Abstract
OBJECTIVE
The insulinotropic action of the incretin glucose-dependent insulinotropic polypeptide (GIP) is impaired in type 2 diabetes, while the effect of glucagon-like peptide-1 (GLP-1) is preserved. To evaluate the role of impaired GIP function in glucose homeostasis and development of the endocrine pancreas in a large animal model, we generated transgenic pigs expressing a dominant-negative GIP receptor (GIPRdn) in pancreatic islets.
RESEARCH DESIGN AND METHODS
GIPRdn transgenic pigs were generated using lentiviral transgenesis. Metabolic tests and quantitative stereological analyses of the different endocrine islet cell populations were performed, and β-cell proliferation and apoptosis were quantified to characterize this novel animal model.
RESULTS
Eleven-week-old GIPRdn transgenic pigs exhibited significantly reduced oral glucose tolerance due to delayed insulin secretion, whereas intravenous glucose tolerance and pancreatic β-cell mass were not different from controls. The insulinotropic effect of GIP was significantly reduced, whereas insulin secretion in response to the GLP-1 receptor agonist exendin-4 was enhanced in GIPRdn transgenic versus control pigs. With increasing age, glucose control deteriorated in GIPRdn transgenic pigs, as shown by reduced oral and intravenous glucose tolerance due to impaired insulin secretion. Importantly, β-cell proliferation was reduced by 60% in 11-week-old GIPRdn transgenic pigs, leading to a reduction of β-cell mass by 35% and 58% in 5-month-old and 1- to 1.4-year-old transgenic pigs compared with age-matched controls, respectively.
CONCLUSIONS
The first large animal model with impaired incretin function demonstrates an essential role of GIP for insulin secretion, proliferation of β-cells, and physiological expansion of β-cell mass.
The incretin hormones glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) are secreted by enteroendocrine cells in response to nutrients like fat and glucose and enhance glucose-induced release of insulin from pancreatic β-cells (1). The effects of GIP and GLP-1 are mediated through specific receptors, GIPR and GLP-1R, respectively. Both receptors belong to the family of seven transmembrane-domain heterotrimeric G-protein–coupled receptors (2). Activation of the GIPR or GLP-1R leads to enhanced exocytosis of insulin-containing granules (3). Interestingly, variation in the GIPR gene influences glucose and insulin responses to an oral glucose challenge in humans (4). Furthermore, findings in insulinoma cells (5–7) and rodent models (8,9) indicate that activation of incretin receptors promotes proliferation and survival of β-cells. Type 2 diabetic patients and ∼50% of their first-degree relatives show a reduced incretin effect, mainly due to an impaired insulinotropic action of GIP (10,11). Nearly sustained insulinotropic action of GLP-1 (11) in type 2 diabetic patients revealed its therapeutic potential and initiated the ongoing development of GLP-1R agonists as well as inhibitors of dipeptidyl peptidase-4 (1,12), which rapidly degrades incretin hormones in vivo. The reasons for the reduced response to GIP in type 2 diabetes are unclear (1), but impaired GIP action might be involved in the early pathogenesis of type 2 diabetes (13).
To clarify this point, a mouse model lacking functional GIPR expression was generated by gene targeting (14). Gipr−/− mice displayed only slightly impaired glucose tolerance and did not develop diabetes. Interestingly, double incretin receptor knockout mice exhibited a similar phenotype. As possible explanations for this relatively mild phenotype (rev. in 15), compensatory regulation of the GLP-1 system or other compensatory mechanisms were discussed. In contrast, transgenic mice overexpressing a dominant-negative GIPR (GIPRdn) displayed a severe phenotype (i.e., early-onset diabetes accompanied by a marked fasting hypoinsulinemia and severe reduction of β-cell mass associated with extensive structural alterations of the pancreatic islets) (16).
In light of these discrepant findings in mouse models, we generated a large animal model to address the question whether GIPR signaling plays a role in maintaining pancreatic islet function and structure. Efficient lentiviral vectors (17) were used to generate transgenic pigs expressing a GIPRdn under the control of the rat Ins2 promoter in the pancreatic islets. This novel animal model, in contrast to GIPRdn transgenic mice (16), initially only exhibits a disturbed incretin effect but develops progressive deterioration of glucose control with increasing age, associated with reduced β-cell proliferation and an impairment of physiological age-related expansion of pancreatic β-cell mass.
RESEARCH DESIGN AND METHODS
Generation of RIP II-GIPRdn transgenic pigs.
The expression cassette consisting of the rat Ins2 promoter (RIP II) and the cDNA of a human GIPRdn (16) was cloned into the lentiviral vector LV-pGFP (18) (supplementary Fig. 1 of the online appendix [available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0519/DC1]). Recombinant lentivirus was produced (18) and injected into the perivitelline space of zygotes from superovulated gilts (17). Embryos were transferred into synchronized recipients (19). Offspring were genotyped by PCR and Southern blot analyses using a probe directed toward the RIP II promoter sequence. Expression of GIPRdn mRNA in the pancreatic islets was determined by RT-PCR. A total of 400 ng of total RNA were reverse transcribed into cDNA using SuperScriptII reverse transcriptase (Invitrogen) and random hexamer primers (Invitrogen) after digestion with DNaseI (Roche). For PCR, the following transgene specific primers were used: sense 5′-TTT TTA TCC GCA TTC TTA CAC GG-3′ and antisense 5′-ATC TTC CTC AGC TCC TTC CAG G-3′. All animal experiments were carried out according to the German animal protection law.
Oral/intravenous glucose tolerance test and GIP/exendin-4 stimulation test.
For the oral glucose tolerance test (OGTT), one central venous catheter (Cavafix Certo; B. Braun) was inserted nonsurgically into the external jugular vein. After an 18-h overnight fast, animals were fed 2 g glucose/kg body weight (BW) (20) mixed with 50/100 g (11-week-old/5-month-old) commercial pig fodder. Blood samples were obtained from the jugular vein catheter at the indicated time points. For the intravenous glucose tolerance test (IVGTT) and GIP/exendin-4 stimulation test, two central venous catheters (Cavafix Certo) were surgically inserted into the external jugular vein under general anesthesia (21). For both tests, a bolus injection of 0.5 g glucose/kg BW (22) was administered through the central venous catheter after an 18-h fasting period. For the GIP/exendin-4 stimulation test, 80 pmol/kg BW of synthetic porcine GIP (Bachem) or 40 pmol/kg BW synthetic exendin-4 (Bachem) were administered intravenously in addition to glucose. Blood samples were collected at the indicated time points. Serum glucose levels were determined using an AU 400 autoanalyzer (Olympus). Serum insulin levels were measured using a porcine insulin radioimmunoassay kit (Millipore).
Pancreas preparation and islet isolation.
