Abstract
OBJECTIVE
It is becoming apparent that there is a strong link between taste perception and energy homeostasis. Recent evidence implicates gut-related hormones in taste perception, including glucagon-like peptide 1 and vasoactive intestinal peptide (VIP). We used VIP knockout mice to investigate VIP's specific role in taste perception and connection to energy regulation.
RESEARCH DESIGN AND METHODS
Body weight, food intake, and plasma levels of multiple energy-regulating hormones were measured and pancreatic morphology was determined. In addition, the immunocytochemical profile of taste cells and gustatory behavior were examined in wild-type and VIP knockout mice.
RESULTS
VIP knockout mice demonstrate elevated plasma glucose, insulin, and leptin levels, with no islet β-cell number/topography alteration. VIP and its receptors (VPAC1, VPAC2) were identified in type II taste cells of the taste bud, and VIP knockout mice exhibit enhanced taste preference to sweet tastants. VIP knockout mouse taste cells show a significant decrease in leptin receptor expression and elevated expression of glucagon-like peptide 1, which may explain sweet taste preference of VIP knockout mice.
CONCLUSIONS
This study suggests that the tongue can play a direct role in modulating energy intake to correct peripheral glycemic imbalances. In this way, we could view the tongue as a sensory mechanism that is bidirectionally regulated and thus forms a bridge between available foodstuffs and the intricate hormonal balance in the animal itself.
Taste perception and its relationship to glucose homeostasis begins with stimulation of taste cells located in tongue taste buds. There are five basic taste modalities: bitter, sweet, umami, salty, and sour. Taste cells are clustered into taste buds in the tongue epithelium (1). Mammals have four different types of taste cells (types I, II, III, and IV), exhibiting different molecular phenotypes and functional roles (2–4). Type I cells are glial-like cells that maintain taste bud structure. Type II taste cells transduce sweet, bitter, or umami stimuli (5) and communicate information through G-protein–coupled transduction cascades (6,7). Type III cells synapse directly with afferent nerve fibers from three cranial nerves (8), and most release serotonin upon depolarization (9). Type IV “basal cells” are rapidly dividing progenitor cells that differentiate into type I, II, and III cells (10). Along with biogenic amine neurotransmitters, it is becoming evident that multiple peptide hormones including glucagon-like peptide-1 (GLP-1; [11]), cholecystokinin (12), and neuropeptide Y (13) as well as VIP (14) are located in taste cells, potentially acting as signaling modulators of multiple gustatory stimuli (15).
VIP is a 28–amino acid peptide expressed at multiple sites throughout the body. It was discovered by its potent muscle relaxant vasodilatory activity and as a stimulator of secretory activity (16). VIP exerts its effects through activation of two G-protein–coupled receptors (GPCRs): VPAC1 and VPAC2 (17). These receptors preferentially stimulate adenylate cyclase and increase intracellular cAMP. VIP activity at VPAC1/2 receptors in pancreatic islets is involved in insulin secretion (18). VIP expression has been identified in the taste cells of rat, hamster, carp, and human (12,14,19). In taste cells, VIP colocalizes with the taste transduction markers α-gustducin and T1R2 (20); any role, however, of VIP in gustation has not previously been established. Gustation is vital for locating food sources and determining which foodstuffs to ingest. Gustation is also involved in maintaining body weight and energy balance, that is, there is a strong link between peripheral somatic energy balance and “flavor perception.” This link may be mediated by the coherent use of a cadre of neuropeptide hormones present in both the tongue and the periphery. We have previously shown that the gut incretin, GLP-1, in taste cells profoundly controls sweet and umami taste (11,21). Peripheral leptin (food intake regulator produced primarily in adipose tissue [22–24]), via activation of the leptin receptor (Ob-Rb) on taste cells, can inhibit responses to sweet tastants (25). Interestingly, such selective sweet taste inhibition by peripheral leptin is not present in leptin receptor–deficient db/db mice (26–28), suggesting that leptin, together with GLP-1, acts as a dual-functional sweet-sensation modulator involved in the regulation of food intake and somatic energy regulation.
In this study, we set out to uncover the role of VIP in gustation. We demonstrate, using VIP knockout mice, that VIP plays a specific role in modifying distinct taste modalities and that VIP knockout mice have significantly altered expression levels of other hormones and receptors that are involved in the control of taste modulation and energy balance.
RESEARCH DESIGN AND METHODS
Animal and tissue processing.
Animal testing procedures were approved by the Animal Care and Use Committee of the National Institute on Aging. Methods are described in detail in the supplementary material, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0807/DC1.
Immunohistochemistry.
Methods are described in detail in the supplementary material. Serial sections (10-μm thickness) were cut from tissues containing circumvallate, foliate, and fungiform papillae, using a cryostat (Microm; Thermo Scientific). To obtain systematic samples without bias throughout the papillae, each papilla was sectioned and every tenth section was saved onto a slide. Taste buds are ∼80–100 μm in length, therefore sampling every tenth section ensures that no two sections will be from the same taste bud. Images were collected using a confocal microscope LSM-710 (Carl Zeiss). Approximately 100–120 taste buds per group were analyzed immunohistochemically as described previously (11,29). Only nuclear profile–positive cells were scored as immunoreactive. The total number of cells in the section was determined by counting the number of TO-PRO-3–stained nuclei present in each taste bud. The percentage of immunoreactive taste cells was calculated by: number of immunoreactive taste cells/total number of taste cells in each taste bud.
Immunoassay analyses of gut and appetite hormones in plasma.
Plasma levels of gut and appetite hormones were measured using a mouse multiplex assay kit (Millipore) according to the manufacturer's instructions. Methods are described in detail in the supplementary material.
Pancreatic islet size quantification and Western blot analyses.
