Abstract
Testosterone (T) is lower among fathers and men in committed relationships, suggesting that the hormone mediates the trade-off between mating and parenting effort. The function of T in women, and responses of the hormone to relationships or motherhood, are less well understood. Here we report relationships between T and pairbonding and motherhood in a random sample of 67 women (20.9 to 22.4 years old) participating in a population-based birth cohort study in the Philippines. Testosterone was measured in saliva collected at bedtime and at waking the following morning to capture circadian dynamics. Compared to non-mothers and non-pairbonded women, mothers and pairbonded women had 32% (p<0.0001) and 23% (p<0.004) lower waking T, respectively, but similar evening T. The lower waking T in mothers largely reflected reduced T in mothers of young offspring (<2 years), with mothers of older offspring (2+ years) having intermediate T. These differences were independent of measures of breastfeeding, contraceptive pill use, menstrual cycle, sleep quality, education, employment and socioeconomic status. Our findings highlight a similar relationship between parenting and committed relationships and T in women as documented in men, and suggest that caregiving of dependent young may modulate female T. Future research should clarify whether this cross-sectional association reflects a suppressive effect of motherhood on T, whether these relationships vary across cultures, and the role of T within the endocrine architecture regulating female reproductive and caregiving strategies.
Keywords: reproduction, life history, parenting, sex steroids, behavioral ecology
INTRODUCTION
The role of testosterone (T) as a mediator of reproductive strategies has been the focus of considerable attention, with most work conducted on males. In human males T is proposed to mediate reproductive strategy in part through anabolic effects on sexually dimorphic traits, including skeletal growth, lean mass and strength (Bhasin et al., 2001; Sinha-Hikim et al., 2003), and also through effects on behaviors related to competition (Elias, 1981; Mazur et al., 1992; Salvador et al., 2003) and libido (Anderson et al., 1992; Wang et al., 2000). In many birds and mammals, T is elevated during periods of competition and mating, and is reduced in the context of caregiving in some species with biparental care (Goymann et al., 2007; Wingfield et al., 1990; Wynne-Edwards, 2001). There is evidence for a similar responsiveness to pairbonding and caretaking status among human males. Men who are in stable pairbonds have been shown to have lower T than non-pairbonded men in some (Booth and Dabbs, 1993; Gray et al., 2004; Mazur and Michalek, 1998; van Anders and Watson, 2006) but not all populations investigated (Flinn et al., 1998; Gray, 2003; Gray et al., 2006). Fathers also tend to have lower T than single men (Berg and Wynne-Edwards, 2001; Gray et al., 2002; Muller et al., 2009), and there is evidence that these relationships are stronger than with pairbonding alone in some (Gray et al., 2006; Kuzawa et al., 2009) but not all populations (Gray, 2003; Gray et al., 2004). These findings in males have been interpreted as support for the hypothesis that T mediates the tradeoff between behavioral and energetic effort devoted to mating and parenting in humans, much as it does in other taxa (Goymann et al., 2007; Wingfield et al., 1990).
Comparably few studies have focused on the importance of motherhood or pairbonding status to T levels in females, but evidence to date highlights similar relationships to those observed in males. Females have been found to exhibit differences in T based on relationship status (van Anders et al., 2007b), sexual orientation and pairbonding (van Anders and Watson, 2006) and whether they reside in the same city as their pairbond partner (van Anders and Watson, 2007). Women with multiple, simultaneous romantic partners showed higher T compared to single women or monogamous women (van Anders et al., 2007b), not unlike the finding of higher T in Swahili men with multiple wives (Gray, 2003). There is some evidence that testosterone promotes behaviors related to mating activity in females, including libido (Braunstein et al., 2005; Buster et al., 2005; Davis et al., 2008; Guay, 2001; van Anders et al., 2007a), competition (Edwards et al., 2006; Oliveira et al., 2009), dominance (Grant and France, 2001), and aggression (Harris et al., 1996). Much as has been argued for men, a tendency to reduce expression of these behaviors might provide reproductive benefits to females when priorities have shifted from securing mates to caring for dependent young (Fite et al., 2005).
