Abstract
OBJECTIVE
Type 1 diabetes is a chronic endocrine disorder in which enteroviruses, such as coxsackie B viruses and echoviruses, are possible environmental factors that can trigger or accelerate disease. The development or acceleration of type 1 diabetes depends on the balance between autoreactive effector T-cells and regulatory T-cells. This balance is particularly influenced by dendritic cells (DCs). The goal of this study was to investigate the interaction between enterovirus-infected human pancreatic islets and human DCs.
RESEARCH DESIGN AND METHODS
In vitro phagocytosis of human or porcine primary islets or Min6 mouse insuloma cells by DCs was investigated by flow cytometry and confocal analysis. Subsequent innate DC responses were monitored by quantitative PCR and Western blotting of interferon-stimulated genes (ISGs).
RESULTS
In this study, we show that both mock- and coxsackievirus B3 (CVB3)-infected human and porcine pancreatic islets were efficiently phagocytosed by human monocyte–derived DCs. Phagocytosis of CVB3-infected, but not mock-infected, human and porcine islets resulted in induction of ISGs in DCs, including the retinoic acid–inducible gene (RIG)-I–like helicases (RLHs), RIG-I, and melanoma differentiation–associated gene 5 (Mda5). Studies with murine Min6 insuloma cells, which were also efficiently phagocytosed, revealed that increased ISG expression in DCs upon encountering CVB-infected cells resulted in an antiviral state that protected DCs from subsequent enterovirus infection. The observed innate antiviral responses depended on RNA within the phagocytosed cells, required endosomal acidification, and were type I interferon dependent.
CONCLUSIONS
Human DCs can phagocytose enterovirus-infected pancreatic cells and subsequently induce innate antiviral responses, such as induction of RLHs. These responses may have important consequences for immune homeostasis in vivo and may play a role in the etiology of type 1 diabetes.
Type 1 diabetes, or insulin-dependent diabetes, is a chronic endocrine disorder characterized by the progressive loss of insulin-producing β-cells. In the majority of cases, type 1 diabetes is associated with an autoimmune reaction against β-cell constituents. Genetic predisposition is a major risk factor for the acquisition of type 1 diabetes, but the pairwise concordance between monozygotic twins is limited (<40%), which indicates that other, environmental, factors are involved (1). Other observations (e.g., a gradual rise in the incidence and a 10-fold difference in the occurrence of type 1 diabetes in various parts of Europe) also point to a significant contribution of the environment (1).
Enteroviruses of the human enterovirus B (HEV-B) species of the Picornaviridae, such as coxsackievirus B (CVB) and echovirus (EV), have long been associated with type 1 diabetes (2–4). These small, nonenveloped single-stranded RNA viruses are widespread. Infection usually remains limited to the gastrointestinal tract and causes mild disease or even remains asymptomatic; however, during severe infections it can spread to secondary target organs such as the pancreas, brain, and heart (5). CVB has been implicated in type 1 diabetes on the basis of 1) isolation of virus from patients with acute diabetes, 2) detection of viral RNA in blood at onset, 3) epidemiological surveys, and 4) prospective studies (rev. in 6,7).
Importantly, several recent studies reported detection of HEV-B in the pancreatic islets of type 1 diabetic patients at autopsy, providing evidence that these viruses are able to infect β-cells in vivo (8–10). Inflammation in pancreatic islets is common in type 1 diabetic patients, and several immune cells can be detected in the islets of type 1 diabetic patients. Among these are phagocytes, as well as different subsets of T-cells such as effector CD4+ and CD8+ T-cells and, in some cases, regulatory T-cells (Treg) (8,11,12). The development or acceleration of type 1 diabetes might depend on the balance between autoreactive effector T-cells and Treg (13)—a balance which is predominantly decided by dendritic cells (DCs).
The importance of antigen-presenting cells (APCs), such as macrophages and DCs, in the development of type 1 diabetes has been shown in vivo in mouse studies, where phagocytosis of CVB-infected islet cells was crucial for the development of autoimmune type 1 diabetes. Resident APCs were shown to engulf CVB-infected β-cells and subsequently stimulated antigen-specific T-cell proliferation and induced diabetes upon adoptive transfer (14). Furthermore, mouse studies have revealed that the influx of macrophages and DCs precedes that of effector T-cells in the islets and aberrantly expresses proinflammatory cytokines (15). In human pancreatic islets, DCs are present in low numbers under steady-state conditions; however, as β-cell destruction ensues leading to diabetes, there is an increase in DC infiltrate (16,17), strengthening the hypothesis that these cells likely play an important role in the progression of human type 1 diabetes. To our knowledge, no studies have been performed that examine the interaction between CVB-infected human islets and human DCs.
DCs are the professional APCs of the immune system and play a decisive role in initiating immune responses and maintaining self tolerance. They not only participate in innate immunity but also initiate and control adaptive immunity (18). DCs continuously sample their microenvironment; they phagocytose pathogens or pathogen-associated products such as immune complexes, yet apoptotic cells are also taken up (19). This results in the induction of immunity against invading pathogens and tolerance to self-antigens, respectively (20). Induction of an immune response occurs when DCs detect pathogen-associated danger signals or pathogen-associated molecular patterns (PAMPs) through specialized receptors known as pattern recognition receptors (PRRs). These PRRs include several receptors that sense the presence of viral RNA, such as Toll-like receptors (TLRs) 3, 7, and 8 and the retinoic acid–inducible gene (RIG)-I–like helicases (RLHs), RIG-I, and melanoma differentiation–associated gene 5 (Mda5) (21,22). Polymorphisms and mutations in PRR family members have been associated with disease, including autoimmune diseases such as type 1 diabetes (23–25). The interaction between PRRs and PAMPs facilitates DC maturation, after which the DCs migrate to draining lymph nodes where they present antigen to T-cells (18). During viral infection, the triggering of these receptors induces type I interferons (IFNs). The IFNs are crucial for a first innate line of defense against invading viruses, yet they also influence adaptive immunity by affecting DC cytokine production and maturation (26) and autoimmunity (27).
In this study, we set out to investigate the interaction between human DCs and CVB-infected islets and the immunological consequences that follow. We used monocyte-derived DCs from healthy blood donors and CVB-infected pancreatic islet preparations or CVB-infected Min6 cells, a murine insuloma cell line. We show that both human islets and Min6 cells are phagocytosed by human DCs. CVB-infected cells, but not mock-infected cells, induced DC activation as indicated by the expression of IFN-stimulated genes (ISGs), resulting in protection of the DCs from subsequent enteroviral infection. These innate DC responses depended on the recognition of RNA in the CVB-infected cells and additionally required IFNs produced by the DC itself, but did not depend on soluble factors secreted by the infected cells. These virus-induced alterations in DCs may have important consequences for immune homeostasis in vivo and may play a role in the etiology of type 1 diabetes.
