Abstract
OBJECTIVE
Diabetic dyslipoproteinemia is characterized by low HDL cholesterol and high triglycerides. We examined the association of lipoprotein particle size and concentration measured by nuclear magnetic resonance (NMR) spectroscopy with clinical type 2 diabetes.
RESEARCH DESIGN AND METHODS
This was a prospective study of 26,836 initially healthy women followed for 13 years for incident type 2 diabetes (n = 1,687). Baseline lipids were measured directly and lipoprotein size and concentration by NMR. Cox regression models included nonlipid risk factors (age, race, smoking, exercise, education, menopause, blood pressure, BMI, family history, A1C, and C-reactive protein). NMR lipoproteins were also examined after further adjusting for standard lipids.
RESULTS
Incident diabetes was significantly associated with baseline HDL cholesterol, triglycerides, and NMR-measured size and concentration of LDL, IDL, HDL, and VLDL particles. The associations of these particles differed substantially by size. Small LDLNMR and small HDLNMR were positively associated with diabetes (quintile 5 vs. 1 [adjusted hazard ratios and 95% CIs], 4.04 [3.21–5.09] and 1.84 [1.54–2.19], respectively). By contrast, large LDLNMR and large HDLNMR were inversely associated (quintile 1 vs. 5, 2.50 [2.12–2.95] and 4.51 [3.68–5.52], respectively). For VLDLNMR, large particles imparted higher risk than small particles (quintile 5 vs. 1, 3.11 [2.35–4.11] and 1.31 [1.10–1.55], respectively). Lipoprotein particle size remained significant after adjusting for standard lipids and nonlipid factors.
CONCLUSIONS
In this prospective study of women, NMR lipoprotein size and concentrations were associated with incident type 2 diabetes and remained significant after adjustment for established risk factors, including HDL cholesterol and triglycerides.
The dyslipoproteinemia of insulin resistance and type 2 diabetes is characterized by low HDL cholesterol and high triglycerides, despite normal or near-normal LDL cholesterol levels (1). Under normal physiologic conditions, insulin results in decreased hepatic synthesis and secretion of VLDL particles. However, when hepatic insulin signaling is impaired in insulin-resistant patients, triglyceride-rich VLDL production and secretion are increased (2). This increase in VLDL is typically associated with reduction in HDL cholesterol levels, in part related to the transfer of cholesteryl ester from the triglyceride-rich lipoproteins to HDLs. In addition, when these triglyceride-rich VLDL particles are subjected to further lipolysis, they give rise to small, cholesterol-poor LDL particles and hence the association with low or nonelevated LDL cholesterol levels. It has been proposed that an abundance of these small dense LDL particles should be considered part of this dyslipoproteinemia. However, small LDL particles cluster metabolically with other risk factors, particularly high triglycerides and low HDL cholesterol (3), and it is unclear if small LDL contribute independent information for prediction of type 2 diabetes (4). Even less is known about the predictive value of particle size or subclass concentrations for HDLs or VLDLs.
One method to measure lipoprotein particle size and concentration is nuclear magnetic resonance (NMR) spectroscopy. This technique simultaneously quantifies the size and concentration (“number”) of lipoprotein particles expressed each as an average particle size (in nanometers) or as lipoprotein particle concentration (in particle mol/l) (5–8). By contrast, standard lipid tests quantify the cholesterol or triglyceride content of lipoproteins, without providing size-specific lipoprotein particle information. NMR lipoproteins have been examined in individuals with insulin resistance or type 2 diabetes, with small LDLs, small HDLs, and large VLDLs associated positively and large HDLs associated inversely, with insulin resistance measured by the euglycemic clamp technique (9) or the frequently sampled intravenous glucose tolerance test (9,10).
Given this pathophysiological relationship between insulin resistance and lipoproteins, we hypothesized that NMR-measured lipoproteins would predict incident type 2 diabetes in a primary prevention setting. Therefore, we conducted this prospective study of initially healthy women to determine 1) whether NMR-measured lipoprotein particle size and concentrations are associated with incident type 2 diabetes, 2) how they compare with chemically measured HDL cholesterol and triglycerides, and 3) whether they provide additive risk information to established risk factors for diabetes.
RESEARCH DESIGN AND METHODS
Study participants were drawn from the Women's Health Study (WHS), a completed randomized, double-blinded, placebo-controlled trial of low-dose aspirin and vitamin E in the primary prevention of cardiovascular disease (CVD) and cancer in women (11–13). WHS participants were apparently healthy female health care professionals, aged ≥45 years, who were free of self-reported CVD and cancer at study entry (1992–1995). At the time of enrollment, women gave written informed consent and completed questionnaires on demographics, anthropometrics, medical history, and lifestyle factors. They were also asked to provide a baseline blood sample; 28,345 women did so, and of these, 98.5% (n = 27,909) had NMR measurements. For this study, we excluded women missing other lipids (n = 33), those with self-reported baseline type 2 diabetes (n = 770), and those with baseline A1C ≥6.5% (n = 270), leaving 26,836 women for analysis. We also repeated the analyses after excluding 169 women with A1C ≥6.0 and <6.5%. The study was approved by the institutional review board of the Brigham and Women's Hospital (Boston, MA).
Laboratory measurements.