To our knowledge, no study to date has specifically evaluated whether motherhood predicts T in women. In this journal, we recently documented that fathers living in Cebu City, the Philippines, have lower T than non-fathers (Kuzawa et al., 2009) and that, unlike in other samples investigated (Gray et al., 2002; Burnham et al., 2003; Gray et al., 2004), there was no association with pairbonding independent of fatherhood status in this population. Working with a same-aged sample of women from this population, here we report T in waking and pre-bedtime saliva samples, which we relate to pairbonding and parenting status while adjusting for potentially important confounding factors.
MATERIALS AND METHODS
Study population
Data come from the Cebu Longitudinal Health and Nutrition Survey (CLHNS), a population-based birth cohort study that has tracked a large cohort of men and women who were born in 1983-84 (Adair et al., 2001; Adair et al., 1993; Kuzawa and Adair, 2003). Women were 20-22 years of age at the time of interview and sample collection. Body weight (kg) and height (cm) were measured using standard anthropometric techniques (Lohman et al., 1988). The body mass index (BMI) was calculated as the ratio of weight (kg)/height (m2). Dietary intake was measured using two 24-hour recalls on consecutive days and the mean was used in analyses. Energy intake was calculated using Philippines Food Composition Tables produced by the Food and Nutrition Research Institute of the Philippines (FNRI, 1997). Information on current pregnancy status, self-reported contraceptive use, breastfeeding, and a range of socioeconomic, educational and employment questions were obtained in interviews. Participants in this study have traditionally been compensated for their time with a modest “bother fee” ($2 USD), which was also provided in this study. This research was conducted under conditions of informed consent with human subjects clearance from the Institutional Review Boards of Northwestern University and the University of North Carolina, Chapel Hill.
Motherhood and pairbonding
Women were classified as pairbonded if they reported currently living with a partner and/or were married. Using the household roster, mothers were defined as women who were living with one or more biological offspring, with age of offspring also noted. Although we did not have information on offspring living outside of the household, this was not likely an important issue. First, in Cebu mothers are the primary caregivers for dependent offspring, and offspring raised away from their mothers are likely rare (Liwag et al., 1998). Second, past research has demonstrated the importance of direct interaction and caregiving as an influence on maternal endocrine profile, which is of greatest interest to our research questions; thus, the rare mother who was not living with and raising her own offspring, and thus would be misclassified as a non-mother in our study, might be expected to share more hormonal similarities with non-mothers (e.g. Feldman et al., 2007; Fleming et al., 1997).
Menstrual cycle changes
Circulating T in females originates from adrenal and ovarian sources (Abraham 1974). Although some have concluded that menarcheal changes in T are minor enough to be ignored in behavioral research (Dabbs 1990), the ovarian contribution to circulating T changes across the menstrual cycle, and has generally been found to rise late in the follicular phase (Abraham 1974). In a study that used a sensitive equilibrium dialysis method capable of measuring free T at the low concentrations found in women, free T in 34 healthy women (the closest correlate of the salivary T measured here) was found to reach a pre-ovulatory peak late in the follicular phase, at 3 days prior to the LH peak, then gradually declined (Sinha-Hikim et al., 1998). To account for this potential influence on T, we estimated which women were in the late follicular phase at the time of saliva collection. During in-home interviews, women who did not report being pregnant were asked the timing of their last menstrual period. From this information we classified women as being in the late follicular phase if they reported that their last menstrual period began 7-14 days prior to saliva collection. In the event that saliva collection was more than 28 days after the interview, we assumed a 28-day cycle.
Lactation suppresses gonadotropin secretion (McNeilly, 1980) and has been reported to reduce testosterone in some but not all studies (e.g. Alder et al., 1986; Dada and Laditan, 1982). In addition, lactational suppression of ACTH could reduce production of adrenal androgens, with secondary effects on the adrenal contribution to circulating T. To account for possible lactational influences on T, we tested whether women who were currently breastfeeding had lower T, and if so, the extent to which this contributed to any differences seen in T among mothers, and especially, mothers of infants and young children of breastfeeding age.
Contraceptive pill use can reduce free T via effects on ovarian T production and also by increasing sex hormone binding globulin concentrations (Greco et al., 2007). Although their use is relatively uncommon in this population, we controlled for oral contraceptive use, self-reported during in-home interviews, as a predictor and potential confounding influence on the associations that we investigated.
