Abstract
Survival of the conceptus is dependent on continuous progesterone signaling in the maternal decidua but how this is achieved under conditions of oxidative stress that characterize early pregnancy is unknown. Using primary cultures, we show that modest levels of reactive oxygen species (ROS) increase sumoylation in human endometrial stromal cells (HESCs), leading to enhanced modification and transcriptional inhibition of the progesterone receptor (PR). The ability of ROS to induce a sustained hypersumoylation response, or interfere with PR activity, was lost upon differentiation of HESCs into decidual cells. Hypersumoylation in response to modest levels of ROS requires activation of the JNK pathway. Although ROS-dependent JNK signaling is disabled on decidualization, the cells continue to mount a transcriptional response, albeit distinct from that observed in undifferentiated HESCs. We further show that attenuated JNK signaling in decidual cells is a direct consequence of altered expression of key pathway modulators, including induction of MAP kinase phosphatase 1 (MKP1). Overexpression of MKP1 dampens JNK signaling, prevents hypersumoylation, and maintains PR activity in undifferentiated HESCs exposed to ROS. Thus, JNK silencing uncouples ROS signaling from the SUMO conjugation pathway and maintains progesterone responses and cellular homeostasis in decidual cells under oxidative stress conditions imposed by pregnancy.—Leitao, B., Jones, M. C., Fusi, L., Higham, J., Lee, Y. Takano, M., Goto, T., Christian, M., Lam, E. W.-F., Brosens, J. J. Silencing of the Jnk pathway maintains progesterone receptor activity in decidualizing human endometrial stromal cells exposed to oxidative stress signals.
Keywords: sumoylation, MKP1, reactive oxygen species, pregnancy
The cyclical waves of endometrial proliferation, differentiation, shedding, and regeneration, which occur on average 400 times during the reproductive years, are unparalleled in any other tissue of the body (1). Within this cycle, the differentiation of stromal fibroblast into epitheloid decidual cells represents a tipping point after which the fate of the endometrium becomes irreversibly dependent on progesterone signaling (2). In pregnancy, high levels of progesterone maintain and enhance decidual transformation of the endometrium and ensure the integrity of the fetomaternal interface during the process of trophoblast invasion and placenta formation. In the absence of pregnancy, falling progesterone levels trigger a cascade of events that results in proteolytic breakdown of the decidualizing superficial endometrial layer, resulting in focal bleeding, cell death, and menstruation (3).
Pregnancy is an intense inflammatory process, characterized by an influx of macrophages and specialized uterine natural killer (uNK) cells that surround the invading conceptus (4). In addition, major fluctuations in oxygen concentrations occur at the fetomaternal interface during normal pregnancy as a consequence of the profound vascular adaptations (5). Inflammation, intense tissue remodeling, and profound changes in local perfusion generate reactive oxygen species (ROS), including the superoxide anion, hydroxyl radical, and hydrogen peroxide (H2O2), capable of damaging nucleic acids, proteins, and lipids if levels surpass the cellular antioxidative defenses (6). Compared to undifferentiated human endometrial stromal cells (HESCs), decidualizing cells are remarkably resistant to oxidative cell death (7). This resistance upon differentiation is partly accounted for by induction of various free radical scavengers, most notably mitochondrial superoxide dismutase 2 (SOD2), monoamine oxidases A and B, thioredoxin, glutaredoxin, and peroxiredoxin (8). However, exposure of undifferentiated, but not decidualizing, HESCs to exogenous H2O2 also rapidly induces FOXO3a expression, and silencing of this proapoptotic transcription factor is sufficient to abrogate ROS-dependent apoptosis (7). Thus, undifferentiated HESCs are programmed to self-destruct in response to oxidative stress signals, whereas this mechanism is firmly disabled upon differentiation into decidual cells.
In addition to activating various signaling pathways, including stress MAP kinases and NF-κB (9, 10), ROS can disrupt cellular homeostasis by interfering with the SUMO (small ubiquitin-like modifier) conjugation/deconjugation pathway (11, 12). Sumoylation, an important post-translational protein modification that affects a large number of substrates, is catalyzed through a sequence of enzymatically directed steps. Firstly, proteolytic cleavage of four C-terminal amino acids from the precursor gives rise to mature SUMO-1. The E1 activating enzyme, a heterodimer consisting of SAE1/SAE2, then binds the modifier and transfers it in an ATP-dependent manner to the E2 conjugase, Ubc9. Ubc9 has the ability to convalently conjugate SUMO at lysine residues of target proteins, although usually, the activity of an E3 ligase, which includes the PIAS family of proteins, is also required. SUMO conjugation is highly dynamic and reversed by a family of sentrin-specific proteases (SENPs). The balance between the sumoylation-desumoyaltion in the cell regulates numerous processes, including cell signaling, nuclear transport, transcription, and DNA replication and repair (13,14,15). In addition to ROS, a variety of noxious stimuli, including heat shock, hypoxia, osmotic pressure, and genotoxic stress, affect the SUMO pathway (16,17,18), suggesting that it serves as an overarching mechanism that coordinates the cellular response to environmental stress signals.
We have previously shown that decidualization of HESCs is associated not only with resistance to oxidative cell death but also with profound changes in cellular sumoylation, characterized by global hyposumylation and redistribution of SUMO-1 conjugates to distinct nuclear foci (19). The global decrease in SUMO-1 conjugation upon decidualization is primarily dependent on cAMP signaling, associated with altered expression of various E3 ligases and SENPs, and sensitizes HESCs to progesterone signaling by attenuating ligand-dependent SUMO-modification of PR-A. Whether sumoylation plays a role in coupling ROS to steroid hormone signaling has not yet been addressed. Herein, we demonstrate that mild oxidative stress induced by relatively low levels of H2O2 rapidly activate JNK signaling in HESCs, which, in turn, enhances global and PR-specific SUMO-1 conjugation, thereby inhibiting progesterone responses. We further show that this hitherto unrecognized coupling of JNK stress signaling to the SUMO conjugation pathway is disabled upon decidualization of HESCs, mediated, at least in part, by the induction of MAP kinase phosphatase 1 (MKP1), thereby ensuring that progesterone signaling is unaffected under conditions of oxidative stress.
MATERIALS AND METHODS
Primary cell culture
Endometrial samples were obtained from premenopausal women without uterine pathology, and HESCs were isolated, established in culture, and maintained as described previously (20). For decidual differentiation, cultures were treated with 0.5 mM 8-br-cAMP (Sigma) and 1 μM MPA (Sigma, St. Louis, MO, USA). For other treatments, H2O2 was freshly prepared from 30% solution (Fluka, Buchs, Switzerland), JNKVIII inhibitor was purchased from Calbiochem (San Diego, CA, USA), and 4-hydroxytamoxifen (Sigma) was used at 100 nM.
Transient transfections and luciferase reporter assays
Primary HESCs were transfected by the calcium phosphate coprecipitation method using the ProFection Mammalian Transfection kit (Promega, Madison, WI, USA), while HeLa cells were transfected using Fugene HD reagent (Roche Diagnostics, Mannheim, Germany) according to manufacturer’s instructions. The expression vectors for PR-A wild-type/K388R, EGFP-SUMO-1, PRE2-luciferase, SUMO-1 wild-type/-GG have been described previously (19, 21). pBabe-MEKK-1:ER* (22) was a gift from S. Cook (The Babraham Institute, Cambridge, UK), and pSG5-MKP-1-myc [1-314] (23) was kindly provided by A. Bennett (Yale University, New Haven, CT, USA). The amounts of expression vectors transfected per well in each experiment are indicated in figure legends. Cells transfected with the luciferase reporter construct were harvested in Reporter Lysis Buffer (Promega), and luciferase activity was measured using Luciferase Assay Reagent (Promega) and a luminometer.