EDTA blood samples were obtained at the time of enrollment into the WHS and stored in vapor-phase liquid nitrogen (−170°C). In a laboratory certified by the National Heart, Lung, and Blood Institute/Centers for Disease Control and Prevention Lipid Standardization Program, baseline samples were thawed and analyzed for standard lipids. Direct determination of concentrations of total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides was simultaneously performed on the Hitachi 917 analyzer using reagents and calibrators from Roche Diagnostics (Indianapolis, IN). Total cholesterol was assayed enzymatically. LDL cholesterol was determined by a homogenous direct method from Roche Diagnostics. HDL cholesterol was determined using a direct enzymatic colorimetric assay. Triglycerides were measured enzymatically with correction for endogenous glycerol. Coefficients of variation (CVs) for these lipids were <3%.
Samples for lipoprotein particle analysis by proton NMR spectroscopy were thawed, aliquoted (200 μl), refrozen, and shipped on dry ice to LipoScience (Raleigh, NC). Particle concentrations of lipoproteins of different sizes were calculated from the measured amplitudes of their spectroscopically distinct lipid methyl group NMR signals (7,14). Weighted-average lipoprotein particle sizes are derived from the sum of the diameter of each subclass multiplied by its relative mass percentage based on the amplitude of its methyl NMR signal. The NMR lipoprotein variables that we examined are those that are provided when ordering an NMR lipoprotein profile for clinical use (7). CVs ranged from 0.4 to 7.1%, except for IDLNMR (13.1%) and medium HDLNMR particle concentration (<30%). A1C was measured with turbidimetric immunoinhibition using packed erythrocytes (Roche Diagnostics). High-sensitive C-reactive protein (hsCRP) was measured using a high-sensitivity immunoturbidimetric assay on the Hitachi 917 analyzer (Roche Diagnostics), using reagents and calibrators from Denka Seiken.
Ascertainment of type 2 diabetes.
Incident clinical type 2 diabetes in WHS participants was ascertained by self-report on annual follow-up questionnaires through March 2008 as previously described (15,16). Confirmation of diabetes was conducted using American Diabetes Association diagnostic criteria (17). Self-reported cases were then further investigated either by telephone interview with a physician or by a previously validated self-administered supplemental questionnaire that inquired about symptoms, diagnostic diabetes testing, and use of diabetes medications. The response rate was high, with >90% response rate to either telephone interview or supplemental questionnaire by women who self-reported diabetes. Glucose screening rates in this population were similar to contemporaneous screening rates in the general population (18), with 68.2% of nondiabetic women having reported a screening fasting glucose performed in the prior 3 years.
Statistical analysis.
Statistical analyses were performed using STATA version 10.1 (STATA, College Station, TX). Statistical comparisons were obtained from Student t tests for continuous variables expressed as means, from Wilcoxon rank-sum tests for variables expressed as medians, and χ2 tests for categorical variables.
Following guidelines from the Department of Health and Human Services (19), lipids and lipoproteins were divided into quintiles based on the distribution among women not taking hormone replacement. Cox proportional hazard regression models were used to calculate the hazard ratios (HRs) and 95% CIs according to these quintiles. The proportional hazard assumption was tested using Schoenfeld residuals and the natural logarithm of follow-up time. Some of the variables did not satisfy the proportionality assumption; hence, we also divided the follow-up time into the first and second 6 years, finding no substantial differences within each 6-year period. Stronger associations were noted for NMR lipoproteins with diabetes during the first 6 years compared with the second 6 years of follow-up, but the relative magnitude of associations was generally similar within each 6-year period; therefore, we report the main results for the overall follow-up period unless otherwise specified.
We initially considered two levels of adjustment for 1) age, race, and randomized treatment assignment (minimally adjusted; model 1); and 2) covariates in model 1 plus smoking status, exercise, education, menopausal status, hormone use, blood pressure, BMI, family history of diabetes, A1C, and hsCRP (nonlipid risk factors; model 2). To determine the magnitude of association of NMR lipoproteins with diabetes independent of standard lipids, we additionally adjusted model 2 for triglycerides and HDL and LDL cholesterol and evaluated the association of NMR lipoproteins with diabetes using likelihood ratio χ2 tests. Since lipoprotein particles are metabolically interrelated (7,20), NMR lipoproteins were also analyzed in a single model that included the nine NMR lipoprotein subclasses (two LDLNMR, one IDLNMR, three HDLNMR, and three VLDLNMR lipoprotein subclasses) in addition to the nonlipid risk factors, in order to estimate the independent associations of these correlated lipoproteins with diabetes.
Based on prior work from this cohort suggesting that nonfasting concentrations of certain lipids may be superior to fasting concentrations for risk prediction (21,22), we examined whether fasting status modified the association of NMR lipoproteins with diabetes. Statistical tests for interaction between fasting status and lipoproteins in relation to diabetes were obtained using likelihood ratio tests.
We repeated the analyses after excluding 169 women with A1C ≥6.0 and <6.5%, with similar results. P value for linear trend was obtained using the quantile number as a predictor. All P values were two tailed.
RESULTS
During a median follow-up of 13.3 years (interquartile range 12.3–13.8), a total of 1,687 incident cases of clinical type 2 diabetes occurred. Table 1 shows the baseline characteristics of participants according to the development of diabetes during follow-up. In comparison with the small differences noted in LDL cholesterol between case subjects and noncase subjects, the NMR-measured concentration of total LDLNMR particles was much higher in case subjects. This resulted from case subjects having more small LDLNMR particles and IDLNMR particles but fewer large LDLNMR particles. Case subjects also had significantly less HDLNMR particles (total) due to having fewer large HDLNMR particles, despite having more small HDLNMR particles. VLDLNMR particles were higher in case subjects (both large and small). In accordance with these results, average particle size in case versus control subjects was smaller for LDLNMR and HDLNMR, and larger for VLDLNMR.
TABLE 1.