RESEARCH DESIGN AND METHODS
Virus stocks and purification.
Reference strain EV9 Hill (EV9) was obtained from the National Institute for Public Health and the Environment (RIVM, Bilthoven, the Netherlands). CVB3 Nancy (CVB3) was provided by R. Kandolf (University of Tübingen, Tübingen, Germany). Production of virus stocks and virus titrations were performed on buffalo green monkey cells as previously described. Serial 10-fold dilutions were tested in 96-well microtiter plates, and 50% tissue culture infective doses were calculated as previously described (28).
Islet and Min6 cell culture.
Human and porcine pancreatic islets were isolated in Pittsburgh as previously described (29). Human islets were obtained from deceased anonymous donors procured by the Center for Organ Recovery and Education (CORE) (Pittsburgh, PA), and islets were isolated using a modification of the semiautomated method described by Ricordi (29,30).
Batches of islets used in this study were obtained from four adult human pancreata and three porcine donors, cultured for a minimum of 3–6 days in Connaught Medical Research Laboratories (CMRL)-1066 medium containing 10% FCS, 2 mmol/l l-glutamine, 100 units/ml penicillin, and 0.1 mg/ml streptomycin (complete CMRL) at 37°C in an atmosphere of 5% CO2. Islet viability was estimated by dual-fluorescence viability dyes (calcein-AM and propidium iodide; Invitrogen, Eugene, OR) and was higher than 80% in all batches. Glucose-stimulated insulin release was carried out by dynamic perifusion (31). After culture for 3–6 days, the islets were sent to Nijmegen as free-floating islets and cultured in complete CMRL in ultra-low attachment culture plates (Corning) at 37°C in 5% CO2. Islets were cultured in Nijmegen for a maximum of 2 days before the start of the experiments.
Min6 cells (32) were a gift from Dr. Merja Roivainen and Dr. Per Bendix Jeppesen and were cultured in Dulbecco's modified Eagle's medium (Gibco) supplemented with 15% FCS, ciproxin, and 50 μmol/l β-mercaptoethanol at 37°C in 5% CO2. Medium was refreshed every other day.
Infection of islets and Min6 cells.
Islets and Min6 cells were infected in a small volume of CMRL-1066 or Dulbecco's modified Eagle's medium, respectively, at indicated multiplicity of infection (MOI) for 1 h at 37°C. Subsequently, cells were washed and viral titers were determined at different time points as described above. In some experiments, supernatant from infected cultures was harvested at indicated time postinfection and cleared from cell debris by centrifugation before stimulation of other cells.
Stimulation of monocyte-derived DCs.
Monocyte-derived DCs were generated as described previously (28). Mature DCs were obtained by stimulating cells with poly(I:C) (20 μg/ml). Stimulation of DCs with supernatant from Min6 cells or from DC/Min6 co-cultures was performed using a 1:2 dilution of supernatant. To block the actions of type I IFNs, cells were stimulated in the presence or absence of neutralizing anti-human IFN antibodies (1:75, Iivari, Kaaleppi, or bovine serum; courtesy of Dr. Julkunen, National Public Health Institute, Helsinki, Finland) (33). For infection, stimulated or unstimulated DCs were harvested using cold PBS and infected in RPMI. After 60 min incubation at 37°C, cells were washed and viral titers were determined as described above.
Uptake of human pancreatic islets and Min6 cells.
Human and porcine pancreatic islets or Min6 cells were labeled using PKH26 (Sigma-Aldrich) according to the manufacturer's instructions and infected with CVB3 at an MOI of 10. DCs were labeled using carboxyfluorescein diacetate succinimidyl ester (CFSE) (Invitrogen) according to the manufacturer's instructions. PKH-labeled cells were added to CFSE-labeled DC cultures at a ratio of 1:1. Alternatively, labeled, infected Min6 cells were incubated for 48 h and subsequently harvested and resuspended in fresh medium at a density of 5 × 106 cells/ml prior to placing them at −20°C until further use. Freeze-thawed cell preparations were subsequently used in DC co-cultures at a 1:1 ratio and resulted in similar inductions of ISGs and innate responses compared with co-culture with viable cells (supplemental Fig. 1, available in the online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1071) (34). These cell preparations were also used for ribonuclease (RNase) treatment prior to DC stimulation. For that purpose, Min6 cell preparations were exposed to a mixture of RNase A, RNase VI, and RNase I (all Ambion) or an equal volume of PBS for a period of 30 min at 37°C prior to addition to DCs. Uptake of islets or Min6 cells by DCs was analyzed using flow cytometry and confocal microscopy. In some experiments, phagocytosis was inhibited using cytochalasin D (CytD) (2.5 μg/ml), or endosomal acidification was inhibited using chloroquine (CQ) (10 μmol/l). DCs were pretreated for 30 min with CytD or CQ, and subsequently stimuli were added. Both treatments had no effect on cell viability as assessed by trypan blue exclusion 8 h after stimulation.
Confocal microscopy.
Staining and visualization of DCs have been previously described (34). Human islets were adhered onto fibronectin-coated coverslips for 2 h at 37°C in islet medium and subsequently fixed in 2% paraformaldehyde. Cells were blocked in PBS containing 100 mmol/l glycine and 2% goat serum, permeabilized using TX-100, and stained with mouse anti-VP1 (Dakocytomation) and rabbit anti-3A (35) followed by incubation with goat anti-mouse IgG alexa 488 and goat anti-rabbit IgG alexa 594. Cells were analyzed using a Leica DMR microscope.
RNA isolation, quantitative PCR, Western blot, and flow cytometry.
These techniques have been previously described (34).
Statistical analysis.
Statistical analysis was performed using Student t test (two-tailed distribution). A P value <0.05 was considered a significant difference.
RESULTS
Human DCs phagocytose human pancreatic islets and induce innate immune responses.
Human pancreatic islets were found to be susceptible to infection by CVB3 as indicated by the profound increase in virus titer, cytopathic effects, and immunofluorescence staining against viral proteins 3A and VP1 (Fig. 1A–C). The latter revealed that only a small number of cells were infected. Infected cells mainly resided at the outer layers of the islets, where cells might be more accessible for the virus (Fig. 1C). Previously it was shown that such a productive infection results in impaired islet function (36).
