Abstract
Objective
We investigated the effect of estrogen replacement on the structure and function of penetrating brain arterioles (PA) and blood-brain barrier (BBB) permeability.
Methods
Female ovariectomized Sprague Dawley rats were replaced with estradiol (E2) and estriol (E3) (OVX+E; N=13) and compared to ovariectomized animals without replacement (OVX; N=14) and intact controls (CTL, proestrous; N=13). Passive and active diameters, percent tone and passive distensibility of pressurized PA were compared. In addition, BBB permeability to Lucifer Yellow, a marker of transcellular transport, was compared in cerebral arteries.
Results
Ovariectomy increased myogenic tone in PA compared to CTL that was not ameliorated by estrogen treatment. Percent tone at 75 mmHg for CTL vs. OVX and OVX+E was 44 ± 3% vs. 51 ± 1% and 54 ± 3% (p<0.01 vs. CTL for both). No differences were found in passive diameters or distensibility between the groups. BBB permeability increased 500% in OVX vs. CTL animals, however, estrogen replacement restored barrier properties: flux of Lucifer Yellow for CTL, OVX and OVX+E was (ng/mL): 3.4 ± 1.2, 20.2 ± 5.3 (p<0.01 vs. CTL) and 6.15 ± 1.2 (n.s.).
Conclusions
These results suggest that estrogen replacement may not be beneficial for small vessel disease in the brain, but may limit BBB disruption and edema under conditions that cause it.
Keywords: estrogen, brain arterioles, myogenic tone, blood-brain barrier
Introduction
The incidence and severity of cerebrovascular disease and stroke increases in postmenopausal women, suggesting a protective effect of endogenous estrogen that is lost during menopause [39,32]. Estrogen receptors (α and β) are expressed on cerebral endothelium and vascular smooth muscle of cerebral arteries and arterioles and are thought to mediate most actions of estrogen [15,27,47]. Numerous studies have shown a beneficial influence of 17β-estradiol (E2) on the cerebral circulation, including effects on vascular tone, inflammation and reactive oxygen species [15,27]. For example, E2 has been shown to mitigate basal resting tone through increased production of nitric oxide (NO) and prostacyclin and reduce production of reactive oxygen species [15,17,33,38]. E2 also decreases inflammatory cytokines that are increased during atherosclerosis and reduces leukocyte adhesion in the brain that is increased due to chronic estrogen depletion [37,45]. The loss of these protective actions of estrogen is thought to promote cerebrovascular disease in postmenopausal women and worsen stroke outcome.
The protective effects of estrogen on the cerebrovasculature have been questioned after report from the Women's Health Initiative (WHI) study found that estrogen replacement increased the risk for stroke [49]. While the majority of studies have investigated the actions of E2 on cerebrovascular function, other estrogen metabolites such as the ER ligand estriol (E3) are less studied. E3 is considered a “weak estrogen” and is widely used in Japan because it has similar beneficial effects as E2 in menopausal women for prevention of osteoporosis and atherosclerotic disease, without endometrial proliferation [35]. Although less studied, E3 has been shown to suppress inflammatory cytokines in vascular smooth muscle and decrease atheromas in high cholesterol-fed rabbits [24,25]. In addition, E3 was shown to be as protective as E2 of cardiac lesions in spontaneously hypertensive stroke-prone rats [18]. To our knowledge, the effect of E3 on cerebrovascular function has not been investigated.
The majority of studies on the effects of estrogen on the cerebral circulation have investigated the response of pial arteries and arterioles to ovariectomy, with or without replacement of E2 [6,15,17,33,38,48]. Pial arteries contribute ∼50% to total cerebrovascular resistance (CVR) and are therefore important for controlling CBF and protecting downstream microvessels from damage during elevated hydrostatic pressure [16]. However, little is known about how ovariectomy or estrogen replacement affects other segments of the cerebrovasculature such as the penetrating parenchymal arterioles (PA). PA are long and unbranched vessels that connect the pial circulation to the microcirculation [8]. These vessels are structurally and functionally distinct from pial vessels in that they possess greater basal tone, lose extrinsic innervation upon entering the brain tissue, and have different receptor profiles, including decreased or absent serotonin and α-adrenergic receptors [10,28]. In addition, these arterioles have prominent EDHF production that may be more important than NO for controlling CBF under conditions in which NO is compromised, such as stroke [11,51]. However, despite their importance for controlling flow in the cerebral cortex, it is not known how ovariectomy or estrogen replacement affects the structure and function of PA.
Another target of estrogen is the cerebral endothelium that forms the blood-brain barrier (BBB). The cerebral endothelium is unique compared to peripheral endothelium in that it contains specialized tight junctions that limit paracellular transport and ionic flux, has limited transcellular transport, and has very low hydraulic conductivity [21,26]. Disruption of these barrier properties causes vasogenic edema, one of the most detrimental consequences of stroke and other cerebrovascular diseases [46]. In addition to its effects on vascular tone and anti-inflammatory properties, E2 has been shown to be protective of the BBB and limit edema formation after conditions such as traumatic brain injury and ischemic stroke [5,36]. However, it is not known if estrogen protects the BBB during acute increases in hydrostatic pressure that are associated with conditions such as posterior reversible encephalopathy syndrome (PRES) and eclampsia [7]. In the present study, we investigated the effect of ovariectomy and estrogen replacement with combined E2 and E3 replacement on the structure and function of PA and BBB permeability in response to acutely elevated hydrostatic pressure. The combined treatment of E2 and E3 was used for this study because we were not certain that there would be any effect of either estrogen on PA structure or function and this initial approach provided a more efficient means of assessing the effects that could be dissected in subsequent studies.