Baseline characteristics of participants according to incident type 2 diabetes
| No diabetes | Diabetes | P* | |
|---|---|---|---|
| n | 25,149 | 1,687 | |
| Age (years) | 54.6 ± 7.10 | 54.6 ± 6.55 | 0.96 |
| Current smoking (%) | 11.5 | 13.2 | 0.04 |
| Hypertension (%) | 22.4 | 47.0 | <0.001 |
| Postmenopausal status (%) | 54.0 | 55.6 | <0.001 |
| Postmenopausal hormone use (%) | 44.3 | 40.4 | 0.002 |
| Fasting (%) | 75.8 | 78.6 | 0.01 |
| BMI (kg/m2) | 25.4 ± 4.6 | 30.6 ± 5.9 | <0.001 |
| A1C (%) | 4.98 (4.83–5.15) | 5.28 (5.07–5.53) | <0.001 |
| Family history of diabetes (%) | 23.4 | 43.9 | <0.001 |
| hsCRP (mg/l) | 1.84 (0.74–3.98) | 4.42 (2.26–7.34) | <0.001 |
| Lipid concentrations (mg/dl) | |||
| Total cholesterol | 208 (184–235) | 213 (187–242) | <0.001 |
| LDL cholesterol | 121 (100–144) | 126 (104–152) | <0.001 |
| HDL cholesterol | 53 (44–63) | 42 (36–50) | <0.001 |
| Triglycerides | 115 (82–167) | 175 (126–247) | <0.001 |
| NMR lipoprotein particle concentrations | |||
| LDLNMR (nmol/l) | |||
| Total | 1,260 (1,024–1,570) | 1,587 (1,288–1,944) | <0.001 |
| Large | 551 (414–692) | 424 (268–589) | <0.001 |
| Small | 632 (382–972) | 1,075 (714–1,502) | <0.001 |
| IDLNMR | 32 (10–66) | 51 (22–93) | <0.001 |
| HDLNMR (μmol/l) | |||
| Total | 35.1 (31.2–39.5) | 34.2 (30.1–39.0) | <0.001 |
| Large | 7.8 (5.3–10.5) | 4.6 (3.0–6.8) | <0.001 |
| Medium | 2.7 (0.8–6.0) | 2.7 (0.8–5.8) | 0.68 |
| Small | 23.6 (19.9–27.2) | 25.5 (22.0–28.8) | <0.001 |
| VLDLNMR (nmol/l) | |||
| Total | 68.0 (48.9–90.1) | 73.8 (55.1–94.6) | <0.001 |
| Large | 1.3 (0.3–3.6) | 3.0 (1.4–5.5) | <0.001 |
| Medium | 20.8 (11.1–31.8) | 20.9 (11.9–32.6) | 0.13 |
| Small | 44.5 (32.2–57.8) | 48.2 (36.7–59.9) | <0.001 |
| NMR average particle size (nm) | |||
| LDLNMR size | 21.4 (20.9–21.9) | 20.7 (20.1–21.3) | <0.001 |
| HDLNMR size | 9.0 (8.7–9.4) | 8.6 (8.4–8.9) | <0.001 |
| VLDLNMR size | 46.3 (42.0–51.6) | 51.1 (46.6–56.7) | <0.001 |
Data are median (interquartile range) or means ± SD, unless otherwise indicated.
*P values were obtained from Student t test for continuous variables expressed as means, from Wilcoxon rank-sum tests for variables expressed as medians, and χ2 tests for categorical variables.
LDL measures.
HRs for diabetes according to quintiles of LDL cholesterol and LDLNMRparticle concentration and size are shown in Table 2. In fully adjusted models, neither total cholesterol (data not shown) nor LDL cholesterol was associated with diabetes (P for trend 0.53 and 0.64, respectively), but other LDL measures, such as LDLNMR particle concentration and size, were significantly associated with diabetes (P for trend <0.001).
TABLE 2.
Association of LDL measures with incident type 2 diabetes
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P for trend | |
|---|---|---|---|---|---|---|
| LDL cholesterol (mg/dl) | ≤97.3 | 97.4–115.2 | 115.3–131.9 | 132.0–153.7 | >153.7 | |
| Model 1 | Referent | 0.99 (0.85–1.16) | 1.14 (0.98–1.33) | 1.10 (0.94–1.29) | 1.57 (1.35–1.82) | <0.001 |
| Model 2 | Referent | 0.94 (0.80–1.11) | 0.94 (0.80–1.10) | 0.85 (0.72–1.00) | 1.08 (0.93–1.26) | 0.64 |
| LDLNMR particle concentrations | ||||||
| Total LDLNMR (nmol/l) | ≤957 | 958–1,155 | 1,156–1,373 | 1,374–1,680 | >1,680 | |
| Model 1 | Referent | 1.31 (1.02–1.68) | 2.29 (1.83–2.87) | 3.78 (3.05–4.67) | 6.49 (5.29–7.96) | <0.001 |
| Model 2 | Referent | 1.07 (0.83–1.38) | 1.48 (1.18–1.87) | 1.83 (1.47–2.28) | 2.53 (2.04–3.13) | <0.001 |
| Large LDLNMR (nmol/l) | ≤361 | 362–476 | 477–577 | 578–698 | >698 | |
| Model 1 | 4.25 (3.64–4.97) | 1.90 (1.59–2.26) | 1.55 (1.29–1.86) | 1.27 (1.05–1.53) | Referent | <0.001 |
| Model 2 | 2.50 (2.12–2.95) | 1.44 (1.20–1.73) | 1.37 (1.14–1.66) | 1.27 (1.04–1.54) | Referent | <0.001 |
| Small LDLNMR (nmol/l) | ≤346 | 347–553 | 554–774 | 775–1,134 | >1,134 | |
| Model 1 | Referent | 1.85 (1.42–2.40) | 3.00 (2.35–3.83) | 5.20 (4.13–6.55) | 10.19 (8.17–12.72) | <0.001 |
| Model 2 | Referent | 1.54 (1.18–2.02) | 2.09 (1.63–2.68) | 2.62 (2.06–3.32) | 4.04 (3.21–5.09) | <0.001 |