Saliva collection
Each participant was provided with instructions and two tubes for saliva collection. The first sample was collected immediately prior to bed. After collection, the participant sealed the tube and kept it at room temperature. Participants were instructed to place the second tube next to their bed and to collect the second sample immediately upon waking the following morning. Respondents reported time of saliva collection, and at waking, wake time, usual wake time, and a 5-level self-rated scale reflecting sleep quality the prior night. Both tubes were collected later that day by an interviewer, who placed the tubes on ice packs in a cooler. They were then transported to a freezer where they were stored at -35 C until shipment on dry ice to the Laboratory for Human Biology Research at Northwestern University, where they were stored at -80 C. They were thawed, centrifuged, supernatant separated, and aliquoted into smaller tubes for analysis of individual analytes.
Testosterone measurement
Salivary T concentrations were determined in the Laboratory for Human Biology Research at Northwestern University using an enzyme immunoassay protocol specifically developed for use with saliva samples (Salimetrics No. 1-2402, State College, PA). All samples were run in duplicate, and low and high controls were included with each assay. The intra-assay coefficients of variation were 2.5% and 6.7% for high and low controls, respectively. The inter-assay coefficients of variation were 5.6% and 6.7%, respectively.
Sample selection
During the 2005 survey, 893 females of the original cohort of 1477 liveborn females, ages 21.5 years (range 20.8-22.6 years), were located and interviewed. Of these, 835 individuals agreed to participate in saliva collection. From these samples, we randomly selected saliva samples from 67 non-pregnant women for testosterone measurement. Baseline characteristics of the final sub-sample of 67 women who were included in the testosterone analyses were compared with those who were in the sample at baseline but excluded from the analysis. There were no significant or borderline significant differences (all p>0.1) in baseline characteristics between the testosterone analysis sub-sample and the remaining original baseline cohort, respectively, in household income (311 vs. 312 pesos), birth weight (3005 vs. 2993 g), mother’s height (150.4 vs. 150.5 cm), mother’s weight at parturition (50.1 vs. 50.1 kg), mother’s age (27.1 vs. 26.0 years), mother’s highest completed grade (6.5 vs. 7.1) or father’s highest completed grade (6.9 vs. 7.3), suggesting that sample inclusion was not biased.
Statistical analysis
All analyses were performed with version 10 of Stata (Stata Corporation, College Station, TX). Anthropometric, evening and waking T and daily energy intake (kcal) were all analyzed as continuous variables. We first used linear regression to test for significant differences in waking and evening T in relation to pairbonding and motherhood status and also to identify important potential confounding influences on T, adjusting only for time of sample collection, wake time that day and usual wake time. Next, we used multiple linear regression to evaluate relationships between waking and evening T and both motherhood and pairbonding while adjusting for covariates and potential confounders. Final models considered the age of the youngest offspring as a predictor, with mothers categorized as having young offspring (<2 years) and older offspring (2+ years) adjusting for time of sample collection, wake time that day and usual wake time, contraceptive pill use and menstrual cycle phase.
RESULTS
The sample included 67 women with an average age of 21.5 years (range 20.9 to 22.4 years), of whom 36% were mothers. Only 7% of non-mothers lived in households headed by themselves (and their partner), while more than half of mothers lived in their own households. Consistent with this, mothers were more likely to be employed, were less likely to be currently enrolled in school, and lived in lower income households. They were also more likely to be pairbonded or to report currently using oral contraceptives (Table 1). When compared to non-mothers, mothers had lower unadjusted waking T but not lower evening T.
Table 1.
