Abstract
Fijian infants aged 6 weeks were stratified by ethnicity and randomized to receive 0, 1, 2, or 3 PCV-7 doses with or without the 23-valent pneumococcal polysaccharide vaccine (PPV-23) at 12 months. Strong booster effects for all 7 PCV-7 serotypes were elicited, and for 4/7 serotypes these responses were highest in the single PCV-7 group. There were fourfold rises in GMC for all non-PCV-7 serotypes. By 17 months the PPV-23 group still had significantly higher GMC (each p<0.001) for all serotypes. The PPV-23 was well tolerated and induced excellent responses for all serotypes which were greatest in the single PCV-7 group.
Keywords: Pneumococcal, polysaccharide, booster
Introduction
Streptococcus pneumoniae is the most common cause of bacterial pneumonia in children worldwide. It is the leading vaccine preventable cause of serious infection in infants [1]. A recent review estimated that over 14 million episodes of serious pneumococcal disease occurred worldwide in the year 2000, with over 800,000 deaths in children under 5 years [2]. The case fatality rate is particularly high in infants less than 6 months old [3]. At least 48 serogroups comprising over 90 serotypes of pneumococcus have been identified [4]. Within serogroups, some serotypes cross-react immunologically, and in some cases this translates into cross-protection such as antibodies against 6B which provide cross-protection against 6A [5]. The association of particular serotypes with disease varies according to age, geography, and clinical presentation [6]. In general, the range of serotypes causing invasive pneumococcal disease (IPD) in affluent countries like the United States and in Europe is relatively narrow and largely confined to the serotypes found in the 7-valent pneumococcal conjugate vaccine (PCV-7, Prevenar™, Wyeth Vaccines). In contrast, the range of serotypes causing disease in low-income countries is wider.
The 10-valent pneumococcal conjugate vaccine has recently been licensed in some countries, and a 13-valent vaccine is likely to be licensed by 2010. Some health authorities have decided or are considering a combination of an infant PCV-7 primary series with a booster of the 23-valent pneumococcal polysaccharide vaccine (PPV-23) in the second year of life to address the limited serotype coverage offered by PCV-7. There have been several studies involving children in a number of countries using different pneumococcal conjugate formulations and schedules, comparing the immunogenicity of a PPV-23 or PCV-7 booster following a pneumococcal conjugate vaccine primary series. The majority of studies have shown that serotype-specific antibody concentrations are generally higher following PPV-23 than PCV-7 booster [7-12]. The higher response may be due to the higher dose of pneumococcal polysaccharide in the PPV-23, compared to PCV-7, enhancing the stimulation of memory B cells or by stimulating a greater number of B cells overall [13]. Despite this, only pneumococcal conjugate vaccines provide mucosal immunity and have shown a reduction in nasopharyngeal (NP) carriage (NP carriage being an antecedent event for all pneumococcal disease) for conjugate serotypes, compared with unvaccinated infants [14-16] or toddlers [17-19]. In contrast, pneumococcal polysaccharide vaccines have shown no effect on pneumococcal carriage [20-24]. Most studies evaluating the impact of pneumococcal polysaccharide immunization in the absence of additional PCV-7 in infants or children have not shown any impact on pneumococcal disease or carriage [25-27].
Data from Fiji shows that the 7 serotypes included in PCV-7, plus the cross reactive serotype 6A, would potentially cover 63.3% of invasive pneumococcal disease (IPD) cases in children under 5 years [28]. This coverage would potentially increase to 83% if the PPV-23 was used, and would increase to 87% if the new 13-valent pneumococcal conjugate vaccine produced by Wyeth Vaccines (which includes serotypes 1, 3, 5, 6A, 7F and 19A) was used, largely due to the inclusion of 6A which is not included in the PPV-23 [28]. The aim of this study was to find an optimal vaccination strategy suitable for resource poor countries in terms of serotype coverage, flexibility, and affordability. To address these issues, we undertook a Phase II vaccine trial in Fiji to document the safety, immunogenicity and impact on pneumococcal carriage of various pneumococcal vaccination regimens combining 1, 2, or 3 doses of PCV-7 in infancy. In order to broaden the serotype coverage, the additional benefit of a PPV-23 booster at 12 months of age was also assessed. Presented are the geometric mean serotype-specific IgG antibody concentrations (GMC) prior to and 2 weeks following the 12 month PPV-23, and at 17 months of age.
Methods
Study participants
The study was a single blind, open-label randomized Phase II vaccine trial undertaken in Suva, the capital of Fiji. Healthy infants aged between six and eight weeks were eligible for enrolment. Details of the selection criteria and the randomization procedure have been reported elsewhere [29].
The study was conducted and monitored according to Good Clinical Practice. It was approved by the Fiji National Research Ethics Review Committee and the University of Melbourne Human Research Ethics Committee.
Study procedures and vaccines
Infants were stratified by ethnicity and randomized into one of eight groups. The seven-valent CRM197 protein-polysaccharide conjugate vaccine containing polysaccharide antigen from pneumococcal serotypes 4, 6B, 9V, 14, 18C, 19F, 23F (Prevenar™, Wyeth Vaccines) was used. The vaccine contains 2 μg of each serotype, except serotype 6B which contains 4μg. The three dose group received PCV-7 at 6, 10, and 14 weeks of age, the 2 dose group received PCV-7 at 6 and 14 weeks of age and the single dose group received PCV-7 at 14 weeks of age. Routine vaccines (Hiberix™ mixed with Tritanrix™-HepB™, GlaxoSmithKline) and oral polio were given with the primary series. Hiberix™ contains 10μg of purified Hib capsular polysaccharide covalently bound to approximately 30μg tetanus toxoid mixed with Tritanrix™-HepB™ which contains not less than 30 IU of adsorbed D toxoid, not less than 60 IU of adsorbed T toxoid, not less than 4 IU of whole cell Pertussis, and 10μg of recombinant HBsAg protein. The children in all primary series groups were further randomized to receive a dose of PPV-23 (Pneumovax™, Merck & Co., Inc., which consists of a purified mixture of 25μg of capsular polysaccharide from 23 pneumococcal serotypes) or no vaccine at 12 months of age (window: 12 months plus 4 weeks). In addition, all children received Measles-Rubella vaccine at 12 months of age co-administered with PPV-23. The children randomized to receive 0 or 1 PCV-7 dose in infancy had a single dose of PCV-7 administered at 2 years of age.
Children were reviewed on day 1, 2 and 7 following PPV-23 and assessed for any adverse event (AE). An AE was defined as any unfavorable and unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with the use of PPV-23, whether or not related to PPV-23. A severe non-serious AE was defined as an event which prevented normal activities but did not meet the criteria of a serious AE (SAE). A SAE was defined as an AE meeting one of the following conditions: death in the 2 year follow up period; a life threatening event; hospitalization or prolongation of existing hospitalization during the 2 year period; or resulting in a persistent or significant disability/incapacity.
SAEs were sourced from parent interview at each study visit and via a search of computerized hospital discharge data. Causality of any non-serious AE were assigned by the study doctor and reviewed by a pediatrician (FR). Causality of SAEs were assigned by the study doctor and assessed by an independent external safety monitor and regularly reviewed by the study's Data Safety and Monitoring Board.