Western blot analysis
Whole-cell protein extracts were obtained by direct lysis in Laemmli buffer heated to 85°C, followed by sonication. Proteins resolved by SDS-PAGE were transferred to a PVDF membrane and probed with antibodies raised against SUMO-1 (33-2400; Zymed, Burlingame, CA, USA), β-actin (ab6276; Abcam, Cambridge, MA, USA), PR (NCL-L-PGR-312; Novocastra Laboratories, Milton Keynes, UK), Ubc9 (sc-5231; Santa Cruz Biotechnology, Santa Cruz, CA, USA), SEA2 (U10820; BD Biosciences, San Jose, CA, USA), Akt (9272; Cell Signaling, Beverly, MA, USA), phospho-Akt (Cell Signaling,), JNK (9252; Cell Signaling), phospho-JNK (4671; Cell Signaling), p38 (4058; Cell Signaling), phosho-p38 (9215; Cell Signaling), ERK (9102; Cell Signaling), phosho-ERK (9101; Cell Signaling), c-jun (9165; Cell Signaling), phospho-c-jun (9164; Cell Signaling), GFP (8371; BD Biosciences), myc (ab9106; Abcam), MKP1 (sc-370; Santa Cruz Biotechnology). Following incubation with HRP-conjugated secondary antibodies (Roche), chemiluminesce was visualized using the ECL+ kit (Amersham). The positive control samples for unprocessed and matured SUMO-1 were obtained by in vitro expression of the respective expression vectors using the TNT T7 Quick Coupled Transcription/Translation System (Promega). Notably, the appearance of SUMO-1 smears on Western blot analysis varied between experiments, reflecting on the one hand differences in the abundance of SUMO-1 conjugates between primary cultures and, on the other, differences in resolution of SUMO-1 modified proteins on SDS-PAGE.
Real-time quantitative PCR
Total RNA was extracted from primary HESC cultures or tissue samples using STAT-60 reagent (AMS Biotech). Following treatment with amplification grade DNaseI (Invitrogen, Carlsbad, CA, USA), cDNA was generated using the Superscript first-strand synthesis system for RT-PCR kit (Invitrogen). Template quantification was performed with an ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) using SYBR Green (Applied Biosystems) as dye layer and the relative standard curve calculation method. RNA input variances were normalized against the levels of the L19 housekeeping gene, which encodes a ribosomal protein. All measurements were performed in triplicate. Specific primer pairs were designed using Primer Express software (Applied Biosystems).
Electrophoretic mobility shift assay (EMSA)
Five micrograms of nuclear protein extracts was incubated on ice for 1 h with nonradiolabeled nonspecific (Oct1) and specific oligonucleotide (NF-κB) in binding buffer (20% v/v glycerol; 5 mM MgCl2; 2 mM EDTA; 50 mM Tris-HCL, pH 7.5; 250 mM NaCl; and 2 mM DTT), followed by a 45-min incubation with 0.035 pmol [γ-32P]ATP end-labeled oligonucleotide probes. DNA-protein complexes were separated from unbound DNA probe on a 4% nondenaturating acrylamide gel in TBE running buffer (9 mM Tris-borate and 0.2 mM EDTA, pH 8.0). Gels were vacuum dried and exposed to Amersham MP films at −80°C for 16–48 h. For supershift analysis, samples were incubated with 2 μg anti-p65 antibody (sc-8008; Santa Cruz Biotechnology) for 1 h on ice prior to incubation with the probe. The consensus oligonucleotides were purchased from Promega Life Science, the sequences of which are as follows (factor binding sites are underscored): NF-κB, 5′-AGTTGAGGGGACTTTCCCAGGC-3′; Oct-1, 5′-TGTCGAATGCAAATCACTAGAA-3′.
Immunohistochimestry
Paraffin-embedded, formalin-fixed endometrial specimens were examined for in vivo MKP-1 immunoreactivity. All specimens were obtained from cycling premenopausal women, free of intrauterine diseases, such as endometrial hyperplasia or polyps, and histologically dated to the secretory phase of the cycle, using standard criteria. Five-micrometer sections, placed on 1% w/v polylysine slides, were deparaffinized, dehydrated, and exposed to 0.3% v/v H2O2 for 15 min. Immunostaining was performed using the Vectastain Elite ABC kit (Vector Laboratories) according to manufacturer’s instructions. The sections were incubated for 1 h with anti-MKP1 antibody (sc-370; Santa Cruz) diluted 1:100. For negative controls, sections were incubated with 1% w/v bovine serum albumin (Sigma) instead of primary antibody.
Microarray gene expression analysis
HESC cultures were set up from biopsies obtained from 3 different patients, and all experiments were performed with passage 1 cultures. Following treatments, total RNA was extracted using STAT-60 reagent (AMS Biotech). To ensure the cells responded adequately to treatment, RNA aliquots were used for first-strand cDNA synthesis followed by RTQ-PCR to determine the levels of FOXO3a mRNA. In accordance with published findings (7), in all three sets of samples, H2O2 treatment induced FOXO3a in undifferentiated cells, while in decidualized cells expression was low and unaltered by H2O2. Microarray analysis on total RNA samples was performed by Almac Diagnostics using GeneChip human genome U133 Plus 2.0 arrays (Affymetrix, Santa Clara, CA, USA). The raw data were normalized, quality controlled, and filtered by Almac Diagnostics to produce gene lists and further analyzed with Ingenuity Pathway Analysis software (Ingenuity Systems, Redwood City, CA, USA).
RESULTS
SUMO-1 conjugation in undifferentiated and decidualizing HESCs exposed to ROS
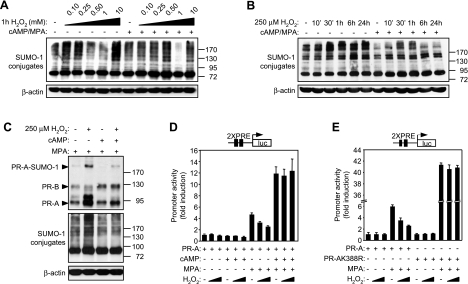
To determine whether the decidual process affects the coupling of the SUMO pathway to cellular stress, primary cultures, either untreated or differentiated with 8-br-cAMP and MPA for 3 d, were exposed to increasing concentrations of H2O2, a source of ROS, for 1 h. Modest levels of H2O2 (100–250 μM) increased SUMO-1 conjugation in undifferentiated but not decidual HESCs (Fig. 1A). At higher concentrations, however, the effects of ROS on the abundance of SUMO-1 conjugates were comparable in undifferentiated and decidualizing cells. An intermediate concentration of H2O2 (1 mM) decreased sumoylation, whereas supraphysiologically, concentrations (10 mM) generated an hypersumoylation response. The latter effects have been observed in other cell types and ascribed to oxidative modification and inhibition of E1/E2 enzymes (11) and SUMO-specific proteases (SENPs) (12), respectively, but the hypersumoylation response on exposure of HESCs to relatively low levels of ROS has not yet been described. To further characterize this cellular response, undifferentiated and decidualized HESCs were treated with 250 μM H2O2 over a time course lasting 24 h. An increase in the levels of high-molecular weight SUMO-1 conjugates became evident in undifferentiated cells within 30 min of H2O2 treatment and was maintained for 24 h (Fig. 1B). In decidualizing HESCs, however, the response to H2O2 was either transient, characterized by only minor increases in conjugate levels at 30- and 60-min time points (Fig. 1B), or entirely absent (data not shown).