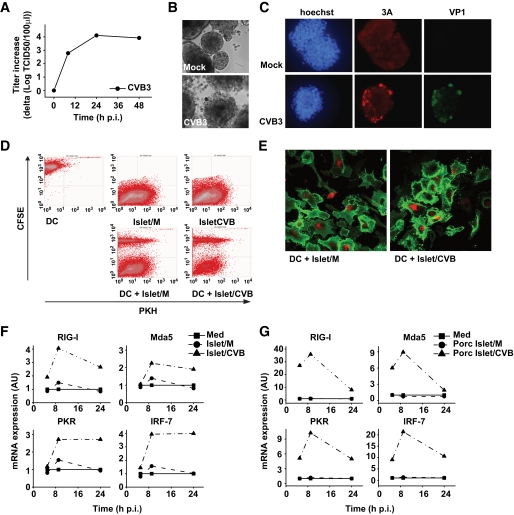
FIG. 1.
CVB replicates in human and porcine pancreatic islets, and DCs can phagocytose islets, resulting in induction of ISGs. A and B: Human islet cells were infected with CVB3 at an MOI of 10, and at indicated time points replication was analyzed (A) and at 48 h postinfection (p.i.) images were taken (B). C: Human islets were infected with CVB3 at an MOI of 10. After 24-h incubation, islets were adhered onto fibronectin-coated coverslips and stained using 3A (red)- and VP1 (green)-specific antibodies. Hoechst stain is included to visualize cell nuclei. D: Human islets were PKH labeled and infected with CVB3 at an MOI of 10 and cultured for 48 h before addition to CFSE-labeled DCs. Uptake of islets either mock-infected (M) or CVB-infected (CVB) was analyzed by flow cytometry 24 h after co-culture. E: Human islets were PKH labeled and infected as in D and co-cultured with unlabeled DCs for 24 h. Subsequently, DCs were harvested, stained using CD86-specific antibodies (green), and analyzed using confocal microscopy. F: Human islets were infected with CVB3 at an MOI of 10 for 48 h prior to addition to DCs. Expression of RIG-I, Mda5, and PKR mRNA in DCs was analyzed using qPCR. G: Porcine islets were prepared and co-cultured as in F and ISG-induction was analyzed at indicated times. In some experiments (F and G), freeze-thawed preparations of islets were used, but these yielded similar results compared with using viable cells. Experiments are representative of 2, 3, or more than 3 independent experiments. Islet/CVB, CVB-infected human islets; Islet/M, mock-infected human islets; Med, medium, i.e., unstimulated DCs; porc Islet/CVB, CVB-infected porcine islets; porc Islet/M, mock-infected porcine islets. (A high-quality color representation of this figure is available in the online issue.)
To investigate whether human DCs can phagocytose human pancreatic islets, PKH-labeled human islets were mock or CVB3 infected for 48 h and subsequently co-cultured with CFSE-labeled DCs. CVB3 has no direct effect on human DCs and is incapable of infecting DCs (28). Mock- and CVB3-infected islets were taken up with equal efficiency as indicated by the number of DCs that became PKH positive (Fig. 1D). Confocal analysis confirmed uptake of human pancreatic islets material by DCs. The DC plasma membrane was stained with CD86-specific antibodies (green), and PKH-positive islet cells (red) were observed within the DCs (Fig. 1E).
To examine whether and how DCs respond to virus-infected human islets, we studied activation of innate immune response pathways by measuring levels of ISGs, such as RIG-I, Mda5, protein kinase R (PKR), and IRF-7 following phagocytosis of infected cells. Quantitative PCR (qPCR) revealed induction of ISGs after engulfment of CVB3-infected islets; moreover, increased expression was observed for all ISGs tested (Fig. 1F). Importantly, stimulation of DCs with mock-infected human islets alone did not induce any ISGs (Fig. 1F). Furthermore, stimulation with CVB3 alone also did not induce ISGs (28), suggesting that the induction depends on the presence of virus or viral products within phagocytosed cells. CVB3 was not able to replicate in DCs upon entry via phagocytosis, as determined by end point titration at several times after the start of co-cultures (data not shown). In some experiments, freeze-thawed mock- or CVB-infected cells were used. This had no effect on either phagocytosis or ISG induction (see below).
At this stage, we were unable to discriminate whether the ISGs were upregulated in DCs or in the islets themselves. Therefore, we performed experiments using porcine islets, which have been shown as useful models for studies on the interaction of CVBs with β-cells (37). To discriminate between ISGs in porcine islets and human DCs, primers were used that specifically recognize human, but not porcine, ISG sequences. Infection and uptake of porcine islets by DC was similar as described above for the human islets (data not shown). Phagocytosis of CVB-infected porcine islets resulted in a profound increase in human ISGs (Fig. 1G). Thus, using porcine islets, we confirmed induction of ISGs in human DCs upon uptake of CVB3-infected human islets, and, additionally revealed that ISG expression is increased in the human DC population.
Phagocytosis of CVB3-infected Min6 cells by human DCs results in an antiviral state that protects DCs from subsequent enterovirus infection.
To further investigate the underlying mechanism of DC responses upon uptake of islets, we selected the murine Min6 cell line as a model for pancreatic β-cells. Min6 cells retain the physiological characteristics of normal β-cells and respond to glucose within the physiological range (32). These islet-like cells grow in patches (Fig. 2B, left panel) and were confirmed to express several β-cell–associated mRNAs (e.g., GAD65, islet-specific glucose-6-phosphatase catalytic subunit-related protein [IGRP], insulinoma-associated protein 2, insulin, [data not shown]). Analogous to human and porcine islets, Min6 cells were susceptible to infection with CVB3 as shown by the increase in virus titer (Fig. 2A). Cytopathic effects were observed in nearly 100% of the cells (Fig. 2B), suggesting that the majority of cells were infected. Uptake of mock- and CVB3-infected Min6 cells by DCs was very efficient (up to 75%) as shown by flow cytometry and confocal analysis (Fig. 2C and D).