Characteristics of sample.
| All (n=67) | Non-mothers (n=43) | Mothers (n=24) | pa | |
|---|---|---|---|---|
| Biological variables | ||||
| Waking testosterone (pg/ml) | 82.5 (33.0) | 93.1 (32.3) | 63.4 (25.1) | 0.0002 |
| Evening testosterone (pg/ml) | 45.0 (20.1) | 47.6 (20.3) | 40.3 (19.3) | 0.156 |
| Collection time (waking) | 6.8 (2.3) | 6.8 (2.3) | 6.5 (1.1) | 0.576 |
| Collection time (evening) | 20.6 (4.8) | 20.2 (5.9) | 21.5 (1.4) | 0.261 |
| Height (cm) | 150.3 (5.6) | 150.7 (5.8) | 149.6 (5.3) | 0.434 |
| BMI (kg/m2) | 20.5 (3.1) | 20.2 (3.0) | 21.0 (3.2) | 0.281 |
| Energy intake (kcal/day) | 1609 (860) | 1528 (680) | 1755 (1115) | 0.502 |
| Triceps skinfold (mm) | 20.2 (5.9) | 20.4 (6.2) | 20.0 (5.5) | 0.921 |
| Social context | ||||
| Age (years) | 21.5 (0.3) | 21.5 (0.3) | 21.6 (0.3) | 0.319 |
| Education (highest grade) | 11.1 (3.1) | 11.9 (3.1) | 9.9 (2.7) | 0.011 |
| Household size (persons) | 6.5 (2.9) | 6.6 (2.7) | 6.2 (3.3) | 0.470 |
| Per capita weekly | ||||
| household income (pesos) | 93 (104) | 109 (122) | 66 (52) | 0.030 |
| Lives in own household (%) | 23.1% | 7.0% | 54.6% | 0.0001 |
| Reproductive status | ||||
| Uses oral contraceptives (%) | 13.4% | 2.3% | 33.3% | 0.0001 |
| Pairbonded (%) | 37.3% | 11.6% | 83.3% | 0.0001 |
| Currently breastfeeding (%) | 13.4% | - | 37.5% | - |
| Mother (%) | 35.8% |
Mean (SD) unless otherwise noted
p-value from 2-sided t-test or chi-square comparing mothers and non-mothers
Table 2 reports mean waking and evening T stratified on motherhood and pairbonding status in addition to three potential confounding factors known to influence T levels in females: using contraceptive pills, breastfeeding and being in the late follicular phase of the menstrual cycle. Measures of socioeconomic status (per capita household income, assets scale, currently employed), education (highest grade completed), sleep quality the night prior to waking sample collection (5-level self-rated scale) and daily caloric intake were not significant or borderline significant as predictors of waking or evening T (all p>0.3) and are therefore not shown. Mothers had lower waking T but not evening T compared to non-mothers. When mothers were stratified according to the age of their youngest offspring, mothers with young offspring (< 2 years of age) had significantly lower waking T and evening T compared to non-mothers, while mothers of older offspring (2+ years) only had borderline significantly lower T and only in the morning. Pairbonded women had lower waking T but their evening T was similar to that of non-pairbonded women. As expected, women late in the follicular phase of the menstrual cycle had higher waking T compared to women in other phases of the menstrual cycle, while contraceptive pill users had lower T at both times of day compared to non pill users.
Table 2.
Mean waking and evening testosterone (pg/ml) by motherhood, pairbonding, pill use, breastfeeding and menstrual cycle phase.
| Variable (n) | Waking T | pa | Evening T | pa |
|---|---|---|---|---|
| Non-mother (43) | 93.1 (4.5) | 47.6 (3.0) | ||
| Mother - all (24) | 63.4 (6.0) | 0.0001b | 40.3 (4.0) | 0.200b |
| Young offspring (14) | 54.7 (7.6) | 0.0001b | 30.6 (4.9) | 0.004b |
| Older offspring (10) | 75.6 (9.0) | 0.096b | 53.8 (5.8) | 0.236b |
| Non-pairbonded (42) | 90.2 (4.7) | 47.7 (3.0) | ||
| Pairbonded (25) | 69.6 (6.2) | 0.004 | 40.5 (3.9) | 0.180 |
| Non pill use (58) | 87.0 (4.0) | 47.8 (2.4) | ||
| Pill user (9) | 53.7 (10.2) | 0.003 | 26.9 (6.2) | 0.003 |
| Not breastfeeding (58) | 85.5 (4.2) | 46.8 (2.5) | ||
| Breastfeeding (9) | 63.2 (10.6) | 0.043 | 33.6 (6.4) | 0.057 |
| Other phase (45) | 76.5 (4.7) | 42.3 (2.9) | ||
| Late follicular (22) | 94.7 (6.7) | 0.018 | 50.4 (4.2) | 0.173 |
Mean (SEM) testosterone adjusted for time of saliva collection, usual wake time, and wake time that day.
p-value for significant difference in T measured at same time of day (all linear regression).
