Abstract
OBJECTIVE
Although interleukin-1 receptor antagonist (IL-1Ra) treatment is associated with improved β-cell function and glycemic control in patients with type 2 diabetes, its role in the development of type 2 diabetes remains unclear. We used repeated measurements to characterize IL-1Ra trajectories in individuals who developed type 2 diabetes.
RESEARCH DESIGN AND METHODS
This case-cohort study, nested within the Whitehall II cohort, was based on 335 incident type 2 diabetes cases and 2,475 noncases. We measured serum IL-1Ra levels at up to three time points per individual and estimated retrospective trajectories of IL-1Ra before diabetes diagnosis (case subjects) or end of follow-up (control subjects) using multilevel analysis. Models were adjusted for age, sex, and ethnicity.
RESULTS
IL-1Ra levels were already higher in the case than control subjects 13 years before diabetes diagnosis/end of follow-up (mean [95% CI] 302 [290–314] vs. 244 [238–249] pg/ml). In control subjects, IL-1Ra levels showed a modest linear increase throughout the study period. In case subjects, IL-1Ra trajectories were parallel to those in control subjects until 6 years (95% CI 7.5–4.5) before diagnosis and then rose steeply to 399 (379–420) pg/ml at the time of diagnosis (P < 0.0001 for slope difference). Adjustment for BMI and waist circumference as time-varying covariates had little impact on these trajectories.
CONCLUSIONS
We show elevated IL-1Ra levels for 13 years and an accelerated increase during the last 6 years before type 2 diabetes diagnosis, indicating the presence of an anti-inflammatory response that may act to counterbalance the metabolic and immunologic disturbances that precede type 2 diabetes.
Type 2 diabetes is characterized by insulin resistance and impaired insulin secretion, the latter being due to a reduction in β-cell mass and an increase in β-cell apoptosis (1). Prospective cohort studies have shown elevated circulating levels of acute-phase proteins, cytokines, and chemokines to predict incident type 2 diabetes (2–6). Interleukin-1β (IL-1β) may be one of the most important immune mediators in this context because this cytokine triggers various proinflammatory events, inhibits β-cell function, and promotes β-cell apoptosis (7,8). The deleterious effects of IL-1β can be inhibited by its naturally occurring antagonist, IL-1 receptor antagonist (IL-1Ra), produced by adipose and other tissues (9). IL-1Ra competitively binds to the IL-1 receptor without inducing a cellular response (7,10,11).
Animal models and evidence from humans support the association between IL-1Ra and diabetes. In mice, IL-1Ra injections have been demonstrated to protect from diabetes induced by high-fat diet (12). A double-blind, parallel-group trial of 70 patients with type 2 diabetes showed that the administration of exogenous IL-1Ra (anakinra) improved glycemia, β-cell function, and surrogate markers of systemic inflammation (13,14).
A nested case-control study within the Whitehall II study found that high levels of IL-1Ra were associated with an increased risk of incident type 2 diabetes. This finding suggests that increased IL-1Ra levels may reflect a reaction to counterregulate immunologic and metabolic disturbances before the onset of type 2 diabetes—a reaction that eventually fails (15).
Recent advances in analysis of longitudinal risk factor trajectories support a multistage model of diabetes development. Although the levels of homeostasis model assessment insulin sensitivity (HOMA-2S) and HOMA β-cell function (HOMA-2B) differed between incident cases and noncases more than a decade before the diagnosis of type 2 diabetes, a substantial divergence in HOMA trajectories, accompanied by a marked acceleration in the dysregulation of glucose metabolism, became evident during the last 5 years before diagnosis (16). Extending the risk factor trajectory approach to inflammatory biomarkers could improve our understanding of the pathogenesis of type 2 diabetes by providing insight into inflammatory changes that may be causally related to disease onset (17).
In this report from the Whitehall II study, we examine the trajectories of serum IL-1Ra in individuals who develop type 2 diabetes and those who remained diabetes-free. We hypothesize that an accelerated increase in circulating IL-1Ra levels would precede diabetes development by ∼5 years before the diagnosis, coinciding with the previously described changes in insulin sensitivity and β-cell function (16). Changes in IL-1Ra would indicate an anti-inflammatory reaction to counterregulate the proinflammatory environment and preserve insulin sensitivity and β-cell function.
RESEARCH DESIGN AND METHODS
Study population.