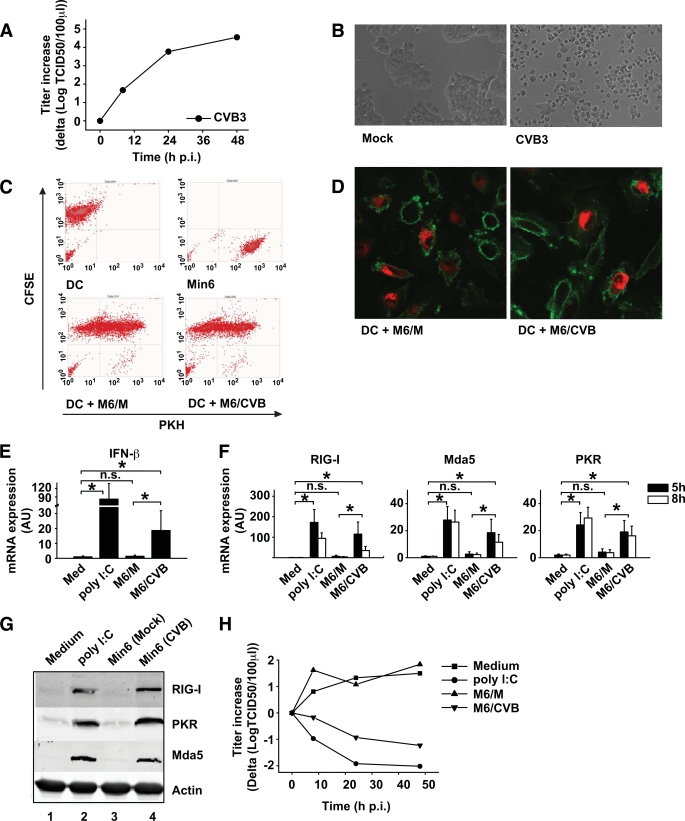
FIG. 2.
Min6 cells are a good model for primary pancreatic cells, are phagocytosed by DCs, and induce innate antiviral immune responses. A and B: Min6 cells were infected with CVB3 at an MOI of 10, and replication was analyzed at indicated time points (A) and images were taken at 48 h postinfection (p.i.) (B). C: Min6 cells were PKH-labeled and infected with CVB3 at an MOI of 10. After 24-h incubation, cells were harvested and added to CFSE-labeled DCs at a 1:1 ratio. After 24-h co-culture, cells were harvested and uptake was determined using flow cytometry. D: Min6 cells were prepared as in C and co-cultured with unlabeled DCs for 24 h, after which DCs were harvested, stained as in Fig. 1E, and analyzed using confocal microscopy. E and F: Min6 cells were prepared as in C, and after 48-h incubation, cells were harvested and added to DCs at a 1:1 ratio or DCs were stimulated with 20 μg/ml poly (I:C) or left untreated. At 5 h after addition (E), or 5 h and 8 h after addition (F), mRNA induction of ISGs was determined as described. G: Protein expression of RIG-I, Mda5, and PKR was analyzed by Western blot 24 h after stimulation of DCs as described for panel E. H: DCs were stimulated as in E, and after 24-h co-culture cells were harvested and infected with EV9 at an MOI of 1. At indicated times postinfection, EV9 replication was analyzed. Poly (I:C) was used as a positive control (34). In some experiments, freeze-thawed cell populations were used but these yielded similar results compared with using viable cells (supplemental Fig. 1). Data shown are representative (A–D, G, and H) or averages (E and F) of at least three independent experiments. Med, medium, i.e., unstimulated cells; M6/CVB, CVB-infected Min6 cells; M6/M, mock-infected Min6 cells; n.s., not significant. *P < 0.05. (A high-quality color representation of this figure is available in the online issue.)
Upon engulfment of CVB-infected Min6 cells, IFN-β and ISGs were strongly induced in DCs (Fig. 2E and F), reaching a greater than 100-fold increase compared with unstimulated DCs in the case of RIG-I, while Mda5 and PKR showed increases of up to 20- and 10-fold, respectively (Fig. 2F). The induction of ISGs following uptake of infected Min6 cells was confirmed at the protein level by Western blotting (Fig. 2G). No ISG signal was observed in mock- or CVB3-infected Min6 cells alone (data not shown), excluding that the observed protein induction in DC/Min6 co-cultures is due to detection of Min6 cell proteins. In some experiments, freeze-thawed mock- or CVB-infected cells were used. This had no effect on either phagocytosis or ISG induction (supplemental Fig. 1).
IFNs and ISGs induce an antiviral state that restricts virus replication (38). To determine whether upregulation of ISGs following uptake of CVB3-infected Min6 cells protects DCs from subsequent infection, DCs that had taken up Min6 cells were infected with EV9, an enterovirus strain that replicates in DCs (28) but not in Min6 cells (data not shown). Uptake of mock-infected cells had no influence on replication of EV9 in DCs. In contrast, phagocytosis of CVB-infected Min6 cells strongly protected the DCs from EV9 replication (Fig. 2H).
Innate responses in DCs that phagocytosed CVB3-infected Min6 cells depend on autocrine IFN-α/β signaling.
ISGs can be upregulated through both IFN-dependent and IFN-independent pathways (39,40). To investigate whether secreted cytokines such as IFN-α/β were involved in ISG induction, we stimulated fresh DCs with cell-free supernatant from DC/Min6 co-cultures. An increase in ISGs was observed upon stimulation of DCs with supernatant from co-cultures of DCs plus CVB-infected Min6 cells but not of DCs plus mock-infected Min6 cells (Fig. 3A). Moreover, the induction of ISGs after uptake of CVB-infected cells was markedly reduced in the presence of neutralizing IFN-α/β antibodies at both the mRNA (Fig. 3B) and protein levels (Fig. 3C [compare lanes 6 and 7]), implying that ISG induction strongly depends on IFNs.
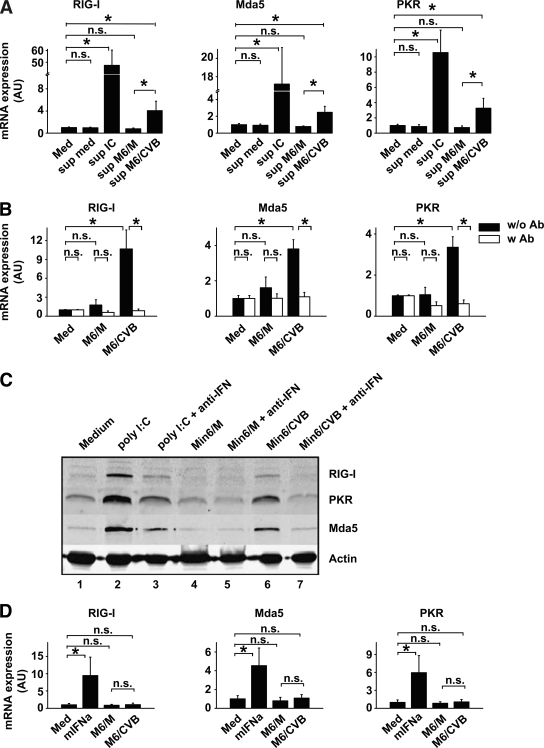
FIG. 3.