Materials and Methods
Animals
Virgin nonpregnant female Sprague-Dawley rats (250-300g;N=22) (Charles River, St. Constant, QU, Canada) were used for all experiments. All animals were housed in the University of Vermont Animal Care Facility, an American Association for the Accreditation of Laboratory Animal Care (AAALAC) accredited facility. Animals had access to food and water ad libitum and were maintained at a 12 hour light-dark cycle. All the procedures were approved by the University of Vermont Institutional Animal Care and Use committee and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Three groups of female rats were used: virgin female controls (CTL; proestrus stage by vaginal smear; n=7 for BBB permeability experiments and n=6 for PA experiments), ovariectomized with estrogen therapy (OVX+E; n=7 for BBB permeability experiments and n=6 for PA experiments), and ovariectomized (OVX; n=6 for BBB permeability experiments and n=8 for PA experiments). For the OVX+E animals, ovariectomy was performed 30 days before experimentation and pellets (Innovative Research of America, Sarasota, FL, USA) containing 17β-estradiol (0.5 mg 21-day release) and estriol (5.0 mg 21-day release) were subcutaneously implanted in the lateral side of the neck at the time of ovariectomy. For the OVX animals, placebo pellets (Innovative Research of America) were implanted at the time of ovariectomy. All surgical procedures were performed by Charles River (Kingston, NY, USA).
Preparation of cerebral arteries and arterioles
The animals were anesthetized with isoflurane in oxygen and decapitated. The brain was quickly removed and placed in physiologic salt solution (HEPES solution). For BBB permeability experiments, a third-order branch of the posterior cerebral artery (PCA) was carefully dissected, cleared of connective tissue, and placed in an arteriograph chamber (Living Systems, Burlington, VT, USA). For OVX and OVX+E animals the abdomen was opened to verify complete removal of ovaries and to assess the morphologic appearance of the uterus (e.g., edema). For myogenic tone and reactivity measurements of PA, arterioles were identified as branches off the middle cerebral artery that penetrate at right angles into the brain parenchyma, as previously described [9,10]. Once identified, surrounding brain tissue was carefully cleared and the vessel removed and placed in the arteriograph chamber.
Pressurized Arteriograph Chamber
A segment of the dissected artery or arteriole was mounted on two glass cannulas with nylon ties and pressurized, as previously described [10,11]. The arteriograph chamber was attached to a heat exchanger to maintain the temperature of the HEPES solution at 37.0 ± 0.5ºC and pH at 7.40 ± 0.05. The proximal cannula was attached to an in-line pressure transducer with a peristaltic pump and controller that adjusted intravascular pressure. The distal cannula was closed off during reactivity experiments to eliminate flow-induced responses. Lumen diameter was measured via video microscopy.
Experimental Protocols
Myogenic Reactivity of Parenchymal Arterioles
PA were equilibrated for 60 minutes at 25 mm Hg after which the HEPES buffer was replaced by fresh HEPES buffer. Intravascular pressure was then increased in 25 mm Hg steps to 200 mm Hg and lumen diameter measured at each pressure after ∼10 minutes, once stable. To obtain fully relaxed diameters, papaverine (0.1 mmol/L) was added to the chamber to inactivate smooth muscle. In the presence of papaverine, diameter was measured at pressures from 5-200 mm Hg.
BBB Permeability Measurements
Arteries were equilibrated for 45 minutes at 60 mm Hg after which the HEPES buffer was replaced by fresh HEPES buffer. After perfusing the vessel with 0.5 mg/ml Lucifer Yellow-CH (LY; MW 522 da; Molecular Probes, Eugene, OR, USA) in HEPES buffer for three minutes, the HEPES buffer outside the vessel wall was sampled to measure baseline fluorescence intensity using a fluorescent spectrophotometer (Photon Technology International, Birmingham, NJ, USA). After 15 minutes at 60 mm Hg the buffer was sampled again to determine the change in fluorescence. The HEPES buffer was then replaced by fresh buffer. This procedure, except for the equilibration step, was repeated at intravascular pressures from 80-200 mm Hg in steps of 20 mm Hg. The concentration of LY in the buffer outside the vessel wall was quantified from a linear standard curve plotted from known amounts of LY in HEPES. For each animal, intravascular pressure was graphed versus permeability (concentration change of LY) and a regression line was drawn using Sigmaplot graphing software (Systat Software Inc., Chicago, IL), the slope of which is the flux of LY in response to pressure. For each group, the permeability at each pressure and the flux of LY were compared.
Determination of Estrogen Levels
Immediately after the animals were decapitated, trunk blood was collected in PST lithium-heparin tubes and serum isolated by centrifuging for 10 minutes at 2500 rpm. Serum was stored at -80 ºC until analysis. Serum E2 and E3 levels were determined using commercial ELISA kits (Cayman Chemical, Ann Arbor, MI, USA).
Statistical Analysis
Data are presented as mean ± SEM. Differences in body weight, E2 and E3 serum levels, vessel diameter, percent tone, percent distensibility and rate of permeability to LY between CTL, OVX and OVX+E animals were determined by one-way ANOVA and a post hoc Student-Newman-Keuls test for multiple comparisons. Differences were considered significant at P<0.05. A repeated measures ANOVA was used to determine the pressure at which forced dilatation occurred, i.e., the pressure at which the diameter was significantly increased compared to baseline. A two-way ANOVA was used to determine the effect of pressure and estrogen replacement on permeability with a posthoc Bonferroni test for multiple comparisons.
