G5 isoform in invasive cervical carcinoma (ICC), stratifying patients according to the presence [ICC with metastasis (ICCW)] and absence [ICC without metastasis (ICCWT)] of metastasis, correlating these findings with interference of HPV and demographic and clinical variables. Seventy-nine patients with a diagnosis of ICC were stratified into two groups: ICCWT (n=52 patients) and ICCW (n=27). Two biopsies were collected from each patient (one from the tumor lesion and one from a lymph node). Immunohistochemistry analyses were performed for the HLA-G5 isoform, for HPV detection, and virus typing. HLA-G5 isoform molecules were detected in 25 cases (31.6%), 17 (32.7%) without metastasis and 8 (29.6%) with metastasis. HPV was detected in the cervical lesions of 74 patients (93.7%), but low expression of the HLA-G5 isoform was observed in all HPV-related cases. These findings are important; however, additional studies are necessary to identify the influence of HPV with HLA-G5 isoform expression on invasive cervical malignancies. (J Histochem Cytochem 58:405–411, 2010)
Keywords: invasive cervical carcinoma, HLA-G5 isoform, HPV, immunohistochemistry, PCR
Cervical cancer is the second most common cancer among women and is responsible for 400,000 deaths worldwide every year (Kondo 2009). Tumors have developed different strategies to escape immune cell recognition, downregulating classical human leukocyte antigen (HLA) class I antigens, and impairing TCD8 cell activity. In addition, studies have shown aberrant expression of non-classical molecules, including HLA-G on the cell surface, that have immunomodulatory effects on natural killer cells, T lymphocytes, and antigen-presenting cells and may further inhibit the immune response (Seliger and Schlaf 2007).
HLA-G expression may occur in normal tissues, including the placenta, where the molecule can protect the fetus against attack by the maternal immune system (Rouas-Freiss et al. 2005). On the other hand, HLA-G expression may occur in pathological states, as beneficial or deleterious depending on the condition involved. In transplantation and autoimmune disorders, HLA-G expression has been associated with a better prognosis, because the molecule may control the immune response against alloantigens or autoantigens. In contrast, HLA-G expression in virus-infected or malignant cells has been associated with tumor progression and metastasis (Carosella et al. 2003; Rouas-Freiss et al. 2005).
The expression of HLA-G in cancer was first described in melanoma cells (Paul et al. 1998), and the relationship between HLA-G expression and tumor progression has been a rapidly expanding field of cancer research, in which HLA-G expression has been observed in several tumor cells (Chen and Liao 2005; El-Chennawi et al. 2005; Adithi et al. 2006; Gros et al. 2006; Ishigami et al. 2006; Kleinberg et al. 2006; Rebmann et al. 2007; Ye et al. 2007; Yie et al. 2007) and at times used as a prognosis marker (Rouas-Freiss et al. 2007).
Overall, increased HLA-G expression in gynecological cancer cells has been associated with lesion progression, particularly in ovarian cancer (Sheu and Shih 2007) and endometrial adenocarcinoma (Barrier et al. 2006). However, the expression of the HLA non-classical molecule in premalignant and malignant cervical lesions caused by human papillomavirus (HPV) infection is increased in low-grade squamous intraepithelial lesions, later decreasing as the lesions progress to high-grade or invasive cancer (Zhou et al. 2006; Goncalves et al. 2008). In this study, we performed a cross-sectional study, systematically comparing the qualitative expression of the HLA-G5 isoform in invasive cervical carcinoma (ICC), stratifying patients according to the presence [ICC with metastasis (ICCW)] or absence [ICC without metastasis (ICCWT)] of metastasis and correlating these findings with interference of HPV infection and demographic and clinical variables.