| IDLNMR (nmol/l) | ≤5 | 6–20 | 21–39 | 40–72 | >72 | |
| Model 1 | Referent | 1.27 (1.04–1.56) | 1.75 (1.45–2.12) | 2.22 (1.85–2.66) | 3.07 (2.58–3.65) | <0.001 |
| Model 2 | Referent | 1.16 (0.94–1.42) | 1.35 (1.11–1.65) | 1.40 (1.16–1.68) | 1.66 (1.39–1.99) | <0.001 |
| LDLNMR average size (nm) | ≤20.5 | 20.6–21.0 | 21.1–21.5 | 21.6–21.9 | >21.9 | |
| Model 1 | 9.99 (8.03–12.44) | 5.54 (4.41–6.96) | 3.07 (2.44–3.88) | 1.83 (1.41–2.38) | Referent | <0.001 |
| Model 2 | 4.16 (3.30–5.24) | 3.04 (2.40–3.86) | 2.21 (1.74–2.81) | 1.63 (1.25–2.13) | Referent | <0.001 |
Data are HR (95% CI) and (ranges minimum–maximum) and are given for each quintile. P for trend obtained from using median quantile as a dependent variable in Cox regression models. Model 1: adjusted for age, race, and randomized treatment assignment. Model 2: adjusted for model 1 variables plus smoking, exercise, education, menopausal status, hormone use, blood pressure, BMI, family history of diabetes, A1C, and hsCRP.
LDLNMR particles differed substantially in their association with diabetes according to their size. Large LDLNMR particles were inversely associated (adjusted HR 2.50 [95% CI 2.12–2.95]) for quintile 1 vs. 5, while small LDLNMR particles were positively associated with diabetes (4.04 [3.21–5.09]) for quintile 5 vs. 1. The concentration of IDLNMR particles, a subclass of LDL particles whose density and size are intermediate between small VLDL and large LDL, was positively associated with diabetes, similar in association to small VLDLNMR (shown in Table 4) but different from the inverse association of large LDLNMR. Total LDLNMR particle concentration (IDLNMR + large LDLNMR + small LDLNMR) was positively associated with diabetes, and the smaller the average LDLNMR particle size the higher the risk. Associations obtained from the minimally adjusted model 1 were generally stronger than model 2, which had further adjustment for other factors.
TABLE 4.
Association of VLDL measures with incident type 2 diabetes
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P for trend | |
|---|---|---|---|---|---|---|
| Triglycerides (mg/dl) | ≤70 | 71–95 | 96–125 | 126–178 | >178 | |
| Model 1 | Referent | 1.73 (1.30–2.31) | 2.47 (1.88–3.25) | 4.91 (3.81–6.33) | 8.88 (6.94–11.37) | <0.001 |
| Model 2 | Referent | 1.36 (1.01–1.83) | 1.57 (1.18–2.08) | 2.57 (1.98–3.35) | 3.71 (2.87–4.80) | <0.001 |
| VLDLNMR particle concentrations | ||||||
| Total VLDLNMR (nmol/l) | ≤45.6 | 45.7–61.8 | 61.9–77.2 | 77.3–96.8 | >96.8 | |
| Model 1 | Referent | 1.41 (1.19–1.67) | 1.62 (1.38–1.91) | 1.70 (1.44–2.00) | 1.81 (1.54–2.13) | <0.001 |
| Model 2 | Referent | 1.18 (0.99–1.41) | 1.16 (0.98–1.38) | 1.25 (1.05–1.48) | 1.26 (1.06–1.50) | 0.01 |
| Large VLDLNMR (nmol/l) | ≤0.1 | 0.2–0.5 | 0.6–1.8 | 1.9–3.8 | >3.8 | |
| Model 1 | Referent | 1.58 (1.16–2.14) | 3.57 (2.71–4.70) | 5.41 (4.12–7.09) | 6.66 (5.10–8.70) | <0.001 |
| Model 2 | Referent | 1.49 (1.09–2.05) | 2.54 (1.91–3.39) | 2.98 (2.24–3.96) | 3.11 (2.35–4.11) | <0.001 |
| Medium VLDLNMR (nmol/l) | ≤8.2 | 8.3–15.9 | 16.0–23.8 | 23.9–34.1 | >34.1 | |
| Model 1 | Referent | 1.11 (0.95–1.30) | 1.08 (0.92–1.27) | 1.07 (0.91–1.26) | 1.15 (0.98–1.35) | 0.19 |
| Model 2 | Referent | 1.03 (0.88–1.22) | 0.99 (0.84–1.18) | 0.89 (0.75–1.05) | 1.04 (0.88–1.23) | 0.73 |
| Small VLDLNMR (nmol/l) | ≤31.5 | 31.6–42.1 | 42.2–51.5 | 51.6–63.1 | >63.1 | |
| Model 1 | Referent | 1.50 (1.28–1.76) | 1.69 (1.44–1.98) | 1.79 (1.53–2.11) | 1.87 (1.59–2.20) | <0.001 |
| Model 2 | Referent | 1.13 (0.96–1.34) | 1.11 (0.94–1.31) | 1.22 (1.03–1.44) | 1.31 (1.10–1.55) | 0.001 |
| VLDLNMR average size (nm) | ≤40.6 | 40.7–43.8 | 43.9–47.3 | 47.4–52.0 | >52.0 | |
| Model 1 | Referent | 1.23 (0.96–1.58) | 1.96 (1.56–2.46) | 3.31 (2.68–4.09) | 4.93 (4.03–6.04) | <0.001 |
| Model 2 | Referent | 1.25 (0.97–1.62) | 1.67 (1.32–2.10) | 2.22 (1.78–2.76) | 2.80 (2.27–3.46) | <0.001 |
Data are adjusted HR (95% CI) and (ranges minimum–maximum) and are given for each quintile. See Table 2 legend for model adjustments.