Figure 1.
ROS effects on sumoylation and PR-A activity in undifferentiated and decidualizing HESCs. A, B) Primary HESC cultures, either maintained undifferentiated or decidualized with 8-br-cAMP and MPA for 3 d, were exposed to 0.1, 0.25, 0.5, 1, and 10 mM of H2O2 for 1 h (A), or to 250 μM H2O2 over a time course of 24 h (B). Whole-cell protein extracts were analyzed by Western blotting with anti-SUMO-1 and anti-β-actin antibodies. C) HESCs were treated with 8-br-cAMP and MPA as indicated for 3 d, while being transfected with PR-A (500 ng) and EGFP-SUMO-1 (50 ng) expression vectors and exposed to 0 or 250 μM H2O2 for the last 24 h. Whole-cell extracts were subjected to Western blot analysis with anti-PR, GFP, and β-actin antibodies. D, E) Primary HESCs were transfected with expression vectors for PR-A wild-type (D) or sumoylation-deficient K388R mutant (400 ng) (E) and PRE2-luciferase reporter (100 ng), and treated with 8-br-cAMP and MPA in the indicated combinations, for 3 d. In the last 24 h, cells were exposed to 0, 100, or 250 μM H2O2, and harvested for luciferase assay. Data are expressed as fold induction with regard to control-treated sample and represent means ± sd of triplicate experiments.
Sumoylation regulates PR activity in response to ROS
Like other steroid receptors, the activity of the liganded PR is modulated by post-translational modifications, including sumoylation. The effect of ROS on PR sumoylation was investigated by cotransfecting primary HESC cultures with expression vectors encoding PR-A and EGFP-tagged-SUMO-1. As PR sumoylation is ligand sensitive (21), all cultures were treated with MPA, either alone or with 8-Br-cAMP, for 72 h. For the last 24 h, some cultures were pulsed with 250 μM H2O2. The levels of EGFP-SUMO-1-modified receptor increased on exposure to 250 μM H2O2 in undifferentiated cultures but much less so in decidualizing HESCs, thus mimicking the global sumoylation pattern in the cells (Fig. 1C). PR-A activity was monitored by cotransfecting primary cultures with a luciferase reporter gene driven by two consensus palindromic PR response elements (PREs), followed by treatment with 8-br-cAMP, MPA, or both for 3 d in the presence or absence of 100 or 250 μM H2O2 for the last 24 h. As shown in Fig. 1D, H2O2 inhibited PR activity in a dose-dependent manner in cells treated with MPA alone. As expected, 8-br-cAMP enhanced the activity of the liganded PR-A (24), but strikingly, it also abolished the inhibitory effects of ROS. Next, we examined the effect of H2O2 on PR-AK388R, a PR-A mutant in which the lysine required for covalent binding of SUMO-1 is replaced by an arginine (21). This SUMO-deficient mutant was not only 7-fold more active in the presence of ligand than wild-type PR-A but also insensitive to H2O2 treatment (Fig. 1E). The results demonstrate that modest levels of ROS disrupt the cellular sumoylation-desumoylation equilibrium in undifferentiated cells, leading to increased PR sumoylation and attenuated progesterone signaling, whereas this sequence of events does not take place in decidualizing HESCs.
SUMO conjugation and deconjugation in response to ROS
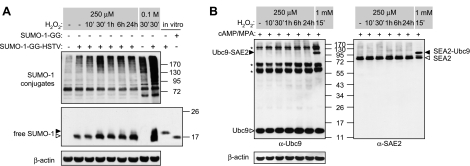
HESC differentiation is characterized by enhanced expression of several free radical scavengers, including superoxide dismutase 2 (SOD2) (7). We reasoned that 100–250 μM of H2O2 may be sufficient to trigger reversible oxidation and inactivation of SENPs, resulting in a global shift in protein sumoylation, in undifferentiated but not decidualized HESCs. To test this hypothesis, SENP activity was assessed by taking advantage of the role of these enzymes in converting unconjungatable SUMO-1 precursor to mature SUMO-1 by proteolytic cleavage of 4 C-terminal amino acids (HSTV). SUMO-1 precursor (SUMO-1-GG-HSTV) was overexpressed in undifferentiated HESCs, the cultures then treated with 250 μM H2O2 for various times, and total cell lysates were subjected to Western blot analysis for the detection of unprocessed and mature SUMO-1 modifier. Acute oxidative stress (0.1 M H2O2) rapidly and substantially increased the levels of SUMO-1 modified proteins, with an expected concomitant appearance of unprocessed SUMO-1 (Fig. 2A). Treatment with 250 μM H2O2 also generated a hypersumoylation response, albeit less pronounced. However, this shift in global sumoylation was not accompanied by accumulation of unprocessed SUMO-1, indicating that oxidative inactivation of SENPs is not the underpinning mechanism. We also tested the reverse scenario, i.e., that oxidative cross-linking and inactivation of the E1 enzyme subunit SAE2 and E2 (Ubc9) would prevent hypersumoylation in decidualizing HESCs subjected to oxidative stress conditions. Primary cultures decidualized with 8-br-cAMP and MPA for 3 d were treated with either 250 μM H2O2 for various times or with 1 mM H2O2 for 15 min. As expected, there was no evidence of cross-linking of Ubc9-SAE2 in decidual cells, treated with 250 μM H2O2 or not, although oxidative cross-linking of these conjugating enzymes occurred in response to 1 mM H2O2 (Fig. 2B) (11). Thus, the sensitivity of the SUMO-1 conjugation pathway to modest levels of ROS in undifferentiated HESCs, and the resistance upon decidualization, operate through mechanisms that do not involve known regulatory oxidative modifications of SUMO enzymes.
Figure 2.
Modest levels of ROS do not trigger oxidative modifications of SUMO enzymes. A) HESCs were transfected with full-length immature SUMO-1 (SUMO-1-GG-HSTV) (500 ng), and at 24 h post-transfection were treated with 250 μM H2O2 for the indicated times. Some cultures were exposed for 30 min to a concentration of 0.1 M H2O2, known to inactivate SUMO proteases. Whole-cell protein extracts were resolved on a 7.5% SDS-polyacrylamide gel and immunoprobed with anti-SUMO-1 antibody to visualize SUMO-1 conjugates. For detection of free SUMO-1, samples were separated on a 12% gel. In vitro expression using vectors encoding the immature (SUMO-1-GG-HSTV) and matured form (SUMO-1-GG) of SUMO-1 were used as positive controls. Open arrowhead indicates the position of C-terminally processed SUMO-1; solid arrowhead indicates the immature form. B) HESCs were decidualized with 8-br-cAMP and MPA for 3 d and subjected to time-course treatment with 250 μM H2O2 over a 24-h period. As a positive control for Ubc9-SAE2 cross-linking, some cultures were treated with 1 mM H2O2 for 15 min. Total protein harvested in Laemmli buffer not containing β-mercaptoethanol was analyzed by Western blotting with anti-Ubc9, SAE2, and β-actin antibodies. Open arrowheads indicate positions of the uncoupled proteins; solid arrowheads show Ubc9-SAE2 cross-linked species; asterisks indicate nonspecific bands detected by the anti-Ubc9 antibody.