Data are from a nested case-cohort study within the prospective Whitehall II cohort study of 10,308 British civil servants aged 35–55 years at phase 1 (1985–1988). Details regarding study design, baseline characteristics, and source population for the case-cohort study have been described previously (18,19). The study was approved by the University College London Medical School Committee on the Ethics of Human Research and conducted according to the Declaration of Helsinki. Written informed consent was obtained at baseline and renewed at each contact.
Phase 3 (1991–1994), when a 75-g oral glucose tolerance test (OGTT) was administered for the first time, served as baseline for the current study. Participants were followed through postal questionnaires at 2.5-year intervals (phase 4: 1995–1996, phase 6: 2001, phase 8: 2006–2007) and through clinical examinations including an OGTT at phase 5 (1997–1999) and phase 7 (2003–2004). For the case-cohort study, we drew a random sample from the source population of 6,058 men and 2,758 women who had attended the phase 3 examination. We excluded participants with prevalent type 2 diabetes at phase 3 (n = 42), missing follow-up data on diabetes (n = 552), or missing data for key variables (C-reactive protein [CRP; limited to subjects with CRP <10 mg/l], weight, waist circumference, cholesterol, triglycerides, fasting glucose, fasting insulin) at baseline (n = 2,018) or during follow-up (phases 5 and 7; n = 3,049), leading to a case cohort of 2,810 subjects: 335 subjects with incident type 2 diabetes and 2,475 subjects without incident type 2 diabetes.
Measurements.
Diabetes was defined by a fasting glucose of 7.0 mmol/l or more, or a 2-h postload glucose of 11.1 mmol/l or more (20). Type 2 diabetes was diagnosed by OGTT (56.4%), self-report (13.1%), or the use of glucose-lowering medication (30.4%).
We measured blood glucose with the glucose oxidase method on a YSI model 23A glucose analyzer (phase 3: mean coefficient of variation [CV] 2.9–3.3%) and YSI model 2300 STAT PLUS analyzer (phases 5 and 7: mean CV 1.4–3.1%) (YSI, Yellow Springs, OH) (16). Serum insulin was determined with an in-house human insulin radioimmunoassay (phase 3: mean CV 7%) and a DAKO insulin ELISA kit (Dako Cytomation, Ely, U.K.) (phases 5 and 7: mean CV 4.2–9.3%) (16).
IL-1Ra serum concentrations were measured with the Quantikine ELISA kit (R&D Systems, Wiesbaden, Germany). Although serum samples were from three different study phases, blood collection, processing, and storage at −80°C followed the same standard operating procedures. All assays were performed consecutively in the same laboratory (German Diabetes Center), and samples from different study phases of the same study participant were always measured using the same enzyme-linked immunosorbent assay (ELISA) plate to minimize assay imprecision. Mean intra-assay and interassay CVs were 2.6 and 7.9%, respectively. The limit of detection was 14 pg/ml. All samples gave values above the limit of detection.
The following variables were included as time-invariant covariates: sex, age at the end of follow-up, and ethnicity (0 = white, 1 = nonwhite). Data for these were derived from phase 3 and phase 1 questionnaires. Given that adipose tissue is a major producer of IL-1Ra (9), BMI and waist circumference (assessed in medical examinations contemporaneously with IL-1Ra measurements) were included as additional covariates and were coded as time-varying covariates.
Statistical analysis.
Statistical analyses were performed using SPSS 14.0 statistical software (SPSS, Chicago, IL). The participants were divided into case subjects (i.e., individuals who developed type 2 diabetes during the follow-up) and control subjects (i.e., people who remained diabetes-free). Initial analyses compared the characteristics of case and control subjects and tested the differences using t tests and χ2 tests. The unadjusted associations between IL-1Ra and potential confounding variables were examined and tested using ANOVA for stratified measures and Spearman rank correlation together with unstandardized regression coefficients (95% CIs) of log2(IL-1Ra) for the continuous variables. For subsequent analyses, year 0 of the observation period was the year of diabetes diagnosis for case subjects and a randomly selected time point during follow-up for control subjects to approximate the follow-up time distribution of case subjects. IL-1Ra levels were traced backwards to participants' first participation in clinical screening (i.e., phase 3, which represents the baseline of the current analysis—a maximum of 13 years previously). Multilevel models were fitted to the data to assess changes in IL-1Ra during these preceding 13 years (21). Of a total of 8,233 serum measurements (964 in case subjects; 7,269 in control subjects), 2,383 measurements after year 0 were excluded and thus the analyses were based on 755 serum samples in case subjects and 5,095 serum samples in control subjects. Data were structured so that measurement times (observations) were nested within subjects, and the standard errors were calculated by taking into account the nonindependence of the observations, that is, that the same individuals contributed to more than one observation in the dataset. We treated observation time as one period (a nonpiecewise approach) for control subjects and as two distinct periods (a piecewise approach) for case subjects. In the latter approach, we created two time variables: a continuous variable, scaled to take the value zero at the start of the second period, and a dummy variable indicating the period (0 = first period and 1 = second period). We first established the most parsimonious model for each piecewise model and chose the position of the start of the second period (from −9 to 0) that had the lowest information criteria for the final model. We estimated the likelihood-based 95% CI for the position of the start of the second period. The selected model was in close agreement with locally weighted scatter plot smoothers displaying the association graphically (data not shown). All analyses were adjusted for age, sex, ethnicity, and study phase. Additional models included BMI, waist circumference, or insulin as time-varying covariates. To provide figures adjusted for baseline characteristics, trajectories were fitted for a hypothetical population of 72.0% male and 91.2% white at age 62.9 years at the end of follow-up. Statistical significance was inferred at a two-tailed P < 0.05.