HDL measures.
HDL cholesterol was inversely associated with diabetes (Table 3), with quintile 1 vs. 5 associated with fourfold increased risk. While total HDLNMR particle concentration was also inversely associated with diabetes (quintile 1 vs. 5, adjusted HR 1.20 [95% CI 1.03–1.40]), it was only large HDLNMR particles that were inversely associated, with quintile 1 (≤4 μmol/l) imparting 4.5-fold increased risk of diabetes compared with quintile 5. Interestingly, this inverse association noted for large HDLNMR particles was not noted for smaller HDLNMR particles. Instead, there was nearly twofold increased risk associated with the highest concentration of small HDLNMR particles. This was also reflected in HDLNMR average particle size, with smaller HDLNMR size having 4.5-fold higher risk of diabetes.
TABLE 3.
Association of HDL measures with incident type 2 diabetes
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P for trend | |
|---|---|---|---|---|---|---|
| HDL cholesterol (mg/dl) | ≤39.8 | 39.9–46.3 | 46.4–52.8 | 52.9–61.8 | >61.8 | |
| Model 1 | 9.52 (7.89–11.48) | 5.32 (4.37–6.48) | 2.94 (2.38–3.63) | 1.84 (1.47–2.30) | Referent | <0.001 |
| Model 2 | 4.01 (3.28–4.92) | 3.14 (2.56–3.86) | 2.05 (1.65–2.55) | 1.54 (1.23–1.93) | Referent | <0.001 |
| HDLNMR particle concentrations | ||||||
| Total HDLNMR (μmol/l) | ≤29.0 | 29.1–31.9 | 32.0–34.3 | 34.4–37.3 | >37.3 | |
| Model 1 | 1.60 (1.39–1.83) | 1.21 (1.04–1.40) | 1.07 (0.92–1.24) | 1.01 (0.88–1.17) | Referent | <0.001 |
| Model 2 | 1.20 (1.03–1.40) | 1.07 (0.91–1.25) | 0.91 (0.77–1.07) | 0.88 (0.75–1.02) | Referent | 0.008 |
| Large HDLNMR (μmol/l) | ≤4 | 4.1–5.8 | 5.9–7.7 | 7.8–10.0 | >10.0 | |
| Model 1 | 9.89 (8.20–11.93) | 5.36 (4.39–6.55) | 3.31 (2.69–4.08) | 1.84 (1.47–2.31) | Referent | <0.001 |
| Model 2 | 4.51 (3.68–5.52) | 3.19 (2.58–3.94) | 2.54 (2.04–3.15) | 1.72 (1.36–2.17) | Referent | <0.001 |
| Medium HDLNMR (μmol/l) | ≤0.2 | 0.3–1.4 | 1.5–3.0 | 3.1–5.6 | >5.6 | |
| Model 1 | Referent | 1.19 (1.01–1.41) | 1.23 (1.04–1.46) | 1.20 (1.01–1.41) | 1.11 (0.95–1.30) | 0.47 |
| Model 2 | Referent | 1.14 (0.96–1.35) | 1.01 (0.84–1.20) | 1.04 (0.87–1.23) | 1.03 (0.87–1.21) | 0.72 |
| Small HDLNMR (μmol/l) | ≤18.8 | 18.9–21.9 | 22.0–24.4 | 24.5–27.3 | >27.3 | |
| Model 1 | Referent | 1.46 (1.20–1.77) | 1.69 (1.40–2.04) | 2.21 (1.85–2.65) | 2.68 (2.25–3.18) | <0.001 |
| Model 2 | Referent | 1.19 (0.97–1.45) | 1.36 (1.12–1.65) | 1.51 (1.25–1.81) | 1.84 (1.54–2.19) | <0.001 |
| HDLNMR average size (nm) | ≤8.5 | 8.6–8.7 | 8.8–9.0 | 9.1–9.4 | >9.4 | |
| Model 1 | 12.01 (9.31–15.50) | 9.04 (6.95–11.75) | 4.78 (3.67–6.23) | 2.10 (1.59–2.79) | Referent | <0.001 |
| Model 2 | 4.56 (3.50–5.93) | 3.97 (3.03–5.21) | 3.08 (2.35–4.03) | 1.72 (1.29–2.29) | Referent | <0.001 |
Data are adjusted HR (95% CI) and (ranges minimum–maximum) and are given for each quintile. See Table 2 legend for model adjustments.
VLDL measures.
Higher concentrations of triglycerides and triglyceride-rich VLDL particles were associated with higher risk of diabetes (Table 4). Large VLDLNMR particles, which carry more triglycerides than smaller particles and correlate more with insulin resistance (9), had the strongest association of the VLDL particles with diabetes, with more than threefold increased risk for quintile 5 vs. 1. Small VLDLNMR also showed positive association with diabetes but less than large particles. Thus, larger average VLDLNMR size correlated with higher risk of diabetes, although not to the same extent as smaller LDLNMR or HDLNMR size, both of which had higher absolute and relative risk.