Materials and Methods
Specimens
The study protocol was approved by the Ethics Committee of the University Hospital, Faculty of Medicine of Ribeirão Preto, University of São Paulo (process #5507/06). This was a retrospective study (1994 to 2004) conducted on 79 human immunodeficiency virus (HIV)-1–negative patients exhibiting ICC without (ICCWT, n=52 patients) or with (ICCW, n=27 patients) lymph node metastasis. In addition, 79 biopsies of lymph nodes from all patients were evaluated. All samples were collected from the archives of the Pathology Department, University Hospital, Faculty of Medicine of Ribeirão Preto, University of São Paulo and from the Anatomic Pathology Department, Faculty of Medical Sciences, State University of Campinas, São Paulo. Clinicopathological information about the patients, such as age and history of smoking and of alcohol consumption, was obtained from the patients' medical records.
The number of patients was apparently small because the archives (1994–2004) of the Faculty of Medicine of Ribeirão Preto and Campinas presented only 79 biopsies with ICCWT and ICCW. In addition, HIV-positive patients were excluded to isolate only patients who were HPV infected.
All biopsies were reviewed and evaluated by two experienced histopathologists in a double-blind protocol. One cervical biopsy and one lymph node specimen obtained from each patient were embedded in paraffin blocks and stained with hematoxylin-eosin. Thin (5-μm) sections were cut, placed on organosilane-pretreated slides, and subjected to immunohistochemical assays (HLA-G5 isoform). Additionally, a 10-μm section was cut for DNA extraction and HPV typing.
Immunohistochemistry Assay for HLA-G5 Isoform
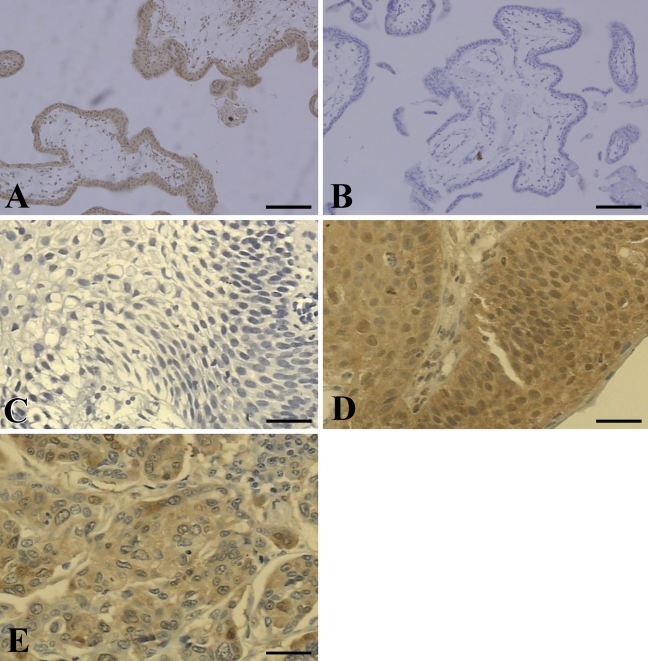
Sections of 5 μm were cut, placed on organosilane-pretreated slides, and subjected to immunohistochemical assay for the HLA-G5 isoform using the streptavidin-biotin system (LSAB; DAKO, Glostrup, Denmark). The cervical specimens were dewaxed in xylene, rehydrated in a graded alcohol series, and rinsed in water. For antigen retrieval, the sections were immersed in 10 mM sodium citrate buffer, pH 6.2. Endogenous peroxidase blocking was performed with three successive incubations of 15 min each in hydrogen peroxide plus absolute methanol. Nonspecific binding was performed with 3% low-fat dried milk diluted 1:100 in PBS. Slides were incubated with the primary monoclonal antibody (MAb) for the HLA-G5 isoform (5A6G7, diluted 1:50; EXBIO, Vestec, Czech Republic) in a humidified chamber at 4C overnight. Then slides were incubated with biotinylated anti-rabbit antibody and the streptavidin–peroxidase complex at 37C for 30 min. Finally, samples were incubated with 3,3-diaminobenzidine (DAB, Gibco; Gaithersburg, MD) diluted in 0.01% H2O2 for 10 min, lightly counterstained with Carrazzi's hematoxylin without acid for 60 sec, exhaustively rewashed with tap water, air dried, and mounted with Permount mounting medium (MERCK; Darmstadt, Germany). To validate the anti-HLA-G MAb and the immunohistochemical method, we systematically analyzed a paraffin-embedded section of trophoblastic tissue (positive control, Figure 1A). A negative control was prepared by omitting the primary antibody (Figure 1B). Basal HLA-G expression was evaluated in seven normal cervical biopsies from autopsies of previously healthy individuals who had died from violent trauma.