RESULTS
Study population.
The comparison of the study participants (n = 2,810) and excluded subjects (n = 6,006) at baseline showed the study participants to be, on average, 1.4 years younger than those excluded, but otherwise revealed only small differences between the groups (supplementary Table 1, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1199/DC1). Characteristics of case and control subjects at baseline (phase 3) are shown in Table 1. Case subjects (n = 335) were older and more overweight than control subjects (n = 2,475). Case subjects also had higher blood pressure, fasting and 2-h blood glucose, and fasting insulin and were more likely to be nonwhite. Mean follow-up time (± SD) was 8.8 ± 3.4 years.
TABLE 1.
Characteristics of incident diabetes case and control subjects at baseline (phase 3)
| Control subjects | Case subjects | P | |
|---|---|---|---|
| n | 2,475 | 335 | |
| Age (years) | 49.1 ± 5.8 | 51.0 ± 6.1 | <0.0001 |
| BMI (kg/m2) | 25.0 ± 3.3 | 27.1 ± 4.2 | <0.0001 |
| Waist circumference (cm) | 83.5 ± 10.8 | 89.2 ± 12.2 | <0.0001 |
| Systolic blood pressure (mmHg) | 119.5 ± 12.7 | 123.8 ± 14.3 | <0.0001 |
| Diastolic blood pressure (mmHg) | 79.3 ± 9.0 | 82.2 ± 9.6 | <0.0001 |
| Fasting blood glucose (mmol/l) | 5.2 ± 0.4 | 5.5 ± 0.5 | <0.0001 |
| 2-h blood glucose (mmol/l) | 5.3 ± 1.4 | 6.7 ± 1.9 | <0.0001 |
| Fasting insulin (pmol/l) | 36.2 ± 28.8 | 61.5 ± 46.4 | <0.0001 |
| Sex, male/female (%) | 73.3/26.7 | 69.9/30.1 | 0.191 |
| Ethnicity, white/nonwhite (%) | 92.9/7.1 | 80.6/19.4 | <0.0001 |
Data are means ± SD or %.
Determinants of serum concentrations of IL-1Ra (univariate analyses).
Unadjusted baseline levels of IL-1Ra were higher in case than control subjects (Table 2). In addition, IL-1Ra levels were higher in women than men. No differences in IL-1Ra were found among ethnic groups. In univariate analyses, IL-1Ra was positively correlated with age, BMI, waist circumference, blood pressure, 2-h glucose, and fasting insulin (Table 3).
TABLE 2.
IL-1Ra levels in subgroups of the study population at baseline (phase 3)
| Stratification variable | IL-1Ra (pg/ml) | P |
|---|---|---|
| Diabetes incidence | ||
| Cases | 308 (293−323) | |
| Noncases | 248 (244−252) | <0.0001 |
| Sex | ||
| Male | 246 (242−251) | |
| Female | 278 (269−287) | <0.0001 |
| Ethnicity | ||
| White | 255 (251−259) | |
| Nonwhite | 253 (238−268) | 0.78 |
Data are means (95% CI).
TABLE 3.