Laboratory procedures
Children who received the 12 month PPV-23 had blood drawn immediately prior to and 14 days following the PPV-23 (window: 10-21 days post PPV-23). All children had blood drawn at 17 months of age. Blood was separated by centrifugation in the health centre, kept chilled and transported to the Colonial War Memorial Hospital laboratory, Suva, where it was divided into aliquots and stored at -20°C on the same day, until transported to the Pneumococcal Laboratory, Murdoch Childrens Research Institute, Melbourne, on dry ice for analysis.
Anticapsular pneumococcal antibody levels were assayed for all PPV-23 serotypes (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, 33F), using a modified 3rd generation ELISA based on current WHO recommendations [30]. In brief, microtiter wells were coated with pneumococcal polysaccharide diluted in phosphate buffered saline by incubating at room temperature overnight. To neutralise non-specific antibodies to cell wall polysaccharide (C-PS), serum samples for all serotypes (except serotype 22F) were diluted 1/100 in pre-absorption buffer containing C-PS (10μg/mL) and serotype 22F (30μg/mL) and incubated overnight at 4°C. Absorption with 30 μg/ml serotype 22F overnight has been reported previously [31, 32] and unpublished data from our laboratory have shown this to further improve the specificity of the pneumococcal ELISA. The reference serum standard 89-SF (Food and Drug Administration, Bethesda MD) and samples for measurement of specific IgG to serotype 22F were pre-absorbed with C-PS at 10μg/mL and incubated overnight at 4°C. Horseradish peroxidase conjugated anti-human IgG and a TMB (3.3′, 5.5′-tetramethylbenzidine) substrate solution was used for detection. A high, medium, and low control serum were used on each plate to assess assay performance and inter-assay variation. Results from an inter-laboratory comparison between the Pneumococcal Laboratory, Murdoch Childrens Research Institute, (Melbourne, Australia), Wyeth Vaccine Research Laboratory (USA) and the KTL laboratory (Finland) demonstrated a good correlation of serotype-specific antibody concentrations [33]. Laboratory staff members were blinded to the group allocation of each serum sample.
Statistical analysis
This manuscript reports analytic results concerning the secondary purpose of the trial. Cleaned data were exported to Stata version 9.0 (Stata Corporation, College Station, Texas) for analysis. Serotype-specific antibody concentrations by ELISA were log (base e) transformed to calculate GMC. Comparisons of serotype-specific GMC between 0-3 dose PCV-7 groups were performed using a two sample t-test. Comparisons of serotype-specific GMC before and after the PPV-23 were performed using the paired t test. Comparisons of the proportion of infants between groups with serotype-specific antibody concentrations ≥0.35 and ≥1μg/mL were performed using Fisher's exact test. Comparisons of serotype-specific antibody concentrations ≥0.35 and ≥1μg/mL before and after the PPV-23 were performed using exact McNemar's test. A p-value of <0.01 was considered statistically significant due to the multiple comparisons.
Results
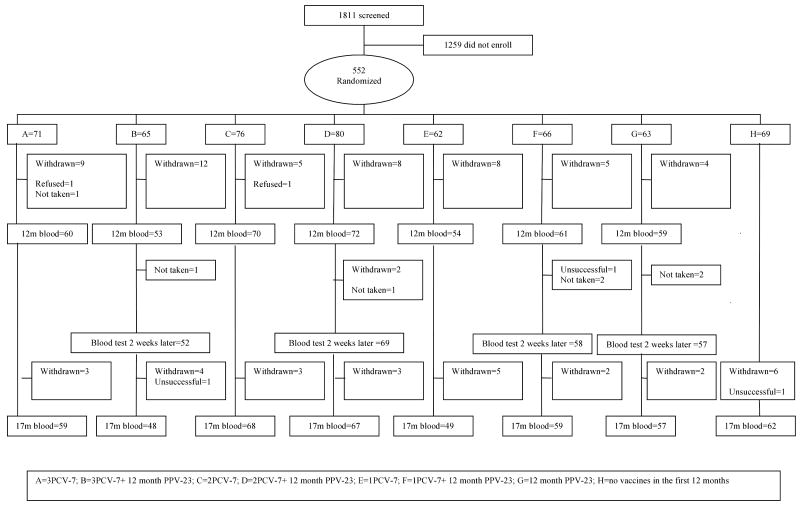
There were 552 infants enrolled in the study (Figure 1) and the characteristics of the randomized infants have been described elsewhere (15). The 552 participants represent a consent rate of 30.5%, of which 10% had withdrawn by 12 months and 15% by 17 months of age. The commonest reason for withdrawal was relocation outside the study area. No participant was withdrawn due to a reaction to any of the vaccines. The 12 month PPV-23 was administered to 245 children with all groups having blood drawn a median of 14 days (IQR 14-15 days) post booster.
Figure 1. CONSORT chart of the screened and enrolled children to 17 months of age.
Immunogenicity to PCV-7 serotypes
Two weeks following the PPV-23, GMC were significantly higher (each p<0.001) for all PCV-7 serotypes for children that had received either 1, 2, or 3 PCV-7 doses in the primary series compared to levels prior to receiving PPV-23 (Table 1). For 4 of 7 serotypes (4, 9V, 18C, 19F) this response was most profound in the single PCV-7 dose group. There were no significant differences in GMC 2 weeks following the PPV-23 for any PCV-7 serotype between the 3 and 2 PCV-7 dose groups. GMC were significantly higher (each p<0.001) 2 weeks following the PPV-23 compared with the pre-PPV-23 levels, for all PCV-7 serotypes in the group that had not received PCV-7 in infancy (Table 1).
Table 1. Serotype-specific IgG geometric mean concentrations (GMC and 95% confidence intervals) to PCV-7 serotypes before and 14 days following the 12 month PPV-23 and by number of PCV-7 doses administered in the primary series.