Type I IFNs produced by DCs themselves are required for ISG induction. A: DCs were stimulated with cleared supernatants from stimulated DC and DC/Min6 co-cultures (harvested 24 h after co-culture started and used at a 1:2 dilution) and mRNA induction of RIG-I, Mda5, and PKR were determined using qPCR 8 h after stimulation. B: Min6 cells were infected with CVB3 at an MOI of 10 and incubation cells were harvested and added to DCs at a 1:1 ratio after 48 h. Stimulations were performed in the absence or presence of neutralizing antibodies (Iivari, Kaaleppi, and bovine anti–IFN-α/β; see research design and methods). After 8 h, mRNA expression levels of RIG-I, Mda5, and PKR were determined using qPCR. C: DCs were treated as in B and protein expression of RIG-I, Mda5, and PKR was analyzed by Western blotting after 24 h. D: DCs were stimulated with 100 units/ml mIFNα or cleared supernatants from Min6 cells (harvested 48 h postinfection and used at a 1:2 dilution), and ISG mRNA induction was determined after 8 h. Data shown are representative of two (C) or average of three (A, B, and D) independent experiments. IC, poly (I:C); Med, medium, i.e., unstimulated cells; M6/CVB, CVB-infected Min6 cells; M6/M, mock-infected Min6 cells; mIFNa, murine recombinant IFN-α; n.s., not significant; Sup, supernatant; w/o Ab or w Ab, without or with neutralizing anti–IFN-α/β antibodies, respectively. *P < 0.05.
To determine whether IFNs produced by DCs or the infected Min6 cells were responsible for ISG upregulation, supernatants from infected Min6 cultures were added to DCs, after which ISG induction was monitored. Although recombinant murine IFN-α induced ISG expression in DCs, supernatant of either mock- or CVB-infected Min6 cells did not (Fig. 3D). Thus, cytokines potentially produced by infected Min6 cells are not responsible for ISG expression in DCs.
Collectively, these data imply that DCs induce an innate immune response upon uptake of CVB3-infected Min6 cells and that this induction depends on IFN induction by DCs themselves.
Induction of ISGs in DCs following uptake of CVB3-infected Min6 cells requires endosomal acidification and recognition of viral RNA.
Both PRRs located on the cell surface and those with intracellular localization may mediate the observed responses in DCs. To investigate whether phagocytosis of cells is required for the induction of ISGs or whether extracellular exposure to infected cells might initiate ISG responses in DCs (e.g., via TLRs on the plasma membrane), phagocytosis was inhibited using CytD. DCs pretreated with CytD and subsequently stimulated with CVB3-infected Min6 cells did not show any induction of ISGs, indicating that phagocytosis is required for ISG induction. Similar decreases in ISG induction were observed when using CVB-infected primary porcine islets (data not shown). Poly (I:C)-induced ISGs were also reduced upon CytD treatment, as has been previously reported (41). Importantly, lipopolysaccharide (LPS)-induced responses were not abrogated in the presence of CytD, proving that CytD has no adverse effects on DC viability or ISG induction (Fig. 4A) (42).
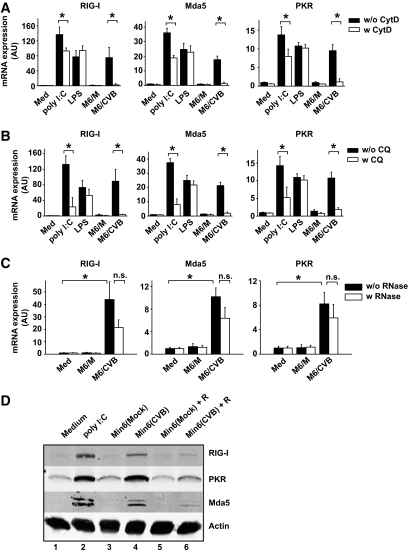
FIG. 4.
Induction of innate immune responses in DCs after Min6/CVB uptake requires (viral) RNA within Min6 cells. A: DCs were pretreated with CytD for 30 min prior to stimulation with TLR ligands or Min6 cells. After 8 h, mRNA expression levels of RIG-I, Mda5, and PKR were determined using qPCR. B: DCs were pretreated with CQ for 30 min, stimulated as in A, and mRNA expression was determined as in A. C: Min6 cell preparations were exposed to a mixture of RNase A, RNase VI, and RNase I prior to addition to DC cultures as described. Expression of RIG-I, Mda5, and PKR in DCs was analyzed using qPCR 5 h after addition of Min6-cell preparations. D: DCs were co-cultured with Min6-cell preparations as described for panel A, and protein expression of RIG-I, Mda5, and PKR was analyzed by Western blotting 24 h after the start of co-culture. Data are representative of two (D) or the average of two (A–C) independent experiments. In these experiments, freeze-thawed cell populations were used. IC, poly (I:C); Med, medium unstimulated cells; M6/CVB, CVB-infected Min6 cells; M6/M, mock-infected Min6 cells; n.s., not significant; w/o RNase or w RNase, without or with RNase treatment of Min6 cell preparations prior to co-culture. *P < 0.05.
Phagocytosed cells are localized to so-called phagosomes, which subsequently “mature” via fusion with the endosomal/lysosomal compartments, followed by progressive decrease in pH (20,43). Intracellular TLRs (TLR3, 7, 8, and 9) are also recruited to these compartments, enabling interaction with potentially released PAMPs such as dsRNA, which occurs in a pH-dependent fashion (44). Alternatively, phagocytosed material might be translocated into the cytoplasm, where, for example, the RLHs (RIG-I and Mda5) can interact with viral RNA. To determine whether endosomal acidification is required for the induction of ISGs following uptake of infected cell preparations, we pretreated DCs with CQ, a chemical that blocks acidification of these compartments. Pretreatment of DCs with CQ markedly decreased CVB-infected Min6-induced mRNA expression of RIG-I, Mda5, and PKR (Fig. 4B), suggesting that endosomal acidification is critical for DC responses upon phagocytosis of infected cells. Similar decreases in ISG induction were observed when using CVB-infected primary porcine islets (data not shown). Flowcytometric analysis showed that phagocytosis of Min6 cells was not inhibited due to CQ treatment (data not shown). Poly (I:C) induction, known to require endosomal acidification, was also decreased (41,44). LPS-induced responses do not require endosomal acidification (41,44). As expected, LPS-induced ISG and interleukin-6 expression was not decreased, indicating that viability and intracellular signaling to induce cytokines were unaltered upon CQ treatment (Fig. 4B and data not shown).