Nonparametric correlations and parametric regression coefficients for the association of anthropometric and metabolic factors with IL-1Ra (log2) at baseline (phase 3)
| Spearman r | P | β (95% CI) | P | |
|---|---|---|---|---|
| Age (years) | 0.066 | 0.0005 | 0.007 (0.003−0.011) | 0.0004 |
| BMI (kg/m2) | 0.370 | <0.0001 | 0.070 (0.065−0.076) | <0.0001 |
| Waist circumference (cm) | 0.278 | <0.0001 | 0.016 (0.014−0.018) | <0.0001 |
| Systolic blood pressure (mmHg) | 0.075 | 0.0001 | 0.003 (0.001−0.005) | 0.0005 |
| Diastolic blood pressure (mmHg) | 0.104 | <0.0001 | 0.007 (0.004−0.009) | <0.0001 |
| Fasting blood glucose (mmol/l) | 0.035 | 0.063 | 0.079 (0.030−0.128) | 0.0016 |
| 2-h blood glucose (mmol/l) | 0.210 | <0.0001 | 0.086 (0.071−0.100) | <0.0001 |
| Fasting insulin (mmol/l) | 0.283 | <0.0001 | 0.005 (0.005−0.006) | <0.0001 |
Trajectories of IL-1Ra in case and control subjects.
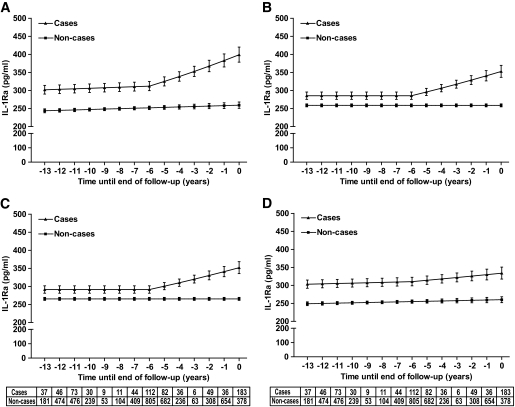
Multilevel models adjusted for age, sex, and ethnicity showed that serum IL-1Ra levels were already higher in case than control subjects 13 years before diagnosis or end of follow-up. Mean IL-1Ra levels (95% CI) were 302 (290–314) pg/ml in case subjects and 244 (238–249) pg/ml in noncase subjects (Fig. 1A).
FIG. 1.
A–D: Model-predicted IL-1Ra trajectories in nondiabetic and incident diabetic subjects. Year 0 denotes the time of diagnosis for case subjects and a randomly selected time point during follow-up for control subjects (noncases). All models were adjusted for age, sex, and ethnicity. Additional adjustment was performed for BMI (B), waist circumference (C), and fasting insulin (D). The table below the panels shows the number of measurements at each time point for both case and control subjects. Error bars represent 95% CIs around the values estimated by the fixed effect part of the mixed model presented in Table 4.
From years −13 to −6 (95% CI −4.5 to −7.5), the trajectories of IL-1Ra of case and control subjects were parallel and increased marginally (<1.5 pg/ml per year) over time. An interaction effect among caseness, time period, and time indicated that the IL-1Ra trajectories of case and control subjects began to separate after year −6 (Fig. 1A). From years −6 to 0, IL-1Ra levels in case subjects increased steeply (13–16 pg/ml per year) and reached 399 (379–420) pg/ml at the time of diagnosis, whereas the slope of the IL-1Ra trajectory in the control subjects remained unaltered (P < 0.0001 for the slope difference during the final 6 years; model 1 in Table 4).
TABLE 4.
Fixed effects for multilevel models of changes over time in log2(IL-1Ra) serum concentrations before diagnosis of type 2 diabetes or the end of follow-up
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Time (per year) | 0.004 (0.002)* | NS | NS | 0.005 (0.002)* |
| Case (incident type 2 diabetes) | 0.31 (0.03)† | 0.14 (0.03)† | 0.13 (0.03)† | 0.28 (0.029)† |
| Case × time | NS | NS | NS | NS |
| Case × time × 2nd period | 0.052 (0.006)† | 0.051 (0.006)† | 0.045 (0.006)† | 0.013 (0.006)* |
| BMI (per kg/m2) | — | 0.065 (0.002)† | — | — |
| Waist circumference (per cm) | — | — | 0.025 (0.001)† | — |
Data are regression coefficients (SE). Time = continuous variable scaled so that time = 0 at 6 years before diagnosis or the end of follow-up. Case = incident type 2 diabetes case. 2nd period = dummy variable (1 for positive values in the time variable, i.e., later than 6 years before diagnosis or the end of follow-up; 0 for nonpositive values). Trajectories in 335 case subjects with incident type 2 diabetes were compared with those in 2,475 control subjects. Log2(IL-1Ra) was the outcome variable of the multilevel longitudinal modeling, and data were adjusted for age, sex, ethnicity, and study phase. Only models with the lowest information criteria are shown.