| Pre-PPV-23 at 12 months of age | 14 days post-12 month PPV-23 | |||||||
|---|---|---|---|---|---|---|---|---|
| 3 PCV-7 (n=52) |
2 PCV-7 (n=69) |
1 PCV-7 (n=58) |
0 PCV-7 (n=57) |
3 PCV-71,2 (n=52) |
2 PCV-71,2 (n=69) |
1 PCV-71-3 (n=58) |
0 PCV-71,3 (n=57) |
|
| 4 | 0.46 (0.37-0.57) |
0.42 (0.34-0.52) |
0.76 (0.53-1.08) |
0.08 (0.06-0.09) |
14.68 (11.10-19.42) |
14.70 (11.72-18.43) |
46.47 (36.65-58.92) |
2.36 (1.76-3.16) |
| 6B | 0.85 (0.64-1.12) |
0.79 (0.59-1.07) |
0.69 (0.49-0.98) |
0.14 (0.12-0.17) |
29.58 (21.58-40.54) |
23.83 (17.99-31.57) |
18.07 (13.55-24.11) |
0.31 (0.23-0.42) |
| 9V | 0.59 (0.49-0.73) |
0.62 (0.53-0.71) |
0.67 (0.49-0.89) |
0.09 (0.07-0.11) |
14.10 (10.60-18.75) |
15.20 (12.03-19.21) |
34.84 (27.03-44.90) |
1.20 (0.90-1.61) |
| 14 | 2.00 (1.48-2.72) |
1.55 (1.21-1.99) |
1.36 (1.01-1.83) |
0.19 (0.16-0.24) |
15.73 (10.48-23.60) |
13.91 (9.87-19.60) |
20.08 (12.19-33.05) |
0.41 (0.29-0.58) |
| 18C | 0.37 (0.27-0.49) |
0.23 (0.19-0.27) |
0.22 (0.17-0.29) |
0.06 (0.05-0.08) |
8.71 (6.70-11.34) |
10.51 (8.21-13.46) |
16.03 (11.33-22.67) |
1.22 (0.88-1.69) |
| 19F | 0.86 (0.64-1.16) |
1.20 (0.93-1.55) |
1.05 (0.79-1.39) |
0.47 (0.36-0.61) |
27.91 (19.98-38.99) |
24.77 (18.21-33.68) |
84.47 (55.80-127.87) |
1.13 (0.80-1.59) |
| 23F | 0.52 (0.39-0.69) |
0.41 (0.32-0.53) |
0.31 (0.23-0.42) |
0.19 (0.14-0.25) |
10.87 (7.41-15.96) |
10.29 (7.19-14.73) |
8.33 (5.25-13.20) |
0.42 (0.31-0.57) |
P-values were <0.001 for all serotypes comparing GMC pre/post 12 month PPV-23 for all PCV-7 dosage groups.
For GMC comparisons between a single or 2 doses of PCV-7 with 3 PCV-7 doses, the p-values were not significant for all serotypes except for the 3 versus single dose comparison for serotypes 4, 9V, 18C, and 19F (each p<0.01).
P-values were <0.001 for all serotypes comparing GMC between 0 or a single dose of PCV-7 with 3 PCV-7 doses.
Two weeks following the 12 month PPV-23, there was no significant difference between the 3 and 2 dose PCV-7 groups or between the 3 and single dose groups in the proportion of children with antibody concentrations ≥0.35 and ≥1μg/mL for the PCV-7 serotypes (Table 2).
Table 2. Proportions of children with antibody concentrations ≥0.35 and ≥1μg/mL to PCV-7 serotypes before and 14 days post-12 month PPV-23 and by number of PCV-7 doses administered in the primary series.
| Pre-PPV-23 at 12 months of age | 14 days post-12 month PPV-23 | |||||||
|---|---|---|---|---|---|---|---|---|
| 3 PCV-7 (n=52) |
2 PCV-7 (n=69) |
1 PCV-7 (n=58) |
0 PCV-7 (n=57) |
3 PCV-7 (n=52) |
2 PCV-7 (n=69) |
1 PCV-7 (n=58) |
0 PCV-7 (n=57) |
|
| ≥0.35μg/mL | ||||||||
| 4 | 63.5 | 60.9 | 64.9 | 7.0 | 98.1 | 1002 | 1002 | 94.72,3 |
| 6B | 78.8 | 72.5 | 70.2 | 12.3 | 100 | 1002 | 1002 | 38.6 |
| 9V | 73.1 | 81.2 | 75.4 | 8.8 | 100 | 1002 | 1002 | 87.72 |
| 14 | 92.3 | 92.8 | 89.5 | 12.3 | 94.21 | 95.71,2 | 93.11,2 | 43.9 |
| 18C | 44.2 | 27.5 | 31.6 | 5.3 | 100 | 1002 | 98.32 | 82.5 |
| 19F | 86.5 | 91.3 | 84.2 | 54.4 | 1001 | 1001,2 | 1002 | 84.2 |
| 23F | 59.6 | 53.6 | 42.1 | 21.1 | 98.1 | 98.62 | 93.12 | 50.9 |
| ≥1μg/mL | ||||||||
| 4 | 13.5 | 8.7 | 36.8 | 0 | 98.1 | 1002 | 1002 | 84.2 |
| 6B | 46.2 | 36.2 | 33.3 | 3.5 | 98.1 | 1002 | 1002 | 14.01 |
| 9V | 26.9 | 27.5 | 21.1 | 1.8 | 98.1 | 1002 | 1002 | 54.4 |
| 14 | 76.9 | 72.5 | 63.2 | 8.8 | 92.3 | 95.72 | 89.72 | 21.11 |
| 18C | 13.5 | 2.9 | 3.5 | 0 | 98.1 | 95.72 | 96.62 | 57.9 |
| 19F | 34.6 | 57.9 | 52.6 | 24.6 | 98.1 | 98.62 | 96.62 | 50.9 |
| 23F | 30.8 | 15.9 | 15.8 | 5.3 | 94.2 | 88.42 | 86.22 | 17.51 |
A significant difference was observed for all comparisons except those marked:
Comparison of proportions pre/post 12 month PPV-23.
Comparison of proportions following 0, 1, or 2 doses with 3 doses of PCV-7.
Comparison of proportions following a single and no dose of PCV-7.
At 17 months of age the groups that had received the 12 month PPV-23 continued to have significantly higher GMC (each p<0.001) for all PCV-7 serotypes compared to those that had not received the 12 month PPV-23 but the same number of PCV-7 doses (Table 3). The single PCV-7 dose group which received the PPV-23 continued to have higher GMC compared to the 2 or 3 dose PCV-7 groups which did or did not receive the PPV-23. There were significantly higher proportions with antibody concentrations ≥1μg/mL for the PCV-7 serotypes in those groups that had received the 12 month PPV-23 compared with those that had not received the PPV-23 (Table 3).
Table 3. Serotype-specific IgG GMC (and 95% confidence intervals) and proportions of children with antibody concentrations ≥0.35 and ≥1μg/mL to PCV-7 serotypes at 17 months in those who did or did not receive the 12 month PPV-23 and by number of PCV-7 doses in the primary series.