Pancreatic islets were isolated from three 12- to 13-month-old GIPRdn transgenic pigs and three littermate control animals (23). After explantation of the pancreas in toto, the left pancreatic lobe was separated from the rest of the organ (supplementary Fig. 2). The left pancreatic lobe was digested using a modification of the half-automated digestion-filtration method as previously described (24). Purification of the isolated islets was performed with the discontinuous OptiPrep density gradient (Progen) in the COBE 2991 cell processor (COBE) (25). Islet numbers were determined using dithizone-stained islet samples (26), which were counted under an Axiovert 25 microscope (Zeiss) with a calibrated grid in the eyepiece. For determination of islet vitality, fluorescein diacetate/propidium iodide (Sigma-Aldrich) staining was performed (27).
Immunohistochemistry and quantitative stereological analyses.
After prefixation, the pancreas was cut into 1-cm-thick slices. Slices were tilted to their left side and covered by a 1-cm2 point-counting grid. Tissue blocks were selected by systematic random sampling, fixed in 10% neutral buffered formalin, routinely processed, and embedded in paraffin. The volume of the pancreas [V(Pan)] before embedding was calculated by the quotient of the pancreas weight and the specific weight of pig pancreas (1.07 g/cm3). The specific weight was determined by the submersion method (28). Paraffin sections were routinely prepared, and insulin, glucagon, somatostatin, and pancreatic polypeptide containing cells were stained, using the indirect immunoperoxidase technique (16) and the antibodies described in the online appendix. The volume densities of α-, β-, δ-, and pp-cells in the islets [Vv(α-cell/Islet), Vv(β-cell/Islet), Vv(δ-cell/Islet), and Vv(pp-cell/Islet)], the total volumes of α-, β-, δ-, and pp-cells in the islets [V(α-cell, Islet), V(β-cell, Islet), V(δ-cell, Islet), and V(pp-cell, Islet)] as well as the total volume of β-cells in the pancreas [referring to β-cells in the islets and isolated β-cells, V(β-cell, Pan)], and the total volume of isolated β-cells in the pancreas [V(isoβ-cell, Pan)], a parameter indicative of islet neogenesis (29–31), were determined as described previously (32). Volume densities of the various endocrine cell types in the islets refer to the volume fraction of the particular endocrine cell type in relation to the cumulative volume of the various endocrine islet cells, thus excluding capillaries and other interstitial tissues in the islets.
Proliferation/apoptosis rates of β-cells were determined by double immunohistochemical staining for insulin and the proliferation marker Ki67 (33) or the apoptosis marker cleaved caspase-3 (34) as detailed in the online appendix. A minimum of 104 β-cells per animal was included in the quantification of β-cell proliferation and apoptosis. Cell proliferation/apoptosis index was defined as the number of immunolabeled cell nuclei divided by the total number of cell nuclei counted and expressed as the number of immunolabeled (Ki67+/Casp-3+) cell nuclei per 105 nuclei. GIPR and GLP-1R were detected in pancreas sections using the streptavidin-biotin complex technique and the antibodies described in the online appendix.
Statistics.
All data are presented as means ± SE. The results of glucose tolerance tests and incretin stimulation tests were statistically evaluated by ANOVA (linear mixed models; SAS 8.2; PROC MIXED), taking the fixed effects of group (wild type, transgenic), time (relative to glucose or hormone application), and the interaction group × time as well as the random effect of animal into account (35). Results of the linear mixed models analysis are shown in supplementary Table 1. The same model was used to compare body weight gain of GIPRdn transgenic and control pigs. Pancreas weight and the results of quantitative stereological analyses were evaluated by the general linear models procedure (SAS 8.2) taking the effects of group (wild type, transgenic), age (11 weeks, 5 months, or 1–1.4 years), and the interaction group × age into account. Results of the general linear models analysis are shown in Table 1. Calculation of areas under the curve (AUCs) was performed using Graph Pad Prism 4 software. Statistical significance of differences between transgenic and wild-type pigs was tested using the Mann-Whitney U test in combination with an exact test procedure (SPSS 16.0, Chicago, IL). P values <0.05 were considered significant.
TABLE 1.
Quantitative stereological analyses of the endocrine pancreas of GIPRdn transgenic pigs (tg) and wild-type control pigs (wt)
| Parameter | 11 weeks (n = 5 wt, 5 tg) |
5 months (n = 4 wt, 4 tg) |
1–1.4 years (n = 5 wt, 5 tg) |
ANOVA |
||
|---|---|---|---|---|---|---|
| Means ± SE | Means ± SE | Means ± SE | Group | Age | Group × age | |
| Pancreas weight (g) | ||||||
| Wild type | 34.5 ± 4.2 | 115.3 ± 5.6 | 183.4 ± 13.8 | NS | <0.0001 | NS |
| Transgenic | 32.7 ± 3.1 | 125.7 ± 6.1 | 206.1 ± 4.9 | |||
| VV(β-cell/islet) (%) | ||||||
| Wild type | 69.8 ± 2.2 | 89.0 ± 1.5 | 90.2 ± 1.2 | 0.0066 | <0.0001 | 0.0024 |
| Transgenic | 70.8 ± 1.3 | 87.0 ± 1.2 | 76.4 ± 3.2* | |||
| VV(α-cell/islet) (%) | ||||||
| Wild type | 14.1 ± 1.2 | 5.0 ± 0.8 | 5.0 ± 0.7 | 0.0122 | <0.0001 | 0.0008 |
| Transgenic | 12.2 ± 0.7 | 6.5 ± 0.7 | 13.8 ± 2.3* | |||
| VV(δ-cell/islet) (%) | ||||||
| Wild type | 13.5 ± 2.8 | 4.3 ± 0.8 | 2.2 ± 0.6 | NS | <0.0001 | NS |
| Transgenic | 13.0 ± 1.0 | 5.5 ± 0.9 | 5.8 ± 0.9† | |||
| VV(pp-cell/islet) (%) | ||||||
| Wild type | 2.7 ± 0.7 | 1.8 ± 0.3 | 2.8 ± 0.8 | NS | 0.0355 | NS |
| Transgenic | 3.9 ± 0.5 | 1.3 ± 0.3 | 3.8 ± 1.1 | |||
| V(β-cell/islet) (mm3) | ||||||
| Wild type | 168.7 ± 29.5 | 1,088.2 ± 82.0 | 1,694.6 ± 251.7 | 0.0002 | <0.0001 | 0.0024 |
| Transgenic | 152.1 ± 17.1 | 664.1 ± 74.5† | 663.7 ± 130.5* | |||
| V(α-cell/islet) (mm3) | ||||||
| Wild type | 32.6 ± 8.7 | 58.4 ± 6.3 | 95.7 ± 17.4 | NS | <0.0001 | NS |
| Transgenic | 24.5 ± 1.7 | 47.7 ± 4.5 | 112.8 ± 14.5 | |||
| V(δ-cell/islet) (mm3) | ||||||
| Wild type | 26.6 ± 3.9 | 49.5 ± 6.1 | 36.8 ± 6.3 | NS | 0.0014 | NS |
| Transgenic | 25.9 ± 1.8 | 39.6 ± 3.5 | 47.5 ± 5.2 | |||
| V(pp-cell/islet) (mm3) | ||||||
| Wild type | 6.0 ± 1.8 | 20.7 ± 2.9 | 52.4 ± 12.6 | NS | <0.0001 | NS |
| Transgenic | 8.2 ± 1.7 | 9.3 ± 2.1 | 30.3 ± 7.9 | |||
Data were analyzed by the general linear models procedure (SAS Institute) taking the effects of group (wild type, transgenic), age (11 weeks, 5 months, 1–1.4 years), and the interaction group × age into account. For significant effects of these factors P values are indicated in the last three columns. In addition, significant differences between groups within age classes are marked by:
*P < 0.01;
†P < 0.05;
NS, not significant.