*P < 0.05;
†P < 0.0001.
Additional adjustment for BMI or waist circumference as time-varying covariates reduced, but did not remove, the differences between case and control subjects (Fig. 1B and C). From years −13 until −6, IL-1Ra levels remained constant in both case and control subjects after adjustment. From years −6 until 0, IL-1Ra levels in case subjects increased steeply (∼11–12 pg/ml per year in BMI-adjusted and 9–11 in waist-adjusted models), whereas IL-1Ra levels in control subjects remained unaltered (P < 0.001 for the slope difference during the final 6 years; models 2–3 in Table 4). Adjustment for fasting insulin had hardly any impact on the difference between case and control subjects from years −13 to −6, but substantially reduced the increase of IL-1Ra from years −6 to 0 in case subjects (∼4 pg/ml per year; P < 0.05 for the slope difference during the final 6 years; model 4 in Table 4, Fig. 1D).
Estimated trajectories for fasting glucose (supplementary Fig. 1A), 2-h glucose (supplementary Fig. 1B), HOMA insulin sensitivity (supplementary Fig. 1C), and HOMA β-cell function (supplementary Fig. 1D) are provided for direct comparisons with the IL-1Ra trajectories. Apart from an earlier start of the compensatory increase in β-cell function in the current analysis (year −5 vs. −4), the shapes of the trajectories are almost identical to those observed in the larger Whitehall II cohort (16).
DISCUSSION
This case-cohort study of a middle-aged metabolically healthy population at baseline has three main findings. First, circulating concentrations of IL-1Ra were elevated in cases of incident type 2 diabetes 13 years in advance of diagnosis compared with individuals who remained diabetes-free. Second, in control subjects, longitudinal changes could be described by a linear trajectory with only a slight increase over time, whereas in case subjects, IL-1Ra increased rapidly starting 6 (95% CI 4.5–7.5) years before diagnosis. Third, changes in obesity explained the slight linear increase in control subjects throughout the observation period and in case subjects up to 6 years before diabetes diagnosis, but did not account for the steep increase in the IL-1Ra trajectory among case subjects in the last 6 years.
Our findings extend current knowledge on the association between inflammation and type 2 diabetes development, as this is the first study to characterize cytokine trajectories before type 2 diabetes. Our previous report on IL-1Ra and incident diabetes in a nested case-control study was limited to a single IL-1Ra measurement at study baseline (phase 3) (15) and therefore did not provide information on the time course of IL-1Ra levels preceding diabetes diagnosis.
Our findings are also novel from a pathophysiological perspective because they support the hypothesis that the pre-diabetic stage is characterized not only by proinflammatory alterations, but also by the presence of an anti-inflammatory response. Several mechanisms could explain the upregulation of IL-1Ra before the diagnosis of type 2 diabetes. The steep increase of IL-1Ra in pre-diabetic individuals occurs within the same time window in which indicators of insulin sensitivity, β-cell function, and glycemia deteriorate (supplementary Fig. 1). This may indicate that these unfavorable changes in glucose metabolism and the increase of IL-1Ra production are closely connected. In vitro studies show that both human islets and monocytes respond to high glucose concentrations with an upregulation of IL-1β (22–24), so that increased IL-1Ra concentrations before type 2 diabetes may represent a response to glucose-mediated IL-1β upregulation. The balance between IL-1β and IL-1Ra has been postulated to be a major determinant of the time course and severity of inflammatory diseases (25,26), and it is conceivable that the local and/or systemic ratio of these cytokines could also be relevant in the pathogenesis of type 2 diabetes. We could not test this hypothesis here because the physiological concentrations of IL-1β in individuals without severe inflammatory diseases are so low that they are mostly undetectable with currently available assays (23,27). However, it has been suggested that elevation of only IL-1β may not be sufficient to increase risk of type 2 diabetes; instead, increased IL-1β in combination with elevated levels of other proinflammatory cytokines may be required (28), thus limiting the predictive relevance of the IL-1Ra/IL-1β ratio without consideration of other risk factors.