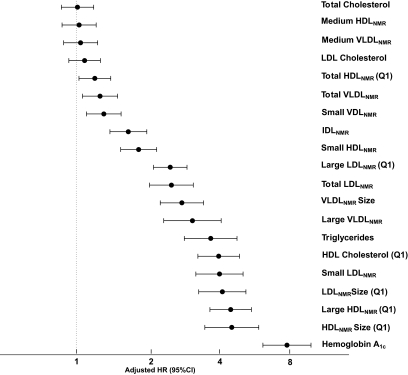
Figure 1 summarizes the adjusted HRs and 95% CIs for incident diabetes associated with extreme quintiles of the NMR lipoproteins, standard lipids, and A1C, ranked according to the magnitude of the HRs.
FIG. 1.
Adjusted HRs and 95% CIs for quintile 5 vs. 1, unless otherwise noted, adjusted for nonlipid risk factors (age, race, randomized treatment assignment, smoking, exercise, education, menopausal status, hormone use, blood pressure, BMI, family history of diabetes, A1C, and hsCRP). A1C results were adjusted for age, race, randomized treatment assignment, smoking, exercise, education, menopausal status, hormone use, blood pressure, BMI, family history of diabetes, hsCRP, and standard lipids.
Other analyses.
When we repeated the analyses using continuous variables instead of quintiles, similar results were obtained. Similar results were also found after additionally excluding 169 women with A1C ≥6.0%. A similar pattern of findings was noted when analyses were stratified by median follow-up time into the first and second 6 years. Overall, stronger associations were noted for A1C and NMR lipoproteins with diabetes during the first 6 years compared with the second 6 years of follow-up, but the relative magnitude of associations was generally similar and significant both early and late in follow-up. For example, the adjusted HRs (95% CIs) for extreme quintile values of LDLNMR, HDLNMR, and VLDLNMR size were 6.56 (3.92–10.99), 7.48 (4.05–13.83), and 2.87 (1.93–4.26), respectively, early in follow-up; and 3.74 (2.88–4.85), 4.12 (3.07–5.53), and 2.90 (2.26–3.73), respectively, late in follow-up.
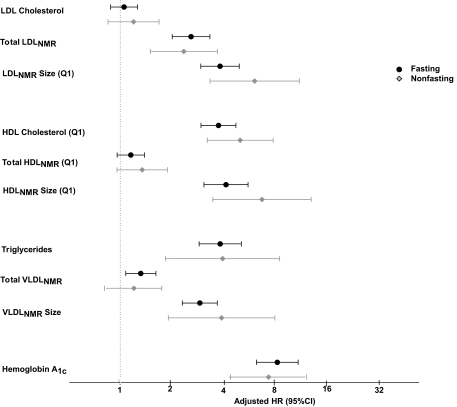
We also examined the effect of fasting status (Fig. 2), noting generally similar results for fasting and nonfasting lipoprotein measurements (all P for interaction with fasting status >0.05). However, there were several borderline significant interactions noted for fasting status with each of small LDLNMR particles, LDLNMR size, and HDLNMR size in relation to diabetes (P for interaction 0.08, 0.06, and 0.05, respectively). Moreover, while the P value for interaction was nonsignificant, nonfasting large VLDLNMR particles carried much higher risk for diabetes than fasting measurements.
FIG. 2.
HRs and 95% CIs were adjusted similar to Fig. 1 and stratified according to fasting (black circles) or nonfasting (gray diamonds) status.
When we evaluated all nine NMR-measured lipoprotein particle concentrations in one model that also adjusted for nonlipid risk factors, we found that large and small LDLNMR, large and small HDLNMR, and large VLDLNMR remained associated with diabetes. IDLNMR and small VLDLNMR particles were no longer significant (P = 0.38 and 0.62, respectively). Medium HDLNMR and medium VLDLNMR particles now showed inverse associations with diabetes.
Incremental value of NMR lipoproteins.
Since NMR lipoproteins are correlated with standard lipids, in particular HDL cholesterol and triglycerides, we performed Cox models that adjusted for triglycerides and HDL and LDL cholesterol in addition to the nonlipid (model 2) risk factors. Although the associations were attenuated, smaller particle size for LDLNMR and HDLNMR remained significant (quintile 1 vs. 5, HR 1.79, [95% CI 1.37–2.33] and 2.39 [1.75–3.28], respectively; P for trend <0.001 for both), as did larger VLDLNMR particle size (quintile 5 vs. 1, 2.04 [1.63–2.56]; P for trend <0.001). The change in the likelihood ratio χ2 tests was significant for adding either LDLNMR, HDLNMR, or VLDLNMR particle size to models that already included standard lipids and nonlipid risk factors (change in χ2 24.53, 48.56, and 59.51, respectively; P < 0.0001 for all three).
Finally, we identified 8,101 women (number of incident diabetes cases = 132) who had normal values of both triglycerides and HDL cholesterol using median values as cut points (triglycerides <117 mg/dl and HDL cholesterol >52 mg/dl). Compared with the rest of the cohort, these women were more likely to be hypertensive and hormone users. We then examined the association of small LDLNMR with incident diabetes in these women after adjusting for nonlipid risk factors (including hypertension and hormone use). Higher concentration of small LDLNMR particles was significantly associated (P for trend 0.003) with incident diabetes, despite that these women had normal levels of triglycerides and HDL cholesterol (fully adjusted HR for the top versus bottom quintile of small LDLNMR 3.95 [95% CI 1.63–9.55]).