Data Calculations
Percent tone was calculated as the percent decrease in diameter from the fully relaxed diameter in papaverine at each intravascular pressure by the equation: [1 - (φtone / φpapaverine) ] × 100%, where φtone is the diameter of the vessel with tone and φpapaverine is the diameter of the vessel in papaverine. Percent distensibility was calculated at each pressure in fully relaxed vessels in papaverine by determining diameter changes as a function of pressure. The following equation was used: [ (φpressure / φ5mm Hg) − 1] × 100%, where φpressure is the diameter of the vessel at a certain pressure and φ5mm Hg is the diameter of the vessel at 5 mm Hg.
Results
A significant difference in body weight was found between all groups, with OVX animals being the heaviest (367±10 g; P<0.01 vs. CTL and OVX+E) and OVX+E animals the lightest (239±6 g; P<0.01 vs. CTL and OVX) compared to CTL (280±6 g; P<0.01 vs. OVX and OVX+E). The mean serum E2 level of OVX+E animals was 97±23 pg/mL, which was significantly higher than that of the OVX animals which was 23±1 pg/mL (P<0.01) and CTL animals which was 38 ± 5 pg/mL (P<0.01). The mean serum E3 level was 778 ± 151 pg/mL in the OVX+E animals and 3 ± 0.7 pg/mL in the OVX animals (P<0.001) and undetectable in CTL animals.
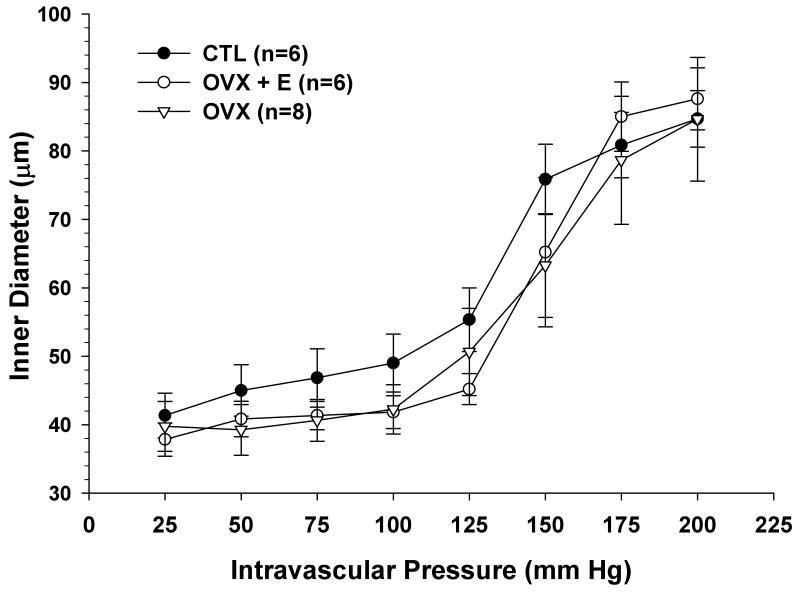
Figure 1 shows the active diameter versus pressure curves of PA for all three groups. At pressures within the autoregulatory range, diameters did not change significantly with increases in intravascular pressure, demonstrating myogenic reactivity in all groups. There were no significant differences in active inner diameters between CTL, OVX and OVX+E animals at any of the pressures studied, suggesting that neither ovariectomy nor estrogen replacement affected myogenic reactivity or active diameters of PA. In addition, the pressure at which forced dilatation occurred was the same for all groups, namely 150 mm Hg.
Figure 1.
Active lumen diameter vs. intravascular pressure for parenchymal arterioles (PA) from control (CTL), ovariectomized (OVX) and estrogen treated ovariectomized (OVX+E) Sprague-Dawley rats. No significant differences in active lumen diameters were found between the groups. Forced dilation occurred at 150 mm Hg for all groups.
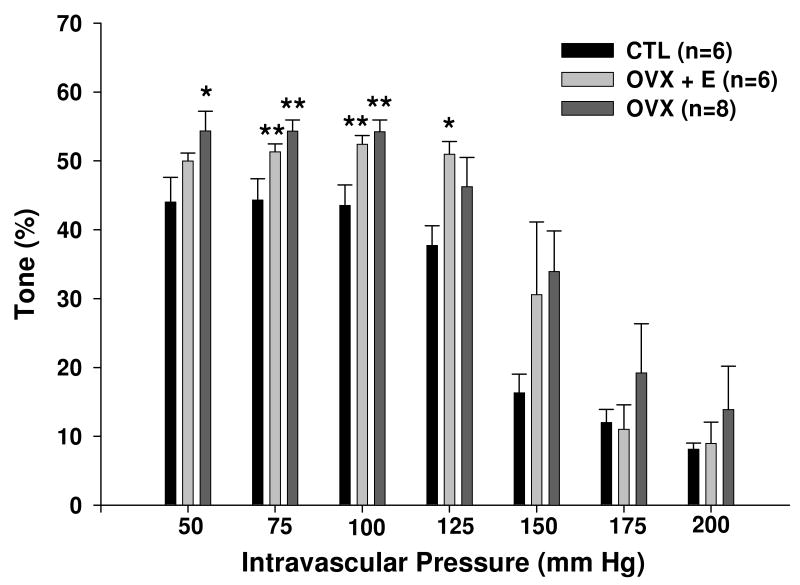
Figure 2 shows the percent myogenic tone of PA for all groups. At intravascular pressures of 75 and 100 mm Hg OVX animals had significantly increased tone compared to CTL animals (P<0.01). Estrogen replacement did not restore tone to CTL levels as OVX+E animals also had increased tone between pressures of 75 and 125 mm Hg compared to CTL animals.
Figure 2.