Figure 1.
Human leukocyte antigen-G (HLA-G) expression in patients with invasive cervical carcinoma (ICC). Cervical biopsies obtained from patients according to the presence of ICC with metastasis (ICCW) or ICC without metastasis (ICCWT) were analyzed by immunohistochemistry. (A) HLA-G expression in trophoblast tissue obtained from a recent abortion (positive control). (B) Absence of anti-HLA-G 5A6G7, negative control. (C) Normal cervix from a healthy woman (autopsy). Note the absence of staining in squamous epithelial cells. (D) Intense HLA-G staining in ICC; and (E) intense HLA-G staining in lymph node metastasis. Bars: A,B = 250 μm; C–E = 100 μm.
Qualitative Classification of HLA-G
All scoring and qualitative interpretation of immunohistochemical results were carried out by an experienced pathologist and classified as negative, discrete, moderate, or intense immunostaining. In the present study, we considered the expression of HLA-G to be low when no signal or discrete immunostaining was observed. HLA-G expression was classified as high when the pathologist observed moderate or intense immunostaining.
Quantitative Classification of HLA-G
Positive cytoplasm was automatically quantified by a computer-assisted system (Image-Pro Plus; Media Cybernetics, Inc., Bethesda, MD) consisting of a microscope, a digital camera, and a software package. A mean of ten random microscope fields were selected to analyze 1000 cytoplasm sites (HLA-G) per biopsy in all patient sections. The image acquisition of the sections of 79 patients (cervical lesion and lymph nodes with and without metastasis) was performed on an electron photomicrograph, and the image was processed and analyzed by the software. For each slide, the digitized image segmentation was controlled interactively by the red/green/blue color filter existing in the software program. The automatic cytoplasm count was established and expressed as percentage.
HPV Detection and Typing
Sections of 10 μm were cut from paraffin blocks and used for DNA extraction and HPV typing. HPV DNA obtained from paraffin blocks (Frank et al. 1996) was PCR amplified using 12.5 pmol of deoxynucleoside triphosphate, 25 pmol of each primer, 1.5 U Taq DNA polymerase (Gibco; Gaithersburg, MD), 5 μl of 10× enzyme buffer, 20 μg of genomic DNA, and distilled deionized H2O to complete a total volume of 25 μl. The mixture was processed in a thermocycler apparatus (MJ Research; Waltham, MA) under the following cycling conditions: 1 cycle at 95C for 5 min; 30 cycles at 95C for 30 sec, 55C for 30 sec, and 72C for 1 min, and finally, 1 cycle at 72C for 10 min, and then 4C indefinitely.
The primers GP5+ and GP6+ (Clifford et al. 2003), which amplify small DNA fragments (150 bp), were used for generic HPV amplification. The specific HPV-type amplification was performed with the set of primers HPV16E7.667/HPV16E7.774 for HPV-16, HPV18E7.696/HPV18E7.799 for HPV-18, HPV31E7.811/HPV31E7.890 for HPV-31, and HPV33E7.671/HPV33E7.761 for HPV-33 (Walboomers et al. 1999). All DNAs were amplified with a set of primers for a β-globin housekeeping gene (Saiki et al. 1988) as an internal control of amplification. The HPV positive control and beta-globin control were cervical biopsies that were tested for HPV DNA. For HPV-16 and HPV-18 controls, DNA was extracted from SiHa and HeLa cell lines, respectively, whereas HPV-31 and HPV-33 were from patient samples. All controls (HPV-16, -18, -31, and -33) were kindly provided by Dr. Luisa Lina Villa, Ludwig Institute for Cancer Research, São Paulo, Brazil. As a negative control, all PCR reagents were added to an Eppendorf tube containing no DNA sample. HPV-16, -18, -31, and -33 were chosen because they are most frequently associated with ICC. PCR amplicons were electrophoresed on 10% polyacrylamide gels at 200 V for 1.45 hr and stained with AgNO3 (Sanguinetti et al. 1994). It is important to note that the absence of HPV-16, -18, -31, or -33 did not exclude the presence of HPV types other than -16, -18, -31, and -33.