Using RNases, we investigated the contribution of (viral) RNA in our CVB-infected cells to DC responses. For this, freeze-thawed Min6 cell preparations were used because viable cells with an intact plasma membrane will make degradation of intracellular RNA impossible. RNase treatment of freeze-thawed Min6 cell preparations prior to addition to DCs reduced upregulation of RIG-I, Mda5, and PKR at both the mRNA and protein levels (Fig. 4C and D [compare lane 4 and lane 6]), demonstrating the important role of viral RNA present in infected cells for the induction of innate immunity. Together, our data show that phagocytosis of CVB-infected cells is required and that subsequent signaling requires endosomal acidification and depends on the presence of viral RNA.
DISCUSSION
DCs play a critical role in inducing immunity and preventing autoimmunity. Although diabetes pathogenesis and the possible role of APCs such as DCs therein have been investigated in mice (14,15), to our knowledge, no studies have been performed that examined the interaction between islets and DCs in humans. In this study, we show for the first time that CVB-infected human islets are efficiently phagocytosed by human DCs resulting in a rapid RNA- and IFN-dependent innate antiviral response by DCs.
The response of DCs was further characterized with use of porcine islets and murine Min6 cells. Mock-infected cells did not induce innate responses, even though, surprisingly, their phagocytosis was as efficient. The reason for equal uptake of mock- and CVB-infected cells is unknown; islets/β-cells may display enhanced molecular signals that mediate phagocytosis (“eat me” signals, such as phosphatidyl serines [PSs]) (45), possibly caused by endoplasmic reticulum stress inherent to massive insulin production (46). Preliminary data revealed that PSs are higher expressed on the outer cell surface of steady-state insulin–producing Min6 cells when compared with other steady-state cell lines that are not efficiently phagocytosed (e.g., HeLa, L929, BGM, and Vero) (data not shown). Whether PSs or other “eat me” signals are present on isolated primary human pancreatic islets and facilitate their engulfment requires further investigation. Nevertheless, equal uptake of mock- and CVB-infected cells enabled us to make a good comparison of DC responses because only the infection status of islets or Min6 cells or islets differed. Phagocytosis of CVB-infected islets and Min6 cells invariably resulted in an increase in ISGs. Importantly, this IFN-dependent ISG increase was sufficient to completely protect DCs from subsequent EV9 infection. This reveals a mechanism by which DCs may protect themselves when attracted to an environment with ongoing infection, ensuring their functional integrity.
Viral RNA is known to be an important inducer of antiviral immunity by triggering PRRs from the TLR and RLH family. In this study, we show that induction of innate responses in DCs requires endosomal acidification and is largely dependent on the presence of RNA within the infected cells. Some residual ISG induction is still observed after RNase treatment. Possibly part of the RNA might be shielded within intact virus particles and therefore may be inaccessible for RNases. This residual viral RNA may have triggered ISG induction when recognized in endosomes and/or lysosomes following phagocytosis. Although we favor the idea that viral RNA present in CVB-infected cells triggers antiviral immunity, we cannot exclude that viral proteins or modified host proteins contribute to the observed antiviral responses.
Interestingly, engulfment of CVB-infected cells resulted in the development of type 1 diabetes in a susceptible mouse model (14). In that study, resident APCs in the pancreas were shown to engulf CVB-infected β-cells, and these APCs were able to subsequently stimulate antigen-specific T-cell proliferation and trigger diabetes, demonstrating that the (infectious) microenvironment may drive innate, as well as adaptive, and autoimmune responses in vivo. We studied human DC maturation in vitro and our preliminary data on the induction of co-stimulatory molecules showed no consistent upregulation, even though ISG induction was consistently observed. In some donors, upregulation of CD80 and CD86 and production of tumor necrosis factor (TNF)-α and interleukin-12 were observed in DCs upon uptake of CVB3-infected Min6 cells; however, in the majority of DC donors, no increases were observed. The reason for these inconsistent outcomes is unknown and requires further investigation.
Obviously, during phagocytosis of infected cells by DCs in vivo, other cell populations are present that can interact with DCs. These cells, including macrophages, plasmacytoid DCs, and natural killer cells, and the cytokines they produce can greatly influence the microenvironment and DC responses. For instance, IFNs can, besides their function in innate immunity, also influence adaptive immunity. The amount of IFNs, timing of encountering IFNs, but also possible synergy with other PRR stimuli can greatly influence DC maturation (47,48). Moreover, other pancreas constituents, such as duct cells, may influence DC function. For example, duct cells produce TNF-α upon CVB3 infection (G. Vreugdenhil, F.J.M.v.K., J.M.D.G., and D. Pipeleers, unpublished observations), and duct cell–derived TNF-α has been shown to influence DC maturation (49). Further in-depth investigation of adaptive immune responses in DCs from healthy control subjects upon encountering CVB-infected islets and the interplay with other cell types and cytokines would be extremely valuable.
Most human infections with enteroviruses are efficiently controlled due to adequate antiviral immune responses. Prolonged or successive enterovirus infections have been suggested to play a role in the development of type 1 diabetes (50,51), raising the possibility that individuals susceptible to type 1 diabetes may have impaired antiviral defenses. The genetic background of susceptible individuals greatly influences disease development. For example, polymorphisms in Mda5, the RNA sensor that recognizes picornavirus RNA (52), have been associated with type 1 diabetes development (25,53). Alterations in Mda5 function may affect adequate sensing of viral infections and thus hamper antiviral immunity. CVB infection in a genetically susceptible individual might therefore progress to chronic inflammation in the pancreas. In this proinflammatory environment where self-antigens and viral antigens are encountered by DCs, autoimmunity might develop or accelerate, ultimately resulting in type 1 diabetes.
In conclusion, this study shows that CVB-infected human islets, porcine islets, and Min6 cells are phagocytosed by human DCs and that this results in an RNA- and IFN-dependent antiviral state in DCs. These events may alter the programming of DCs and thus influence the development of Treg and/or effector T-cell populations. These novel findings provide important new insights into the possible role of DCs during human type 1 diabetes development.
ACKNOWLEDGMENTS
This work was supported by Grants DFN 2001.00.047 from the Netherlands Diabetes Foundation (to J.M.D.G. and G.J.A.); NWO-VIDI-917.46.305 from The Netherlands Organization for Scientific Research (to F.J.M.v.K.) and NOW-912-02-034 (to G.J.A.); 2005-8 from the Radboud University Medical Centre Nijmegen (to F.J.M.v.K. and J.M.D.G.); and 24-2008-949 from the Juvenile Diabetes Research Foundation (to G.J.A. and F.J.M.v.K.). No potential conflicts of interest relevant to this article were reported.