Percent myogenic tone of parenchymal arterioles (PA) from control (CTL), ovariectomized (OVX) and estrogen treated ovariectomized (OVX+E) Sprague-Dawley rats at pressure from 50-200 mm Hg. OVX animals had increased tone compared to CTL animals, that was not restored by estrogen replacement. *P < 0.05 versus CTL, ** P < 0.01 versus CTL
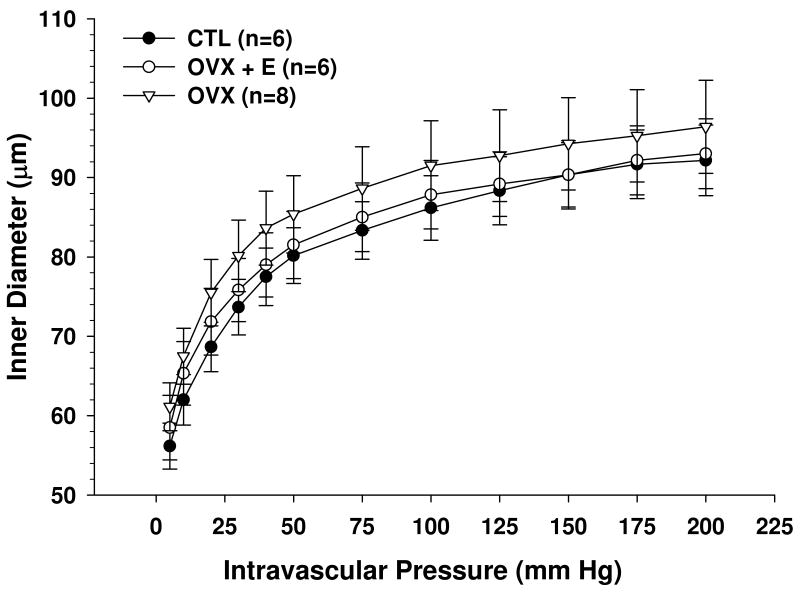
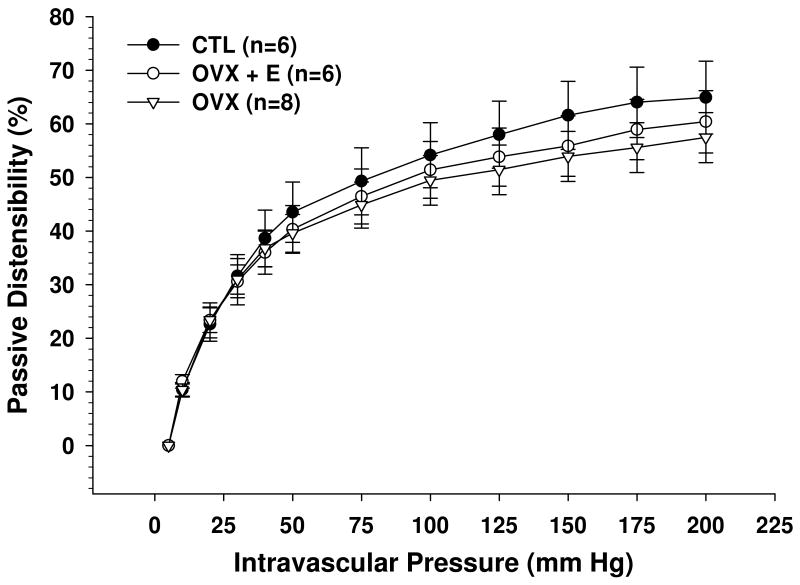
To determine if estrogen affected structural remodeling of PA, passive diameter and distensibility measurements in papaverine were performed (Figures 3 and 4). No differences were found in passive lumen diameters, suggesting that neither ovariectomy nor estrogen caused remodeling of the PA. In addition, there was no difference in distensibility between CTL, OVX and OVX+E animals, suggesting that estrogen did not affect structural components of the vascular wall.
Figure 3.
Passive lumen diameter vs. pressure of parenchymal arterioles (PA) from control (CTL), ovariectomized (OVX) and estrogen replaced ovariectomized (OVX+E) Sprague-Dawley rats. No significant differences were found between groups.
Figure 4.
Percent passive distensibility vs. pressure of parenchymal arterioles (PA) from control (CTL), ovariectomized (OVX) and estrogen replaced ovariectomized (OVX+E) Sprague-Dawley rats. No significant differences were found between groups.
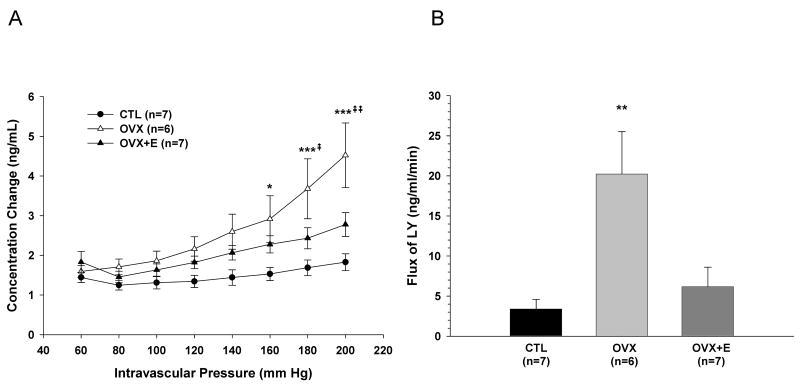
Figure 5A shows BBB permeability to LY in response to pressure for all groups of animals. Permeability changed little in response to increased pressure in CTL animals, however, OVX animals had a significantly higher permeability to LY compared to CTL animals at pressures >160 mm Hg. Estrogen replacement decreased BBB permeability such that it was not significantly different from CTL at any pressure. Figure 5B shows the average flux of LY in response to pressure, calculated from the slope of the regression line of the pressure vs. permeability curves for each group. There was a 500% increase in BBB permeability in response to elevated hydrostatic pressure in OVX compared to CTL animals that was ameliorated by estrogen replacement, as OVX+E animals had permeability similar to CTL animals (P<0.01 vs OVX).