The finding that the difference in trajectories between case and control subjects could not be explained by obesity is important because adipose tissue is a major producer of IL-1Ra (9,29). We replicated strong correlations of IL-1Ra levels with BMI and waist circumference (30–33). However, inclusion of BMI or waist circumference as time-varying covariates had no major effect on the shape of IL-1Ra trajectories, suggesting that the upregulation of IL-1Ra cannot directly be attributed to weight gain. Differences in secretion of leptin, an adipokine strongly upregulated in obesity that can stimulate IL-1β production and lead to increased IL-1Ra release, may be one of the mechanisms involved (34,35).
We did not adjust for glycemia, because our stratifying variable (incident diabetes caseness) already includes fasting and/or postload glucose in its definition, which means that we have already adjusted for glucose values at time 0. Because fasting insulin is not included in the diagnostic criteria, we performed an analysis adjusted for fasting insulin, which substantially attenuated the slope difference between case and control subjects preceding the diagnosis of diabetes. However, a significant acceleration of the slope from years −6 to 0 remains among case subjects, indicating that fasting insulin explains or mediates some, but not all, of the late increase in IL-1Ra. It should be kept in mind that with our study design, we cannot directly establish whether this attenuation is due to confounding, mediation, or shared causation.
Our findings point to the possibility that shape of biomarker trajectories is informative in terms of assessing their predictive value for different time windows. Sequential measurements of cytokines and other biomarkers and the characterization of individual trajectories may improve the estimation of type 2 diabetes risk. Differences in IL-1Ra levels between case and control subjects varied considerably over the lead time such that IL-1Ra may be more useful in predicting short-term diabetes risk, whereas other cytokines may be more strongly associated with long-term risk of type 2 diabetes. To date, attempts to improve diabetes risk prediction in the general population based on biomarker panels measured at a single time point have produced only marginal improvements compared with conventional risk models. It remains to be determined whether it will be possible to improve risk prediction at different pre-diabetic stages in the general population by considering serial biomarker measurements over time.
From a therapeutic point of view, it is noteworthy that recombinant IL-1Ra has been shown to improve metabolic control in patients with type 2 diabetes (13,14). In this light, an upregulation of IL-1Ra may be expected to be protective rather than associated with increased risk. In our study, it appears that the steep increase of IL-1Ra levels by approximately one-third was by far not sufficient to prevent the onset of type 2 diabetes. In the anakinra trial, an improvement of glycemia and β-cell function was accompanied by supraphysiological IL-1Ra peak levels in serum that were >1,000-fold higher in the intervention group than in the placebo group (13).
Our study has some limitations and strengths that should be acknowledged. First, the study design does not enable us to exclude the alternative interpretation that the increase of IL-1Ra before type 2 diabetes contributes to the disease risk and represents a causal factor rather than an anti-inflammatory response. However, data from the anakinra trial (13) as well as preclinical data (12,36) argue strongly against diabetogenic effects of IL-1Ra. Second, we did not determine IL-1β levels and therefore cannot know whether IL-1β increases before type 2 diabetes and whether such an increase precedes the increase in IL-1Ra. In addition, we did not have data from all three study phases for other proinflammatory markers, such as CRP and IL-6, for a comparison of trajectories. We did not adjust for the available CRP or IL-6 levels at baseline, because this adjustment has the capacity to influence only the intercept of the model, not the shape of the trajectories, and therefore would not help to answer the question of temporal sequences. Third, the Whitehall II study is an occupational cohort and as such is not population based. Although the cohort was healthy at recruitment, as in a community-based sample, the cohort overrepresents white, male, and middle-aged individuals. Fourth, due to missing data, approximately two-thirds of the source population at phase 3 was excluded from the present analysis. However, more than 300 incident cases and a total of almost 6,000 measurement points for the trajectories led to sufficient statistical power to detect differences between case and control subjects. In addition, our dropout analysis (supplementary Table 1) indicates that exclusions are unlikely to have affected internal validity.
The study has notable strengths. It is based on a well-phenotyped cohort, and more than half of incident diabetes cases were diagnosed based on the gold standard oral glucose tolerance test. We applied a sophisticated methodology that considered the interrelation among repeated measurements from the same individual at different time points during the follow-up and based our analysis on a large sample, as noted above.
In conclusion, we characterized cytokine trajectories to understand the evolution of the association between IL-1Ra levels in the circulation and type 2 diabetes before the diagnosis of diabetes. IL-1Ra levels showed an accelerated increase during the last 6 years preceding diagnosis. We showed that crude measures of adiposity did not explain the IL-1Ra trajectory, potentially implicating other factors in disease susceptibility. These data support the hypothesis that an anti-inflammatory response counterbalances metabolic and immunologic disturbances preceding type 2 diabetes. Moreover, these results suggest that multiple measurements of inflammation-related and other biomarkers can markedly improve our understanding of the pathogenesis of type 2 diabetes. The clear temporal characterization shown by our data may help define the optimal clinical use of these biomarkers.