Statistical Analyses
Qualitative differences in HLA-G expression between groups determined according to clinical parameters were evaluated by means of the two-sided Fisher's exact test for 2 × 2 contingency tables, with the aid of the GraphPad InStat software (San Diego, CA), which was also used to estimate the odds ratio and its 95% confidence interval. Age, reported as arithmetic mean and standard deviation, was also compared between groups by means of the two-sided Student's unpaired t-test. The mean expression levels of HLA-G between ICC and metastatic cells of the draining lymph nodes were compared using the Wilcoxon matched-pair signed-rank test. Quantitative comparisons of HLA-G expression among groups were performed using the Mann-Whitney test. Correlations were calculated using the Spearman test. Statistical analyses were performed using the GraphPad Instat 3.05 software, with p values ≤0.05 considered to be significant.
Results
In the qualitative evaluation of the HLA-G5 isoform in all biopsies (n=79), HLA-G molecules were detected in only 25 patients (31.6%). In ICCWT patients, HLA-G was observed in 17 out of 52 (32.7%) cervical specimens. In the ICCW group, this molecule was detected in 8 out of 27 (29.6%) cervical specimens. HLA-G expression was found to be low in both groups (Table 1).
Table 1.
Qualitative classification of HLA-G expression in ICCs according to the presence or absence of lymph node metastasis
| Low expression |
High expression |
|||
|---|---|---|---|---|
| Negative n (%) | Discrete n (%) | Moderate n (%) | Intense n (%) | |
| ICCWTN=52 | 35 (67.3) | 9 (17.3) | 6 (11.5) | 2 (3.8) |
| Lymph node of ICCWTN=52 | 52 (100) | 0 | 0 | 0 |
| ICCWN=27 | 19 (70.4) | 5 (18.5) | 2 (11.5) | 1 (3.8) |
| Lymph node of ICCWN=27 | 21 (77.8) | 4 (14.8) | 2 (11.5) | 0 |
HLA-G, human leukocyte antigen-G; ICC, invasive cervical carcinoma; ICCWT, ICC without metastasis; ICCW, ICC with metastasis.
Normal cervical specimens obtained from healthy patients at autopsy (Figure 1C) and negative controls (Figure 1B) did not present any immunostaining in squamous cells, whereas the positive control (Figure 1A) showed specific immunostaining. In the patient sample, HLA-G expression was primarily detected in the cytoplasm of malignant cervical cells (Figure 1D) and in lymph node metastatic cells (Figure 1E).
In the quantitative analysis of the ICCW group, HLA-G expression in cervical lesions exhibited a positive correlation (r = 0.922, p<0.0001) with that observed in the corresponding lymph node metastatic cells (Table 2).
Table 2.
Quantitative immunoexpression of HLA-G in ICCs stratified according to presence or absence of lymph node metastasis
| HLA-G (Mean ± SEM) | |
|---|---|
| ICCWT (n=52) | 27.98 ± 5.66 |
| ICCW (n=27) | 22.64 ± 7.28 |
| Lymph nodes of ICCW (n=27) | 13.83 ± 5.82 |
Analysis of variance (Mann-Whitney Test): p values: ICCWT vs ICCW (p=0.7771); Spearman correlation: ICCW cervical lesions vs corresponding lymph nodes (p<0.0001, r = 0.922); Wilcoxon matched-pairs signed-ranks test ICCW (p=0.0078). Data are reported as mean and standard error of the mean (SEM).