Figure 5.
Blood-brain barrier permeability to Lucifer Yellow (LY). A, Graph showing permeability at each pressure for posterior cerebral arteries (PCA) from control (CTL), ovariectomized (OVX) and estrogen replaced ovariectomized (OVX+E) Sprague-Dawley rats. B, Flux of LY calculated from the average slope (m) of the regression lines for permeability vs. intravascular pressure. OVX animals had a significantly higher permeability to LY compared to CTL animals, while OVX+E animals showed permeability similar to CTL animals. ***P < 0.001 versus CTL; **P < 0.01 versus CTL; *P < 0.05 versus CTL; ⇞⇞ P<0.01 vs. OVX+E; ⇞ P<0.05 vs. OVX+E.
Discussion
In the present study, we investigated the effect of estrogen replacement with a combination of E2 and E3 on PA structure and function. These arterioles are the site of small vessel resistance in the brain and connect the pial vessels to the microcirculation [8,10,16]. If estrogen replacement diminished myogenic tone of these arterioles as has been shown in pial vessels [17], this could benefit the brain during conditions such as ischemic stroke and small vessel occlusive disease by increasing CBF and limiting ischemia. In addition, we investigated how combined E2/E3 treatment affected BBB permeability in response to increased hydrostatic pressure. We found that while estrogen replacement with E2/E3 did not affect myogenic tone or structural components of PA, it had a profound effect on BBB permeability. Permeability to LY was significantly increased in OVX animals, however, estrogen replacement restored barrier properties to the level of CTL. These results suggest that estrogen replacement may not be beneficial for small vessel disease in the brain or protective of infarction, but may limit vasogenic edema during elevations in pressure.
The present study found that, similar to pial vessels, ovariectomy significantly increased myogenic tone compared to CTL animals. However, in contrast to pial arteries, replacement of E2/E3 did not decrease myogenic tone in PA. In fact, tone was similarly increased in both groups of animals with ovariectomy, regardless of the presence of estrogen. While the level of tone was increased in ovariectomized animals, regardless of estrogen replacement, reactivity to increased intravascular pressure or myogenic reactivity was similar between groups. Myogenic reactivity is the vasoactive response to pressure [22]. It is prominent in brain arteries and arterioles that underlies cerebrovascular resistance and contributes significantly to autoregulation of CBF [31]. PA from all groups maintained diameter within the pressure range from 25 to 125 mmHg, after which forced dilatation occurred, suggesting that the increase in the level of tone did not affect the pressure at which forced dilatation occurred. Previous studies have shown that increased myogenic tone in cerebral pial arteries from ovariectomized rats was endothelium-dependent and due to the loss of estrogen's enhanced NO production and a switch from vasodilating to vasoconstricting prostaglandins [15,17,38]. In contrast, Chrissobolis found that basilar arteries from female rats had greater basal NO production that was estrogen-independent [6]. In that study, the vasoprotective effects of estrogen was thought to be due to suppression of Rho-kinase function. In the present study, the mechanism by which ovariectomy increased myogenic tone in PA was not investigated and further studies are needed at assess the mechanism by which OVX affected myogenic tone in these arterioles.
There are several possible explanations for the different responsiveness to estrogen between pial arteries and PA. Since the vasodilatory effect of estrogen is likely receptor mediated [29,30], differential expression of estrogen receptors between pial arteries and PA may account for the different responsiveness to estrogen replacement. In fact, differences in receptor number and density of α- and β-adrenoreceptors, and serotonin (5HT) receptors have been found between pial and PA [10,28]. However, differential expression of estrogen receptors by the cerebral endothelium of these vessel types is not known. In addition, PA develop greater myogenic tone at lower pressures compared to pial arteries, suggesting that the vascular smooth muscle is more depolarized at lower pressures. Therefore, the differential effect of estrogen on myogenic tone between pial and PA may be related to the basal level of tone that is more resistant to the dilatory effects of estrogen. In addition to increased tone at lower pressures, PA appear to have basal EDHF production that is not present in pial arteries [11]. It is possible that ovariectomy inhibits basal EDHF that is not affected by estrogen.
This study also found that E2/E3 did not affect the passive structure of PA. Passive lumen diameters, determined in the presence of papaverine, were not different between groups, suggesting that ovariectomy and estrogen replacement did not cause remodeling of PA. In addition, no differences between the groups were found in passive distensibility, an indirect measure of the collagen:elastin ratio of the vascular wall [13]. This suggests that estrogen replacement did not have an effect on the acellular components of the vessel wall of PA, which is in contrast to findings in the peripheral circulation. For example, estrogen treatment in aging female rats has been shown to increase distensibility of mesenteric arteries [52]. Also, hormonal replacement therapy in postmenopausal women increased carotid artery distensibility [4]. The lack of an effect of estrogen on brain arterioles may be related to an effect of estrogen on large arteries vs. small arterioles, or may be specific to brain vessels. In fact, our results are consistent with other studies in which distensibility of cerebral pial arteries was not different in pregnancy, a state of high E2/E3 levels [12].