JNK signaling couples ROS to the SUMO conjugation pathway
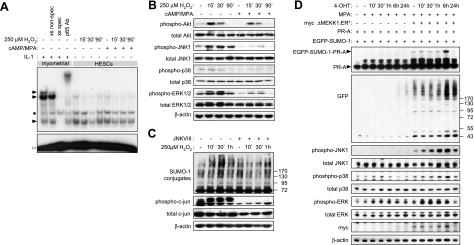
ROS are important regulators of various signal transduction cascades, including NF-κB (25), phosphoinositide-3-kinase (PI3K)/AKT (26), and MAP kinase (MAPK) pathways (9). As there was no evidence that modest levels of ROS trigger oxidative modifications of SUMO enzymes in HESCs, we decided to investigate ROS-dependent signaling events in undifferentiated and decidualizing HESCs. NF-κB activity in nuclear extracts derived from undifferentiated or decidualized HESCs treated with 250 μM H2O2 over a time course of 90 min was assessed by EMSA. As shown in Fig. 3A, NF-κB DNA binding activity was low in both undifferentiated and decidualizing HESCs and largely unaffected by H2O2. The levels of AKT, JNK, p38, and ERK1/2 and their activation status were determined in parallel cultures using Western blot analysis with pan- and phospho-specific antibodies, respectively. H2O2 rapidly activated the AKT, JNK, and ERK1/2 but not p38 pathways in undifferentiated cells (Fig. 3B). H2O2 also strongly activated the PI3K/AKT pathway in decidualizing HESCs. In contrast, ROS-dependent JNK and, to a lesser extent, ERK1/2 phosphorylation was markedly blunted on decidualization of HESCs, although total levels of these kinases were comparable to those in undifferentiated cells. Pretreatment with U0126, an inhibitor of ERK1/2 signaling, had no effect on H2O2-induced hypersumoylation response in HESCs (data not shown). In contrast, silencing of the JNK pathway, assessed by the phosphorylation levels of the downstream target c-JUN, was sufficient to attenuate global hypersumoylation in HESCs treated with H2O2 (Fig. 3C).
Figure 3.
JNK signaling mediates ROS-dependent hypersumoylation. A) Undifferentiated and decidualizing primary HESCs were exposed to 250 μM H2O2 for the indicated times, and DNA binding of NF-κB contained in the nuclear extracts was analyzed by EMSA. Nuclear lysate from myometrial cells stimulated with 1 ng/ml IL-1β for 30 min was used as a positive control. Myometrial lysates were also incubated with 100-fold excess of unlabeled NF-κB (xs spec) and Oct1 (xs nonspec) oligonucleotides prior to the addition of the [32P]-labeled probe, as DNA binding competitors to verify binding specificity. For supershift analysis, myometrial nuclear extracts were incubated with an antibody against p65 prior to addition of the hot probe. Solid arrowheads indicate position of specific complexes; solid bullet indicates position of nonspecific complexes; open arrowhead indicates the position of uncomplexed DNA probe. B) Primary HESC cultures were kept undifferentiated or decidualized with 8-br-cAMP and MPA for 3 d and then exposed to 250 μM H2O2 for 0, 15, 30, or 90 min. Whole-cell extracts were subjected to Western blot analysis with antibodies against the total and phosphorylated forms of Akt, JNK, p38, and ERK1/2. C) Undifferentiated HESC cultures were either kept untreated or treated with 36 μM of JNKVIII inhibitor for 2 h before exposure to 250 μM H2O2 for 10, 30, or 60 min. Whole-cell extracts were subjected to Western blot analysis with antibodies against SUMO-1, total and phosphorylayed forms of c-jun, and β-actin as loading control. D) HeLa cells were transfected with PR-A (500 ng) and EGFP-SUMO-1 (50 ng), with or without ΔMEKK1:ER*-myc (500 ng). Twenty-four hours later, cells were treated with MPA and 4-hydroxytamoxifen (4-OHT) for the times indicated. Western blotting was performed on whole-cell protein extracts by using antibodies against PR, GFP, myc tag, β-actin, and total or phosphorylated forms of JNK, p38, and ERK1/2.
Next, we examined whether JNK activation in the absence of primary cellular stress would trigger a global hypersumoylation response and enhanced PR-A modification. To induce JNK activity, we made use of a 4-hydroxytamoxifen (4-OHT)-inducible chimeric protein kinase composed of the constitutively active kinase domain of rat MEKK1 (ΔMEKK1) fused to a modified form of the ligand binding domain of estrogen receptor (ER*). HeLa cells were transfected with ΔMEKK1:ER*, PR-A, and EGFP-SUMO-1 and then treated with MPA in combination with 4-OHT for various times. As previously reported (22), activation of ΔMEKK1:ER* with 4-OHT predominantly activates JNK, with only marginal effects on p38 and ERK1/2 phosphorylation (Fig. 3D). In addition, global and PR-A sumoylation were strongly enhanced after 6 h treatment with 4-OHT, corresponding with the time point showing greatest levels of JNK activation. Taken together, the results establish that JNK signaling, independently of cellular stress, is a major regulator of the SUMO conjugation pathway.
ROS regulate distinct gene networks in undifferentiated and decidualized HESCs
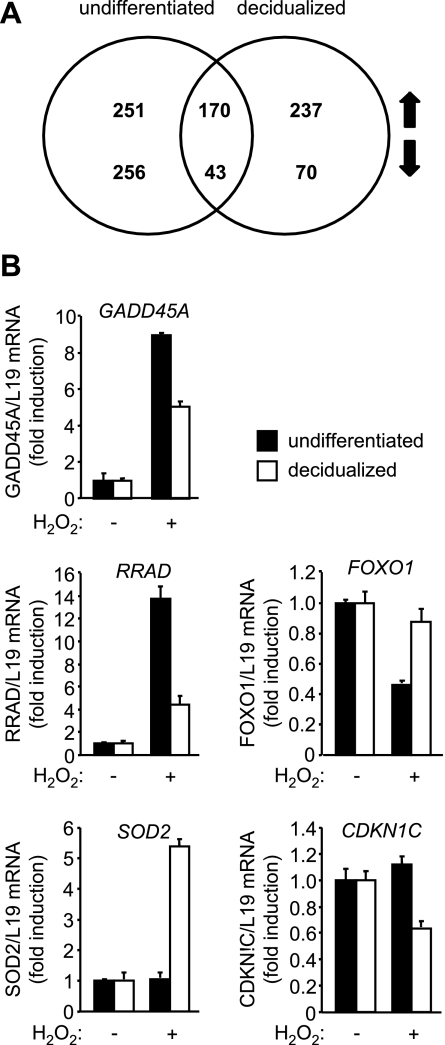
We reasoned that heightened oxidative defenses upon differentiation may render decidual cells largely unresponsive to environmental ROS, thus accounting for the restricted activation of MAPK pathways in response to H2O2. It appeared equally plausible that decidualization is associated with reprogramming of various signal transduction pathways (27), which would lead to a cellular response to ROS that is qualitatively different from that observed in undifferentiated cells. To distinguish between these possibilities, three different primary cultures were decidualized with 8-br-cAMP and MPA for 3 d or not and then treated with 250 μM H2O2 for 8 h. Total RNA was extracted and processed for whole genome microarray analysis. The Venn diagram in Fig. 4A depicts the numbers of genes up- and down-regulated by H2O2 in undifferentiated and decidualized cells. Only 213 genes, representing 21% of the total 1027 genes identified, were common, demonstrating that the transcriptional networks responsive to stress signals profoundly change upon differentiation of HESCs. Further, the number of H2O2-repressed, but not H2O2-induced, genes in decidualizing cells was significantly lower compared to undifferentiated HESCs (P<0.05). Supplemental Tables S1–S3 list the 100 most regulated genes by H2O2 in undifferentiated and/or decidual HESCs. Notably, the expression of highly sensitive progesterone-dependent decidual marker genes, such as PRL, IGFBP1, and WNT4, was not effected by H2O2. RTQ-PCR analysis of an independent sample set was used to validate the array data. As expected, ROS induced GADD45A in both undifferentiated and decidualizing HESCs but selectively repressed FOXO1 in undifferentiated cells and CDKN1C, encoding tumor suppressor p57KIP2, in decidualizing cultures. SOD2 expression was further enhanced upon treatment of decidualizing but not undifferentiated HESCs with H2O2. Overall, the RTQ-PCR analysis was in agreement with the array data, perhaps with the exception of ROS-dependent regulation of RRAD, which was found also to be induced in decidualizing HESCs, albeit much more modestly than in undifferentiated cells. Thus rather than being insensitive to ROS, decidualizing cells mount a transcriptional response that is distinct from undifferentiated HESCs.