RESULTS
Generation of GIPRdn transgenic pigs.
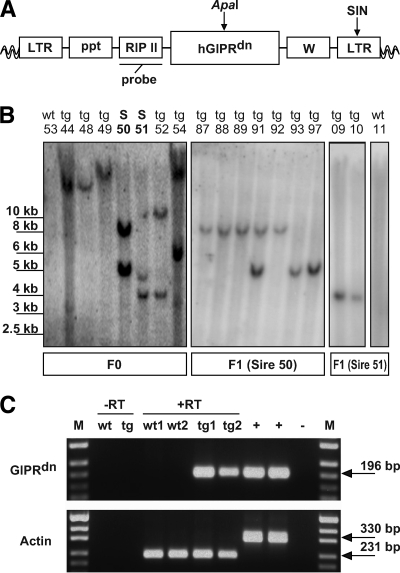
A lentiviral vector was cloned that expresses a dominant-negative GIPR (GIPRdn) under the control of the rat insulin 2 gene promoter (RIP II) (Fig. 1A). The GIPRdn has an eight–amino acid deletion (positions 319–326) and an Ala→Glu exchange at amino acid position 340 in the third intracellular loop, which is essential for signal transduction (16). Lentiviral vectors were injected into the perivitelline space of pig zygotes (17). A total of 113 injected zygotes were transferred laparoscopically into the oviducts of three cycle-synchronized recipient gilts. Nineteen piglets (17% of the transferred zygotes) were born. Southern blot analysis identified nine founder animals (47% of the born animals) carrying one or two lentiviral integrants (Fig. 1B), confirming the high efficiency of lentiviral transgenesis in large animals (17).
FIG. 1.
Lentiviral vector, Southern blot analyses, and transgene expression. A: The lentiviral vector (LV-GIPRdn) carrying the cDNA of the dominant-negative GIPR (GIPRdn) under the control of the rat Ins2 gene promoter (RIP II). ApaI, restriction site of ApaI; LTR, long terminal repeat; ppt, polypurine tract; SIN, self-inactivating mutation; W, woodchuck hepatitis posttranscriptional regulatory element; probe, probe used for Southern blot analyses; wavy lines, pig genome. B: Southern blot analyses of ApaI-digested genomic DNA isolated from EDTA blood of piglets generated by subzonal injection of LV-GIPRdn (transgenic [tg]) and two nontransgenic littermates (wild type [wt]). Pigs of the F0 generation show either one or two single-copy integration sites of the transgene. Sires 50 and 51 (S 50/S 51) were selected to establish two transgenic lines. Note that pigs of the F1 generation show segregation of the integrants according to the Mendelian rules. C: Analysis of transgene expression (GIPRdn) in isolated porcine islets of Langerhans of transgenic (tg) and nontransgenic littermates (wt) by RT-PCR. β-Actin RT-PCR used for confirmation of reverse transcription efficiency. Due to the use of intron-spanning primers to detect β-actin, two different-sized bands are visible differentiating cDNA and genomic DNA. M: pUC Mix Marker; −RT wt: minus RT wild-type pigs; −RT tg: minus RT GIPRdn transgenic pigs (no signals were obtained from islets of transgenic offspring after omission of the RT step, demonstrating that expressed rather than integrated sequences were detected); +, genomic DNA of GIPRdn transgenic pig; −, aqua bidest.
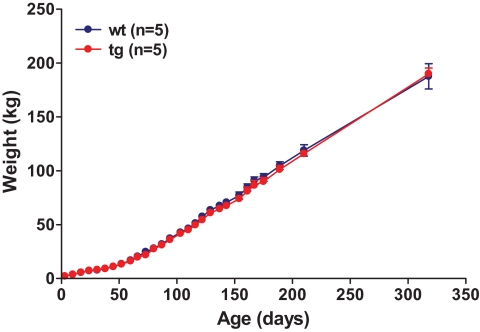
Two male founder animals (nos. 50 and 51) were mated to nontransgenic females (Fig. 1B). The resulting offspring demonstrated germ line transmission and segregation of the integrants according to Mendelian rules (Fig. 1B). To analyze expression of GIPRdn mRNA, pancreatic islets were isolated from transgenic and nontransgenic offspring and analyzed by RT-PCR. Expression of the GIPRdn was detected in the islets of all transgenic animals but not in the islets of nontransgenic control animals (Fig. 1C). GIPRdn transgenic pigs developed normally and did not show any deviation in body weight gain compared with controls (Fig. 2).
FIG. 2.
Body weight gain of GIPRdn transgenic pigs (tg) compared with control pigs (wt). Data are means ± SE. (A high-quality digital representation of this figure is available in the online issue.)
Normal fasting glucose and fructosamine levels in GIPRdn transgenic pigs.
To evaluate effects of GIPRdn expression on glucose homeostasis, fasting blood glucose and serum fructosamine levels were determined in regular intervals from 1 to 7 months of age. No significant differences in blood glucose levels and serum fructosamine levels were detected between GIPRdn transgenic and control pigs (supplementary Fig. 3). Fasting blood glucose levels, determined in irregular intervals up to an age of 2 years, were unaltered in GIPRdn transgenic pigs (data not shown).
Reduced insulinotropic effect of GIP but enhanced insulinotropic effect of exendin-4 in GIPRdn transgenic pigs.
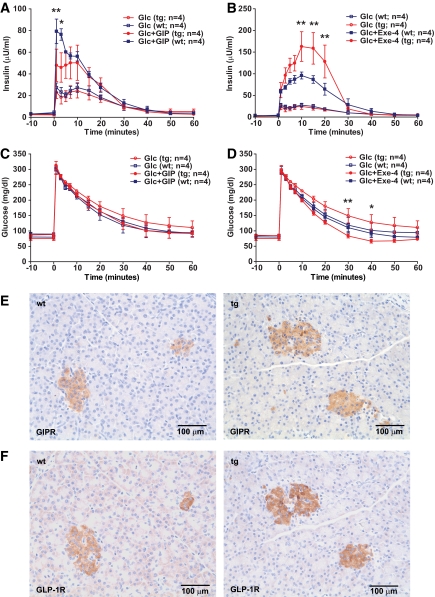
To evaluate whether expression of a GIPRdn specifically impairs the function of GIP, we performed stimulation tests with GIP and the GLP-1 receptor agonist exendin-4 (1). The insulinotropic effect of GIP, intravenously administered as a bolus, was significantly diminished (Fig. 3A), while insulin secretion in response to exendin-4 was increased in GIPRdn transgenic versus control pigs (Fig. 3B), leading to a faster decrease of serum glucose levels (Fig. 3D). These findings demonstrate that expression of GIPRdn specifically reduces the insulinotropic action of GIP and does not impair the function of a related G-protein–coupled receptor, namely the GLP-1R. Further, the enhanced insulinotropic effect of exendin-4 in GIPRdn transgenic versus control pigs indicates a compensatory hyperactivation of the GLP-1/GLP-1R system, which has also been observed in Gipr−/− mice (rev. in 15). To clarify, whether compensatory mechanisms involve altered expression of incretin receptors, we performed immunohistochemical staining of pancreas sections for GIPR (Fig. 3E) and GLP-1R (Fig. 3F), which revealed no apparent difference in the abundance and spatial distribution of both receptors comparing GIPRdn transgenic and control pigs.