| 3 PCV-7, no PPV-23 (n=59) |
3 PCV-7, PPV-23 (n=48) |
2 PCV-7, no PPV-23 (n=68) |
2 PCV-7, PPV-23 (n=67) |
1 PCV-7, no PPV-23 (n=49) |
1 PCV-7, PPV-23 (n=59) |
0 PCV-7, no PPV-23 (n=62) |
0 PCV-7, PPV-23 (n=57) |
|
|---|---|---|---|---|---|---|---|---|
| GMC (95%CI) | ||||||||
| 4 | 0.35 (0.29-0.43) | 2.19 (1.79-2.69) | 0.43 (0.33-0.56) | 2.03 (1.70-2.42) | 0.56 (0.39-0.80) | 6.36 (4.94-8.19) | 0.11 (0.09-0.13) | 0.70 (0.53-0.91) |
| 6B | 0.91 (0.69-1.21) | 4.35 (3.22-5.89) | 0.78 (0.58-1.04) | 3.85 (3.10-4.79) | 0.62 (0.47-0.83) | 3.88 (2.87-5.25) | 0.20 (0.16-0.24) | 0.21 (0.17-0.26) 1 |
| 9V | 0.41 (0.34-0.49) | 2.25 (1.74-2.91) | 0.49 (0.39-0.62) | 2.50 (2.02-3.08) | 0.51 (0.36-0.71) | 5.49 (4.23-7.12) | 0.14 (0.11-0.17) | 0.38 (0.31-0.47) |
| 14 | 1.78 (1.42-2.24) | 4.31 (3.00-6.19) | 1.12 (0.86-1.46) | 3.58 (2.72-4.71) | 0.93 (0.66-1.32) | 4.85 (3.27-7.19) | 0.31 (0.25-0.38) | 0.67 (0.47-0.96) |
| 18C | 0.21 (0.18-0.26) | 1.28 (1.07-1.53) | 0.20 (0.16-0.25) | 1.13 (0.92-1.38) | 0.15 (0.12-0.19) | 1.79 (1.37-2.34) | 0.10 (0.08-0.12) | 0.50 (0.39-0.66) |
| 19F | 1.19 (0.84-1.67) | 5.55 (4.24-7.26) | 1.06 (0.82-1.38) | 4.53 (3.54-5.79) | 0.92 (0.66-1.26) | 13.47 (9.89-18.33) | 0.59 (0.47-0.75) | 0.79 (0.61-1.04) 1 |
| 23F | 0.57 (0.43-0.75) | 1.68 (1.29-2.20) | 0.43 (0.32-0.58) | 1.41 (1.06-1.86) | 0.32(0.22-0.48) | 1.78 (1.28-2.49) | 0.19 (0.16-0.23) | 0.27 (0.21-0.34) 1 |
| Proportion ≥0.35μg/mL | ||||||||
| 4 | 52.5 | 100 | 51.5 | 100 | 63.3 | 98.3 | 6.5 | 73.7 |
| 6B | 83.1 | 97.92 | 72.1 | 100 | 73.5 | 98.3 | 21.0 | 24.62 |
| 9V | 61.0 | 97.9 | 60.3 | 98.5 | 61.2 | 100 | 12.9 | 56.1 |
| 14 | 98.3 | 95.82 | 91.2 | 97.02 | 83.7 | 94.92 | 41.9 | 73.7 |
| 18C | 22.0 | 97.9 | 22.1 | 94.0 | 10.2 | 93.2 | 9.7 | 66.7 |
| 19F | 89.8 | 1002 | 91.2 | 1002 | 87.8 | 98.32 | 72.6 | 82.52 |
| 23F | 64.4 | 93.8 | 54.4 | 88.1 | 34.7 | 89.3 | 17.7 | 43.9 |
| Proportion ≥1μg/mL | ||||||||
| 4 | 5.1 | 85.4 | 23.5 | 88.1 | 30.6 | 96.6 | 1.6 | 38.6 |
| 6B | 39.0 | 89.6 | 41.2 | 95.5 | 34.7 | 84.7 | 3.2 | 5.32 |
| 9V | 10.2 | 85.4 | 16.2 | 82.1 | 22.4 | 91.5 | 1.6 | 5.32 |
| 14 | 69.5 | 89.6 | 47.1 | 85.1 | 42.9 | 81.4 | 9.7 | 28.12 |
| 18C | 3.4 | 66.7 | 5.9 | 59.7 | 2.0 | 69.5 | 3.2 | 31.6 |
| 19F | 40.7 | 95.8 | 47.1 | 94.0 | 42.9 | 94.9 | 29.0 | 29.82 |
| 23F | 22.0 | 75.0 | 22.1 | 65.7 | 10.2 | 72.9 | 1.6 | 5.32 |
All comparisons were significant except:
Comparison of GMC for 3, 2, 1 or 0 PCV-7 doses with or without the 12 month PPV-23.
Comparison of the proportions with antibody concentrations ≥0.35 and ≥1μg/mL for 3, 2, 1 or 0 PCV-7 doses with or without the 12 month PPV-23.
Immunogenicity to non-PCV-7 serotypes
Two weeks following the 12 month PPV-23, GMC and the proportions with antibody concentrations ≥0.35 and ≥1μg/mL for all non-PCV-7 serotypes in the PPV-23 were significantly higher (each p<0.001) than pre-PPV-23 levels (Table 4). To assess for non-specific effects, the proportion of children with antibody concentrations ≥0.35μg/mL were compared between the 3, 2, and single PCV-7 dose groups with the group that had received no prior PCV-7. There were no significant differences in responses to the non-PCV-7 serotypes following the 12 month PPV-23 between the 3 and 0 PCV dose groups (data not shown). However for serotypes 15B and 19A, the proportion of children with antibody concentrations ≥0.35μg/mL were significantly higher in the 2 and single dose groups compared with the 0 PCV dose group (data not shown).”
Table 4. Serotype-specific IgG GMC (and 95% confidence intervals) and proportions of children with antibody concentrations ≥0.35 and ≥1μg/mL to non-PCV-7 serotypes before and 14 days post-12 month PPV-23.
| Pre-PPV-23 at 12 months of age (n=235) 1 | 14 days post-12 month PPV-23 (n=235) 1 | |||||
|---|---|---|---|---|---|---|
| Serotype | GMC2 (95%CI) | % ≥0.35μg/mL 3 | % ≥1μg/mL 3 | GMC2 (95%CI) | % ≥0.35μg/mL 3 | % ≥1μg/mL 3 |
| 1 | 0.17 (0.15-0.19) | 17.9 | 6.4 | 1.59 (1.38-1.82) | 91.9 | 65.9 |
| 2 | 0.41(0.36-0.46) | 54.5 | 13.6 | 10.73 (9.50-12.11) | 100 | 98.7 |
| 3 | 0.27 (0.23-0.32) | 34.9 | 10.6 | 8.28 (7.26-9.44) | 99.1 | 96.6 |
| 5 | 0.26 (0.23-29) | 35.7 | 11.5 | 2.26 (2.01-2.55) | 97.9 | 78.7 |
| 7F | 0.09 (0.08-0.10) | 11.5 | 2.9 | 1.73 (1.51-1.99) | 92.3 | 73.6 |
| 8 | 0.24 (0.21-0.28) | 30.6 | 7.7 | 8.88 (7.82-10.09) | 98.7 | 97.4 |
| 9N | 0.23 (0.19-0.26) | 27.7 | 7.7 | 8.31 (7.04-9.82) | 98.3 | 93.6 |
| 10A | 0.21 (0.19-0.24) | 22.9 | 6.4 | 0.76 (0.66-0.89) | 73.6 | 38.7 |
| 11A | 0.09 (0.09-0.11) | 12.8 | 7.2 | 1.51 (1.28-1.77) | 87.2 | 68.9 |
| 12F | 0.07 (0.07-0.08) | 7.2 | 1.3 | 0.37 (0.31-0.43) | 50.6 | 23.4 |
| 15B | 0.29 (0.27-0.34) | 37.9 | 9.4 | 2.15 (1.84-2.51) | 91.1 | 76.2 |
| 17F | 0.10 (0.09-0.11) | 5.1 | 1.3 | 0.81 (0.68-0.96) | 73.2 | 43.2 |
| 19A | 0.46 (0.40-0.51) | 56.6 | 17.9 | 1.93 (1.60-2.32) | 86.8 | 63.8 |
| 20 | 0.09 (0.09-0.10) | 4.3 | 1.3 | 0.68 (0.57-0.83) | 66.1 | 39.1 |
| 22F | 0.40 (0.36-0.46) | 54.5 | 15.3 | 4.73 (3.81-5.87) | 94.9 | 81.3 |
| 33F | 0.13 (0.12-0.14) | 7.7 | 2.9 | 1.66 (1.40-1.97) | 84.3 | 69.4 |
235 pairs available for comparison. One pre-PPV-23 sample was not available for testing.