Methods are described in detail in the supplementary material. Quantification of pancreatic immunohistochemistry images was performed in Matlab (Mathworks) as described previously (30). Hypothalamic protein levels of various factors involved in energy regulation were determined by Western blot analyses.
Taste behavioral tests.
Taste behavioral testing was performed as previously described (11). VIP knockout and wild-type (n = 8) mice were habituated to the laboratory environment for 30 min each day prior to testing. The same animals were used for both the taste behavioral tests and analyses of physiological parameters (pancreatic function, gut/appetite plasma hormone analyses). Test stimuli consisted of various concentrations of sucrose (25, 75, 150, 300, and 600 mmol/l; Fisher Scientific), sodium chloride (NaCl: 100, 200, 400, 600, 800, and 1,000 mmol/l; Sigma), denatonium benzoate (0.001, 0.01, 0.1, 0.3, 1, 5, and 10 mmol/l; Sigma), and citric acid (0.01, 0.1, 1, 3, 5, 10, 30, and 100 mmol/l; Fisher Scientific). Brief-access taste testing took place in a Davis MS-160 gustometer (DiLog Instruments) as previously described (11,31–34). Brief-access procedures minimize postingestive effects that may confound other assays such as intake tests (33). To test salty, sour, and bitter stimuli responses, mice were water deprived prior to testing. For testing of the sweet stimulus, mice were both food and water restricted (2.5 ml of water, 1 g of food) prior to testing. All mice were tested with a single tastant at various concentrations per trial, to prevent the mice from being affected by prior experience. Data analysis is described in detail in the supplementary material.
RESULTS
VIP knockout mice possess normal taste bud morphology.
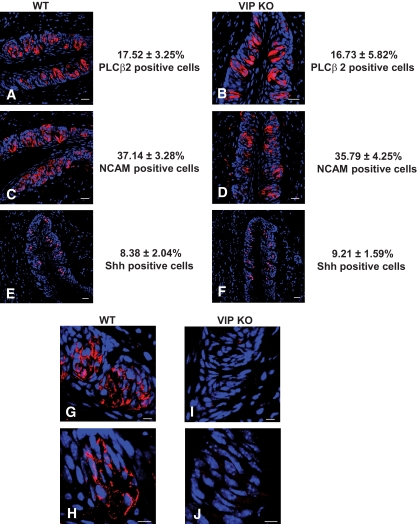
Gross taste bud morphology and taste cell number were assessed in wild-type and VIP knockout mice using classical taste cell type–specific immunocytochemical markers (supplementary Tables 1 and 2). Compared with wild-type mice, VIP knockout mice showed no gross taste bud abnormalities and expressed similar distribution patterns of taste cell–specific markers (Fig. 1A–F, supplementary Table 2). VIP immunoreactivity was present in the taste cells of wild-type animals (Fig. 1G and H) but absent in the VIP knockout mice (Fig. 1I and J). The lack of VIP, therefore, did not significantly affect initial taste cell development and number.
FIG. 1.
Expression of taste cell markers and VIP in circumvallate papillae of wild-type (WT) and VIP knockout (KO) mice. Type II cell marker (PLCβ2) (A and B), type III cell marker (NCAM) (C and D), and type IV cell marker (Shh) (E and F) are expressed in taste cells of both wild-type and VIP knockout mice. Scale bars, 20 μm. Blue is TO-PRO-3 nuclear stain. G and H: VIP (red) is expressed in a subset of taste cells of wild-type mice. I and J: VIP is not expressed within the VIP knockout mouse taste bud. Scale bars, 20 μm. Sections are representative of three mice. (A high-quality digital representation of this figure is available in the online issue.)
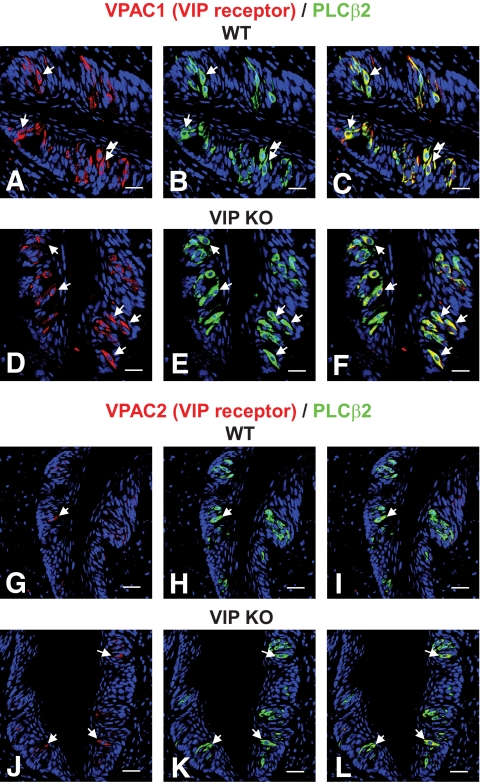
VPAC1/2 receptors are expressed in taste cells.
Both VPAC1/2 receptors were primarily expressed in phospholipase Cβ2 (PLCβ2)-positive taste cells (type II cells: Fig. 2; supplementary Figs. 2 and 3). The presence of VIP and its cognate receptors in type II taste cells suggests that VIP signaling is local to the taste bud and could likely affect tongue-localized gustation.
FIG. 2.
Coexpression of VIP receptors (VPAC1/VPAC2) and PLCβ2 in circumvallate papillae of wild-type (WT) and VIP knockout (KO) mice. A–C: VPAC1 (red) and PLCβ2 (green) are colocalized in a subset of PLCβ2-positive cells (yellow cells) in wild-type mice. The arrows denote cells expressing both. D–F: VPAC1 (red) and PLCβ2 (green) are colocalized in a subset of PLCβ2-positive cells (yellow cells) in VIP knockout mice. G–I: VPAC2 (red) and PLCβ2 (green) are colocalized in a subset of PLCβ2-positive cells (yellow cells) in wild-type mice. The arrows denote cells expressing both. J–L: VPAC2 (red) and PLCβ2 (green) are colocalized in a subset of PLCβ2-positive cells (yellow cells) in VIP knockout mice. The arrows denote cells expressing both. Scale bars, 20 μm. Blue is TO-PRO-3 nuclear stain. (A high-quality digital representation of this figure is available in the online issue.)