DISCUSSION
Consistent with prior studies in individuals with insulin resistance, we found that in initially healthy women followed prospectively for incident clinical type 2 diabetes, both triglycerides and HDL cholesterol were independently associated with diabetes but not LDL or total cholesterol. Furthermore, NMR-measured size and concentrations of LDL, HDL, and VLDL particles were also associated with diabetes, independent of triglycerides, HDL cholesterol, and other factors. The associations of lipoprotein particles differed markedly by size. Smaller average size of LDLNMR and HDLNMR particles, as well as the concentration of small LDLNMR and HDLNMR particles, was associated with increased risk, while the concentration of large LDLNMR and HDLNMR particles carried lower risk. Large VLDLNMR particles carried higher risk than small particles. LDLNMR, HDLNMR, and VLDLNMR particle size remained associated with diabetes in models that already included standard lipids and nonlipid risk factors, adding incremental risk information beyond that obtained from established risk factors.
A uniting feature of these lipoprotein alterations and their association with type 2 diabetes may be a state of insulin resistance. The associations we found in this study in relation to the NMR-measured lipoproteins have been previously linked to insulin resistance as measured by the euglycemic clamp (9). Garvey et al. (9) demonstrated a progressive increase in insulin resistance associated with larger VLDLNMR size, smaller LDLNMR size, and smaller HDLNMR size, all of which are consistent with our findings in relation to predicting incident type 2 diabetes. In 830 subjects with insulin resistance followed in the Insulin Resistance Atherosclerosis Study over a 5-year period, NMR-measured larger VLDLNMR size and smaller HDLNMR particles were independently associated with increased risk of type 2 diabetes (14), while LDLNMR size and LDLNMR particles were not significant independent of other risk factors. Factor analysis revealed a single factor that correlated with insulin resistance accounted for nearly half the variance in these lipoprotein measures (10).
Our study, which was conducted in a large population of healthy women, found independent associations for incident diabetes with baseline LDLNMR size and concentration, with larger LDLNMR particles associated with lower risk and smaller LDLNMR particles associated with higher risk. Moreover, small LDLNMR imparted higher risk of diabetes even in women with normal triglyceride and HDL cholesterol levels. The inverse association of large LDLNMR particles with type 2 diabetes contrasts with the positive association noted previously in relation to incident CVD in this population of women (23).
Risk factors for type 2 diabetes may differ from those for CVD (24). For CVD risk, we reported that both small and large LDLNMR particles had similar increase in risk, which contrasts with the inverse association of large LDLNMR, and positive association of small LDLNMR, with risk of diabetes in the current study. For CVD events, NMR lipoprotein profiles in this cohort of women were comparable but not superior to standard lipids, as recently reported (23). This is in contrast to the current findings for type 2 diabetes, where NMR-measured lipoprotein classification by size provided additive and independent risk information to standard lipids and other risk factors.
For HDL particles, the inverse association of HDLNMR size with risk of type 2 diabetes was also noted previously in relation to risk of CVD in this population of women (23). Of the HDLNMR particles, only large particles were associated with lower risk of diabetes, to a magnitude similar to the association of HDL cholesterol with diabetes, while small particles carried higher risk. Furthermore, adjusting for HDL cholesterol and other risk factors attenuated the association, but larger HDLNMR size remained associated with more than twofold increased risk. Previous studies have found strong inverse relationships between insulin resistance and the large HDLNMR subclass as measured by NMR (9,10,14) or the corresponding HDL2 subclass as measured by ultracentrifugation (25).
For VLDL particles, large particles had a greater magnitude of association with diabetes compared with smaller particles, which we explain by large VLDL carrying more triglycerides than small VLDL and correlating more with the severity of insulin resistance (9). Hepatic overproduction of large VLDL particles is a key feature of the dyslipoproteinemia of insulin resistance and type 2 diabetes, with evidence for independent regulation of large and small VLDL particles (26).
In addition, our finding of similar lipoprotein associations with diabetes both early and late in follow-up suggests that these lipoprotein alternations may occur years before the onset of overt hyperglycemia and clinical diagnosis of diabetes, providing a potential opportunity for the early detection and prevention of type 2 diabetes and its complications.
This study has potential limitations. Several of the risk factors were assessed by self-report. Since our study is largely limited to Caucasian women, these data may not be generalizable to men or other patient groups. We studied an apparently healthy cohort at low overall risk for diabetes. While our study found incremental predictive information for NMR lipoproteins, further studies should be performed in the appropriate patient settings to determine whether a strategy using NMR lipoprotein testing is cost-effective for prevention of type 2 diabetes and related metabolic disorders. Undetected diabetes at study entry is unlikely to have biased our results, since we excluded women with baseline A1C levels ≥6.5% from our primary sample and found similar results when we excluded those with A1C ≥6.0%. In addition, similar results during the first 6 years of follow-up compared with the second 6 years.
We conclude that the size and concentration of NMR-measured LDL, HDL, and VLDL particles were associated with clinical type 2 diabetes, independent of other risk factors, particularly chemically measured HDL cholesterol and triglycerides. The associations of LDLNMR and HDLNMR particles with diabetes differed according to size, with larger particles carrying lower risk and smaller particles carrying higher risk. For VLDLNMR, large particles were associated with higher risk. LDLNMR, HDLNMR, and VLDLNMR particle size remained significant in models that already included standard lipids and risk factors, adding incremental risk information beyond that obtained from established risk factors for type 2 diabetes.