P-values were <0.001 for all pre/post PPV-23 GMC comparisons for all serotypes.
P-values were <0.001 for comparisons of proportions with antibody concentrations ≥0.35μg/mL and ≥1μg/mL for all serotypes.
By 17 months of age, GMC and the proportion with antibody concentrations ≥0.35μg/mL were still significantly higher (each p<0.001) for all non-PCV-7 serotypes in the groups that had received the PPV-23 vaccine at 12 months compared to the groups that had not (Table 5).
Table 5. Serotype-specific IgG GMC (and 95% confidence intervals) and proportions of children with antibody concentrations ≥0.35 and ≥1μg/mL to non-PCV-7 serotypes at 17 months of age in those that did or did not receive the 12 month PPV-23.
| No PPV-23 at 12 months of age (n=238) | PPV-23 at 12 months of age (n=231) | |||||
|---|---|---|---|---|---|---|
| GMC1 (95%CI) | % ≥0.35μg/mL2 | % ≥1μg/mL3 | GMC1 (95%CI) | % ≥0.35μg/mL2 | % ≥1μg/mL 3 | |
| 1 | 0.23 (0.21-0.25) | 25.6 | 3.4 | 0.63 (0.56-0.71) | 77.5 | 27.3 |
| 2 | 0.51 (0.45-0.57) | 65.5 | 22.3 | 2.88 (2.60-3.18) | 99.1 | 92.2 |
| 3 | 0.30 (0.26-0.34) | 34.9 | 10.1 | 1.46 (1.30-1.63) | 96.1 | 69.3 |
| 5 | 0.31 (0.28-0.34) | 41.6 | 7.1 | 0.77 (0.69-0.86) | 81.4 | 39.4 |
| 7F | 0.12 (0.10-0.13) | 11.3 | 3.8 | 0.51 (0.45-0.57) | 63.6 | 22.9 |
| 8 | 0.29 (0.26-0.33) | 34.9 | 8.4 | 2.31 (2.08-2.56) | 99.1 | 86.6 |
| 9N | 0.23 (0.20-0.26) | 24.4 | 4.6 | 1.90 (1.64-2.19) | 92.6 | 74.0 |
| 10A | 0.19 (0.17-0.21) | 16.4 | 2.5 | 0.27 (0.25-0.31) | 36.8 | 6.5 |
| 11A | 0.14 (0.12-0.16) | 19.7 | 11.3 | 0.35 (0.31-0.41) | 51.5 | 15.6 |
| 12F | 0.09 (0.08-0.10) | 5.0 | 0.8 | 0.18 (0.16-0.21) | 23.8 | 6.1 |
| 15B | 0.30 (0.27-0.34) | 37.4 | 9.7 | 0.74 (0.66-0.84) | 79.2 | 39.8 |
| 17F | 0.12 (0.11-0.13) | 6.3 | 0.4 | 0.36 (0.31-0.41) | 48.5 | 16.9 |
| 19A | 0.59 (0.53-0.67) | 71.4 | 24.4 | 1.14 (1.00-1.31) | 87.0 | 57.1 |
| 20 | 0.13 (0.12-0.15) | 12.6 | 4.2 | 0.26 (0.23-0.30) | 36.4 | 10.0 |
| 22F | 0.46 (0.41-0.51) | 59.7 | 18.9 | 1.43 (1.22-1.68) | 91.3 | 59.7 |
| 33F | 0.18 (0.17-0.20) | 15.1 | 3.4 | 0.62 (0.54-0.71) | 70.6 | 36.4 |
P-values were <0.001 for all serotypes.
P-values were <0.001 comparing proportions ≥0.35μg/mL for all serotypes.
P-values were <0.01 comparing proportions ≥1μg/mL for all serotypes except serotypes 10A and 11A.
Adverse events
Following PPV-23 at 12 months of age, low grade fever was common (28.2%) while high grade fever occurred in 6.1%. The description of other general reactions are shown in Table 6. Local injection site reactions occurred in a minority of recipients. All events resolved within 48 hours. There were 101 SAEs throughout the 2 year follow up period, with none attributable to receipt of any of the study vaccines. One child who had received 2 doses of PCV-7 at 6 and 14 weeks of age died at 9 months of age from dehydration secondary to acute gastroenteritis. For children over 12 months of age, there were 14 SAEs in children who had received the 12 month PPV-23 and 22 SAEs in children who had not received PPV-23, during the follow up period up to 2 years of age. For children over 12 months of age, there were 4 cases of inpatient pneumonia in children who had received the 12 month PPV-23 compared with 7 cases in those that had not during the same follow up period. There were no cases of IPD throughout the study period.
Table 6. Non-serious adverse events1 in those children who received PPV-23 at 12 months of age (n=245).
| Systemic adverse events | Number (%) |
|---|---|
| High fever (≥38°C per axilla) | 15 (6.1) |
| Low grade fever (>37°C & <38°C per axilla) | 69 (28.2) |
| Diarrhoea | 2 (0.8) |
| Vomiting | 4 (1.6) |
| Severe vomiting2 | 1 (0.4) |
| Anorexia | 11 (4.5) |
| Severe anorexia2 | 2 (0.8) |
| Drowsiness | 13 (5.3) |
| Severe drowsiness2 | 2 (0.8) |
| Irritability | 23 (9.4) |
| Severe irritability3 | 2 (0.8) |
| Local reactions | |
| Erythema | 15 (6.1) |
| >30mm2 | 3 (1.2) |
| Tenderness | 13 (5.3) |
| Induration | 13 (5.3) |
| >30mm2 | 3 (1.2) |
All cases were classified as mild or moderate unless otherwise stated
Resolved within 2 days
Resolved within 1 day
Discussion
This study has shown that 1, 2, or 3 doses of PCV-7 in infancy primed infants sufficiently elicit an excellent booster response to the PPV-23 at 12 months of age for all PCV-7 serotypes. Furthermore, there were good antibody responses to the 16 non-PCV-7 serotypes following PPV-23 at 12 months. The antibody concentrations for all 23 serotypes remained significantly higher at 17 months of age in the PPV-23 group compared to the group that had not received PPV-23.
In addition, this study has shown that priming with a single PCV-7 dose in infancy produced the greatest booster (memory) response for most serotypes following PPV-23 at 12 months compared with 2 or 3 PCV-7 doses. Responses following the PPV-23 were similar for those children that had received either 2 or 3 PCV-7 doses in infancy and lower than that in children who received a single PCV-7 dose. The immunological explanation for the single PCV-7 dose having a better booster response is not clear. Post booster antibody concentrations are usually higher in those that have had a stronger primary response [34]. One study found that a stronger primary response was more likely following higher doses of antigen and/or a higher concentration of carrier protein, possibly through the enhanced induction of antibody producing plasma cells [35]. However this would not explain the findings in our study of a better booster response in the single dose group as our previously published data has shown that a single PCV-7 dose (lower antigen dose) administered at 14 weeks of age induced a weaker primary response [29]. In that previous study, a significant immunological response was found in the single dose group compared with an unvaccinated control group, but significantly lower GMC for all PCV-7 serotypes compared to 2 or 3 PCV-7 doses [29].