In the present study, BBB permeability was measured using LY, a polar compound that does not pass through the high electrical resistance tight junctions of the BBB [21] and is a marker of transcellular transport [9,19]. We used cerebral pial arteries that have BBB properties [1,26] to investigate changes in permeability in response to ovariectomy and estrogen replacement in vitro. In cerebral endothelium that comprised the BBB, transcellular transport is low under normal conditions, but increases substantially under pathologic conditions in which barrier permeability is enhanced leading to vasogenic edema [19,34,50]. One of the earliest microscopic findings in an experimental animal model of acute hypertension (i.e., hypertensive encephalopathy) is an increased rate of pinocytosis in the cerebrovascular endothelium of arteries and arterioles, which allows significant passage of fluid and molecules into the brain [19,34]. Our own studies have shown in cerebral pial arteries that pressure alone is a potent stimulus for pinocytosis and transcellular transport [9]. In the present study, we found that ovariectomy significantly increased BBB permeability to LY in response to pressure, that was prevented by estrogen replacement. To our knowledge this is the first study to suggest that ovariectomy and estrogen affect transcellular transport.
Increased cerebrovascular permeability is considered the most important factor for development of cerebral edema and is determined by the cerebral endothelial cells that form the blood-brain barrier [26,46]. While contrary to some studies, the finding that estrogen is protective of the BBB during brain injury and limits edema formation has been found. Estrogen treatment in male and ovariectomized female rats attenuated edema formation after traumatic brain injury [36]. Estrogen also reduced BBB disruption in ovariectomized rats during focal cerebral ischemia [5]. In addition, Bake et al. reported decreased Evans Blue extravasation in the olfactory bulb and hippocampus in estrogen treated ovariectomized animals vs. untreated ovariectomized animals [3]. Further, increased BBB permeability was found in the cortex, brain stem, and cerebellum in ovariectomized animals compared to animals in proestrus stage when estrogen is the lowest [44]. Together with the results from the current study, it appears that estrogen replacement with E2/E3 is protective of the BBB that is increased in response to ovariectomy. However, it is worth noting that because E2 and E3 were given in combination, it is not clear which estrogen had a greater effect and further studies are needed to assess the efficacy of different estrogens on BBB permeability.
While the mechanism by which estrogen is protective of the BBB and limits cerebral edema is currently unknown, several possibilities have been postulated. Estrogen is a known antioxidant [15,32,33] and has been reported to inhibit membrane lipid peroxidation [2], thereby possibly attenuating the increased BBB permeability caused by free radical generation that is known to occur during acute hypertension and other brain injuries [41,50]. However, given that the level of estrogen in the OVX+E group was greater than CTL, estrogen replacement would be expected in this case to have a greater antioxidant effect and restore permeability to control levels. Although not statistically different, the permeability of the OVX+E group was not completely restored, suggesting the antioxidant effect of estrogen was not likely to be the only contributor to improved barrier function. In addition to an effect on cerebral endothelium, estrogen may also indirectly limit edema formation in the intact brain through regulation of aquaporins, as has been shown in peripheral tissues [42]. These transmembrane water channels have been proposed to have a role in the development and resolution of cerebral edema [26,40]. Lastly, estrogen has been shown to upregulate expression of the tight junction protein occludin that is decreased during ovariectomy [23]. Although LY is a polar compound that does not pass through the high electrical resistance tight junctions, it is possible that ovariectomy and estrogen have similar transcriptional effects on the machinery of pinocytotic vesicles that are important for transcellular transport (e.g., dynamin).
In conclusion, this study demonstrates that estrogen replacement with combined E2/E3 did not affect myogenic tone of brain PA. In fact, myogenic tone was significantly elevated in PA from ovariectomized animals, regardless of estrogen replacement. Ovariectomy also significantly increased BBB permeability to LY, suggesting increased transcellular transport. However, unlike the effect on myogenic tone, E2/E3 treatment was effective at protecting the BBB from disruption under these conditions.
Acknowledgments
We gratefully acknowledge the support of American Heart Association Established Investigator Award 0540081N and the NIH National Institutes of Neurologic Disorders and Stroke grant NS045940. We also would like to thank the Totman Medical Research Trust for their continued support.
Grant support: NINDS grants RO1 NS045940, RO1 NS043316, American Heart Association Established Investigator Award 0540081N and the Totman Medical Research Trust.
References
- 1.Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002;200:629–38. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayres S, Abplanalp W, Liu JH, Subbiah MT. Mechanisms involved in the protective effect of estradiol-17beta on lipid peroxidation and DNA damage. Am J Physiol. 1998;274:E1002–1008. doi: 10.1152/ajpendo.1998.274.6.E1002. [DOI] [PubMed] [Google Scholar]