HPV Detection and Typing
HPV DNA was detected in 74 of 79 (93.7%) specimens analyzed. In the ICCWT group, HPV virus was positive in 47 out of 52 (90.4%) patients, and in the ICCW group, this virus was detected in 27 (100%) of patients (Table 3). There was no correlation between HLA-G expression and HPV infection.
Table 3.
HPV DNA detection and typing in invasive cervical carcinomas stratified according to the presence or absence of lymph node metastasis
| HPV Positive n (%) | HPV Negative n (%) | HPV-16 n (%) | HPV-18 n (%) | HPV-31 n (%) | HPV-33 n (%) | Multiple types n (%) | Other types n (%) | |
|---|---|---|---|---|---|---|---|---|
| ICCWTN=52 | 47 (90.4) | 5 (9.6) | 1 (1.9) | 3 (5.8) | 2 (3.8) | 5 (9.6) | 24 (46.1) | 12 (23.1) |
| Lymph node of ICCWTN=52 | 23 (44.2) | 29 (55.8) | 3 (5.8) | 1 (1.9) | 4 (7.7) | 6 (11.5) | 3 (5.8) | 6 (11.5) |
| ICCWN=27 | 27 (100) | 0 | 4 (14.8) | 5 (18.5) | 3 (11.1) | 1 (3.7) | 7 (25.9) | 7 (25.9) |
| Lymph node of ICCWN=27 | 18 (66.7) | 9 (33.3) | 2 (7.4) | 1 (3.7) | 2 (7.4) | 0 | 7 (25.9) | 6 (22.2) |
HLA-G5 Isoform Expression According to Demographic and Clinical Variables
No significant associations were observed regarding HLA-G expression in relation to age, alcohol use, and smoke exposure. However, the patients were predominantly not exposed to tobacco or alcohol (Table 4).
Table 4.
Demographic and epidemiologic findings observed in HLA-G–positive patients with ICC stratified according to presence or not of lymph node metastasis
| ICCWT | ICCW | p value | |
|---|---|---|---|
| Demography | |||
| Age (years) | (n=17) | (n=8) | |
| Mean | 19 ± 1.473 | 16.875 ± 0.6928 | 0.3485 (two-sided t-test)a |
| Smoke exposure | (n=17) | (n=8) | 0.0870 (two-sided Fisher)b |
| Yes/No | 4/13 | 4/4 | OR, 0.1846 (0.02996-1.138) |
| Alcohol exposure | (n=17) | (n=8) | 0.2313 (two-sided Fisher)b |
| Yes/No | 1/16 | 2/6 | OR, 0.1875 (0.01424-2.469) |
Unpaired t-test.
Fisher's exact test.
OR = odds ratio.
Discussion
Tumor cells can efficiently evade the antitumor immune response by modulating antigen expression, producing immunosuppressive cytokines, or inducing peripheral tolerance (Smyth et al. 2006). Tumor cells that have lost classical HLA molecules that are important for immune recognition tend to express the immune-tolerant HLA-G molecule on their cell surfaces (Rouas-Freiss et al. 2005). Cancers of different origin show preferential upregulation of HLA-G in advanced disease, rather than in the initial tumor lesions, supporting its tolerogenic role in the final phase of immunoediting (Urosevic and Dummer 2008).
In this study, we reported that HLA-G expression was low in cervical cancer specimens. Therefore, HLA-G expression in tumor cells may vary according to the type of cancer. A recent study stratified tumors according to the magnitude of HLA-G expression, i.e., lesions exhibiting no expression, less than 30% expression, and more than 30% expression (Carosella et al. 2008). Among some tumors affecting women, ovarian carcinoma expressed HLA-G in 61% of specimens (45/74) (Sheu and Shih 2007), endometrial adenocarcinoma in 55% (24/44) (Barrier et al. 2006), and breast cancer in 39% (14/36) (Lefebvre et al. 2002).