FIG. 3.
Functional analysis of GIPRdn expression by GIP/exendin-4 stimulation test. Reduced insulinotropic action of GIP but enhanced insulinotropic action of exendin-4 in 11-week-old GIPRdn transgenic pigs (tg) compared with nontransgenic control animals (wt). A: Serum insulin levels of GIPRdn transgenic (tg) and control (wt) pigs after intravenous administration of glucose (Glc) ± GIP. B: Serum insulin levels of GIPRdn transgenic (tg) and control (wt) pigs after intravenous administration of glucose (Glc) ± exendin-4 (Exe-4). C and D: Corresponding serum glucose levels for the GIP (C) and exendin-4 (D) stimulation test. 0 min = point of Glc/GIP/exendin-4 administration. Data are means ± SE. *P < 0.05 vs. control; **P < 0.01 vs. control. E and F: Immunohistochemical staining of GIPR (E) and GLP-1R (F) in pancreas sections from 11-week-old GIPRdn transgenic pigs (tg) and nontransgenic control animals (wt) does not provide evidence for differences in receptor abundance. (A high-quality digital representation of this figure is available in the online issue.)
Disturbed incretin function in young GIPRdn transgenic pigs.
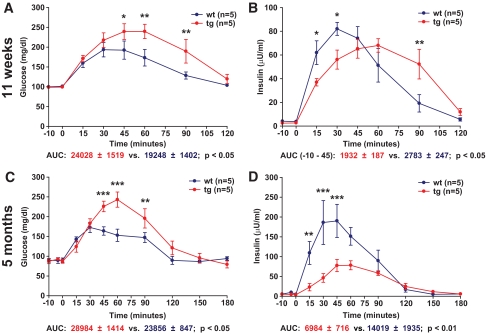
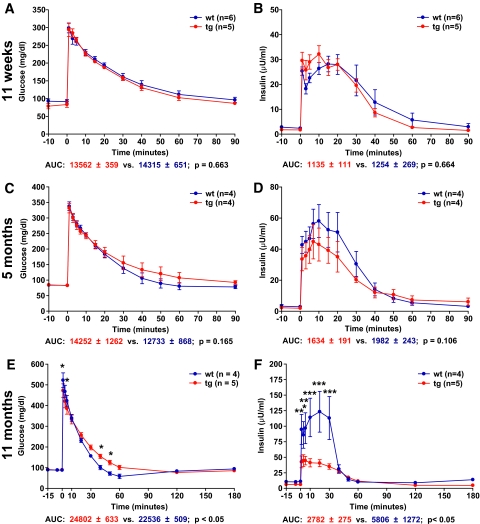
An OGTT (2 g glucose/kg BW) was performed in 11-week-old GIPRdn transgenic pigs (n = 5) and controls (n = 5) originating from founder boars nos. 50 and 51. GIPRdn transgenic pigs exhibited elevated (P < 0.05) serum glucose levels (Fig. 4A) as well as a distinct delay in insulin secretion (Fig. 4B) after glucose challenge. The area under the insulin curve (AUC insulin) during the first 45 min following glucose challenge was 31% (P < 0.05) smaller in GIPRdn transgenic pigs than in age-matched controls (Fig. 4B); however, the total amount of insulin secreted during the experimental period (i.e., total AUC insulin until 120 min following glucose load) was not different between the two groups (5,155 ± 763 vs. 5,698 ± 625; P = 0.351). These findings indicate that expression of a GIPRdn in the pancreatic islets of transgenic pigs is sufficient to interfere with the incretin effect but does not initially affect the total AUC insulin. This assumption is supported by the fact that intravenous glucose tolerance was not reduced in GIPRdn transgenic pigs (Fig. 5A), and the time course and amount of insulin secreted in response to an intravenous glucose load were not different between the two groups (Fig. 5B). Quantitative stereological investigations of the pancreas (32) revealed that the total volume of β-cells in the pancreas was not different between GIPRdn transgenic pigs and controls (Fig. 6A). Further, the total volume of isolated β-cells (single insulin-positive cells and small clusters of insulin-positive cells not belonging to established islets) was equal in the two groups (49 ± 4 vs. 49 ± 6 mm3; P = 0.695). These findings clearly demonstrate that expression of a GIPRdn does not exhibit a toxic effect on pancreatic islets and further suggest that pancreatic islet neogenesis is not disturbed.
FIG. 4.
Oral glucose tolerance in 11-week-old and 5-month-old GIPRdn transgenic pigs (tg) compared with nontransgenic littermates (wt). A and C: Serum glucose levels; 0 min = point of glucose administration. B and D: Serum insulin levels. AUC glucose/insulin for transgenic pigs (red) and wild-type pigs (blue). Data are means ± SE. *P < 0.05 vs. control; **P < 0.01 vs. control; ***P < 0.001 vs. control. Note that in 11-week-old transgenic pigs, there is a delay in insulin secretion leading to a significant reduction of insulin secretion during the first 45 min following oral glucose load, although the total amount of insulin secreted over 120 min is not different from controls. In contrast, 5-month-old transgenic pigs display not only delayed but also reduced insulin secretion. A high-quality digital representation of this figure is available in the online issue.
FIG. 5.
Intravenous glucose tolerance in GIPRdn transgenic pigs (tg) compared with nontransgenic controls (wt). A, C, and E: Serum glucose levels; 0 min = point of glucose administration. B, D, and F: Serum insulin levels. AUC glucose/insulin for transgenic pigs (red) and wild-type pigs (blue). Data are means ± SE. *P < 0.05 vs. control; **P < 0.01 vs. control; ***P < 0.001 vs. control. Note that intravenous glucose tolerance (A) and insulin secretion (B) are not altered in 11-week-old transgenic pigs. In 5-month-old transgenic pigs, a tendency of reduced insulin secretion (D) is observed, while 11-month-old transgenic pigs display a significantly reduced intravenous glucose tolerance (E) due to a significantly reduced insulin secretion (F). (A high-quality digital representation of this figure is available in the online issue.)
FIG. 6.