We thank Dr. Merja Roivainen and Dr. Per Bendix Jeppesen for providing Min6 cells. Furthermore, we thank Dr. Paul Fisher and Dr. Iekka Julkunen for providing us with anti-Mda5 and anti–type I IFN antibodies, respectively.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying commentary, p. 1126.
REFERENCES
- 1.Knip M, Veijola R, Virtanen SM, Hyoty H, Vaarala O, Akerblom HK. Environmental triggers and determinants of type 1 diabetes. Diabetes 2005;54(Suppl. 2):S125–S136 [DOI] [PubMed] [Google Scholar]
- 2.Gamble DR, Kinsley ML, FitzGerald MG, Bolton R, Taylor KW. Viral antibodies in diabetes mellitus. Br Med J 1969;3:627–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gamble DR, Taylor KW, Cumming H. Coxsackie viruses and diabetes mellitus. Br Med J 1973;4:260–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon JW, Austin M, Onodera T, Notkins AL. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med 1979;300:1173–1179 [DOI] [PubMed] [Google Scholar]
- 5.Whitton JL. Immunopathology during coxsackievirus infection. Springer Semin Immunopathol 2002;24:201–213 [DOI] [PubMed] [Google Scholar]
- 6.Varela-Calvino R, Peakman M. Enteroviruses and type 1 diabetes. Diabete Metab Res Rev 2003;19:431–441 [DOI] [PubMed] [Google Scholar]
- 7.Hyöty H, Taylor KW. The role of viruses in human diabetes. Diabetologia 2002;45:1353–1361 [DOI] [PubMed] [Google Scholar]
- 8.Dotta F, Censini S, van Halteren AG, Marselli L, Masini M, Dionisi S, Mosca F, Boggi U, Muda AO, Prato SD, Elliott JF, Covacci A, Rappuoli R, Roep BO, Marchetti P. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci U S A 2007;104:5115–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ylipaasto P, Klingel K, Lindberg AM, Otonkoski T, Kandolf R, Hovi T, Roivainen M. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia 2004;47:225–239 [DOI] [PubMed] [Google Scholar]
- 10.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia 2009;52:1143–1151 [DOI] [PubMed] [Google Scholar]
- 11.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol 2009;155:173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velthuis JH, Unger WW, van der Slik AR, Duinkerken G, Engelse M, Schaapherder AF, Ringers J, van Kooten C, de Koning EJ, Roep BO. Accumulation of autoreactive effector T cells and allo-specific regulatory T cells in the pancreas allograft of a type 1 diabetic recipient. Diabetologia 2009;52:494–503 [DOI] [PubMed] [Google Scholar]
- 13.Arif S, Tree TI, Astill TP, Tremble JM, Bishop AJ, Dayan CM, Roep BO, Peakman M. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest 2004;113:451–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horwitz MS, Ilic A, Fine C, Balasa B, Sarvetnick N. Coxsackieviral-mediated diabetes: induction requires antigen-presenting cells and is accompanied by phagocytosis of beta cells. Clin Immunol 2004;110:134–144 [DOI] [PubMed] [Google Scholar]
- 15.Dahlén E, Dawe K, Ohlsson L, Hedlund G. Dendritic cells and macrophages are the first and major producers of TNF-alpha in pancreatic islets in the nonobese diabetic mouse. J Immunol 1998;160:3585–3593 [PubMed] [Google Scholar]
- 16.Lu W, Pipeleers DG, Klöppel G, Bouwens L. Comparative immunocytochemical study of MHC class II expression in human donor pancreas and isolated islets. Virchows Arch 1996;429:205–211 [DOI] [PubMed] [Google Scholar]
- 17.Uno S, Imagawa A, Okita K, Sayama K, Moriwaki M, Iwahashi H, Yamagata K, Tamura S, Matsuzawa Y, Hanafusa T, Miyagawa J, Shimomura I. Macrophages and dendritic cells infiltrating islets with or without beta cells produce tumour necrosis factor-alpha in patients with recent-onset type 1 diabetes. Diabetologia 2007;50:596–601 [DOI] [PubMed] [Google Scholar]
- 18.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998;392:245–252 [DOI] [PubMed] [Google Scholar]
- 19.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol 2000;18:767–811 [DOI] [PubMed] [Google Scholar]
- 20.Blander JM, Medzhitov R. On regulation of phagosome maturation and antigen presentation. Nat Immunol 2006;7:1029–1035 [DOI] [PubMed] [Google Scholar]
- 21.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol 2006;7:131–137 [DOI] [PubMed] [Google Scholar]
- 22.Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity 2007;27:370–383 [DOI] [PubMed] [Google Scholar]
- 23.Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, Picard C, Chapgier A, Plancoulaine S, Titeux M, Cognet C, von Bernuth H, Ku CL, Casrouge A, Zhang XX, Barreiro L, Leonard J, Hamilton C, Lebon P, Héron B, Vallée L, Quintana-Murci L, Hovnanian A, Rozenberg F, Vivier E, Geissmann F, Tardieu M, Abel L, Casanova JL. TLR3 deficiency in patients with herpes simplex encephalitis. Science 2007;317:1522–1527 [DOI] [PubMed] [Google Scholar]
- 24.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nuñez G, Cho JH. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 2001;411:603–606 [DOI] [PubMed] [Google Scholar]
- 25.Smyth DJ, Cooper JD, Bailey R, Field S, Burren O, Smink LJ, Guja C, Ionescu-Tirgoviste C, Widmer B, Dunger DB, Savage DA, Walker NM, Clayton DG, Todd JA. A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat Genet 2006;38:617–619 [DOI] [PubMed] [Google Scholar]
- 26.Fitzgerald-Bocarsly P, Feng D. The role of type I interferon production by dendritic cells in host defense. Biochimie 2007;89:843–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang KS, Recher M, Junt T, Navarini AA, Harris NL, Freigang S, Odermatt B, Conrad C, Ittner LM, Bauer S, Luther SA, Uematsu S, Akira S, Hengartner H, Zinkernagel RM. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat Med 2005;11:138–145 [DOI] [PubMed] [Google Scholar]
- 28.Kramer M, Schulte BM, Toonen LW, de Bruijni MA, Galama JM, Adema GJ, van Kuppeveld FJ. Echovirus infection causes rapid loss-of-function and cell death in human dendritic cells. Cell Microbiol 2007;9:1507–1518 [DOI] [PubMed] [Google Scholar]