Figure 4.
ROS triggers distinct gene expression regulation in undifferentiated and decidualizing HESCs. Primary HESCs were maintained undifferentiated or decidualized with 8-br-cAMP and MPA for 3 d and exposed or not to 250 μM H2O2 for 8 h. RNA extracts were utilized for gene expression profiling using genome-wide arrays (A) or RTQ-PCR (B). A) Venn diagram shows numbers of significantly down-regulated and up-regulated genes in undifferentiated and decidualizing cells. B) Data are depicted as fold induction relative to the transcript levels of undifferentiated and decidualizing untreated samples and represent means ± sd of triplicate determinations.
Decidualization reprograms the JNK pathway
The microarray analysis also identified 2105 genes regulated >2-fold upon treatment of HESCs with 8-br-cAMP and MPA for 72 h, 46 and 54% of which were up- and down-regulated, respectively. Most of these genes have been identified in previous studies (8, 28), and hence, they were not subjected to further analysis. However, Ingenuity Pathway Analysis was employed to analyze the expression of signal intermediates and modulators of the JNK pathway upon decidualization of HESCs and in response to H2O2 (Supplemental Fig. S1). Decidualization inhibited several genes encoding for components upstream of JNK, including MAP4K5, GAB1, ZAK, and MAP3K1 (MEKK1), while simultaneously up-regulating DUSP1 (MKP1). Treatment with 8-br-cAMP and MPA also inhibited the abundance of JNK1 (MAPK8) transcripts but increased JNK3 (MAPK10) mRNA levels. DUSP10, encoding for the JNK/p38 phosphatase MKP5, was repressed 2.7-fold on differentiation of HESCs, but the levels were restored on exposure of decidual cells to H2O2 (3.1-fold induction). In undifferentiated cells, ROS had little or no effect on the expression levels of key intermediates or phosphatases that determine JNK activity, but it increased the transcript levels of the downstream effector c-JUN ∼2-fold. Overall, the expression profile suggests that signal transduction involving stress MAP kinases, and specifically JNK, is actively dampened upon differentiation of HESCs into decidual cells.
MKP1 uncouples ROS signaling from the SUMO conjugation pathway
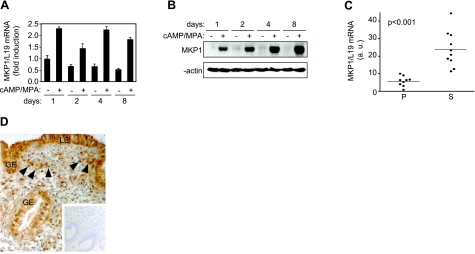
MKP1, which preferentially dephosphorylates and inactivates both JNK and p38, plays a key role in metabolic homeostasis and resistance to cellular stress (29, 30), including protection against ischemia/reperfusion injury (31). This prompted us to further characterize the expression of this phosphatase on decidualization of HESCs and to explore its role in oxidative stress resistance. In keeping with the array data, MKP1 mRNA levels increased ∼2-fold on decidualization of HESCs, a response apparent within 1 d of treatment with 8-br-cAMP and MPA and maintained over a 8-d time course (Fig. 5A). However, the induction of MKP1 at protein level was much more pronounced and the levels continued to rise on prolonged treatment with 8-br-cAMP and MPA (Fig. 5B). Analysis of 19 snap-frozen tissue biopsies showed that MKP1 transcript levels are 5-fold higher in secretory compared to proliferative endometrium (Fig. 5C). Immunohistochemistry demonstrated that MKP1 is expressed in secretory endometrium, not only in the luminal and glandular epithelial cell compartments but also in a subpopulation of stromal cells (Fig. 5D).
Figure 5.
MKP1 expression is induced upon decidualization. Primary HESC cultures were maintained undifferentiated or decidualized with 8-br-cAMP and MPA for 1, 2, 4, and 8 d. Cells were harvested for RNA or protein, and MKP1 expression was profiled by RTQ-PCR (A) or Western blot analysis (B), respectively. A) Data are presented as mean ± sd fold induction relative to transcript levels in untreated cells at d 1 of triplicate experiments. C) RNA was extracted from proliferative (P; n=9) and secretory (S; n=10) endometrial biopsies, and RTQ–PCR was used to determine levels of MKP1 transcripts. Statistical significance was determined using Student’s t test. D) MKP1 expression in secretory endometrium. MKP1 immunoreactivity was apparent not only in luminal and glandular epithelial cells (LE and GE, respectively) but also in the stromal compartment, with staining intensity varying in different cell populations from weak to intense (arrowheads; original view: ×20). Inset: negative control (no primary antibody).
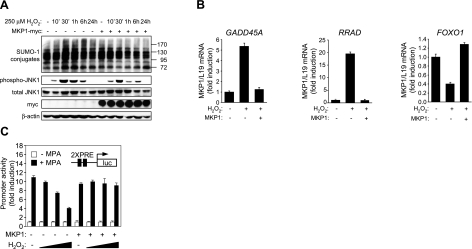
To define the role of MKP1 in regulating oxidative stress responses, undifferentiated HESCs were transfected with a plasmid encoding myc-tagged MKP1 or an empty expression vector and treated with 250 μM H2O2, and total cell lysates were harvested at different time points over 24 h. As expected, expression of MKP1 blunted both the magnitude and duration of JNK activation in response to ROS, which was sufficient to abolish the hypersumoylation response. Overexpression of MKP1 was also sufficient to prevent the induction of GADD45A and RRAD and to reverse the repression of FOXO1 in undifferentiated HESCs treated with H2O2 for 8 h. Finally, we tested whether MKP1 plays a role in maintaining progesterone signaling in HESCs exposed to modest levels of ROS. Primary cultures transfected with the PRE-luciferase reporter, PR-A, and MKP1, or the empty pSG5 vector, were treated for 24 h with or without MPA in the presence or absence of increasing concentrations of H2O2. As shown in Fig. 6, ROS inhibited PR-A activity in a dose-dependent manner but not in cells cotransfected with MKP1. Together, the data suggest that induction of MKP1 upon decidualization of HESCs is sufficient to dampen JNK activity, to uncouple ROS signaling from the SUMO conjugation pathway, and to maintain progesterone responses under oxidative stress conditions.
Figure 6.