In the present study, concerning the immunological response, the loss of HLA class I molecules was consistently related to the occurrence of cervical lesions. We hypothesize that, similarly to other viral models, HPV infection may downregulate major histocompatibility complex class I molecules. Although definitive evidence of HPV influence on these cancers is not available, HPV infection is the hallmark in cervical cancer (Clifford et al. 2003; Fule et al. 2006), and HPV DNA was observed in patients of this series. Whether HPV is implicated in the magnitude of HLA-G expression remains to be elucidated; however, it is possible that HPV may be involved in the modulation of HLA-G surface expression, because it has been reported that high-risk HPV oncoproteins may inhibit the promotor gene of HLA class I heavy chain and may modulate latent membrane protein-2 and transporter associated with antigen processing-1 (TAP-1) proteins, responsible for processing and transporting peptides into the groove of HLA class I molecules (Georgopoulos et al. 2000). In addition, HPV E5 protein may downregulate HLA class I molecules by retaining HLA class I heavy chain in the Golgi complex (Ashrafi et al. 2002,2005). It is also interesting to observe the behavior of HLA-G expression in HPV-associated cervical lesions. HLA-G expression appeared in atypical glandular cells of undetermined significance and progressively decreased from cervical intraepithelial neoplasia-1 (CIN1) to CIN2–3 and ICC (Goncalves et al. 2008).
Several mechanisms have been proposed to explain the immunomodulatory effects of HLA-G in cancer. HLA-G may induce Th2 cytokine (IL-4, IL-5, IL-10) polarization, which is not effective on viral depletion (Nakanishi et al. 2001), permitting viral maintenance and lesion progression (Clerici et al. 1997; Nguyen et al. 2005). In our findings, low expression of HLA-G in cervical invasive lesions was observed, as demonstrated in Goncalves et al. (2008). Perhaps the genetic alterations of cervical invasive lesions could modify HLA-G expression in cancer cells in different tumor types (Carosella et al. 2008).
Particularly in cervical cancer, low expression of HLA-G could be attributed to other factors, such as genetic mutations in only one HLA allele in the 6p2.3 region (Vermeulen et al. 2005). Premalignant and malignant cervical lesions may originate from the same clonal process; therefore, the loss of HLA-G expression could be an initial event in cervical carcinogenesis (Vermeulen et al. 2005), and the loss of heterozygosis in the TAP region could cause failure of the transport of the HLA-G molecule (Vermeulen et al. 2007).
Overall, it remains to be determined why some cervical cancer patients express HLA-G, whereas others do not. These findings are important; however, additional studies are necessary to identify the influence of HPV on HLA-G5 isoform expression in invasive cervical malignancies.
In conclusion, further studies are needed to clarify the molecular aspects of the relationship between HLA-G expression and HPV infection, with potential applications for cervical cancer prediction.
Acknowledgments
This study was supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-PROCAD # 0210/05-6) and Fundação de Apoio ao Ensino, Pesquisa e Assistência (FAEPA) HCFMRP-USP. M.C.M.G. was supported by a doctoral fellowship from CAPES/Brazil.