ACKNOWLEDGMENTS
The Whitehall II study was funded by the Medical Research Council, the Economic and Social Research Council, the British Heart Foundation, the Health and Safety Executive, and the Department of Health (all U.K.); the National Heart Lung and Blood Institute (HL-36310), the National Institutes of Health, the National Institute on Aging (AG-13196), the Agency for Health Care Policy Research (HS-06516), and the John and Catherine MacArthur Foundation. This case-cohort study was funded by a Medical Research Council New Investigator Grant (G0501184), the Federal Ministry of Health (Berlin, Germany), and the Ministry of Innovation, Science, Research, and Technology of the state North Rhine-Westphalia (Düsseldorf, Germany). M.K. was supported by the BUPA Foundation Specialist Research Grant, U.K., and the Academy of Finland. M.J.S. was supported by a grant from the British Heart Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
C.H. has received a consulting honorarium from XOMA (U.S.). D.R.W. holds shares in Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
We thank Ulrike Poschen and Karin Röhrig (German Diabetes Center) and Enas El-Safa (Whitehall study) for their excellent technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-cell deficient and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 2.Kolb H, Mandrup-Poulsen T. An immune origin of type 2 diabetes? Diabetologia 2005;48:1038–1050 [DOI] [PubMed] [Google Scholar]
- 3.Herder C, Baumert J, Thorand B, Koenig W, de Jager W, Meisinger C, Illig T, Martin S, Kolb H. Chemokines as risk factors for type 2 diabetes: results from the MONICA/KORA Augsburg study, 1984–2002. Diabetologia 2006;49:921–929 [DOI] [PubMed] [Google Scholar]
- 4.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–867 [DOI] [PubMed] [Google Scholar]
- 5.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006;116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 2008;118:2992–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinarello CA. The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med 2000;343:732–734 [DOI] [PubMed] [Google Scholar]
- 8.Donath MY, Schumann DM, Faulenbach M, Ellingsgaard H, Perren A, Ehses JA. Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care 2008;31(Suppl. 2):S161–S164 [DOI] [PubMed] [Google Scholar]
- 9.Juge-Aubry CE, Somm E, Giusti V, Pernin A, Chicheportiche R, Verdumo C, Rohner-Jeanrenaud F, Burger D, Dayer JM, Meier CA. Adipose tissue is a major source of interleukin-1 receptor antagonist: upregulation in obesity and inflammation. Diabetes 2003;52:1104–1110 [DOI] [PubMed] [Google Scholar]
- 10.Seckinger P, Lowenthal JW, Williamson K, Dayer JM, MacDonald HR. A urine inhibitor of interleukin 1 activity that blocks ligand binding. J Immunol 1987;139:1546–1549 [PubMed] [Google Scholar]
- 11.Seckinger P, Williamson K, Balavoine JF, Mach B, Mazzei G, Shaw A, Dayer JM. A urine inhibitor of interleukin 1 activity affects both interleukin 1α and 1β but not tumor necrosis factor α. J Immunol 1987;139:1541–1545 [PubMed] [Google Scholar]
- 12.Sauter NS, Schulthess FT, Galasso R, Castellani LW, Maedler K. The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology 2008;149:2208–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 2007;356:1517–1526 [DOI] [PubMed] [Google Scholar]
- 14.Larsen CM, Faulenbach M, Vaag A, Ehses JA, Donath MY, Mandrup-Poulsen T. Sustained effects of interleukin-1 receptor antagonist treatment in type 2 diabetes mellitus. Diabetes Care 2009;32:1663–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herder C, Brunner EJ, Rathmann W, Strassburger K, Tabák AG, Schloot NC, Witte DR. Elevated levels of the anti-inflammatory interleukin-1 receptor antagonist precede the onset of type 2 diabetes: the Whitehall II study. Diabetes Care 2009;32:421–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tabák AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimäki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet 2009;373:2215–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunner EJ, Kivimäki M, Witte DR, Lawlor DA, Davey Smith G, Cooper JA, Miller M, Lowe GD, Rumley A, Casas JP, Shah T, Humphries SE, Hingorani AD, Marmot MG, Timpson NJ, Kumari M. Inflammation, insulin resistance, and diabetes: Mendelian randomization using CRP haplotypes point upstream. PLoS Med 2008;5:e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunner EJ, Marmot MG, Nanchahal K, Shipley MJ, Stansfeld SA, Juneja M, Alberti KG. Social inequality in coronary risk: central obesity and the metabolic syndrome. Evidence from the Whitehall II study. Diabetologia 1997;40:1341–1349 [DOI] [PubMed] [Google Scholar]