Compared to non-mothers
Although women who were currently breastfeeding had lower waking T and borderline lower evening T compared to women not currently breastfeeding (Table 2), breastfeeding did not appear to be an important influence on the lower T found in mothers. Although mothers as a group had lower T than non-mothers, of the 14 mothers with young offspring, the 7 who were breastfeeding had modestly higher waking T (61.5 pg/ml) compared to the 7 who were not breastfeeding (47.9 pg/ml). Consistent with this, breastfeeding was no longer associated with lower T in the morning (-0.7 pg/ml, p<0.954 compared to non-breastfeeders) or in the evening (-2.3 pg/ml, p<0.775 compared to non-breastfeeders) after adjusting for being a mother of a young offspring (<2 years).
We next ran a series of regression models designed to disentangle the independent associations of motherhood and pairbonding status with T. Given the low frequency in our sample of mothers who were not pairbonded (n=4) and of pairbonded non-mothers (n=5), we caution that these results should be interpreted as preliminary. Adjusting for usual wake time, wake time that day and time of saliva collection, waking T was similar in the 5 non-mothers who were pairbonded and the 38 non-mothers who were not pairbonded (86.5 pg/ml vs. 94.0 pg/ml, respectively, p<0.4). Of the 24 mothers, the 4 who were not pair-bonded had similar mean waking T to the 20 mothers who were pairbonded (53.9 pg/ml vs. 65.3 pg/ml, p<0.7). When motherhood and pairbonding were considered simultaneously in a regression model that adjusted for circadian time variables, mothers had a significant 28.1 pg/ml (SE 10.9 pg/ml) reduction in waking T compared to non-mothers (t = -2.58, p<0.012), while pairbonding status was no longer a significant predictor of waking T after adjusting for motherhood (-3.6 pg/ml, SE 10.8 pg/ml, p<0.737).
Final regression models evaluated relationships between motherhood status and waking and evening T adjusting for potential confounding influences identified above. In final models (Table 3) mothers of young offspring had lower T in the morning (significant) and evening (not significant) compared to non-mothers, adjusting for time of saliva collection, usual wake time, and wake time that day, contraceptive pill use and being in the late follicular phase of the menstrual cycle (Figure 1). Mothers of older offspring had non-significantly lower waking T than non-mothers, and a statistically significant but modest (~6 pg/ml) increase in evening T compared to non-mothers.
Table 3.
Multiple regression models predicting waking and evening testosterone.
| Waking T | p | Evening T | p | |
|---|---|---|---|---|
| Mother (offspring < 2 years)b | -33.6 (10.2) | 0.002 | -9.1 (6.2) | 0.149 |
| Mother (offspring 2+ years)b | -8.5 (10.6) | 0.428 | 13.7 (6.6) | 0.044 |
| Contraceptive use | -14.0 (11.4) | 0.226 | -15.5 (7.2) | 0.035 |
| Late follicular | 13.6 (7.9) | 0.092 | 7.4 (5.0) | 0.142 |
| Model R2 | 0.360 | 0.321 | ||
β (SE) predicting waking testosterone (pg/ml). Also adjusted for time of saliva collection, wake time and usual wake time (not shown).
Compared to non-mothers
Fig. 1.