We thank Ana Maria Rocha for excellent technical assistance.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Adithi M, Kandalam M, Ramkumar HL, Subramanian A, Venkatesan N, Krishnakumar S (2006) Retinoblastoma: expression of HLA-G. Ocul Immunol Inflamm 14:207–213 [DOI] [PubMed] [Google Scholar]
- Ashrafi GH, Haghshenas MR, Marchetti B, O'Brien PM, Campo MS (2005) E5 protein of human papillomavirus type 16 selectively downregulates surface HLA class I. Int J Cancer 113:276–283 [DOI] [PubMed] [Google Scholar]
- Ashrafi GH, Tsirimonaki E, Marchetti B, O'Brien PM, Sibbet GJ, Andrew L, Campo MS (2002) Down-regulation of MHC class I by bovine papillomavirus E5 oncoproteins. Oncogene 21:248–259 [DOI] [PubMed] [Google Scholar]
- Barrier BF, Kendall BS, Sharpe-Timms KL, Kost ER (2006) Characterization of human leukocyte antigen-G (HLA-G) expression in endometrial adenocarcinoma. Gynecol Oncol 103:25–30 [DOI] [PubMed] [Google Scholar]
- Carosella ED, Moreau P, Le Maoult J, Le Discorde M, Dausset J, Rouas-Freiss N (2003) HLA-G molecules: from maternal-fetal tolerance to tissue acceptance. Adv Immunol 81:199–252 [DOI] [PubMed] [Google Scholar]
- Carosella ED, Moreau P, Lemaoult J, Rouas-Freiss N (2008) HLA-G: from biology to clinical benefits. Trends Immunol 29:125–132 [DOI] [PubMed] [Google Scholar]
- Chen CL, Liao QP (2005) [Role of human leukocyte antigen-G small interference RNA in a choriocarcinoma cell line] Zhonghua Fu Chan Ke Za Zhi 40:549–552 [PubMed] [Google Scholar]
- Clerici M, Merola M, Ferrario E, Trabattoni D, Villa ML, Stefanon B, Venzon DJ, et al. (1997) Cytokine production patterns in cervical intraepithelial neoplasia: association with human papillomavirus infection. J Natl Cancer Inst 89:245–250 [DOI] [PubMed] [Google Scholar]
- Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S (2003) Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer 88:63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Chennawi FA, Auf FA, El-Diasty AM, El-Daim MA, El-Sherbiny SM, Ali A, El-Baz M, et al. (2005) Expression of HLA-G in cancer bladder. Egypt J Immunol 12:57–64 [PubMed] [Google Scholar]
- Frank TS, Svoboda-Newman SM, Hsi ED (1996) Comparison of methods for extracting DNA from formalin-fixed paraffin sections for nonisotopic PCR. Diagn Mol Pathol 5:220–224 [DOI] [PubMed] [Google Scholar]
- Fule T, Csapo Z, Mathe M, Tatrai P, Laszlo V, Papp Z, Kovalszky I (2006) Prognostic significance of high-risk HPV status in advanced cervical cancers and pelvic lymph nodes. Gynecol Oncol 100:570–578 [DOI] [PubMed] [Google Scholar]
- Georgopoulos NT, Proffitt JL, Blair GE (2000) Transcriptional regulation of the major histocompatibility complex (MHC) class I heavy chain, TAP1 and LMP2 genes by the human papillomavirus (HPV) type 6b, 16 and 18 E7 oncoproteins. Oncogene 19:4930–4935 [DOI] [PubMed] [Google Scholar]
- Goncalves MA, Le Discorde M, Simoes RT, Rabreau M, Soares EG, Donadi EA, Carosella ED (2008) Classical and non-classical HLA molecules and p16(INK4a) expression in precursors lesions and invasive cervical cancer. Eur J Obstet Gynecol Reprod Biol 141:70–74 [DOI] [PubMed] [Google Scholar]
- Gros F, Sebti Y, de Guibert S, Branger B, Bernard M, Fauchet R, Amiot L (2006) Soluble HLA-G molecules increase during acute leukemia, especially in subtypes affecting monocytic and lymphoid lineages. Neoplasia 8:223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigami S, Natsugoe S, Miyazono F, Nakajo A, Tokuda K, Matsumoto M, Okumura H, et al. (2006) HLA-G expression in gastric cancer. Anticancer Res 26:2467–2472 [PubMed] [Google Scholar]
- Kleinberg L, Florenes VA, Skrede M, Dong HP, Nielsen S, McMaster MT, Nesland JM, et al. (2006) Expression of HLA-G in malignant mesothelioma and clinically aggressive breast carcinoma. Virchows Arch 449:31–39 [DOI] [PubMed] [Google Scholar]