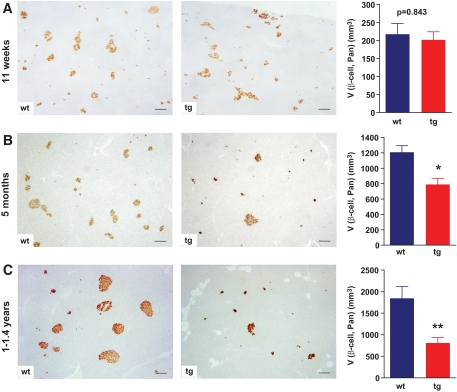
Immunohistochemistry for insulin and total β-cell volume in the pancreas [V(β-cell, Pan)] determined with quantitative stereological methods. A–C: Representative histological sections of pancreatic tissue from a control (wt) and a GIPRdn transgenic pig (tg); scale bar = 200 μm. Unaltered total β-cell volume in 11-week-old GIPRdn transgenic pigs (n = 5 per group) (A) but reduction of the total β-cell volume in 5-month-old (n = 4 per group) (B) and young adult (1–1.4 years old) (n = 5 per group) GIPRdn transgenic pigs (C) compared with controls. Data are means ± SE. *P < 0.05 vs. control; **P < 0.01 vs. control. A high-quality digital representation of this figure is available in the online issue.
Glucose control in GIPRdn transgenic pigs deteriorates with increasing age.
To monitor the long-term consequences of GIPRdn expression, a second collective of animals was repeatedly investigated. First, an OGTT was performed in 5-month-old (20 ± 1 weeks) GIPRdn transgenic pigs (n = 5) and littermate controls (n = 5) originating from founder boars nos. 50 and 51. GIPRdn transgenic pigs exhibited elevated glucose levels (Fig. 4C) as well as a distinct reduction of initial insulin secretion after glucose challenge compared with their nontransgenic littermates (Fig. 4D). In addition, peak insulin levels were clearly reduced compared with controls. The AUC glucose was 26% (P < 0.05) larger (Fig. 4C), whereas AUC insulin was 49% (P < 0.01) smaller in GIPRdn transgenic pigs (Fig. 4D). The latter finding suggests that, in contrast to 11-week-old animals, the overall insulin secretion following an oral glucose load is reduced in 5-month-old GIPRdn transgenic pigs and that their islets may undergo progressive functional and/or structural changes. Additionally, an IVGTT was carried out in 5-month-old (22.5 ± 1.5 weeks) GIPRdn transgenic and control pigs (n = 4 per group; different collective of animals). Intravenous glucose tolerance (Fig. 5C), as well as insulin secretion (Fig. 5D), in GIPRdn transgenic pigs was similar to controls. However, a tendency toward reduced intravenous glucose tolerance and reduced insulin secretion in GIPRdn transgenic pigs was visible.
Next, we performed an IVGTT in 11-month-old (45 ± 2 weeks) GIPRdn transgenic pigs (n = 5) and littermate controls (n = 4) from the same collective of animals used for OGTT at 5 months of age. GIPRdn transgenic pigs exhibited a decelerated decline of blood glucose levels (10% larger AUC glucose; P < 0.05) (Fig. 5E), going along with significantly reduced insulin release (52% smaller AUC insulin; P < 0.05) (Fig. 5F). This observation corroborated the suspicion that impaired GIPR function may cause a general disturbance of insulin secretion and/or alterations in islet structure and/or islet integrity over time.
Impaired age-related expansion of pancreatic β-cell mass in GIPRdn transgenic pigs.
To clarify long-term effects of GIPRdn expression on the islets, we performed quantitative stereological analyses of pancreata from 5-month-old and from 1- to 1.4-year-old GIPRdn transgenic pigs and controls. Pancreas weight did not differ between GIPRdn transgenic pigs and control animals in both age-groups (Table 1).
Qualitative histological assessment revealed that pancreatic islet profiles of 5-month-old and 1- to 1.4-year-old GIPRdn transgenic pigs appeared to be smaller in size and reduced in number (Fig. 6B and C). These findings were confirmed by quantitative stereological investigations. In 5-month-old GIPRdn transgenic pigs (n = 4), the total volume of β-cells [V(β-cell, Pan)] was diminished by 35% (P < 0.05) versus controls (n = 4) (Fig. 6B). In 1- to 1.4-year-old GIPRdn transgenic pigs (n = 5), the reduction of total β-cell volume compared with controls (n = 5) was even more pronounced (58%; P < 0.01) (Fig. 6C). Reduced β-cell mass of young adult GIPRdn transgenic pigs compared with controls was confirmed by islet isolation experiments. The number of islet equivalents recovered from pancreas samples of GIPRdn transgenic pigs (n = 3) was reduced by 93% (P < 0.05) as compared with littermate controls (n = 3) (supplementary Table 2).
In contrast, volume density (data not shown) as well as the total volume of isolated β-cells were not different between GIPRdn transgenic and control pigs, neither at 5 months of age (121 ± 18 vs. 127 ± 15 mm3; P = 0.883) nor at 1–1.4 years of age (77 ± 8 vs. 71 ± 5 mm3; P = 0.844). These data clearly demonstrate an age-related reduction of pancreatic β-cell mass expansion in GIPRdn transgenic pigs, which is in line with previous evidence for a trophic action of GIP on β-cells in vitro (5–7).
Altered cellular composition of islets in GIPRdn transgenic pigs.
To evaluate effects of GIPRdn expression on the volume densities of the various endocrine islet cells and their total volumes, we performed detailed stereological analyses of the pancreatic islets in all three age classes investigated. In control animals, total volumes of α-, β-, δ-, and pp-cells in established islets increased significantly with age (Table 1). In GIPRdn transgenic pigs, a similar age-dependent increase was seen for the total volumes of α-, δ-, and pp-cells. However, in comparison with controls, the increase of total β-cell volume of GIPRdn transgenic pigs was less pronounced from 11 weeks to 5 months of age. Importantly, there was no further augmentation of total β-cell volume in 1- to 1.4-year-old GIPRdn transgenic pigs, demonstrating that impaired GIPR function interferes with the physiological expansion of pancreatic β-cells. In addition, the fractional volume of β-cells in the islets was decreased, while that of α- and δ-cells was increased in 1- to 1.4-year-old GIPRdn transgenic pigs. However, the total volumes of these non–β-cell populations were not different from those of age-matched control pigs (Table 1).
Reduced proliferation rate of β-cells in GIPRdn transgenic pigs.
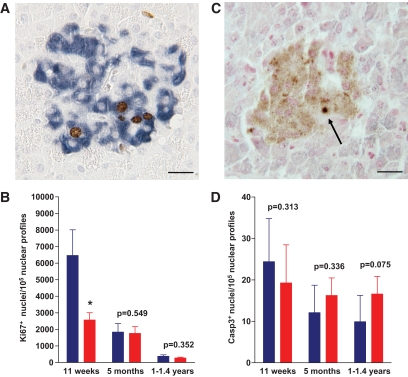
To clarify the mechanism of impaired β-cell expansion in GIPRdn transgenic pigs, we determined β-cell proliferation by double immunohistochemical staining for insulin and the proliferation marker Ki67 in all three age-groups. Indeed, β-cell proliferation was significantly reduced by 60% (P < 0.05) in 11-week-old GIPRdn transgenic pigs (Fig. 7A and B). In addition, we performed double immunohistochemical staining for insulin and the apoptosis marker cleaved caspase-3 to evaluate a potential impact of GIPRdn expression on cell death in the β-cell compartment. Overall, the proportion of cleaved caspase-3 positive cells was very low, with no significant difference between GIPRdn transgenic pigs and controls of all age classes. However, there was a trend (P = 0.075) of more cleaved caspase-3 positive β-cells in 1- to 1.4-year-old GIPRdn transgenic pigs as compared with age-matched controls (Fig. 7C and D).