- 29.Bottino R, Balamurugan AN, Tse H, Thirunavukkarasu C, Ge X, Profozich J, Milton M, Ziegenfuss A, Trucco M, Piganelli JD. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes 2004;53:2559–2568 [DOI] [PubMed] [Google Scholar]
- 30.Balamurugan AN, Chang Y, Fung JJ, Trucco M, Bottino R. Flexible management of enzymatic digestion improves human islet isolation outcome from sub-optimal donor pancreata. Am J Transplant 2003;3:1135–1142 [DOI] [PubMed] [Google Scholar]
- 31.Balamurugan AN, Chang Y, Bertera S, Sands A, Shankar V, Trucco M, Bottino R. Suitability of human juvenile pancreatic islets for clinical use. Diabetologia 2006;49:1845–1854 [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y, Oka Y, Yamamura K. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology 1990;127:126–132 [DOI] [PubMed] [Google Scholar]
- 33.Mogensen KE, Pyhala L, Cantell K. Raising antibodies to human leukocyte interferon. Acta Pathol Microbiol Scand [B] 1975;83:443–450 [DOI] [PubMed] [Google Scholar]
- 34.Kramer M, Schulte BM, Toonen LW, Barral PM, Fisher PB, Lanke KH, Galama JM, van Kuppeveld FJ, Adema GJ. Phagocytosis of picornavirus-infected cells induces an RNA-dependent antiviral state in human dendritic cells. J Virol 2008;82:2930–2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wessels E, Duijsings D, Niu TK, Neumann S, Oorschot VM, de Lange F, Lanke KH, Klumperman J, Henke A, Jackson CL, Melchers WJ, van Kuppeveld FJ. A viral protein that blocks Arf1-mediated COP-I assembly by inhibiting the guanine nucleotide exchange factor GBF1. Dev Cell 2006;11:191–201 [DOI] [PubMed] [Google Scholar]
- 36.Roivainen M, Ylipaasto P, Savolainen C, Galama J, Hovi T, Otonkoski T. Functional impairment and killing of human beta cells by enteroviruses: the capacity is shared by a wide range of serotypes, but the extent is a characteristic of individual virus strains. Diabetologia 2002;45:693–702 [DOI] [PubMed] [Google Scholar]
- 37.Roivainen M, Ylipaasto P, Ustinov J, Hovi T, Otonkoski T. Screening enteroviruses for beta-cell tropism using foetal porcine beta-cells. J Gen Virol 2001;82:1909–1916 [DOI] [PubMed] [Google Scholar]
- 38.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev 2001;14:778–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yount JS, Moran TM, López CB. Cytokine-independent upregulation of MDA5 in viral infection. J Virol 2007;81:7316–7319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Domizio J, Blum A, Gallagher-Gambarelli M, Molens JP, Chaperot L, Plumas J. TLR7 stimulation in human plasmacytoid dendritic cells leads to the induction of early IFN-inducible genes in the absence of type I IFN. Blood 2009;114:1794–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Itoh K, Watanabe A, Funami K, Seya T, Matsumoto M. The clathrin-mediated endocytic pathway participates in dsRNA-induced IFN-beta production. J Immunol 2008;181:5522–5529 [DOI] [PubMed] [Google Scholar]
- 42.Pietrella D, Corbucci C, Perito S, Bistoni G, Vecchiarelli A. Mannoproteins from Cryptococcus neoformans promote dendritic cell maturation and activation. Infect Immun 2005;73:820–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramachandra L, Simmons D, Harding CV. MHC molecules and microbial antigen processing in phagosomes. Curr Opin Immunol 2009;21:98–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Bouteiller O, Merck E, Hasan UA, Hubac S, Benguigui B, Trinchieri G, Bates EE, Caux C. Recognition of double-stranded RNA by human toll-like receptor 3 and downstream receptor signaling requires multimerization and an acidic pH. J Biol Chem 2005;280:38133–38145 [DOI] [PubMed] [Google Scholar]
- 45.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol 2007;7:964–974 [DOI] [PubMed] [Google Scholar]
- 46.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 2008;29:42–61 [DOI] [PubMed] [Google Scholar]
- 47.Nagai T, Devergne O, Mueller TF, Perkins DL, van Seventer JM, van Seventer GA. Timing of IFN-beta exposure during human dendritic cell maturation and naive Th cell stimulation has contrasting effects on Th1 subset generation: a role for IFN-beta-mediated regulation of IL-12 family cytokines and IL-18 in naive Th cell differentiation. J Immunol 2003;171:5233–5243 [DOI] [PubMed] [Google Scholar]
- 48.Walker J, Tough DF. Modification of TLR-induced activation of human dendritic cells by type I IFN: synergistic interaction with TLR4 but not TLR3 agonists. Eur J Immunol 2006;36:1827–1836 [DOI] [PubMed] [Google Scholar]
- 49.Movahedi B, Van de Casteele M, Caluwé N, Stangé G, Breckpot K, Thielemans K, Vreugdenhil G, Mathieu C, Pipeleers D. Human pancreatic duct cells can produce tumour necrosis factor-alpha that damages neighbouring beta cells and activates dendritic cells. Diabetologia 2004;47:998–1008 [DOI] [PubMed] [Google Scholar]
- 50.Oikarinen M, Tauriainen S, Honkanen T, Oikarinen S, Vuori K, Kaukinen K, Rantala I, Mäki M, Hyöty H. Detection of enteroviruses in the intestine of type 1 diabetic patients. Clin Exp Immunol 2008;151:71–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salminen KK, Vuorinen T, Oikarinen S, Helminen M, Simell S, Knip M, Ilonen J, Simell O, Hyöty H. Isolation of enterovirus strains from children with preclinical type 1 diabetes. Diabet Med 2004;21:156–164 [DOI] [PubMed] [Google Scholar]
- 52.Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, Flavell RA, Diamond MS, Colonna M. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci U S A 2006;103:8459–8464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu S, Wang H, Jin Y, Podolsky R, Reddy MV, Pedersen J, Bode B, Reed J, Steed D, Anderson S, Yang P, Muir A, Steed L, Hopkins D, Huang Y, Purohit S, Wang CY, Steck AK, Montemari A, Eisenbarth G, Rewers M, She JX. IFIH1 polymorphisms are significantly associated with type 1 diabetes and IFIH1 gene expression in peripheral blood mononuclear cells. Hum Mol Genet 2009;18:358–365 [DOI] [PMC free article] [PubMed] [Google Scholar]