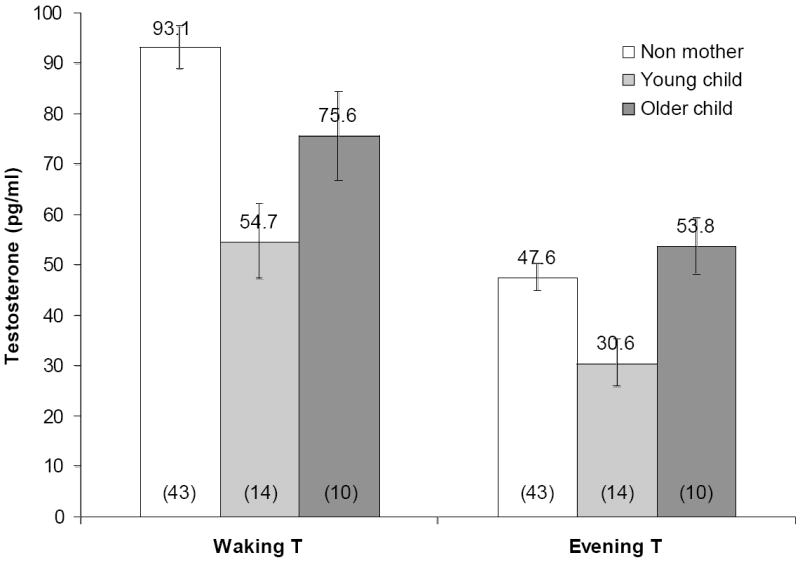
Mean (± SD) waking and evening salivary free testosterone in non-mothers, mothers whose youngest offspring is less than 2 years of age, and mothers of older children (2+ years of age). Sample sizes in parentheses. Adjusted for time of saliva measurement, usual wake time, wake time that day, oral contraceptive use and being in the late follicular stage of the menstrual cycle. Significant differences: Waking T, mothers of young offspring vs. older offspring p<0.051, mothers of young offspring vs. non-mothers p<0.002; Evening T, mothers of older offspring vs. non-mothers (p<0.044) and mothers of young offspring (p<0.004).
DISCUSSION
In this sample of Filipino women, mothers had lower waking T than non-mothers, with differences greatest among mothers with younger offspring. Although the number of breastfeeding women was relatively small, available data suggested minimal contributions of lactation as an influence on these differences. Although pairbonded women had lower T than non-pairbonded women, these differences were more modest than those seen in mothers, and, although small sample size limited strong inferences, were largely accounted for by the association between motherhood and T. The present findings are to our knowledge the first demonstration that T is lower in the context of parenting among women.
Our findings among the females in this sample share similarities to patterns that we recently reported in males from the same cohort (Kuzawa et al., 2009), and that have been documented in males in other populations (Gray et al., 2006). Notably, in both men and women at Cebu, parenting is a strong predictor of lower T levels, while pairbonding status was less important as an independent predictor of T. Similar studies of T in females in other populations have focused on relationship status as a predictor, without accounting for parenting status, and have found mixed evidence for lower T in pairbonded women. Similar to our results, two studies have documented no relationship between pairbonding and T in heterosexual women (van Anders et al., 2007b; van Anders and Watson, 2006). However, another study, which controlled for sexual orientation, showed that women living in the same city as their partner had lower T levels compared to single women and those in long distance relationships (van Anders and Watson, 2007).
Why we find evidence for lower T specific only to motherhood—with no difference by pairbonding status alone—is uncertain, but could trace to recent cultural shifts in the nature of pairbonding in Philippine society. Long-term pairings in which men and women are not legally married are increasingly common in Cebu (NSO, 2004). The growing incidence of couples avoiding legal marriage may reflect an effort of men and women to circumvent the lack of legalized divorce in the Philippines (Gloria, 2007). While it remains speculative, such non-legalized pairings may involve different endocrine responses than are elicited by formalized marital relations that have typically been practiced in the Philippines in the past.
While traditional patterns of marriage and pairbonding may have shifted in the Philippines, levels of maternal involvement with their offspring have remained relatively stable (Liwag et al., 1998; Medina, 2001). Although fathers have traditionally played a relatively minor role in offspring care, this is gradually changing, and sibling care is also quite prevalent (ECD, unpublished data; Harper, 2010; Kuzawa et al., 2009). However, in general, girls are socialized into domestic, parenting roles from a young age and mothers remain the primary source of nurturing and caregiving for their children across socioeconomic strata in the Philippines (Liwag et al., 1998). This remains the case even as millions of women enter the workforce to help support the family financially (Medina, 2001). Our findings suggest cross-cultural differences in the relationship between pairbonding and T, highlighting the need for future comparative work to be couched in a biocultural framework that addresses the similarities and differences in the roles related to pairbonding, marriage and childcare.