MKP1 overexpression in undifferentiated HESCs protects against ROS effects. A) Undifferentiated HESC cultures were either mock or MKP1-myc transfected (500 ng) and exposed to 250 μM H2O2 over a time course of 24 h. Whole-cell extracts were subjected to Western blot analysis with antibodies against SUMO-1, total- and phospho-JNK, myc tag, and β-actin. B) Undifferentiated HESC cultures were either mock or MKP1-myc transfected (500 ng) and exposed to 250 μM H2O2 for 8 h. RNA was extracted, and RTQ-PCR was used to determine the abundance of GADD45A, RRAD, and FOXO1 transcripts, relative to L19. Data are presented as fold induction relative to the transcript levels in untreated mock transfected cells and depict means ± sd triplicate determinations. Results are representative of 3 independent experiments. C) Primary HESCs were transfected with PR-A (400 ng) and PRE2-luciferase reporter (100 ng) with or without MKP1 expression vector (500 ng), and treated for 24 h with MPA and 0, 100, 250, and 500 μM H2O2, as indicated. Cells were harvested, and luciferase activity was assayed. Data are expressed as fold induction with regard to −MPA treatment and represent means ± sd of triplicate determinations.
DISCUSSION
Upon breaching the luminal endometrial epithelium, the implanting blastocyst becomes rapidly encapsulated by decidualizing endometrial stromal cells. From a functional perspective, the decidual process, which denotes the remodeling of the endometrial stromal compartment in response to pregnancy, establishes maternal immunological tolerance to fetal antigens, ensures tissue integrity and hemostasis during the process of trophoblast invasion and placenta formation, and, importantly, protects the conceptus from environmental insults (4, 32). The last few weeks of the first trimester of pregnancy are particularly perilous for the conceptus, as the onset of placental perfusion causes a dramatic increase in local oxygen tension interface, which in turn poses a considerable oxidative challenge (33). Once placental perfusion is established between 9 and 11 wk gestation, the likelihood of pregnancy failure drops significantly, although oxidative injury of the decidua-placental interface at this time of pregnancy leads to subsequent obstetrical complications, such as placental abruption, fetal growth restriction, and preeclampsia (32).
Although differentiation of HESCs requires elevated cAMP levels and sustained activation of the protein kinase A pathway, maintenance of the decidual phenotype depends on continuous progesterone signaling, mediated by PR-A (34). Withdrawal of progesterone after initiation of the decidual process invariably causes a degree of cell death in vitro and, clinically, antiprogestins such as RU486 are widely used for termination of unwanted pregnancies (35). Thus, the integrity of the fetomaternal interface is not only dependent on resistance to oxidative cell death but also on the ability to maintain progesterone responses and cellular homeostasis under adverse environmental conditions. Sumoylation has emerged as a central regulatory post-translational modification, responsive to a variety of environmental stresses (18), which controls the function of numerous proteins and, ultimately, cell fate (36, 37). Several mechanisms have been implicated in redox regulation of the SUMO pathway, including reversible oxidative inhibition of E1-E2 ligases and inactivation of SENPs by intramolecular disulfide linkage (11, 12). Both mechanisms are operational in undifferentiated and decidual cells but only at relatively high H2O2 concentrations, which may be achieved during the respiratory burst in activated macrophages but unlikely to be relevant to pregnancy. At lower concentrations, however, H2O2 induced a global hypersumoylation response in undifferentiated but not decidualized cells, which was not only sustained but also sufficient to modify PR-A and to limit its trans-activation potential. Several lines of evidence implicated JNK activation in this hypersumoylation response, including the observations that pretreatment of undifferentiated HESCs with an inhibitor against JNK (JNKVIII) attenuated ROS-induced hypersumoylation and that induction of JNK activity in the absence of primary stress, using the 4-OHT-inducible ΔMEKK1:ER* construct, was sufficient to enhance global and PR-specific SUMO-1 modification in heterologous systems, such as HeLa and COS-1 cell lines (Fig. 3D and data not shown). Further, the ability of low levels of H2O2 to perturb PR activity or the sumoylation-desumoylation equilibrium was either markedly reduced or absent on decidualization of HESCs with 8-br-cAMP and MPA in concert with attenuated JNK activation. Interestingly, a recent study reported that comparable levels of H2O2 stabilize the SUMO-2/3-specific protease SENP3, limiting the overall abundance of SUMO-2/3 modified proteins (38). Whether or not the increase in SUMO-1 modification is reciprocated by changes in SUMO-2/3 conjugation in H2O2-treated HESCs remains to be determined.
In view of the increased scavenging potential, altered ROS signaling and its uncoupling of the SUMO-1 conjugation pathway, it was anticipated that the cellular response to an oxidative challenge would profoundly change on decidualization of HESCs. Our microarray analysis confirmed this supposition, demonstrating that only a minor subset of genes (21%) is responsive to H2O2 in both undifferentiated and decidualizing cells. Extrapolating to the in vivo situation, this suggests that the oxidative challenge at the end of the first trimester of pregnancy may in fact play a physiological role in reprogramming the decidual response, which perhaps facilitates further trophoblast invasion and placenta formation. Although speculative, this notion is supported by clinical studies demonstrating that dietary antioxidative supplements can adversely affect pregnancy outcome (39). The array also demonstrated that highly sensitive PR-induced genes (e.g., PRL, IGFBP1, CNR1, FKBP5, DKK1, etc.) are not affected on H2O2 treatment of decidual cells, indicating that PR signaling will be maintained at the fetomaternal interface under oxidative stress conditions as long as the endometrium is adequately decidualized. Targeted pathway analysis also yielded important insights into the mechanism of JNK inhibition on decidualization of HESCs. Of particular relevance is the reduced expression of the GAB1 mRNA, which encodes a docking protein involved in selective JNK but not ERK or p38 activation upon H2O2 (40). This is further accompanied by inhibition of genes encoding for MEKK1, MAP4K5, and ZAK, kinases whose activities converge on MKK4/7, which, in turn, is responsible for JNK phosphorylation (41,42,43,44,45). Therefore, it appears that decidualization leads to widespread dampening of the signaling circuitry upstream of JNK, raising the possibility that activation of this pathway by stimuli other than H2O2 will also be diminished. Another interesting observation is the concomitant reduction in JNK1 and increase in JNK3 transcripts on decidualization. Although JNK isoforms can target common substrates, isoform-selective substrates also exist (46), and this would provide a further point of divergence for the differential transcriptional response to stress on differentiation of HESCs.
For functional analysis, we focused on MKP1, the founding member of the family of dual-specificity protein phosphatases and capable of dephosphorylating/deactivating JNK and p38 MAP kinases activated by stress (47, 48). Although the abundance of MKP1 transcripts increased on decidualization of HESCs in vitro, and even more so on secretory transformation of the endometrium in vivo, induction at protein level was far greater. In agreement, MKP1 expression is known to be subject to posttranscriptional regulation, including enhanced translation on binding of RNA-binding proteins, such as HuR, to the 3′-untranslated region of MKP1 transcripts (48, 49). Several studies have shown that MKP1 confers protection against apoptosis induced by a wide range of noxious stimuli, including cisplatin, ethanol, ischemia/reperfusion injury, and H2O2, by attenuating the magnitude and duration of JNK signal (31, 50,51,52). This was also the case in undifferentiated HESCs, where overexpression of MKP1 not only dampened H2O2-induced JNK activation but also prevented an hypersumoylation response and maintained PR-A activity. Interestingly, silencing of MKP1 using siRNA or expression of ΔMEKK1:ER* in decidualizing cells enhanced JNK phosphorylation upon H2O2 or 4-OHT treatment, respectively, but was insufficient to globally enhance SUMO-1 conjugation (data not shown). Why restoring JNK activity fails to elicit an hypersumoylation response in differentiating HESCs is as yet unknown. However, decidualization is associated with down-regulation of PIAS1, PIASL, PIASxa, PIASxb, and RanBP2 in concert with induction of SENP2 (19), suggesting that one of these SUMO enzymes may be a downstream target for H2O2-activated JNK and, upon phosphorylation, triggers the global shift in the sumoylation-desumoylation equilibrium in undifferentiated HESCs. Global changes in cellular sumoylation on post-translational modification of a single SUMO enzyme are not without precedent. For example, S-nitrosation of PIAS3 in response nitric oxide promotes its degradation and has been shown to cause global hyposumoylation in mammalian cells (53).