FIG. 7.
β-Cell proliferation and apoptosis. A and C: Representative histological sections doublestained for insulin (blue) and Ki67 (brown) (A) or for insulin (light brown) plus cleaved caspase-3 (dark blue; see arrow) (C). B and D: Determination of the number of Ki67− (B) and cleaved caspase-3–positive β-cells (D). Wild type: blue bars, transgenic: red bars. Wild type: n = 5, transgenic: n = 5 for 11-week-old and 1- to 1.4-year-old pigs; wild type: n = 4, transgenic: n = 4 for 5-month-old pigs. Data are means ± SE. *P < 0.05 vs. control; scale bar = 20 μm. Note the significantly (P < 0.05) reduced β-cell proliferation rate in 11-week-old GIPRdn transgenic pigs. (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
This study established the first transgenic large animal model of impaired incretin function. The cDNA of the human GIPR was mutated at the third intracellular loop, where a deletion of eight amino acids (positions 319–326) and a point mutation at position 340 was introduced. In stably transfected Chinese hamster lymphoblast (CHL) cells, GIPRdn bound GIP with normal affinity but failed to increase intracellular cAMP levels. Thus, the GIPRdn expressed in transgenic pigs is capable of ligand binding but not of signal transduction (16) and competes with the endogenous GIPR for GIP. Consequently, the insulinotropic effect of GIP is highly reduced but not completely eliminated, mirroring the situation in human type 2 diabetes.
In view of the severe, early-onset diabetes of GIPRdn transgenic mice (16), we tested the oral and intravenous glucose tolerance of young (11-week-old) GIPRdn transgenic and control pigs and determined their pancreatic β-cell mass by quantitative stereological analyses. The finding of reduced oral glucose tolerance associated with delayed insulin secretion is in line with a disturbance of the incretin effect. Reduced oral glucose tolerance in GIPRdn transgenic pigs is also consistent with previous observations in Gipr−/− mice (14). Importantly, normal intravenous glucose tolerance, insulin secretion, and unchanged β-cell mass in 11-week-old GIPRdn transgenic pigs strongly argue against a toxic effect of GIPRdn expression. The reasons for different outcomes in the GIPRdn transgenic pig and the GIPRdn transgenic mouse model remain unclear but may be related to different methods of transgenesis (lentiviral transgenesis versus pronuclear DNA microinjection) or different copy numbers and/or integration sites leading to different expression levels of the transgene.
To evaluate long-term effects of GIPRdn expression in the pancreatic islets, we performed a longitudinal study of a collective of animals, involving OGTTs and IVGTTs. These revealed a progressive deterioration of glucose control in GIPRdn transgenic pigs, although none of our transgenic pigs has developed fasting hyperglycemia up to an age of 2 years. Quantitative stereological investigations of pancreata from 5-month-old and from 1- to 1.4-year-old GIPRdn transgenic pigs showed a reduced pancreatic β-cell mass, which was confirmed by quantitative islet isolation experiments.
Quantitative stereological analyses of the pancreatic islets demonstrated a marked increase of total β-cell volume from 11 weeks to 5 months (6.4-fold) and from 5 months to 1–1.4 years (1.6-fold) of age in control pigs. In contrast, the expansion of total β-cell volume in GIPRdn transgenic pigs was less pronounced between 11 weeks and 5 months of age (4.3-fold), with no further increase in 1- to 1.4-year-old animals. These findings are explained by a markedly reduced proliferation rate of β-cells in 11-week-old GIPRdn transgenic pigs, a developmental stage characterized by massive expansion of β-cells in pigs (36). Staining for cleaved caspase-3 did not show a significantly increased rate of cell death in the β-cell compartment of GIPRdn transgenic pigs versus controls, although a trend of higher numbers of cleaved caspase-3 positive β-cells was visible in 1- to 1.4-year-old GIPRdn transgenic pigs. This may suggest a contribution of apoptosis to the reduction of total β-cell volume in mature GIPRdn transgenic pigs.
Due to the reduced volume fraction of β-cells in the islets of 1- to 1.4-year-old GIPRdn transgenic pigs, the relative volumes of α- and δ-cells in the islets were increased. However, since the islet volume was concomitantly reduced, the absolute volumes of α- and δ-cells were not different between GIPRdn transgenic and control pigs.
The numbers of animals investigated in our study are, in part, smaller than in some rodent studies. However, due to the large size of the pig, several blood-based parameters could be measured repeatedly with short time intervals in the same animals, providing an important advantage for statistical analysis. Further, the stereological data of all animals (n = 28) were evaluated together by ANOVA, demonstrating significant group effects (Table 1) with P values <0.01 for many data/differences supporting our core statements.
Interestingly, Gipr−/−mice provided no evidence that GIPR action is required for the maintenance of islet and β-cell integrity in vivo (15,37). These mice exhibited an increase in relative β-cell area referring to pancreas area (37), leading to the conclusion that in vivo the function of GIP is primarily restricted to that of an incretin (15). However, the relatively mild phenotype of Gipr−/− mice may result from compensatory mechanisms (15). Although mice lacking both GIPR and GLP-1R exhibited more severe glucose intolerance than the individual mutants (38,39) these double mutant animals did not develop diabetes, raising the suspicion of the existence of compensatory mechanisms other than the GIP/GLP-1 system (38). The findings in GIPRdn transgenic pigs suggest that, in addition to its role as an incretin hormone, GIP is necessary for the expansion of β-cell mass and that its partial loss of function cannot be fully compensated by hyperactivation of the GLP-1/GLP-1R system.
In conclusion, GIPRdn transgenic pigs resemble characteristic features of human type 2 diabetic patients very closely in the following ways: disturbed GIP function, glucose intolerance, and reduced pancreatic β-cell mass. Moreover, GIPRdn transgenic pigs may be an attractive animal model for the development and preclinical evaluation of incretin-based therapeutic strategies (40). Another potential application of GIPRdn transgenic pigs is the development of novel techniques for dynamic in vivo monitoring of pancreatic islet mass (41). Due to their size and close physiological and anatomical similarities to humans (42), pigs represent attractive animal models for translating novel therapeutic and diagnostic principles into clinical practice.
ACKNOWLEDGMENTS
This study was supported by the Deutsche Forschungsgemeinschaft (GRK 1029), the Bayerische Forschungsstiftung (492/02), and the Diabetes Hilfs- und Forschungsfonds Deutschland (DHFD).