- 19.Marmot M, Brunner E. Cohort profile: the Whitehall II study. Int J Epidemiol 2005;34:241–256 [DOI] [PubMed] [Google Scholar]
- 20.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications: part 1, diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–553 [DOI] [PubMed] [Google Scholar]
- 21.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurence. New York, Oxford University Press, 2003 [Google Scholar]
- 22.Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest 2002;110:851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Böni-Schnetzler M, Thorne J, Parnaud G, Marselli L, Ehses JA, Kerr-Conte J, Pattou F, Halban PA, Weir GC, Donath MY. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta-cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. J Clin Endocrinol Metab 2008;93:4065–4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dasu MR, Devaraj S, Jialal I. High glucose induces IL-1beta expression in human monocytes: mechanistic insights. Am J Physiol Endocrinol Metab 2007;293:E337–E346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev 2002;13:323–340 [DOI] [PubMed] [Google Scholar]
- 26.Perrier S, Darakhshan F, Hajduch E. IL-1 receptor antagonist in metabolic diseases: Dr Jekyll or Mr Hyde? FEBS Lett 2006;580:6289–6294 [DOI] [PubMed] [Google Scholar]
- 27.Pfleger C, Mortensen HB, Hansen L, Herder C, Roep BO, Hoey H, Aanstoot HJ, Kocova M, Schloot NCHvidøre Study Group on Childhood Diabetes. Association of IL-1ra and adiponectin with C-peptide and remission in patients with type 1 diabetes. Diabetes 2008;57:929–937 [DOI] [PubMed] [Google Scholar]
- 28.Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 2003;52:812–817 [DOI] [PubMed] [Google Scholar]
- 29.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab 2007;92:1023–1033 [DOI] [PubMed] [Google Scholar]
- 30.Meier CA, Bobbioni E, Gabay C, Assimacopoulos-Jeannet F, Golay A, Dayer JM. IL-1 receptor antagonist serum levels are increased in human obesity: a possible link to the resistance to leptin? J Clin Endocrinol Metab 2002;87:1184–1188 [DOI] [PubMed] [Google Scholar]
- 31.Ruotsalainen E, Salmenniemi U, Vauhkonen I, Pihlajamäki J, Punnonen K, Kainulainen S, Laakso M. Changes in inflammatory cytokines are related to impaired glucose tolerance in offspring of type 2 diabetic subjects. Diabetes Care 2006;29:2714–2720 [DOI] [PubMed] [Google Scholar]
- 32.Ybarra J, Lehmann TN, Golay A, Juge-Aubry CE, Roux-Lombard P, Dayer JM, Meier CA. Gender-based dimorphic pattern for interleukin-1 receptor antagonist in type 2 diabetes mellitus. Diabetes Metab 2008;34:75–81 [DOI] [PubMed] [Google Scholar]
- 33.Cartier A, Bergeron J, Poirier P, Almeras N, Tremblay A, Lemieux I, Despres JP. Increased plasma interleukin-1 receptor antagonist levels in men with visceral obesity. Ann Med 2009;41:471–478 [DOI] [PubMed] [Google Scholar]
- 34.Maedler K, Sergeev P, Ehses JA, Mathe Z, Bosco D, Berney T, Dayer JM, Reinecke M, Halban PA, Donath MY. Leptin modulates beta cell expression of IL-1 receptor antagonist and release of IL-1beta in human islets. Proc Natl Acad Sci U S A 2004;101:8138–8143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maedler K, Schulthess FT, Bielman C, Berney T, Bonny C, Prentki M, Donath MY, Roduit R. Glucose and leptin induce apoptosis in human beta-cells and impair glucose-stimulated insulin secretion through activation of c-Jun N-terminal kinases. FASEB J 2008;22:1905–1913 [DOI] [PubMed] [Google Scholar]
- 36.Glas R, Sauter NS, Schulthess FT, Shu L, Oberholzer J, Maedler K. Purinergic P2X7 receptors regulate secretion of interleukin-1 receptor antagonist and beta cell function and survival. Diabetologia 2009;52:1579–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]