- 3.Bake S, Sohrabji F. 17beta-estradiol differentially regulates blood-brain barrier permeability in young and aging female rats. Endocrinology. 2004;145:5471–5475. doi: 10.1210/en.2004-0984. [DOI] [PubMed] [Google Scholar]
- 4.Bui MN, Arai AE, Hathaway L, Waclawiw MA, Csako G, Cannon RO., 3rd Effect of hormone replacement therapy on carotid arterial compliance in healthy postmenopausal women. Am J Cardiol. 2002;90:82–85. doi: 10.1016/s0002-9149(02)02397-4. [DOI] [PubMed] [Google Scholar]
- 5.Chi OZ, Liu X, Weiss HR. Effects of 17beta-estradiol on blood-brain barrier disruption during focal ischemia in rats. Horm Metab Res. 2002;34:530–534. doi: 10.1055/s-2002-34794. [DOI] [PubMed] [Google Scholar]
- 6.Chrissobolis S, Budzyn K, Marley PD, Sobey CG. Evidence that estrogen suppresses rho-kinase function in the cerebral circulation in vivo. Stroke. 2004;35:2200–5. doi: 10.1161/01.STR.0000136951.85586.c8. [DOI] [PubMed] [Google Scholar]
- 7.Cipolla MJ. Cerebrovascular function in pregnancy and eclampsia. Hypertension. 2007;50:14–24. doi: 10.1161/HYPERTENSIONAHA.106.079442. [DOI] [PubMed] [Google Scholar]
- 8.Cipolla MJ, Bullinger LV. Reactivity of brain parenchymal arterioles after ischemia and reperfusion. Microcirculation. 2008;15:495–501. doi: 10.1080/10739680801986742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cipolla MJ, Crete R, Vitullo L, Rix RD. Transcellular transport as a mechanism of blood-brain barrier disruption during stroke. Front Biosci. 2004;9:777–785. doi: 10.2741/1282. [DOI] [PubMed] [Google Scholar]
- 10.Cipolla MJ, Li R, Vitullo L. Perivascular innervation of penetrating brain parenchymal arterioles. J Cardiovasc Pharmacol. 2004;44:1–8. doi: 10.1097/00005344-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Cipolla MJ, Smith J, Kohlmeyer MM, Godfrey JA. SKCa and IKCa Channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: effect of ischemia and reperfusion. Stroke. 2009;40:1451–7. doi: 10.1161/STROKEAHA.108.535435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cipolla MJ, Vitullo L, McKinnon J. Cerebral artery reactivity changes during pregnancy and the postpartum period: a role in eclampsia? Am J Physiol Heart Circ Physiol. 2004;286:H2127–2132. doi: 10.1152/ajpheart.01154.2003. [DOI] [PubMed] [Google Scholar]
- 13.Coulson RJ, Cipolla MJ, Vitullo L, Chesler NC. Mechanical properties of rat middle cerebral arteries with and without myogenic tone. J Biomech Eng. 2004;126:76–81. doi: 10.1115/1.1645525. [DOI] [PubMed] [Google Scholar]
- 14.Drewes L. Molecular architecture of the brain microvasculature: perspective on blood-brain barrier transport. J Mol Neurosci. 2001;16:93–8. doi: 10.1385/JMN:16:2-3:93. [DOI] [PubMed] [Google Scholar]
- 15.Duckles SP, Krause DN. Cerebrovascular effects of oestrogen: multiplicity of action. Clin Exp Pharmacol Physiol. 2007;34:801–8. doi: 10.1111/j.1440-1681.2007.04683.x. [DOI] [PubMed] [Google Scholar]
- 16.Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- 17.Geary GG, Krause DN, Duckles SP. Estrogen reduces myogenic tone through a nitric oxide-dependent mechanism in rat cerebral arteries. Am J Physiol. 1998;275:H292–300. doi: 10.1152/ajpheart.1998.275.1.H292. [DOI] [PubMed] [Google Scholar]
- 18.Gross ML, Ritz E, Korsch M, Adamczak M, Weckbach M, Mall G, Berger I, Hansen A, Amann K. Effects of estrogens on cardiovascular structure in uninephrectomized SHRsp rats. Kidney Int. 2005;67:849–57. doi: 10.1111/j.1523-1755.2005.00149.x. [DOI] [PubMed] [Google Scholar]
- 19.Hansson HA, Johansson BB. Induction of pinocytosis in cerebral vessels by acute hypertension and by hyperosmolar solutions. J Neurosci Res. 1980;5:183–90. doi: 10.1002/jnr.490050303. [DOI] [PubMed] [Google Scholar]
- 20.Hardebo JE, Owman C. Barrier mechanisms for neurotransmitter monoamines and their precursors at the blood-brain barrier. Ann Neurol. 1979;8:1–11. doi: 10.1002/ana.410080102. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 22.Johansson B. Myogenic tone and reactivity: definitions based on muscle physiology. J Hyperten. 1989;7 4:S5–S8. [PubMed] [Google Scholar]
- 23.Kang HS, Ahn HS, Kang HJ, Gye MC. Effect of estrogen on the expression of occludin in ovariectomized mouse brain. Neurosci Lett. 2006;402:30–4. doi: 10.1016/j.neulet.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 24.Kano H, Hayashi T, Sumi D, Matusi-Hirai H, Tsunekawa T, Endo H, Iguchi A. Estriol retards and stabilizes atherosclerosis through an NO-mediated system. Life Sci. 2002;71:31–42. doi: 10.1016/s0024-3205(02)01564-3. [DOI] [PubMed] [Google Scholar]
- 25.Kikuchi N, Urabe M, Iwasa K, Okubo T, Tsuchiya H, Hosoda T, Tatsumi H, Honjo H. Atheroprotective effect of estriol and estrone sulfate on human vascular smooth muscle cells. J Steroid Biochem Mol Biol. 2000;72:71–8. doi: 10.1016/s0960-0760(99)00149-1. [DOI] [PubMed] [Google Scholar]
- 26.Kimelberg HK. Water homeostasis in the brain: basic concepts. Neuroscience. 2004;129:851–60. doi: 10.1016/j.neuroscience.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 27.Krause DN, Duckles SP, Pelligrino DA. Influence of sex steroid hormones on cerebrovascular function. J Appl Physiol. 2006;101:1252–61. doi: 10.1152/japplphysiol.01095.2005. [DOI] [PubMed] [Google Scholar]
- 28.Lincoln J. Innervation of cerebral arteries by nerves containing 5-hydroxytryptamine and noradrenaline. Pharmacol Ther. 1995;68:473–501. doi: 10.1016/0163-7258(95)02017-9. [DOI] [PubMed] [Google Scholar]