Another possible explanation for the better booster response in the single PCV-7 dose group may be that a single antigen challenge rather than multiple antigen exposures, may preferentially drive the induction of memory B cells (which are required for a booster response), rather than plasma cells [36]. Having a greater pool of memory B cells would subsequently elicit a greater booster response. A fewer dose (single PCV-7 dose) primary series may preferentially induce B cell differentiation away from plasma cells, towards memory B cells compared to repeated antigen exposure associated with 2 or 3 PCV-7 dose primary series [8, 11]. In summary, our findings are consistent with the suggestion that 2 or 3 PCV-7 doses in infancy are more effective at producing plasma cells [29] and less effective at producing memory B cells than a single PCV-7 dose. Alternatively, it is speculated that our findings may be explained by some form of immunological tolerance following 2 or 3 PCV-7 doses.
Our findings indicate that PCV-7/PPV-23 compared to the PCV-7 primary series without a booster should offer superior protection from pneumococcal disease lasting at least 5 months following the 12 month PPV-23. A recent study of asthmatic children aged 2-5 years underwent sequential immunization of PCV-7 followed by PPV-23 either 2 or 10 months post PCV-7 [37]. Antibody concentrations for PCV-7 and 2 non-PCV-7 serotypes (5 and 7F) were higher following the PPV-23 booster than after PCV-7 alone [37]. Despite superior antibody concentrations being demonstrated for PCV-7/PPV-23 compared with PCV-7/PCV-7, we would not advise PCV-7/PPV-23 for 3 reasons. Firstly, superior vaccine efficacy using PCV-7/PPV-23 against clinical disease has not been demonstrated. A study of vaccine efficacy against acute otitis media found that a PCV-7/PPV-23 compared to a PCV-7/PCV-7 schedule had similar results despite higher antibodies generated post PCV-7/PPV-23 [12]. This may be due to inferior quality of antibodies being produced following PPV-23. However previous studies have found that the quality of antibody, measured by avidity or opsonophagocytic activity, can differ in those that have received PPV-23 or PCV-7 as a booster, however results have been conflicting and therefore inconclusive [8, 10, 38-40]. Finnish studies have shown the concentration of antibodies required for 50% killing was higher [38] and that the avidity of such antibodies was lower after PCV-7/PPV-23 compared with PCV-7/PCV-7 [8, 39, 41]. In contrast, another study in Finland using the 11-valent pneumococcal conjugate vaccine showed that opsonophagocytic activity was better in the group that received a PPV-23 booster at 12-15 months than those that had the conjugate booster [40]. A study in Israeli children who received 1 dose of the 7-valent pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine followed by either a conjugate or PPV-23 booster, achieved similar opsonic antibody titers in each group for the 1 serotype tested (6B) [8]. Data from the assessment of functional antibody responses in our study documenting the avidity to 23 serotypes and opsonophagocytic activity to 8 serotypes will be forthcoming.
Secondly, conjugate vaccines are the only vaccines that provide mucosal immunity. As nasopharyngeal (NP) carriage is an antecedent event in IPD, the reduction or prevention of NP carriage reduces the transmission of pneumococci and prevents IPD in the vaccinated individual and provides herd immunity [42-44]. In contrast, pneumococcal polysaccharide vaccines have shown no effect on pneumococcal carriage [20-24]. Most studies evaluating the impact of pneumococcal polysaccharide immunization in the absence of additional PCV-7 in infants or children have not shown any impact on pneumococcal disease or carriage [25-27]. This finding may be at least partially explained by the lack of effect that pneumococcal polysaccharide vaccine has on NP carriage. In contrast, one study in Papua New Guinea, where children aged six months to five years of age were given either the 14-valent or PPV-23 in one or two doses according to age, there was a (non-significant) 19% reduction in mortality from any cause, and a 50% reduction in pneumonia mortality (95%CI, 1-75%) [45]. Natural exposure in a population with a high incidence of pneumococcal infections, resulting in regular antigenic stimulation may explain this finding [13].
Thirdly, immunological hyporesponsiveness following PPV-23 at 12 months of age has been demonstrated by reduced responses to a small re-challenge dose of PPV-23 administered at 17 months of age (Russell FM, Carapetis JR, Balloch A, Licciardi PV, Jenney AWJ, Tikoduadua L, Waqatakirewa L, Pryor J, Nelson J, Byrnes GB, Cheung YB, Tang MLK, Mulholland EK, submitted). This attenuated response to the re-challenge dose may be due to depletion of the memory B cell pool [46]. A study documenting immunologic memory 5 years after meningococcal A/C conjugate vaccination in infancy showed that challenge with the meningococcal polysaccharide or conjugate at 2 years of age demonstrated immunologic memory. However subsequent challenge with polysaccharide at 5 years of age resulted in an inability to demonstrate memory in the polysaccharide group. The authors concluded that polysaccharide immunization at 2 years of age interfered with the immune response to subsequent polysaccharide vaccination [46]. One explanation for this is that polysaccharide immunization induces memory B cells to differentiate into plasma cells and secrete antibody but does not replenish the memory B cell pool [47]. Subsequent challenge with PPV-23 may then result in immune hyporesponsiveness. No adverse clinical effects have ever been documented due to repeated exposure to the meningococcal polysaccharide vaccine. In this study we demonstrated no adverse clinical consequences, although the study was not designed to evaluate this effect.
In summary, PPV-23 at 12 months induces an excellent booster response following 1, 2, or 3 doses of PCV-7 in infancy for all PCV-7 and significant responses for non-PCV-7 serotypes up to 5 months following vaccination. Booster responses were greatest for a single PCV-7 dose compared to 2 or 3 doses of PCV-7.