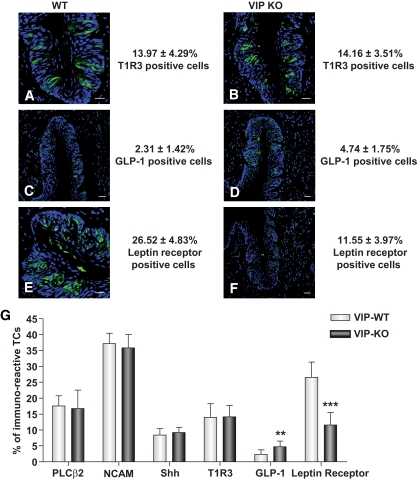
Reduced leptin receptor and increased GLP-1 expression in VIP knockout mice taste cells.
VIP is coexpressed with the taste transduction marker α-gustducin and with the T1R2 GPCR (20). The majority of VIP-immunoreactive taste cells also coexpresses α-gustducin, although a subset also expresses T1R2. α-Gustducin and T1R2 are markers for type II cells, and based on this specific expression pattern, we hypothesized that VIP could be involved in the transduction of sweet and bitter stimuli. To investigate this, we first determined the expression patterns in the wild-type and VIP knockout mice of additional hormones and receptors known to play a functional role in sweet taste coding, that is, the T1R3 sweet stimulant receptor, GLP-1, and leptin receptor (Ob-Rb) (Fig. 3A–F). Both wild-type and VIP knockout mice showed similar expression patterns of T1R3 in the circumvallate papillae (Fig. 3A, B, and G). Interestingly, we found that GLP-1 expression was significantly increased in the taste cells of VIP knockout mice compared with wild type (Fig. 3C, D, and G, P < 0.001). Leptin receptor expression was markedly reduced in VIP knockout mice compared with wild type (Fig. 3E–G, P < 0.001). Peripheral leptin has been shown to suppress sweet taste preference through its action on the leptin receptors in the taste cells (26–28). Elevation in GLP-1 expression and the reduction in leptin receptor expression strongly indicates that VIP could play a role in modulating sweet taste. Taken together, it is apparent that there are significant differences in the numbers of GLP-1– and Ob-Rb–immunopositive cells between wild-type and VIP knockout mice (Fig. 3G, supplementary Table 2). To enhance our understanding of these alterations in the taste cells of VIP knockout mice, we next determined whether other systemic alterations in appetite/energy-regulatory hormones occur in VIP knockout mice.
FIG. 3.
Expression of taste-related proteins in circumvallate papillae of wild-type (WT) and VIP knockout (KO) mice. A and B: Sweet taste receptor (T1R3, green) is expressed in both wild-type and VIP knockout mice. C and D: GLP-1 (green) is expressed in wild-type and VIP knockout mice. E and F: Leptin receptor (green) is expressed in wild-type and VIP knockout mice. Scale bars, 20 μm. Blue is TO-PRO-3 nuclear stain. G: The percentage of immunopositive cells quantified in both wild-type and VIP knockout mice for the following markers: PLCβ2, NCAM, Shh, T1R3, GLP-1, and leptin receptor. Cells were scored as immunoreactive only if a nuclear profile was present within the cell. Values are the means ± SEM. **P < 0.01, ***P < 0.001. TCs, taste cells. (A high-quality digital representation of this figure is available in the online issue.)
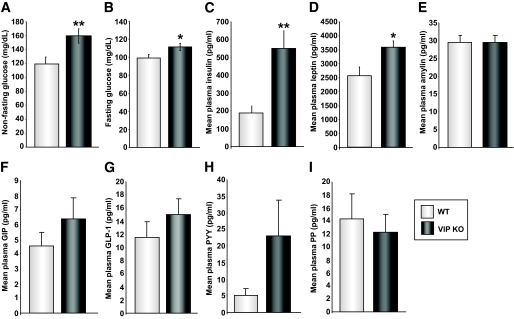
Elevation of plasma leptin and glucose in VIP knockout mice.
As we detected reduced leptin receptor expression in VIP knockout mouse taste cells compared with wild type, we measured plasma leptin levels in these mice. Circulating leptin can bind to and activate the leptin receptor on taste cells, where it acts to specifically inhibit gustatory responses to sweet substances, without affecting responses to sour, salty, and bitter substances in lean mice (26–28). In VIP knockout mice, both nonfasting and fasting glucose levels were elevated compared with wild type (Fig. 4A and B). In addition, we noted a significant elevation in fasting insulin in VIP knockout mice compared with wild type (Fig. 4C). We also found that plasma leptin levels were significantly elevated in VIP knockout mice compared with wild type (Fig. 4D). These data suggest that VIP knockout mice possess a diabetic-like pathology. Plasma levels of amylin (Fig. 4E) were not different between wild-type and VIP knockout mice, whereas VIP knockout mice possessed considerably higher plasma gastrointestinal polypeptide (Fig. 4F), GLP-1 (Fig. 4G), and peptide tyrosine tyrosine (PYY) levels (Fig. 4H). Plasma pancreatic polypeptide (PP) levels between wild-type and VIP knockout mice were not significantly different despite a trend for lower PP levels in the VIP knockout mice (Fig. 4I).
FIG. 4.