The similarities in relationships between T and pairbonding and parenting among males and females in Cebu are striking. Recent comparative studies also document similarities in T response and function in the males and females of other taxa, such as fish and birds (Moller et al., 2005). For instance, in certain fish taxa, the degree of male sexually-selected ornamentation is positively related to peak female androgen levels, suggesting that sexual selection for higher T in males has correlated consequences on T levels in females of that species (Mank, 2007). It is presently unclear how sex differences in T might co-vary across species in mammals or primates, or the degree to which androgen levels, or related behavioral and somatic effects in women, might be a correlated consequence of selection on hormone regulation and target tissue effects in human males (Ketterson et al., 2005). The broad similarities in the relationship between parenting status and T in males and females in our sample underscore the need to investigate this possibility.
It is also possible that T has effects that are adaptive to females, and thus have been shaped by natural selection for a female-specific function. There is evidence from other species that selection has influenced the level of T and its behavioral effects in females independent of effects in males, as suggested for instance by the finding that peak female T is highest in colonial bird species in which females play a prominent role in territorial defense (Moller et al., 2005). Although less research into T has been conducted in female primates, the work to date supports the hormone’s importance as a modulator of female reproductive strategies. For instance, in Wied’s black tufted-ear marmosets (a species of New World monkey), mothers have been found to exhibit reduced caregiving effort in association with elevated T (Fite et al., 2005). In the great apes, T is higher for more of the cycle in chimpanzees than in gorilla or orangutan, which has been related to the longer period of mating during each cycle in chimpanzees (Nadler et al., 1985)
Although conflicting evidence exists (e.g. Davis et al., 2005; Kivlighan et al., 2005; Mazur et al. 1997), it has been speculated that T is associated with a wide range of behaviors in women that may influence mate choice, sexual behavior, and parenting, which could improve mating success among non-mothers (Braunstein et al., 2005; Buster et al., 2005; Davis et al., 2008; Grant and France, 2001; Guay, 2001; Harris et al., 1996; van Anders et al., 2007a). To the extent that the lower T observed among mothers in our sample reflects a hormonal response to motherhood, rather than a trait-level predictor of motherhood status, reduced T in the context of motherhood could help shift female priorities away from mating and in favor of caretaking and parenting, much as has been proposed for men (Gray and Campbell, 2009).
It is important to note, however, that the sources of T are distinct in men and women. In women, the concentration of free T, which is indexed by the salivary measures used in this study, is a function of steroid production in the adrenal and ovaries and binding by sex hormone binding globulin. Lower T in mothers likely traces to a combination of factors that shift across the menstrual cycle and with changing patterns and intensity of offspring investment. For instance, breastfeeding has been associated with suppression of ovarian and adrenal hormone production, which could influence circulating T. To the extent that T motivates mating-related behaviors in women (Buster et al., 2005), such a mechanism could help shift metabolic and behavioral priorities away from initiating a new pregnancy during the most energetically intensive period of investment in the current offspring (Valeggia and Ellison 2009). Although we did find that breastfeeding women had lower T, this association was weaker than the association between T and motherhood, and mothers of young offspring who were not breastfeeding actually had modestly lower T than women who were breastfeeding. In light of our small sample sizes, we emphasize the need to investigate these questions with a larger sample of mothers and with more variation in lactational status.
In addition to any direct effects of reproductive or energetic status on hormone production, past work has demonstrated that exposure to infant cues, such as odors or recorded cries, development of maternal attachment and mother-infant bonding, and rates of affectionate maternal behavior, can directly elicit changes in neuroendocrine regulation and hormonal levels in mothers (Feldman et al., 2007; Fleming et al., 1997a; Fleming et al., 1997b; Storey et al., 2000). These studies have focused primarily on cortisol, oxytocin, and female sex steroids, such as estradiol and progesterone, and comparably little is known about female T responses to offspring cues and caregiving. Most studies of T in mothers have focused on the immediate postpartum period, and results have been inconsistent. Still, in the early post-partum period higher T in mothers has been associated with reduced affectionate contact and positive feelings about the infant (Fleming et al., 1997b), and also with maternal depression and higher anger scores (Hohlagschwandtner, 2001).