ACKNOWLEDGMENTS
The research for this article was supported by research grants to S.M. from the Sandra A. Daugherty Foundation, the American Heart Association (0670007N), and the National Heart, Lung, and Blood Institute (NHLBI) (K08 HL094375). A.P. is supported by a grant from NHLBI (K08 HL082740). The Women's Health Study is supported by grants HL-43851 and CA-47988 from NHLBI and the National Cancer Institute, and the Donald W. Reynolds Foundation, Leducq Foundation, and Doris Duke Charitable Foundation.
J.D.O. is employed by, is a stockholder of, and serves on the board of directors of LipoScience, a diagnostic laboratory company that performed the lipoprotein subclass analyses described in the manuscript. R.S.R. is a stockholder of LipoScience and serves as a member of its scientific advisory board. No other potential conflicts of interest relevant to this article were reported.
Parts of this manuscript were presented in abstract form at the 2008 American College of Cardiology 57th Annual Scientific Session, Chicago, Illinois, 29 March to 1 April 2008.
Footnotes
Clinical trial reg. no. NCT00000479, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Reaven GM. Banting lecture 1988: role of insulin resistance in human disease. Diabetes 1988;37:1595–1607 [DOI] [PubMed] [Google Scholar]
- 2.Santamarina-Fojo S, Haudenschild C, Amar M. The role of hepatic lipase in lipoprotein metabolism and atherosclerosis. Curr Opin Lipidol 1998;9:211–219 [DOI] [PubMed] [Google Scholar]
- 3.Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 4.Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res 2002;43:1363–1379 [DOI] [PubMed] [Google Scholar]
- 5.Otvos JD. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. Clin Lab 2002;48:171–180 [PubMed] [Google Scholar]
- 6.Kuller L, Arnold A, Tracy R, Otvos J, Burke G, Psaty B, Siscovick D, Freedman DS, Kronmal R. Nuclear magnetic resonance spectroscopy of lipoproteins and risk of coronary heart disease in the cardiovascular health study. Arterioscler Thromb Vasc Biol 2002;22:1175–1180 [DOI] [PubMed] [Google Scholar]
- 7.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med 2006;26:847–870 [DOI] [PubMed] [Google Scholar]
- 8.Otvos JD, Jeyarajah EJ, Cromwell WC. Measurement issues related to lipoprotein heterogeneity. Am J Cardiol 2002;90:22i–29i [DOI] [PubMed] [Google Scholar]
- 9.Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, Pugh K, Jenkins AJ, Klein RL, Liao Y. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes 2003;52:453–462 [DOI] [PubMed] [Google Scholar]
- 10.Goff DC, Jr, D'Agostino RB, Jr, Haffner SM, Otvos JD. Insulin resistance and adiposity influence lipoprotein size and subclass concentrations: results from the Insulin Resistance Atherosclerosis Study. Metabolism 2005;54:264–270 [DOI] [PubMed] [Google Scholar]
- 11.Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA 2005;294:47–55 [DOI] [PubMed] [Google Scholar]
- 12.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA 2005;294:56–65 [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005;352:1293–1304 [DOI] [PubMed] [Google Scholar]
- 14.Festa A, Williams K, Hanley AJ, Otvos JD, Goff DC, Wagenknecht LE, Haffner SM. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the Insulin Resistance Atherosclerosis Study. Circulation 2005;111:3465–3472 [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Lee IM, Song Y, Van Denburgh M, Cook NR, Manson JE, Buring JE. Vitamin E and risk of type 2 diabetes in the Women's Health Study randomized controlled trial. Diabetes 2006;55:2856–2862 [DOI] [PubMed] [Google Scholar]
- 16.Pradhan AD, Cook NR, Manson JE, Ridker PM, Buring JE. A randomized trial of low-dose aspirin in the prevention of clinical type 2 diabetes in women. Diabetes Care 2009;32:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 18.Ealovega MW, Tabaei BP, Brandle M, Burke R, Herman WH. Opportunistic screening for diabetes in routine clinical practice. Diabetes Care 2004;27:9–12 [DOI] [PubMed] [Google Scholar]
- 19.Hainline A, Karon J, Lippel K. Manual of Laboratory Operations: Lipid Research Clinics Program and Lipid and Lipoprotein Analysis Bethesda, MD, U.S. Dept of Health and Human Services, 1982 [Google Scholar]
- 20.Mora S, Szklo M, Otvos JD, Greenland P, Psaty BM, Goff DC, Jr, O'Leary DH, Saad MF, Tsai MY, Sharrett AR. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2007;192:211–217 [DOI] [PubMed] [Google Scholar]
- 21.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007;298:309–316 [DOI] [PubMed] [Google Scholar]
- 22.Mora S, Rifai N, Buring JE, Ridker PM. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation 2008;118:993–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation 2009;119:931–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson PWF, Meigs J. Risk of type 2 diabetes mellitus and coronary heart disease: a pivotal role for metabolic factors. Eur Heart J Supplements 2008;10:B11–B15 [Google Scholar]
- 25.Tilly-Kiesi M, Knudsen P, Groop L, Taskinen MR. Hyperinsulinemia and insulin resistance are associated with multiple abnormalities of lipoprotein subclasses in glucose-tolerant relatives of NIDDM patients: Botnia Study Group. J Lipid Res 1996;37:1569–1578 [PubMed] [Google Scholar]
- 26.Adiels M, Olofsson SO, Taskinen MR, Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol 2008;28:1225–1236 [DOI] [PubMed] [Google Scholar]