VIP knockout (KO) mice possess alterations in multiple hormones controlling appetite and energy balance. Multiplex hormone analysis from plasma samples of wild-type (WT) and VIP knockout mice reveal multiple distinctions between the two animal groups. A and B: Significantly higher nonfasting and fasting glucose occurs in the VIP knockout mice. The VIP knockout mice also possessed significantly higher plasma insulin (C) and leptin (D) levels. No significant differences between wild-type and VIP knockout for amylin were noted (E). VIP knockout mice demonstrated higher levels of plasma gastrointestinal polypeptide (F), GLP-1 (G), and peptide tyrosine tyrosine (PYY) (H). No significant difference in the plasma levels of PP were noted between wild-type and VIP knockout mice (I). Each panel depicts means ± SEM from eight animals in each group. *P < 0.05, **P < 0.01.
VIP knockout mice have normal pancreatic islet cell morphology.
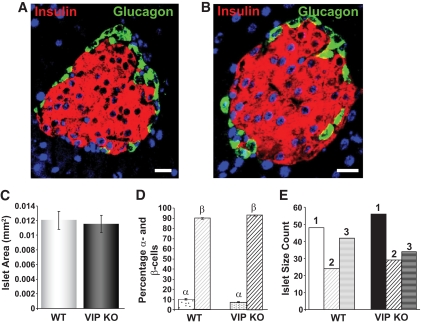
As VIP knockout mice possessed significantly elevated plasma insulin levels compared with wild-type mice (P < 0.01), we next investigated pancreatic islet size and distribution of β-cells and α-cells. VIP knockout mice showed similar total β-cell area compared with wild-type (Fig. 5A–C). No significant difference in the total percentage of α- or β-cells in the islets (Fig. 5D) and the number of small-, middle-, and large-sized islets between wild-type and VIP knockout mice was observed (Fig. 5E).
FIG. 5.
Pancreatic islet sizing in VIP knockout (KO) and wild-type (WT) mice. Representative pancreatic islets from wild-type (A) or VIP knockout (B) mice demonstrate similar insulin (red fluorescence) and glucagon (green fluorescence) expression profiles. C: Mean islet area (mm2) measured in wild-type or VIP knockout pancreata. D: Similar mean α-cell (α) and β-cell (β) percentage in the islets of VIP knockout and wild-type mice. E: Relative distribution of the different islet sizes (1 = small; 2 = middle; 3 = large) for VIP knockout and wild-type mice. Values are expressed as the means ± SEM. (A high-quality digital representation of this figure is available in the online issue.)
VIP in taste cells regulates multiple taste modalities.
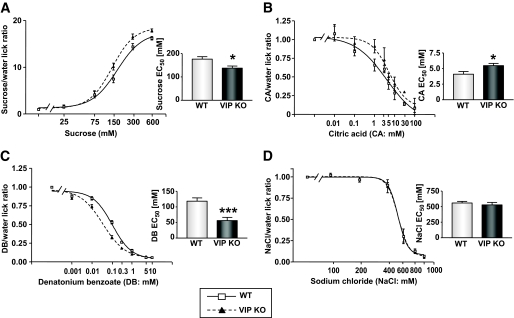
As a considerable amount of our molecular/immunocytochemical data suggested a role for VIP in taste modulation, we next investigated VIP's role in taste perception. We tested the ability of wild-type and VIP knockout mice to detect prototypical taste stimuli, that is, sweet (sucrose), sour (citric acid), salty (sodium chloride), and bitter (denatonium benzoate). VIP knockout mice displayed significantly altered concentration-dependent licking for sucrose (P = 0.0108 between mean half-maximal effective concentration [EC50] values) and denatonium benzoate (P = 0.0003 between mean EC50 values) compared with wild-type mice, demonstrated by their lower EC50 values compared with wild type (Fig. 6A and C, supplementary Fig. 1). VIP knockout mice also showed concentration-dependent changes in licking for citric acid (P = 0.023 between mean EC50 values) (Fig. 6B), but there was no significant alteration for sodium chloride in the VIP knockout mice compared with wild-type mice (Fig. 6D). Taken together, these results indicate that VIP plays a specific functional role in modulating taste perception of sweet, bitter, and sour stimuli.
FIG. 6.
Altered sweet, bitter, and sour taste responses of wild-type (WT) and VIP knockout (KO) mice in brief-access taste tests (A–D). Taste responses, expressed as tastant/water lick ratios and as a function of stimulus concentration, of VIP knockout (closed triangle, dotted line) and wild-type (open square, solid line) to sucrose (A), citric acid (CA; B), denatonium benzoate (DB; C), and sodium chloride (NaCl; D). Values are expressed as means ± SEM. Curves were fit as described in research design and methods. *P < 0.05, ***P < 0.001.
VIP knockout mice attempt to ameliorate their dysglycemia.