Acknowledgments
The authors wish to sincerely thank all the FiPP staff and families participating in the study, the Fiji Ministry of Health, CWMH laboratory and paediatric department, and the many other people who contributed to the study including: Amanda O'Brien, Kathryn Bright, Amy Bin Chen, Timothy Gemetzis, Amy Auge, Katherine Gilbert, Evan Willis, Philip Greenwood, Beth Temple, Vanessa Johnston, Loretta Thorn, Porter Anderson, Brian Greenwood, George Siber, David Klein, Elizabeth Horigan, Farukh Khambaty, and the members of the DSMB. Funding was provided by the U.S. NIAID (grant number R01 AI 52337) and the Australian National Health and Medical Research Council. Pneumovax™ was kindly donated by CSL Biotherapies, Australia. The co-administered Tritanrix™-HepB™ and Hiberix™ vaccines were kindly donated by GlaxoSmithKline. Clinicaltrials.gov number NCT00170612.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Conclusions from the WHO multicenter study of serious infections in young infants. The WHO Young Infants Study Group. Pediatr Infect Dis J. 1999 Oct;18(10 Suppl):S32–4. doi: 10.1097/00006454-199910001-00006. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009 Sep 12;374(9693):893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 3.Denny FW, Loda FA. Acute respiratory infections are the leading cause of death in children in developing countries. Am J Trop Med Hyg. 1986 Jan;35(1):1–2. doi: 10.4269/ajtmh.1986.35.1. [DOI] [PubMed] [Google Scholar]
- 4.Park IH, Pritchard DG, Cartee R, Brandao A, Brandileone MC, Nahm MH. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J Clin Microbiol. 2007 Apr;45(4):1225–33. doi: 10.1128/JCM.02199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003 May 1;348(18):1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 6.GAVI's PneumoADIP, Department of International Health, Health. JHBSoP. Pneumococcal Regional Serotype Distribution for Pneumococcal AMC TPP. 2008. [Google Scholar]
- 7.Ahman H, Kayhty H, Lehtonen H, Leroy O, Froeschle J, Eskola J. Streptococcus pneumoniae capsular polysaccharide-diphtheria toxoid conjugate vaccine is immunogenic in early infancy and able to induce immunologic memory. Pediatr Infect Dis J. 1998 Mar;17(3):211–6. doi: 10.1097/00006454-199803000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Blum MD, Dagan R, Mendelman PM, Pinsk V, Giordani M, Li S, et al. A comparison of multiple regimens of pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine and pneumococcal polysaccharide vaccine in toddlers. Vaccine. 2000 May 8;18(22):2359–67. doi: 10.1016/s0264-410x(00)00021-9. [DOI] [PubMed] [Google Scholar]
- 9.Choo S, Seymour L, Morris R, Quataert S, Lockhart S, Cartwright K, et al. Immunogenicity and reactogenicity of a pneumococcal conjugate vaccine administered combined with a haemophilus influenzae type B conjugate vaccine in United Kingdom infants. Pediatr Infect Dis J. 2000 Sep;19(9):854–62. doi: 10.1097/00006454-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Goldblatt D, Southern J, Ashton L, Richmond P, Burbidge P, Tasevska J, et al. Immunogenicity and boosting after a reduced number of doses of a pneumococcal conjugate vaccine in infants and toddlers. Pediatr Infect Dis J. 2006 Apr;25(4):312–9. doi: 10.1097/01.inf.0000207483.60267.e7. [DOI] [PubMed] [Google Scholar]
- 11.Huebner RE, Mbelle N, Forrest B, Madore DV, Klugman KP. Long-term antibody levels and booster responses in South African children immunized with nonavalent pneumococcal conjugate vaccine. Vaccine. 2004 Jul 29;22(21-22):2696–700. doi: 10.1016/j.vaccine.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Kilpi T, Ahman H, Jokinen J, Lankinen KS, Palmu A, Savolainen H, et al. Protective efficacy of a second pneumococcal conjugate vaccine against pneumococcal acute otitis media in infants and children: randomized, controlled trial of a 7-valent pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine in 1666 children. Clin Infect Dis. 2003 Nov 1;37(9):1155–64. doi: 10.1086/378744. [DOI] [PubMed] [Google Scholar]
- 13.O'Brien KL, Hochman M, Goldblatt D. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue? Lancet Infect Dis. 2007 Sep;7(9):597–606. doi: 10.1016/S1473-3099(07)70210-4. [DOI] [PubMed] [Google Scholar]
- 14.Obaro SK, Adegbola RA, Banya WA, Greenwood BM. Carriage of pneumococci after pneumococcal vaccination. Lancet. 1996 Jul 27;348(9022):271–2. doi: 10.1016/s0140-6736(05)65585-7. [DOI] [PubMed] [Google Scholar]
- 15.Mbelle N, Huebner RE, Wasas AD, Kimura A, Chang I, Klugman KP. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J Infect Dis. 1999 Oct;180(4):1171–6. doi: 10.1086/315009. [DOI] [PubMed] [Google Scholar]
- 16.Lakshman R, Murdoch C, Race G, Burkinshaw R, Shaw L, Finn A. Pneumococcal nasopharyngeal carriage in children following heptavalent pneumococcal conjugate vaccination in infancy. Arch Dis Child. 2003 Mar;88(3):211–4. doi: 10.1136/adc.88.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dagan R, Melamed R, Muallem M, Piglansky L, Greenberg D, Abramson O, et al. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J Infect Dis. 1996 Dec;174(6):1271–8. doi: 10.1093/infdis/174.6.1271. [DOI] [PubMed] [Google Scholar]
- 18.Veenhoven RH, Bogaert D, Schilder AG, Rijkers GT, Uiterwaal CS, Kiezebrink HH, et al. Nasopharyngeal pneumococcal carriage after combined pneumococcal conjugate and polysaccharide vaccination in children with a history of recurrent acute otitis media. Clin Infect Dis. 2004 Oct 1;39(7):911–9. doi: 10.1086/422651. [DOI] [PubMed] [Google Scholar]
- 19.Dagan R, Givon-Lavi N, Zamir O, Sikuler-Cohen M, Guy L, Janco J, et al. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J Infect Dis. 2002 Apr 1;185(7):927–36. doi: 10.1086/339525. [DOI] [PubMed] [Google Scholar]
- 20.Herva E, Luotonen J, Timonen M, Sibakov M, Karma P, Makela PH. The effect of polyvalent pneumococcal polysaccharide vaccine on nasopharyngeal and nasal carriage of Streptococcus pneumoniae. Scand J Infect Dis. 1980;12(2):97–100. doi: 10.3109/inf.1980.12.issue-2.04. [DOI] [PubMed] [Google Scholar]
- 21.Wright PF, Sell SH, Vaughn WK, Andrews C, McConnell KB, Schiffman G. Clinical studies of pneumococcal vaccines in infants. II. Efficacy and effect on nasopharyngeal carriage. Rev Infect Dis. 1981 Mar-Apr;3(Suppl):S108–12. doi: 10.1093/clinids/3.supplement_1.s108. [DOI] [PubMed] [Google Scholar]
- 22.Rosen C, Christensen P, Hovelius B, Prellner K. A longitudinal study of the nasopharyngeal carriage of pneumococci as related to pneumococcal vaccination in children attending day-care centres. Acta Otolaryngol. 1984 Nov-Dec;98(5-6):524–32. doi: 10.3109/00016488409107593. [DOI] [PubMed] [Google Scholar]
- 23.Douglas RM, Hansman D, Miles HB, Paton JC. Pneumococcal carriage and type-specific antibody. Failure of a 14-valent vaccine to reduce carriage in healthy children. Am J Dis Child. 1986 Nov;140(11):1183–5. doi: 10.1001/archpedi.1986.02140250109044. [DOI] [PubMed] [Google Scholar]
- 24.Christensen P, Hovelius B, Prellner K, Rosen C, Christensen KK, Kurl DN, et al. Effects of pneumococcal vaccination on tonsillo-pharyngitis and upper respiratory tract flora. Int Arch Allergy Appl Immunol. 1985;78(2):161–6. doi: 10.1159/000233879. [DOI] [PubMed] [Google Scholar]
- 25.Rosen C, Christensen P, Hovelius B, Prellner K. Effect of pneumococcal vaccination on upper respiratory tract infections in children. Design of a follow-up study. Scand J Infect Dis Suppl. 1983;39:39–44. [PubMed] [Google Scholar]
- 26.Douglas RM, Miles HB. Vaccination against Streptococcus pneumoniae in childhood: lack of demonstrable benefit in young Australian children. J Infect Dis. 1984 Jun;149(6):861–9. doi: 10.1093/infdis/149.6.861. [DOI] [PubMed] [Google Scholar]
- 27.Sloyer JL, Jr, Ploussard JH, Howie VM. Efficacy of pneumococcal polysaccharide vaccine in preventing acute otitis media in infants in Huntsville, Alabama. Rev Infect Dis. 1981 Mar-Apr;3(Suppl):S119–23. doi: 10.1093/clinids/3.supplement_1.s119. [DOI] [PubMed] [Google Scholar]
- 28.Russell F, Chandra R, Carapetis J, Seduadua A, Tikoduadua L, Buadromo E, et al. Epidemiology and Serotypes of Invasive Pneumococcal Disease in all ages in Fiji. 6th International Symposium of Pneumococci and Pneumococal Diseases; 2008 June 8-12; Reykjavik, Iceland. 2008. 2008. [Google Scholar]
- 29.Russell FM, Balloch A, Tang ML, Carapetis JR, Licciardi P, Nelson J, et al. Immunogenicity following one, two, or three doses of the 7-valent pneumococcal conjugate vaccine. Vaccine. 2009 Sep 18;27(41):5685–91. doi: 10.1016/j.vaccine.2009.06.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nahm MH, Goldblatt D. Training manual for enzyme linked immunosorbent assay for the quantitation of Streptococcus pneumoniae serotype specific IgG (Pn PS ELISA) 2006 http://www.vaccine.uab.edu/ELISA%20Protocol.pdf.