- Kondo K (2009) [Development of an HPV vaccine: remaining issues and perspective] Nippon Rinsho 67:62–68 [PubMed] [Google Scholar]
- Lefebvre S, Antoine M, Uzan S, McMaster M, Dausset J, Carosella ED, Paul P (2002) Specific activation of the non-classical class I histocompatibility HLA-G antigen and expression of the ILT2 inhibitory receptor in human breast cancer. J Pathol 196:266–274 [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H (2001) Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol 19:423–474 [DOI] [PubMed] [Google Scholar]
- Nguyen HH, Broker TR, Chow LT, Alvarez RD, Vu HL, Andrasi J, Brewer LR, et al. (2005) Immune responses to human papillomavirus in genital tract of women with cervical cancer. Gynecol Oncol 96:452–461 [DOI] [PubMed] [Google Scholar]
- Paul P, Rouas-Freiss N, Khalil-Daher I, Moreau P, Riteau B, Le Gal FA, Avril MF, et al. (1998) HLA-G expression in melanoma: a way for tumor cells to escape from immunosurveillance. Proc Natl Acad Sci USA 95:4510–4515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebmann V, Wagner S, Grosse-Wilde H (2007) HLA-G expression in malignant melanoma. Semin Cancer Biol 17:422–429 [DOI] [PubMed] [Google Scholar]
- Rouas-Freiss N, Moreau P, Ferrone S, Carosella ED (2005) HLA-G proteins in cancer: do they provide tumor cells with an escape mechanism? Cancer Res 65:10139–10144 [DOI] [PubMed] [Google Scholar]
- Rouas-Freiss N, Moreau P, Menier C, LeMaoult J, Carosella ED (2007) Expression of tolerogenic HLA-G molecules in cancer prevents antitumor responses. Semin Cancer Biol 17:413–421 [DOI] [PubMed] [Google Scholar]
- Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, et al. (1988) Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239:487–491 [DOI] [PubMed] [Google Scholar]
- Sanguinetti CJ, Dias Neto E, Simpson AJ (1994) Rapid silver staining and recovery of PCR products separated on polyacrylamide gels. Biotechniques 17:914–921 [PubMed] [Google Scholar]
- Seliger B, Schlaf G (2007) Structure, expression and function of HLA-G in renal cell carcinoma. Semin Cancer Biol 17:444–450 [DOI] [PubMed] [Google Scholar]
- Sheu JJ, Shih IeM (2007) Clinical and biological significance of HLA-G expression in ovarian cancer. Semin Cancer Biol 17:436–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Dunn GP, Schreiber RD (2006) Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol 90:1–50 [DOI] [PubMed] [Google Scholar]
- Urosevic M, Dummer R (2008) Human leukocyte antigen-G and cancer immunoediting. Cancer Res 68:627–630 [DOI] [PubMed] [Google Scholar]
- Vermeulen CF, Jordanova ES, ter Haar NT, Kolkman-Uljee SM, de Miranda NF, Ferrone S, Peters AA, et al. (2007) Expression and genetic analysis of transporter associated with antigen processing in cervical carcinoma. Gynecol Oncol 105:593–599 [DOI] [PubMed] [Google Scholar]
- Vermeulen CF, Jordanova ES, Zomerdijk-Nooijen YA, ter Haar NT, Peters AA, Fleuren GJ (2005) Frequent HLA class I loss is an early event in cervical carcinogenesis. Hum Immunol 66:1167–1173 [DOI] [PubMed] [Google Scholar]
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, et al. (1999) Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189:12–19 [DOI] [PubMed] [Google Scholar]
- Ye SR, Yang H, Li K, Dong DD, Lin XM, Yie SM (2007) Human leukocyte antigen G expression: as a significant prognostic indicator for patients with colorectal cancer. Mod Pathol 20:375–383 [DOI] [PubMed] [Google Scholar]
- Yie SM, Yang H, Ye SR, Li K, Dong DD, Lin XM (2007) Expression of human leucocyte antigen G (HLA-G) is associated with prognosis in non-small cell lung cancer. Lung Cancer 58:267–274 [DOI] [PubMed] [Google Scholar]
- Zhou JH, Ye F, Chen HZ, Zhou CY, Lu WG, Xie X (2006) Altered expression of cellular membrane molecules of HLA-DR, HLA-G and CD99 in cervical intraepithelial neoplasias and invasive squamous cell carcinoma. Life Sci 78:2643–2649 [DOI] [PubMed] [Google Scholar]