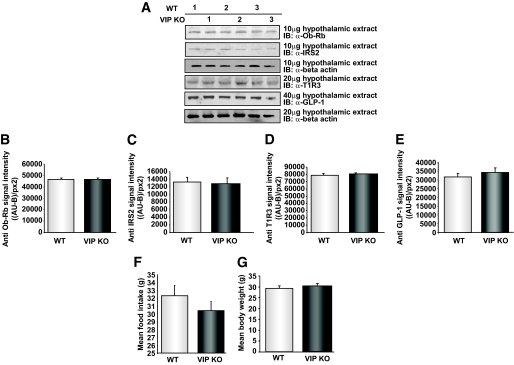
Our data indicate that there is an alteration in the biochemical, but not structural, nature of the tongue of VIP knockout mice, as well as complex alterations in circulating metabolic hormones. One paradoxical finding, however, was the reduction in leptin receptor levels in the tongue. VIP knockout mice demonstrate several features reminiscent of insulin resistance (e.g., high fasting glucose and insulin levels). In addition, VIP knockout animals possessed a heightened preference for appetitive sweet stimuli. Perhaps this change in taste preference—induced by increased tongue GLP-1 levels and reduced leptin receptor levels—could be a compensatory mechanism engendered to ameliorate their dysglycemia by attempting to reduce sugar intake. High circulating levels of leptin would be expected to reduce sweet taste preference (25), but with a significant ablation of the lingual Ob-Rb, this would be prevented. However, if this Ob-Rb ablation was universal, especially in the hypothalamus, then the animal would likely consume even more food and thus exacerbate its dysglycemic state. We, therefore, assessed the expression level of Ob-Rb in the hypothalami of wild-type and VIP knockout mice. In contrast to the tongue, no difference in Ob-Rb expression between wild-type and VIP knockout hypothalami was observed (Fig. 7A and B). In addition, we found no difference in insulin receptor substrate 2 (IRS2), T1R3 receptor, or GLP-1 expression in the hypothalami of wild-type or VIP knockout mice (Fig. 7A and C–E). VIP knockout mice showed a small reduction in food intake compared with wild-type mice (Fig. 7F) and there was no significant difference in body weight between VIP knockout and wild-type mice (Fig. 7G). Thus, it appears that the VIP knockout mice possess a complex physiological reaction to the imposed loss of VIP, one that may, in part, attempt to ameliorate their dysglycemia. VIP knockout mice show an increase in sweet taste preference and have elevated circulating leptin levels, which together could potentially be a mechanism to prevent additional excessive sugary food intake (summarized in Fig. 8). However, this mechanism would succeed only if there was localized lingual loss of the Ob-Rb, which was observed in the VIP-null mice.
FIG. 7.
Normal leptin receptor and IRS2 expression in the hypothalami of wild-type (WT) and VIP knockout (KO) mice. A: Representative Western blots of three wild-type (1–3) or VIP knockout (1–3) mice hypothalami. The tissue extracts were probed with specific antisera for the leptin receptor (Ob-Rb), IRS2, and β-actin as a loading control. For the Ob-Rb blot, a plasma membrane extract was used, whereas cytoplasmic tissue extracts were used for the IRS2 and actin blots. B–E: Mean ± SEM of the relative band intensities for the specific Western blots measured as relative absorbance units minus background absorbance per square pixel (AU-B/px2). F: Mean food intake (g) in wild-type and VIP knockout mice. G: Mean body weight of wild-type and VIP knockout mice. Values are expressed as means ± SEM.
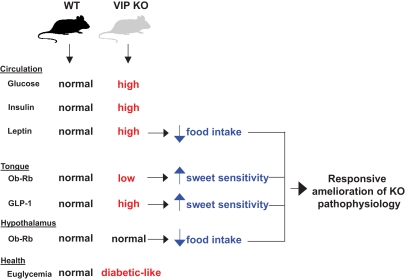
FIG. 8.
A diagrammatic summary of the complex physiological parameters observed in the VIP knockout (KO) mice compared with the wild-type (WT) controls. The VIP knockout mice present with a diabetic-like state, and they actively attempt to ameliorate this condition with a series of physiological alterations (high circulating leptin, low tongue Ob-Rb expression, high tongue GLP-1 expression, normal hypothalamic Ob-Rb expression) that—in concert—could attenuate the animals' euglycemic disruption by enhancing their sweet taste and potentially reducing their desire to consume sweet foods.
DISCUSSION
We have demonstrated that VIP knockout mice possess alterations in their taste cells, circulating metabolic hormones, and taste preferences. Together, these factors appear to contribute to a unique dysglycemic pathophysiology. VIP and its cognate receptors are expressed in the type II taste cells of the circumvallate papillae. VIP knockout mice demonstrated normal gross taste bud morphology (Figs. 1 and 2). However, several key biochemical and pharmacologic alterations in their tongues were seen, that is, VIP knockout compared with wild-type mice exhibited significant increases and decreases in GLP-1 and leptin receptor, respectively, in taste cells (Fig. 3). In light of our previous findings that GLP-1 in taste cells enhances sweet taste perception, this strongly indicated to us that taste cell VIP signaling may be involved in sweet taste perception. We demonstrated that VIP knockouts possess a greater preference for sweet compounds compared with wild-type mice (Fig. 6). VIP knockout mice also exhibited concentration-dependent changes in licking bitter stimuli and sour stimuli, but there was no change in salt perception compared with wild-type mice.
It is interesting to note that VPAC1/2 receptors were expressed on type II taste cells. Receptor systems responsive to bitter, sweet, and umami stimuli use similar signal transduction systems that themselves are narrowly tuned and achieve functional specificity by expression in nonoverlapping subsets of type II cells. Interestingly, in this study, compared with our previous study (11), both VIP knockout and wild-type mice presented much higher sucrose/water lick ratios than GLP-1 receptor knockout (GLP-1R knockout) mice and their wild-type controls. It is unsurprising that distinct strains of mice possess different tastant responses, and this observation may be important for future experimental consideration of specific strains to facilitate efficient and sensitive detection of alterations in different taste modalities.