In summary, maintaining progesterone signaling and cellular homeostasis in the maternal decidua under oxidative stress conditions is essential for survival of the conceptus. Herein, we demonstrate that dampening of the JNK pathway, mediated by induction of MKP1 and altered expression of other pathway intermediates, is a cardinal event on decidual transformation of endometrial cells, which uncouples oxidative stress signals from the SUMO-1 conjugation pathway in general and PR responses in specific. This hitherto unrecognized mechanism may provide further insights into the pathological processes that underpin obstetrical complications, especially early pregnancy loss.
Supplementary Material
Acknowledgments
The authors are grateful to all the women who participated in this study. This work was supported by the Wellcome Trust (084336), the Institute of Obstetrics and Gynaecology (IOG) Trust, and the National Institute for Health Research (NIHR) Biomedical Research Centre funding scheme. The authors declare no conflicts of interest.
References
- Brosens J J, Gellersen B. Death or survival–progesterone-dependent cell fate decisions in the human endometrial stroma. J Mol Endocrinol. 2006;36:389–398. doi: 10.1677/jme.1.02060. [DOI] [PubMed] [Google Scholar]
- Labied S, Kajihara T, Madureira P A, Fusi L, Jones M C, Higham J M, Varshochi R, Francis J M, Zoumpoulidou G, Essafi A, Fernandez de Mattos S, Lam E W, Brosens J J. Progestins regulate the expression and activity of the forkhead transcription factor FOXO1 in differentiating human endometrium. Mol Endocrinol. 2006;20:35–44. doi: 10.1210/me.2005-0275. [DOI] [PubMed] [Google Scholar]
- Critchley H O, Kelly R W, Brenner R M, Baird D T. The endocrinology of menstruation—a role for the immune system. Clin Endocrinol (Oxf) 2001;55:701–710. doi: 10.1046/j.1365-2265.2001.01432.x. [DOI] [PubMed] [Google Scholar]
- Gellersen B, Brosens I A, Brosens J J. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25:445–453. doi: 10.1055/s-2007-991042. [DOI] [PubMed] [Google Scholar]
- Brosens J J, Parker M G, McIndoe A, Pijnenborg R, Brosens I A. A role for menstruation in preconditioning the uterus for successful pregnancy. Am J Obstet Gynecol. 2009;200:615.e1–615.e6. doi: 10.1016/j.ajog.2008.11.037. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin M T, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Kajihara T, Jones M, Fusi L, Takano M, Feroze-Zaidi F, Pirianov G, Mehmet H, Ishihara O, Higham J M, Lam E W, Brosens J J. Differential expression of FOXO1 and FOXO3a confers resistance to oxidative cell death upon endometrial decidualization. Mol Endocrinol. 2006;20:2444–2455. doi: 10.1210/me.2006-0118. [DOI] [PubMed] [Google Scholar]
- Takano M, Lu Z, Goto T, Fusi L, Higham J, Francis J, Withey A, Hardt J, Cloke B, Stavropoulou A V, Ishihara O, Lam E W, Unterman T G, Brosens J J, Kim J J. Transcriptional cross-talk between the forkhead transcription factor FOXO1 and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol Endocrinol. 2007;21:2334–2349. doi: 10.1210/me.2007-0058. [DOI] [PubMed] [Google Scholar]
- McCubrey J A, Lahair M M, Franklin R A. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal. 2006;8:1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- Sanchez-Perez I, Benitah S A, Martinez-Gomariz M, Lacal J C, Perona R. Cell stress and MEKK1-mediated c-Jun activation modulate NF-κB activity and cell viability. Mol Biol Cell. 2002;13:2933–2945. doi: 10.1091/mbc.E02-01-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossis G, Melchior F. Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol Cell. 2006;21:349–357. doi: 10.1016/j.molcel.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Xu Z, Mi Lam L S, Lam L H, Chau S F, Ng T B, Au S W. Molecular basis of the redox regulation of SUMO proteases: a protective mechanism of intermolecular disulfide linkage against irreversible sulfhydryl oxidation. FASEB J. 2007;22:127–137. doi: 10.1096/fj.06-7871com. [DOI] [PubMed] [Google Scholar]
- Zhao J. Sumoylation regulates diverse biological processes. Cell Mol Life Sci. 2007;64:3017–3033. doi: 10.1007/s00018-007-7137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyst M J, Stancheva I. A role for SUMO modification in transcriptional repression and activation. Biochem Soc Trans. 2007;35:1389–1392. doi: 10.1042/BST0351389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergink S, Jentsch S. Principles of ubiquitin and SUMO modifications in DNA repair. Nature. 2009;458:461–467. doi: 10.1038/nature07963. [DOI] [PubMed] [Google Scholar]
- Manza L L, Codreanu S G, Stamer S L, Smith D L, Wells K S, Roberts R L, Liebler D C. Global shifts in protein sumoylation in response to electrophile and oxidative stress. Chem Res Toxicol. 2004;17:1706–1715. doi: 10.1021/tx049767l. [DOI] [PubMed] [Google Scholar]
- Golebiowski F, Matic I, Tatham M H, Cole C, Yin Y, Nakamura A, Cox J, Barton G J, Mann M, Hay R T. System-wide changes to SUMO modifications in response to heat shock. Sci Signal. 2009;2:ra24. doi: 10.1126/scisignal.2000282. [DOI] [PubMed] [Google Scholar]
- Tempe D, Piechaczyk M, Bossis G. SUMO under stress. Biochem Soc Trans. 2008;36:874–878. doi: 10.1042/BST0360874. [DOI] [PubMed] [Google Scholar]
- Jones M C, Fusi L, Higham J H, Abdel-Hafiz H, Horwitz K B, Lam E W, Brosens J J. Regulation of the SUMO pathway sensitizes differentiating human endometrial stromal cells to progesterone. Proc Natl Acad Sci U S A. 2006;103:16272–16277. doi: 10.1073/pnas.0603002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian M, Pohnke Y, Kempf R, Gellersen B, Brosens J J. Functional association of PR and CCAAT/enhancer-binding protein beta isoforms: promoter-dependent cooperation between PR-B and liver-enriched inhibitory protein, or liver-enriched activatory protein and PR-A in human endometrial stromal cells. Mol Endocrinol. 2002;16:141–154. doi: 10.1210/mend.16.1.0763. [DOI] [PubMed] [Google Scholar]
- Abdel-Hafiz H, Takimoto G S, Tung L, Horwitz K B. The inhibitory function in human progesterone receptor N termini binds SUMO-1 protein to regulate autoinhibition and transrepression. J Biol Chem. 2002;277:33950–33956. doi: 10.1074/jbc.M204573200. [DOI] [PubMed] [Google Scholar]
- Molton S A, Todd D E, Cook S J. Selective activation of the c-Jun N-terminal kinase (JNK) pathway fails to elicit Bax activation or apoptosis unless the phosphoinositide 3′-kinase (PI3K) pathway is inhibited. Oncogene. 2003;22:4690–4701. doi: 10.1038/sj.onc.1206692. [DOI] [PubMed] [Google Scholar]
- Bennett A M, Tonks N K. Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science. 1997;278:1288–1291. doi: 10.1126/science.278.5341.1288. [DOI] [PubMed] [Google Scholar]