- 31.Concepcion NF, Frasch CE. Pneumococcal type 22f polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 2001 Mar;8(2):266–72. doi: 10.1128/CDLI.8.2.266-272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henckaerts I, Goldblatt D, Ashton L, Poolman J. Critical differences between pneumococcal polysaccharide enzyme-linked immunosorbent assays with and without 22F inhibition at low antibody concentrations in pediatric sera. Clin Vaccine Immunol. 2006 Mar;13(3):356–60. doi: 10.1128/CVI.13.3.356-360.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balloch A, Mininni T, Nurkka A, Mackenzie G, Leach A, Kayhty H. Interlaboratory comparison of the specific IgG response to serotypes in Prevenar. 5th International Symposium on Pneumococci and Pneumococcal Diseases; 2006 April 2-6; Alice Springs, Australia. 2006. 2006. [Google Scholar]
- 34.Black S, Eskola J, Whitney CG, Shinefield H. Pneumococcal conjugate vaccine and pneumococcal common protein vaccines. In: Plotkin S, Orenstein W, Offit P, editors. Vaccines. 5th. Elsevier Inc.; 2008. pp. 531–67. [Google Scholar]
- 35.Ahman H, Kayhty H, Vuorela A, Leroy O, Eskola J. Dose dependency of antibody response in infants and children to pneumococcal polysaccharides conjugated to tetanus toxoid. Vaccine. 1999 Jun 4;17(20-21):2726–32. doi: 10.1016/s0264-410x(99)00048-1. [DOI] [PubMed] [Google Scholar]
- 36.Siegrist C. Vaccine Immunology. In: Plotkin S, Orenstein W, Offit P, editors. Vaccines. 5th. Elsevier; 2008. pp. 17–36. [Google Scholar]
- 37.Rose MA, Gruendler M, Schubert R, Kitz R, Schulze J, Zielen S. Safety and immunogenicity of sequential pneumococcal immunization in preschool asthmatics. Vaccine. 2009 Aug 20;27(38):5259–64. doi: 10.1016/j.vaccine.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 38.Ekstrom N, Vakevainen M, Verho J, Kilpi T, Kayhty H. Functional antibodies elicited by two heptavalent pneumococcal conjugate vaccines in the Finnish Otitis Media Vaccine Trial. Infect Immun. 2007 Apr;75(4):1794–800. doi: 10.1128/IAI.01673-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anttila M, Eskola J, Ahman H, Kayhty H. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J Infect Dis. 1998 Jun;177(6):1614–21. doi: 10.1086/515298. [DOI] [PubMed] [Google Scholar]
- 40.Nurkka A, Poolman J, Henckaerts Iea. Opsonophagocytic activity of antibodies induced by 11-valent pneumococcal conjugate vaccine. 4th International Symposium on Pneumococci and Pneumococcal Disease; 2004 May 9–13; Helsinki, Finland. 2004. 2004. [Google Scholar]
- 41.Anttila M, Eskola J, Ahman H, Kayhty H. Differences in the avidity of antibodies evoked by four different pneumococcal conjugate vaccines in early childhood. Vaccine. 1999 Apr 9;17(15-16):1970–7. doi: 10.1016/s0264-410x(98)00458-7. [DOI] [PubMed] [Google Scholar]
- 42.Pelton SI, Huot H, Finkelstein JA, Bishop CJ, Hsu KK, Kellenberg J, et al. Emergence of 19A as virulent and multidrug resistant Pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2007 Jun;26(6):468–72. doi: 10.1097/INF.0b013e31803df9ca. [DOI] [PubMed] [Google Scholar]
- 43.Hammitt LL, Bruden DL, Butler JC, Baggett HC, Hurlburt DA, Reasonover A, et al. Indirect effect of conjugate vaccine on adult carriage of Streptococcus pneumoniae: an explanation of trends in invasive pneumococcal disease. J Infect Dis. 2006 Jun 1;193(11):1487–94. doi: 10.1086/503805. [DOI] [PubMed] [Google Scholar]
- 44.Millar EV, Watt JP, Bronsdon MA, Dallas J, Reid R, Santosham M, et al. Indirect effect of 7-valent pneumococcal conjugate vaccine on pneumococcal colonization among unvaccinated household members. Clin Infect Dis. 2008 Oct 15;47(8):989–96. doi: 10.1086/591966. [DOI] [PubMed] [Google Scholar]
- 45.Riley ID, Lehmann D, Alpers MP, Marshall TF, Gratten H, Smith D. Pneumococcal vaccine prevents death from acute lower-respiratory-tract infections in Papua New Guinean children. Lancet. 1986 Oct 18;2(8512):877–81. doi: 10.1016/s0140-6736(86)90409-5. [DOI] [PubMed] [Google Scholar]
- 46.MacLennan J, Obaro S, Deeks J, Lake D, Elie C, Carlone G, et al. Immunologic memory 5 years after meningococcal A/C conjugate vaccination in infancy. J Infect Dis. 2001 Jan 1;183(1):97–104. doi: 10.1086/317667. [DOI] [PubMed] [Google Scholar]
- 47.Granoff DM, Pollard AJ. Reconsideration of the use of meningococcal polysaccharide vaccine. Pediatr Infect Dis J. 2007 Aug;26(8):716–22. doi: 10.1097/INF.0b013e3180cc2c25. [DOI] [PubMed] [Google Scholar]