One unexpected finding in our VIP knockout mice was the significant reduction in leptin receptor (Ob-Rb) expression in the taste cells (Fig. 3F and G). Circulating leptin can activate leptin receptors on taste cells and specifically inhibit gustatory responses to sweet compounds (25). Hence, the significant reduction in taste cell leptin receptor expression in VIP knockout mice may, in part, account for their heightened preference for sweet tastants. Loss of the Ob-Rb would reduce any inhibitory action that circulating leptin would have upon sweet taste. This finding was interesting in light of our data showing that circulating levels of leptin in VIP knockout mice were considerably higher than those in wild type. Therefore, despite the presence of high concentrations of this sweet taste suppressor, the reduction of taste cell leptin receptor was able to facilitate enhanced sweet sensation by preventing inhibitory leptin signaling activity in the tongue. The elevated sweet preference of the VIP knockout mice may also stem from the elevated levels of taste cell GLP-1. We have previously shown that GLP-1's ability to activate the GLP-1R in the tongue strongly modulates sweet taste in mice (11). Increased levels of GLP-1 could, therefore, potentiate GLP-1R activity in the tongue, especially considering our previous findings that the enzyme that degrades GLP-1 in the peripheral circulation (dipeptidyl peptidase-4) is not present in taste cells of mice (11). The precise temporal nature of Ob-Rb loss, peripheral increase in leptin levels, or elevations in taste cell GLP-1 remains to be determined, but our data suggest that there is a strong connection between peripheral energy–regulatory hormones and lingual preference for foodstuffs. From our data, this physiological link may involve VIP. Because of the multiple sites and mechanisms of action of VIP in mammals, it is unsurprising that the metabolic phenotype of VIP knockout mice is complex. Hence, despite increased leptin and PYY levels in VIP knockout mice there was no dramatic diminution of body weight or food intake. This may, in part, be due to removal of an inhibitory action of VIP upon food intake, demonstrated in multiple species including mice (35). Therefore, the resultant phenotype of the VIP knockout mice may be truly an intricate mixture of alterations of metabolic food desire and digestion.
In our study, several lines of evidence indicate a strong local taste cell action of VIP. First, VIP knockout mice demonstrated concentration-dependent changes in licking for sweet, bitter, and sour tastants, but not salt, indicating that there was not a generalized, widespread positive effect on all taste modalities. Second, VIP knockout and wild-type mice responded similarly to the salty taste stimulus; therefore, VIP knockout mice had no difficulty in learning and completing the taste test. Lastly, VIP and VIP receptors are specifically localized within sweet- and bitter-sensitive taste cells, consistent with the specific taste alterations we observed. Further reinforcing a selective local action of VIP, we found no VIP immunoreactivity in type I cells. It has been demonstrated that amiloride-sensitive Na+ channels are expressed in type I cells of the fungiform papillae, suggesting that these taste cells could play a role in transducing salty taste stimuli (36). In line with this finding, we found that there was no difference in salt perception between VIP knockout and wild-type mice. Thus, it would be reasonable to propose that VIP signaling in the taste bud plays a local role in modulating sweet, bitter, and sour taste stimuli.
There are numerous potential mechanisms by which VIP may affect both sweet and bitter stimuli. Sweet and bitter signal transduction is commonly mediated by PLCβ mechanisms involving taste receptor activation, as well as through adenylate cyclase–dependent mechanisms. VPAC1/2 receptors are coupled to multiple G-protein effectors to activate phospholipase D, PLCβ, and adenylate cyclase. VIP stimulation of VPAC receptors would therefore likely compete for availability of the PLCβ and adenylate cyclase signaling pathways, potentially diluting the functional signal transduction efficacy of the primary taste receptors. We have previously demonstrated that receptor stimulation of multiple related receptor systems can result in strong inhibitory effects that are profound and long-lasting (37). Genetic removal of the stimulating ligand will result in a diminution in the number of active VPAC1/2 receptors, reducing any diluting competitive actions upon the taste receptor's signaling capacity. Future experimentation upon the signaling efficiency of taste receptors in such an environment may shed light on this hypothesis.
The ability of VIP ablation to affect not only sweet and bitter taste perception, but also the response to a sour stimulus may be, in part, due to a role of VIP in controlling cell-cell signaling between taste cells and the type III (“presynaptic”) cells possessing the putative sour receptor (PKD2L1). Recent data have shown that although presynaptic cells do not possess tastant-sensitive GPCRs (38), they still respond to tastant stimulation (5). Communication between taste cells and presynaptic cells is thought to involve ATP and serotonin secretion from the taste cells (39). In many areas of the gut, VIP is coreleased with both ATP and nitric oxide (NO) to exert relaxatory effects on smooth muscle (40). NO is commonly secreted from postsynaptic nervous tissue to act upon presynaptic neurons to control neurotransmitter release, and NO has also been associated with taste transduction (41). Therefore, it may be possible that in the tongue VIP may act in an analogous manner as in other parts of the digestive tract (i.e., in a concerted manner with NO and ATP). Therefore, genetic disruption of VIP may affect efficient taste cell–presynaptic cell communication, resulting in the reduction in sour taste we observed in VIP knockout mice.
We have demonstrated a complex physiological pattern of changes in VIP knockout mice that superficially may seem unconnected but together form a coherent and rational reactive mechanism to maintain energy homeostasis. VIP knockout animals demonstrate significantly elevated blood glucose and insulin levels. They also possess significantly higher levels of circulating leptin but a striking reduction in Ob-Rb expression in the taste cells, while maintaining normal levels of Ob-Rb and other energy-regulatory factors (GLP-1, T1R3) in the hypothalamus. In addition, the animals demonstrate an altered preference for sweet tastants. The loss of VIP in our mice induces a dysglycemic state, that is, high resting glucose paired with a high insulin level. With knockout animals that survive birth, it is likely that they have physiologically adapted to counteract their deficits. For example, the lack of alteration of islet β-cell mass in the VIP knockout mice may be compensated for by the nonselective activity of structurally similar peptides, for example, pituitary adenylate cyclase activating polypeptide, which can activate VPAC2 receptors in the VIP knockout pancreata, substituting for VIP activity (18).