- Brosens J J, Hayashi N, White J O. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology. 1999;140:4809–4820. doi: 10.1210/endo.140.10.7070. [DOI] [PubMed] [Google Scholar]
- Gloire G, Legrand-Poels S, Piette J. NF-κB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Barthel A, Klotz L O. Phosphoinositide 3-kinase signaling in the cellular response to oxidative stress. Biol Chem. 2005;386:207–216. doi: 10.1515/BC.2005.026. [DOI] [PubMed] [Google Scholar]
- Cloke B, Huhtinen K, Fusi L, Kajihara T, Yliheikkila M, Ho K K, Teklenburg G, Lavery S, Jones M C, Trew G, Kim J J, Lam E W, Cartwright J E, Poutanen M, Brosens J J. The androgen and progesterone receptors regulate distinct gene networks and cellular functions in decidualizing endometrium. Endocrinology. 2008;149:4462–4474. doi: 10.1210/en.2008-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brar A K, Handwerger S, Kessler C A, Aronow B J. Gene induction and categorical reprogramming during in vitro human endometrial fibroblast decidualization. Physiol Genomics. 2001;7:135–148. doi: 10.1152/physiolgenomics.00061.2001. [DOI] [PubMed] [Google Scholar]
- Keyse S M. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 2008;27:253–261. doi: 10.1007/s10555-008-9123-1. [DOI] [PubMed] [Google Scholar]
- Kuwano Y, Gorospe M. Protecting the stress response, guarding the MKP-1 mRNA. Cell Cycle. 2008;7:2640–2642. doi: 10.4161/cc.7.17.6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P, Guo S, Fan Y, Zhang H, Gu D, Li H. Atrogin-1/MAFbx enhances simulated ischemia/reperfusion-induced apoptosis in cardiomyocytes through degradation of MAPK phosphatase-1 and sustained JNK activation. J Biol Chem. 2009;284:5488–5496. doi: 10.1074/jbc.M806487200. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Poston L, Burton G J. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum Reprod Update. 2006;12:747–755. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauniaux E, Watson A L, Hempstock J, Bao Y P, Skepper J N, Burton G J. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol. 2000;157:2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol. 2003;178:357–372. doi: 10.1677/joe.0.1780357. [DOI] [PubMed] [Google Scholar]
- Lievre M, Sitruk-Ware R. Meta-analysis of 200 or 600 mg mifepristone in association with two prostaglandins for termination of early pregnancy. Contraception. 2009;80:95–100. doi: 10.1016/j.contraception.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Muller S, Ledl A, Schmidt D. SUMO: a regulator of gene expression and genome integrity. Oncogene. 2004;23:1998–2008. doi: 10.1038/sj.onc.1207415. [DOI] [PubMed] [Google Scholar]
- Meinecke I, Cinski A, Baier A, Peters M A, Dankbar B, Wille A, Drynda A, Mendoza H, Gay R E, Hay R T, Ink B, Gay S, Pap T. Modification of nuclear PML protein by SUMO-1 regulates Fas-induced apoptosis in rheumatoid arthritis synovial fibroblasts. Proc Natl Acad Sci U S A. 2007;104:5073–5078. doi: 10.1073/pnas.0608773104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Han Y, Wang Y, Sun X, Yan S, Yeh E T, Chen Y, Cang H, Li H, Shi G, Cheng J, Tang X, Yi J. SENP3 is responsible for HIF-1 transactivation under mild oxidative stress via p300 de-SUMOylation. EMBO J. 2009;28:2748–2762. doi: 10.1038/emboj.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston L, Briley A L, Seed P T, Kelly F J, Shennan A H. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367:1145–1154. doi: 10.1016/S0140-6736(06)68433-X. [DOI] [PubMed] [Google Scholar]
- Holgado-Madruga M, Wong A J. Gab1 is an integrator of cell death versus cell survival signals in oxidative stress. Mol Cell Biol. 2003;23:4471–4484. doi: 10.1128/MCB.23.13.4471-4484.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Diener K, Wang X S, Zukowski M, Matsumoto G, Zhou G, Mo R, Sasaki T, Nishina H, Hui C C, Tan T H, Woodgett J P, Penninger J M. Activation of stress-activated protein kinases/c-Jun N-terminal protein kinases (SAPKs/JNKs) by a novel mitogen-activated protein kinase kinase. J Biol Chem. 1997;272:32378–32383. doi: 10.1074/jbc.272.51.32378. [DOI] [PubMed] [Google Scholar]
- Yan M, Dai T, Deak J C, Kyriakis J M, Zon L I, Woodgett J R, Templeton D J. Activation of stress-activated protein kinase by MEKK1 phosphorylation of its activator SEK1. Nature. 1994;372:798–800. doi: 10.1038/372798a0. [DOI] [PubMed] [Google Scholar]
- Tung R M, Blenis J. A novel human SPS1/STE20 homologue, KHS, activates Jun N-terminal kinase. Oncogene. 1997;14:653–659. doi: 10.1038/sj.onc.1200877. [DOI] [PubMed] [Google Scholar]
- Yang J J. Mixed lineage kinase ZAK utilizing MKK7 and not MKK4 to activate the c-Jun N-terminal kinase and playing a role in the cell arrest. Biochem Biophys Res Commun. 2002;297:105–110. doi: 10.1016/s0006-291x(02)02123-x. [DOI] [PubMed] [Google Scholar]
- Derijard B, Raingeaud J, Barrett T, Wu I H, Han J, Ulevitch R J, Davis R J. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch M A. The isoform-specific functions of the c-Jun N-terminal kinases (JNKs): differences revealed by gene targeting. Bioessays. 2006;28:923–934. doi: 10.1002/bies.20458. [DOI] [PubMed] [Google Scholar]
- Sun H, Charles C H, Lau L F, Tonks N K. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- Charles C H, Abler A S, Lau L F. cDNA sequence of a growth factor-inducible immediate early gene and characterization of its encoded protein. Oncogene. 1992;7:187–190. [PubMed] [Google Scholar]
- Emslie E A, Jones T A, Sheer D, Keyse S M. The CL100 gene, which encodes a dual specificity (Tyr/Thr) MAP kinase phosphatase, is highly conserved and maps to human chromosome 5q34. Hum Genet. 1994;93:513–516. doi: 10.1007/BF00202814. [DOI] [PubMed] [Google Scholar]
- Wang Z, Xu J, Zhou J Y, Liu Y, Wu G S. Mitogen-activated protein kinase phosphatase-1 is required for cisplatin resistance. Cancer Res. 2006;66:8870–8877. doi: 10.1158/0008-5472.CAN-06-1280. [DOI] [PubMed] [Google Scholar]
- Venugopal S K, Chen J, Zhang Y, Clemens D, Follenzi A, Zern M A. Role of MAPK phosphatase-1 in sustained activation of JNK during ethanol-induced apoptosis in hepatocyte-like VL-17A cells. J Biol Chem. 2007;282:31900–31908. doi: 10.1074/jbc.M703729200. [DOI] [PubMed] [Google Scholar]
- Xu Q, Konta T, Nakayama K, Furusu A, Moreno-Manzano V, Lucio-Cazana J, Ishikawa Y, Fine L G, Yao J, Kitamura M. Cellular defense against H2O2-induced apoptosis via MAP kinase-MKP-1 pathway. Free Radic Biol Med. 2004;36:985–993. doi: 10.1016/j.freeradbiomed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Qu J, Liu G H, Wu K, Han P, Wang P, Li J, Zhang X, Chen C. Nitric oxide destabilizes Pias3 and regulates sumoylation. PLoS One. 2007;2:e1085. doi: 10.1371/journal.pone.0001085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.