No potential conflicts of interest relevant to this article were reported.
Parts of this work were presented in abstract form at the 51st Annual Meeting of the German Society of Endocrinology, Salzburg, Austria, 7–10 March 2007; the 42nd Annual Meeting of the German Diabetes Society, Hamburg, Germany, 16–19 May 2007; the 34th Annual Conference of the International Embryo Transfer Society, Denver, Colorado, 5–9 January 2008; the 44th Annual Meeting of the German Diabetes Society, Leipzig, Germany, 20–23 May 2008; the 68th Scientific Sessions of the American Diabetes Association, San Francisco, California, 6–10 June 2008; and the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009.
The authors thank Prof. Dr. Karl Heinritzi, Prof. Dr. Holm Zerbe, and Dr. Birgit Rathkolb for the generous support of this study; Prof. Dr. Helmut Kuechenhoff (StaBLab, Ludwig Maximilians University Munich) for expert help with the statistical analysis of longitudinal data; and Tamara Holy, Lisa Pichl, Bianca Schneiker, Elfi Holupirek, Christian Erdle, and Siegfried Elsner for excellent technical assistance and animal management.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157 [DOI] [PubMed] [Google Scholar]
- 2.Mayo KE, Miller LJ, Bataille D, Dalle S, Goke B, Thorens B, Drucker DJ. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol Rev 2003;55:167–194 [DOI] [PubMed] [Google Scholar]
- 3.Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab 2004;287:E199–E206 [DOI] [PubMed] [Google Scholar]
- 4.Saxena R, Hivert MF, Langenberg C, Tanaka T, Pankow JS, Vollenweider P, Lyssenko V, Bouatia-Naji N, Dupuis J, Jackson AU, Kao WH, Li M, Glazer NL, Manning AK, Luan J, Stringham HM, Prokopenko I, Johnson T, Grarup N, Boesgaard TW, Lecoeur C, Shrader P, O'Connell J, Ingelsson E, Couper DJ, Rice K, Song K, Andreasen CH, Dina C, Kottgen A, Le Bacquer O, Pattou F, Taneera J, Steinthorsdottir V, Rybin D, Ardlie K, Sampson M, Qi L, van Hoek M, Weedon MN, Aulchenko YS, Voight BF, Grallert H, Balkau B, Bergman RN, Bielinski SJ, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Bottcher Y, Brunner E, Buchanan TA, Bumpstead SJ, Cavalcanti-Proenca C, Charpentier G, Chen YD, Chines PS, Collins FS, Cornelis M, G JC, Delplanque J, Doney A, Egan JM, Erdos MR, Firmann M, Forouhi NG, Fox CS, Goodarzi MO, Graessler J, Hingorani A, Isomaa B, Jorgensen T, Kivimaki M, Kovacs P, Krohn K, Kumari M, Lauritzen T, Levy-Marchal C, Mayor V, McAteer JB, Meyre D, Mitchell BD, Mohlke KL, Morken MA, Narisu N, Palmer CN, Pakyz R, Pascoe L, Payne F, Pearson D, Rathmann W, Sandbaek A, Sayer AA, Scott LJ, Sharp SJ, Sijbrands E, Singleton A, Siscovick DS, Smith NL, Sparso T, Swift AJ, Syddall H, Thorleifsson G, Tonjes A, Tuomi T, Tuomilehto J, Valle TT, Waeber G, Walley A, Waterworth DM, Zeggini E, Zhao JH, Illig T, Wichmann HE, Wilson JF, van Duijn C, Hu FB, Morris AD, Frayling TM, Hattersley AT, Thorsteinsdottir U, Stefansson K, Nilsson P, Syvanen AC, Shuldiner AR, Walker M, Bornstein SR, Schwarz P, Williams GH, Nathan DM, Kuusisto J, Laakso M, Cooper C, Marmot M, Ferrucci L, Mooser V, Stumvoll M, Loos RJ, Altshuler D, Psaty BM, Rotter JI, Boerwinkle E, Hansen T, Pedersen O, Florez JC, McCarthy MI, Boehnke M, Barroso I, Sladek R, Froguel P, Meigs JB, Groop L, Wareham NJ, Watanabe RM: Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat Genet 2010;42:142–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehses JA, Casilla VR, Doty T, Pospisilik JA, Winter KD, Demuth HU, Pederson RA, McIntosh CH. Glucose-dependent insulinotropic polypeptide promotes beta-(INS-1) cell survival via cyclic adenosine monophosphate-mediated caspase-3 inhibition and regulation of p38 mitogen-activated protein kinase. Endocrinology 2003;144:4433–4445 [DOI] [PubMed] [Google Scholar]
- 6.Trumper A, Trumper K, Horsch D. Mechanisms of mitogenic and anti-apoptotic signaling by glucose-dependent insulinotropic polypeptide in beta(INS-1)-cells. J Endocrinol 2002;174:233–246 [DOI] [PubMed] [Google Scholar]
- 7.Trumper A, Trumper K, Trusheim H, Arnold R, Goke B, Horsch D. Glucose-dependent insulinotropic polypeptide is a growth factor for beta (INS-1) cells by pleiotropic signaling. Mol Endocrinol 2001;15:1559–1570 [DOI] [PubMed] [Google Scholar]
- 8.Kim SJ, Winter K, Nian C, Tsuneoka M, Koda Y, McIntosh CH. Glucose-dependent insulinotropic polypeptide (GIP) stimulation of pancreatic beta-cell survival is dependent upon phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling, inactivation of the forkhead transcription factor Foxo1, and down-regulation of bax expression. J Biol Chem 2005;280:22297–22307 [DOI] [PubMed] [Google Scholar]
- 9.Yusta B, Baggio LL, Estall JL, Koehler JA, Holland DP, Li H, Pipeleers D, Ling Z, Drucker DJ. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab 2006;4:391–406 [DOI] [PubMed] [Google Scholar]
- 10.Meier JJ, Hucking K, Holst JJ, Deacon CF, Schmiegel WH, Nauck MA. Reduced insulinotropic effect of gastric inhibitory polypeptide in first-degree relatives of patients with type 2 diabetes. Diabetes 2001;50:2497–2504 [DOI] [PubMed] [Google Scholar]
- 11.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 1993;91:301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368:1696–1705 [DOI] [PubMed] [Google Scholar]
- 13.Nauck MA, Baller B, Meier JJ. Gastric inhibitory polypeptide and glucagon-like peptide-1 in the pathogenesis of type 2 diabetes. Diabetes 2004;53(Suppl. 3):S190–S196 [DOI] [PubMed] [Google Scholar]
- 14.Miyawaki K, Yamada Y, Yano H, Niwa H, Ban N, Ihara Y, Kubota A, Fujimoto S, Kajikawa M, Kuroe A, Tsuda K, Hashimoto H, Yamashita T, Jomori T, Tashiro F, Miyazaki J, Seino Y. Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc Natl Acad Sci U S A 1999;96:14843–14847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansotia T, Drucker DJ. GIP and GLP-1 as incretin hormones: lessons from single and double incretin receptor knockout mice. Regul Pept 2005;128:125–134 [DOI] [PubMed] [Google Scholar]