It would be interesting to speculate that reactive actions in VIP knockout mice have attempted to ameliorate their diabetic-like pathology by increasing their sweet preference (through reduced inhibitory leptin receptor activity and increased GLP-1 expression in the tongue) so as not to overconsume carbohydrates, while also trying to dampen their overall appetite (increased peripheral circulating leptin with normal hypothalamic Ob-Rb expression) (Fig. 8). Central nervous system VIP is antagonistic to dopamine and serotonin nervous activity (42,43). Both of these transmitters control food reward responses (44,45), and therefore VIP knockout mice may possess an elevated reward response, curtailing their intake and preventing weight increases. Human analogy of VIP knockout mice may be seen in “supertasters” (possessing increased sweet and bitter sensitivity) who tend to be more lean and perhaps eat less than nonsupertasters (46). However, it is unclear whether the VIP knockout mice would not have gained weight if their chow was sweetened with sucrose, and more studies are needed to explore this further.
Thus, an interesting hypothesis would be that these multiple concerted responses in the VIP knockout mice suggest that the tongue could potentially act as a vessel for the animal to modulate its energy intake to correct any endogenous glycemic imbalances. In this way, we could potentially view the tongue as a sensory mechanism that is bidirectionally regulated, and thus forms a bridge between available foodstuffs and the intricate metabolic hormonal balance in the animal itself. Future studies would be needed to further explore this hypothesis.
To conclude, it is becoming apparent that there is a strong link between gustation and peripheral energy balance. Hormonal signaling within the taste buds of the tongue is likely to influence food intake and peripheral glucose homeostasis (15). For example, hormones such as GLP-1 and leptin, crucial to the control of peripheral somatic energy balance/appetite, are now thought to exert a strong local effect on the tongue with respect to food perception. This activity is mediated by their signaling roles in the gustatory system. Carbohydrates are among the most vital food stuffs for mammalian survival, and it would make evolutionary sense for mammals to have evolved multimodal carbohydrate sensing mechanisms. We feel that gaining a greater appreciation for which endocrine factors are present within taste cells and their putative roles in taste perception will shed much-needed light on how taste perception is linked to peripheral somatic energy balance. This knowledge will be invaluable for finding novel therapeutic targets and agents for the treatment of disorders such as metabolic syndrome and type 2 diabetes.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
No potential conflicts of interest relevant to this article were reported.
We thank Dr. Jacqueline Crawley, Dr. Joanna Hill, and Connor Stack (National Institute of Mental Health) for kindly providing us with VIP knockout mouse breeding pairs.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Dulac C. The physiology of taste, vintage 2000. Cell 2000;100:607–610 [DOI] [PubMed] [Google Scholar]
- 2.Yoshie S, Wakasugi C, Teraki Y, Fujita T. Fine structure of the taste bud in guinea pigs: I, cell characterization and innervation patterns. Arch Histol Cytol 1990;53:103–119 [DOI] [PubMed] [Google Scholar]
- 3.Huang YJ, Lu KS. Unilateral innervation of guinea pig vallate taste buds as determined by glossopharyngeal neurectomy and HRP neural tracing. J Anat 1996;189:315–324 [PMC free article] [PubMed] [Google Scholar]
- 4.Yee CL, Yang R, Böttger B, Finger TE, Kinnamon JC. “Type III” cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol 2001;440:97–108 [DOI] [PubMed] [Google Scholar]
- 5.Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci 2007;27:10840–10848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature 2006;444:288–294 [DOI] [PubMed] [Google Scholar]
- 7.Roper SD. Cell communication in taste buds. Cell Mol Life Sci 2006;63:1494–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang R, Stoick CL, Kinnamon JC. Synaptobrevin-2-like immunoreactivity is associated with vesicles at synapses in rat circumvallate taste buds. J Comp Neurol 2004;471:59–71 [DOI] [PubMed] [Google Scholar]
- 9.Huang YJ, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE, Wu D, Roper SD. Using biosensors to detect the release of serotonin from taste buds during taste stimulation. Arch Ital Biol 2005;143:87–96 [PMC free article] [PubMed] [Google Scholar]
- 10.Stone LM, Tan SS, Tam PP, Finger TE. Analysis of cell lineage relationships in taste buds. J Neurosci 2002;22:4522–4529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin YK, Martin B, Golden E, Dotson CD, Maudsley S, Kim W, Jang HJ, Mattson MP, Drucker DJ, Egan JM, Munger SD. Modulation of taste sensitivity by GLP-1 signaling. J Neurochem 2008;106:455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kusakabe T, Matsuda H, Gono Y, Furukawa M, Hiruma H, Kawakami T, Tsukuda M, Takenaka T. Immunohistochemical localisation of regulatory neuropeptides in human circumvallate papillae. J Anat 1998;192:557–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao FL, Shen T, Kaya N, Lu SG, Cao Y, Herness S. Expression, physiological action, and coexpression patterns of neuropeptide Y in rat taste-bud cells. Proc Natl Acad Sci U S A 2005;102:11100–11105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herness MS. Vasoactive intestinal peptide-like immunoreactivity in rodent taste cells. Neuroscience 1989;33:411–419 [DOI] [PubMed] [Google Scholar]
- 15.Martin B, Maudsley S, White CM, Egan JM. Hormones in the naso-oropharynx: endocrine modulation of taste and smell. Trends Endocrinol Metab 2009;20:163–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickson L, Finlayson K. VPAC and PAC receptors: from ligands to function. Pharmacol Ther 2009;121:294–316 [DOI] [PubMed] [Google Scholar]