- 29.Littleton-Kearney MT, Agnew DM, Traystman RJ, Hurn PD. Effects of estrogen on cerebral blood flow and pial microvasculature in rabbits. Am J Physiol Heart Circ Physiol. 2000;279:H1208–1214. doi: 10.1152/ajpheart.2000.279.3.H1208. [DOI] [PubMed] [Google Scholar]
- 30.McNeill AM, Zhang C, Stanczyk FZ, Duckles SP, Krause DN. Estrogen increases endothelial nitric oxide synthase via estrogen receptors in rat cerebral blood vessels: effect preserved after concurrent treatment with medroxyprogesterone acetate or progesterone. Stroke. 2002;33:1685–1691. doi: 10.1161/01.str.0000016325.54374.93. [DOI] [PubMed] [Google Scholar]
- 31.Mellander S. Functional aspects of myogenic vascular control. J Hyperten. 1989;7 4:S21–S30. [PubMed] [Google Scholar]
- 32.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–11. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 33.Miller AA, Drummond GR, Mast AE, Schmidt HH, Sobey CG. Effect of gender on NADPH-oxidase activity, expression, and function in the cerebral circulation: role of estrogen. Stroke. 2007;38:2142–9. doi: 10.1161/STROKEAHA.106.477406. [DOI] [PubMed] [Google Scholar]
- 34.Nag S, Robertson DM, Dinsdale HB. Quantitative estimate of pinocytosis in experimental acute hypertension. Acta Neuropathol (Berl) 1979;46:107–116. doi: 10.1007/BF00684811. [DOI] [PubMed] [Google Scholar]
- 35.Nishibe A, Morimoto S, Hirota K, Yasuda O, Ikegami H, Yamamoto T, Fukuo K, Onishi T, Ogihara T. Effect of estriol and bone mineral density of lumbar vertebrae in elderly and postmenopausal women. Jap J Geriatrics. 1996;33:353–359. doi: 10.3143/geriatrics.33.353. [DOI] [PubMed] [Google Scholar]
- 36.O'Connor CA, Cernak I, Vink R. Both estrogen and progesterone attenuate edema formation following diffuse traumatic brain injury in rats. Brain Res. 2005;1062:171–174. doi: 10.1016/j.brainres.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Ospina JA, Brevig HN, Krause DN, Duckles SP. Estrogen suppresses IL-1beta-mediated induction of COX-2 pathway in rat cerebral blood vessels. Am J Physiol Heart Circ Physiol. 2004;286:H2010–9. doi: 10.1152/ajpheart.00481.2003. [DOI] [PubMed] [Google Scholar]
- 38.Ospina JA, Duckles SP, Krause DN. 17beta-estradiol decreases vascular tone in cerebral arteries by shifting COX-dependent vasoconstriction to vasodilation. Am J Physiol Heart Circ Physiol. 2003;285:H241–50. doi: 10.1152/ajpheart.00018.2003. [DOI] [PubMed] [Google Scholar]
- 39.Paganini-Hill A, Ross RK, Henderson BE. Postmenopausal oestrogen treatment and stroke: a prospective study. BMJ. 1988;297:519–22. doi: 10.1136/bmj.297.6647.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18:1291–1293. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- 41.Poulet R, Gentile MT, Vecchione C, Distaso M, Aretini A, Fratta L, Russo G, Echart C, Maffei A, De Simoni MG, Lembo G. Acute hypertension induces oxidative stress in brain tissues. J Cereb Blood Flow Metab. 2006;26:253–262. doi: 10.1038/sj.jcbfm.9600188. [DOI] [PubMed] [Google Scholar]
- 42.Richard C, Gao J, Brown N, Reese J. Aquaporin water channel genes are differentially expressed and regulated by ovarian steroids during the periimplantation period in the mouse. Endocrinology. 2003;144:1533–1541. doi: 10.1210/en.2002-0033. [DOI] [PubMed] [Google Scholar]
- 43.Rubin LL, Standdon JM. The cell biology of the blood-brain barrier. Annu Rev Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- 44.Saija A, Princi P, D'Amico N, De Pasquale R, Costa G. Aging and sex influence the permeability of the blood-brain barrier in the rat. Life Sci. 1990;47:2261–2267. doi: 10.1016/0024-3205(90)90157-m. [DOI] [PubMed] [Google Scholar]
- 45.Santizo R, Pelligrino DA. Estrogen reduces leukocyte adhesion in the cerebral circulation of female rats. J Cereb Blood Flow Metab. 1999;19:1061–5. doi: 10.1097/00004647-199910000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–68. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stirone C, Duckles SP, Krause DN. Multiple forms of estrogen receptor-alpha in cerebral blood vessels: regulation by estrogen. Am J Physiol Endocrinol Metab. 2003;284:E184–92. doi: 10.1152/ajpendo.00165.2002. [DOI] [PubMed] [Google Scholar]
- 48.Thorin E, Pham-Dang M, Clement R, Mercier I, Calderone A. Hyper-reactivity of cerebral arteries from ovariectomized rats: therapeutic benefit of tamoxifen. Br J Pharmacol. 2003;140:1187–92. doi: 10.1038/sj.bjp.0705547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ, WHI Investigators Effect of estrogen plus progestin on stroke in postmenopausal women: the Women's Health Initiative: a randomized trial. JAMA. 2003;289:2673–84. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 50.Wei EP, Kontos HA, Christman CW, DeWitt DS, Povlishock JT. Superoxide generation and reversal of acetylcholine-induced cerebral arteriolar dilation after acute hypertension. Circ Res. 1985;57:781–787. doi: 10.1161/01.res.57.5.781. [DOI] [PubMed] [Google Scholar]
- 51.You J, Johnson TD, Marrelli SP, Bryan RM., Jr Functional heterogeneity of endothelial P2 purinoceptors in the cerebrovascular tree of the rat. Am J Physiol. 1999;277:H893–900. doi: 10.1152/ajpheart.1999.277.3.H893. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y, Stewart KG, Davidge ST. Estrogen replacement reduces age-associated remodeling in rat mesenteric arteries. Hypertension. 2000;36:970–974. doi: 10.1161/01.hyp.36.6.970. [DOI] [PubMed] [Google Scholar]