- 16.Herbach N, Goeke B, Schneider M, Hermanns W, Wolf E, Wanke R. Overexpression of a dominant negative GIP receptor in transgenic mice results in disturbed postnatal pancreatic islet and beta-cell development. Regul Pept 2005;125:103–117 [DOI] [PubMed] [Google Scholar]
- 17.Hofmann A, Kessler B, Ewerling S, Weppert M, Vogg B, Ludwig H, Stojkovic M, Boelhauve M, Brem G, Wolf E, Pfeifer A. Efficient transgenesis in farm animals by lentiviral vectors. EMBO Rep 2003;4:1054–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfeifer A, Ikawa M, Dayn Y, Verma IM. Transgenesis by lentiviral vectors: lack of gene silencing in mammalian embryonic stem cells and preimplantation embryos. Proc Natl Acad Sci U S A 2002;99:2140–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klose R, Kemter E, Bedke T, Bittmann I, Kelsser B, Endres R, Pfeffer K, Schwinzer R, Wolf E. Expression of biologically active human TRAIL in transgenic pigs. Transplantation 2005;80:222–230 [DOI] [PubMed] [Google Scholar]
- 20.Larsen MO, Rolin B, Ribel U, Wilken M, Deacon CF, Svendsen O, Gotfredsen CF, Carr RD. Valine pyrrolidide preserves intact glucose-dependent insulinotropic peptide and improves abnormal glucose tolerance in minipigs with reduced beta-cell mass. Exp Diabesity Res 2003;4:93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moritz MW, Dawe EJ, Holliday JF, Elliott S, Mattei JA, Thomas AL. Chronic central vein catheterization for intraoperative and long-term venous access in swine. Lab Anim Sci 1989;39:153–155 [PubMed] [Google Scholar]
- 22.Kobayashi K, Kobayashi N, Okitsu T, Yong C, Fukazawa T, Ikeda H, Kosaka Y, Narushima M, Arata T, Tanaka N. Development of a porcine model of type 1 diabetes by total pancreatectomy and establishment of a glucose tolerance evaluation method. Artif Organs 2004;28:1035–1042 [DOI] [PubMed] [Google Scholar]
- 23.Krickhahn M, Meyer T, Buhler C, Thiede A, Ulrichs K. Highly efficient isolation of porcine islets of Langerhans for xenotransplantation: numbers, purity, yield and in vitro function. Ann Transplant 2001;6:48–54 [PubMed] [Google Scholar]
- 24.Ricordi C, Socci C, Davalli AM, Staudacher C, Baro P, Vertova A, Sassi I, Gavazzi F, Pozza G, Di Carlo V. Isolation of the elusive pig islet. Surgery 1990;107:688–694 [PubMed] [Google Scholar]
- 25.van der Burg MP, Graham JM. Iodixanol density gradient preparation in university of wisconsin solution for porcine islet purification. ScientificWorldJournal 2003;3:1154–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Latif ZA, Noel J, Alejandro R. A simple method of staining fresh and cultured islets. Transplantation 1988;45:827–830 [PubMed] [Google Scholar]
- 27.Jones KH, Senft JA. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J Histochem Cytochem 1985;33:77–79 [DOI] [PubMed] [Google Scholar]
- 28.Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie 1970;26:57–60 [PubMed] [Google Scholar]
- 29.Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE. A second pathway for regeneration of adult exocrine and endocrine pancreas: a possible recapitulation of embryonic development. Diabetes 1993;42:1715–1720 [DOI] [PubMed] [Google Scholar]
- 30.Petrik J, Reusens B, Arany E, Remacle C, Coelho C, Hoet JJ, Hill DJ. A low protein diet alters the balance of islet cell replication and apoptosis in the fetal and neonatal rat and is associated with a reduced pancreatic expression of insulin-like growth factor-II. Endocrinology 1999;140:4861–4873 [DOI] [PubMed] [Google Scholar]
- 31.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both β-cell replication and neogenesis, resulting in increased β-cell mass and improved glucose tolerance in diabetic rats. Diabetes 1999;48:2270–2276 [DOI] [PubMed] [Google Scholar]
- 32.Herbach N, Rathkolb B, Kemter E, Pichl L, Klaften M, de Angelis MH, Halban PA, Wolf E, Aigner B, Wanke R. Dominant-negative effects of a novel mutated Ins2 allele causes early-onset diabetes and severe β-cell loss in Munich Ins2C95S mutant mice. Diabetes 2007;56:1268–1276 [DOI] [PubMed] [Google Scholar]
- 33.Iatropoulos MJ, Williams GM. Proliferation markers. Exp Toxicol Pathol 1996;48:175–181 [DOI] [PubMed] [Google Scholar]
- 34.Hui H, Dotta F, Di Mario U, Perfetti R. Role of caspases in the regulation of apoptotic pancreatic islet beta-cells death. J Cell Physiol 2004;200:177–200 [DOI] [PubMed] [Google Scholar]
- 35.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data New York, Springer, 2001 [Google Scholar]
- 36.Bock T, Kyhnel A, Pakkenberg B, Buschard K. The postnatal growth of the beta-cell mass in pigs. J Endocrinol 2003;179:245–252 [DOI] [PubMed] [Google Scholar]
- 37.Pamir N, Lynn FC, Buchan AM, Ehses J, Hinke SA, Pospisilik JA, Miyawaki K, Yamada Y, Seino Y, McIntosh CH, Pederson RA. Glucose-dependent insulinotropic polypeptide receptor null mice exhibit compensatory changes in the enteroinsular axis. Am J Physiol Endocrinol Metab 2003;284:E931–E939 [DOI] [PubMed] [Google Scholar]
- 38.Hansotia T, Baggio LL, Delmeire D, Hinke SA, Yamada Y, Tsukiyama K, Seino Y, Holst JJ, Schuit F, Drucker DJ. Double incretin receptor knockout (DIRKO) mice reveal an essential role for the enteroinsular axis in transducing the glucoregulatory actions of DPP-IV inhibitors. Diabetes 2004;53:1326–1335 [DOI] [PubMed] [Google Scholar]
- 39.Preitner F, Ibberson M, Franklin I, Binnert C, Pende M, Gjinovci A, Hansotia T, Drucker DJ, Wollheim C, Burcelin R, Thorens B. Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors. J Clin Invest 2004;113:635–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaffer C. Incretin mimetics vie for slice of type 2 diabetes market. Nat Biotechnol 2007;25:263. [DOI] [PubMed] [Google Scholar]
- 41.Medarova Z, Moore A. MRI as a tool to monitor islet transplantation. Nat Rev Endocrinol 2009;5:444–452 [DOI] [PubMed] [Google Scholar]
- 42.Larsen MO, Rolin B. Use of the Gottingen minipig as a model of diabetes, with special focus on type 1 diabetes research. ILAR J 2004;45:303–313 [DOI] [PubMed] [Google Scholar]