- 17.Martin B, Lopez de Maturana R, Brenneman R, Walent T, Mattson MP, Maudsley S. Class II G protein-coupled receptors and their ligands in neuronal function and protection. Neuromolecular Med 2005;7:3–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winzell MS, Ahrén B. Role of VIP and PACAP in islet function. Peptides 2007;28:1805–1813 [DOI] [PubMed] [Google Scholar]
- 19.Witt M. Distribution of vasoactive intestinal peptide-like immunoreactivity in the taste organs of teleost fish and frog. Histochemical J 1995;27:161–165 [DOI] [PubMed] [Google Scholar]
- 20.Shen T, Kaya N, Zhao FL, Lu SG, Cao Y, Herness S. Co-expression patterns of the neuropeptides vasoactive intestinal peptide and cholecystokinin with the transduction molecules α-gustducin and T1R2 in rat taste receptor cells. Neuroscience 2005;130:229–238 [DOI] [PubMed] [Google Scholar]
- 21.Martin B, Dotson CD, Shin YK, Ji S, Drucker DJ, Maudsley S, Munger SD. Modulation of taste sensitivity by GLP-1 signaling in taste buds. Ann N Y Acad Sci 2009;1170:98–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425–432 [DOI] [PubMed] [Google Scholar]
- 23.Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature 1996;379:632–635 [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 1996;84:491–495 [DOI] [PubMed] [Google Scholar]
- 25.Nakamura Y, Sanematsu K, Ohta R, Shirosaki S, Koyano K, Nonaka K, Shigemura N, Ninomiya Y. Diurnal variation of human sweet taste recognition thresholds is correlated with plasma leptin levels. Diabetes 2008;57:2661–2665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawai K, Sugimoto K, Nakashima K, Miura H, Ninomiya Y. Leptin as a modulator of sweet taste sensitivities in mice. Proc Natl Acad Sci U S A 2000;97:11044–11049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ninomiya Y, Sako N, Imai Y. Enhanced gustatory neural responses to sugars in the diabetic db/db mouse. Am J Physiol 1995;269:R930–R937 [DOI] [PubMed] [Google Scholar]
- 28.Shigemura N, Ohta R, Kusakabe Y, Miura H, Hino A, Koyano K, Nakashima K, Ninomiya Y. Leptin modulates behavioral responses to sweet substances by influencing peripheral taste structures. Endocrinology 2004;145:839–847 [DOI] [PubMed] [Google Scholar]
- 29.Ma H, Yang R, Thomas SM, Kinnamon JC. Qualitative and quantitative differences between taste buds of the rat and mouse. BMC Neurosci 2007;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin B, Golden E, Carlson OD, Pistell P, Zhou J, Kim W, Frank BP, Thomas S, Chadwick WA, Greig NH, Bates GP, Sathasivam K, Bernier M, Maudsley S, Mattson MP, Egan JM. Exendin-4 improves glycemic control, ameliorates brain and pancreatic pathologies, and extends survival in a mouse model of Huntington's disease. Diabetes 2009;58:318–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boughter JD, Jr, St John SJ, Noel DT, Ndubuizu O, Smith DV. A brief-access test for bitter taste in mice. Chem Senses 2002;27:133–142 [DOI] [PubMed] [Google Scholar]
- 32.Glendinning JI, Gresack J, Spector AC. A high-throughput screening procedure for identifying mice with aberrant taste and oromotor function. Chem Senses 2002;27:461–474 [DOI] [PubMed] [Google Scholar]
- 33.Nelson TM, Munger SD, Boughter JD., Jr Taste sensitivities to PROP and PTC vary independently in mice. Chem Senses 2003;28:695–704 [DOI] [PubMed] [Google Scholar]
- 34.Dotson CD, Spector AC. The relative affective potency of glycine, L-serine and sucrose as assessed by a brief-access taste test in inbred strains of mice. Chem Senses 2004;29:489–498 [DOI] [PubMed] [Google Scholar]
- 35.Morley JE, Horowitz M, Morley PM, Flood JF. Pituitary adenylate cyclase activating polypeptide (PACAP) reduces food intake in mice. Peptides 1992;13:1133–1135 [DOI] [PubMed] [Google Scholar]
- 36.Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci 2008;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin B, Brenneman R, Golden E, Walent T, Becker KG, Prabhu VV, Wood W, 3rd, Ladenheim B, Cadet JL, Maudsley S. Growth factor signals in neural cells: coherent patterns of interaction control multiple levels of molecular and phenotypic responses. J Biol Chem 2009;284:2493–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci 2006;26:3971–3980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang YA, Dando R, Roper SD. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J Neurosci 2009;29:13909–13918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burnstock G. The journey to establish purinergic signaling in the gut. Neurogastroenterol Motil 2008;20:8–19 [DOI] [PubMed] [Google Scholar]
- 41.Nakamura T, Murata Y, Mashiko M, Okano K, Satoh H, Ozaki M, Amakawa T. The nitric oxide-cyclic GMP cascade in sugar receptor cells of the blowfly, Phormia regina. Chem Senses 2005;30:281–282 [DOI] [PubMed] [Google Scholar]
- 42.Gerhold LM, Sellix MT, Freeman ME. Antagonism of vasoactive intestinal peptide mRNA in the suprachiasmatic nucleus disrupts the rhythm of FRAs expression in neuroendocrine dopaminergic neurons. J Comp Neurol 2002;450:135–143 [DOI] [PubMed] [Google Scholar]
- 43.Karganov M, Romanova G, Braslawsky W, Tarshitz D, Telegdy G. Neuromodulator role of VIP in recovery of rat behavior and brain neurotransmitters level after frontal lobectomy. Ann N Y Acad Sci 1998;865:519–522 [DOI] [PubMed] [Google Scholar]
- 44.Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci 2006;361:1149–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ledonne A, Sebastianelli L, Federici M, Bernardi G, Mercuri NB. The anorexic agents, sibutramine and fenfluramine, depress GABA(B)-induced inhibitory postsynaptic potentials in rat mesencephalic dopaminergic cells. Br J Pharmacol 2009;156:962–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golding J, Steer C, Emmett P, Bartoshuk LM, Horwood J, Smith GD. Associations between the ability to detect a bitter taste, dietary behavior, and growth: a preliminary report. Ann N Y Acad Sci 2009;1170:553–557 [DOI] [PubMed] [Google Scholar]