Although our data are cross-sectional, the patterns that we document are consistent with an effect of caretaking on female T levels. Mothers play a central role as caretakers in the Philippines, and often have primary responsibility for meeting a child’s needs. In our sample, less than 50% of the fathers listed themselves as individuals within the household who were responsible for childcare (Kuzawa et al., 2009). Fathers who reported being involved in childcare had particularly low evening T levels, which we speculated could indicate a direct role of interacting with the child across the day on T suppression. Similarly, our present finding that T was lowest among mothers with young offspring, whereas mothers of older children had intermediate T levels, could indicate that the more frequent and demanding activities of caring for infants and young children, compared to older offspring, contribute to the lower T in these women. Our findings provide a strong rationale to include T measurement in future endocrine studies of maternal caretaking.
Several limitations of this study warrant mention. First, we only have a single waking and evening saliva sample for each participant. Past work on T has shown that collecting samples across several days and using the average in analyses enhances reliability (Dabbs, 1990). Similarly, a validation study that compared serum free and total T to salivary T measured with an enzyme immunoassay protocol similar to that used here found that relationships between salivary measures and total T were similar in males and females (r = 0.54 and 0.56, respectively), but that relationships with serum free T--reflecting the unbound fraction--were weaker in females (r = 0.42) than in males (r = 0.64) (Shirtcliff et al., 2002). Another potential source of error traces to menstrual changes in T, which we adjusted for using each woman’s recalled timing of last menstrual period and assuming a 28-day cycle for each woman. Repeat measurement of blood samples would have allowed more precise characterization of menstrual changes in T, which are secondary to the contribution of ovarian T to the pool of circulating T. Unless these factors influence mothers and non-mothers differently in our sample, these various sources of error are not likely to introduce bias but will merely limit our ability to detect statistically-significant relationships between T and social context (Shirtcliff et al., 2002). It is possible that some of the borderline relationships in our study, such as the lower evening T among mothers, would have reached the level of statistical significance with better characterization of typical T levels and with better statistical control of each woman’s position in the menstrual cycle.
As is the case with most prior studies of T and social context in males or females, our data are cross-sectional and thus do not permit us to distinguish whether women with high T are less likely to become pregnant from the alternative that mothering has a direct suppressive effect on T. Both trait and state effects are possible, and both could occur simultaneously. Although only circumstantial evidence, the finding that T is lowest in mothers of young infants and children, and intermediate in mothers of older children, makes a trait-level relationship less likely, as it suggests that mothers may have relatively high or low levels of T depending on the age of their currently youngest offspring. A longitudinal design that follows women prospectively as they transition from non-mothers into motherhood, and that assesses acute T responses to interacting with offspring, will be necessary to more definitively establish the causal direction of effects underlying the relationships that we document.
In summary, in a population of young Filipino women, T levels were roughly one third lower in mothers than in non-mothers in the morning, but there were no differences in T measured in the evening prior to bed. Differences were larger and more significant among mothers with young infants, perhaps indicating a role of demanding caregiving as an influence on T. Although small sample sizes precluded definitive tests of their independent associations, T was more strongly related to motherhood status than to pairbonding status in these women. These findings are similar to our recent findings among the males in this cohort, in which parenting but not pairbonding predicted lower T (Kuzawa et al., 2009). Future research is needed to clarify cultural variation in these relationships, establish whether motherhood lowers T in a longitudinal design, and assess the possible role of reduced T in female caretaking and reproductive strategies.
Acknowledgments
Linda Adair played an important role in designing and implementing the CLHNS survey from which these data and samples were obtained. Alan Feranil provided helpful assistance with literature related to parenting and marriage norms within Philippine society. Elizabeth Quinn, Katy Sharrock, Iram Azam, Divya Mallampati, Brian Dubin, and Laura Rogers helped with various phases of lab work with these samples. We thank the many researchers at the Office of Population Studies, University of San Carlos, Cebu, the Philippines, for their central role in study design and data collection, and the Filipino participants, who generously provided their time for this study. This study was funded by the Wenner Gren Foundation (Gr. 7356) and the National Science Foundation (BCS-0542182). Fieldwork and sample collection were supported by pilot funds from the Interdisciplinary Obesity Center (RR20649) and the Center for Environmental Health and Susceptibility (ES10126; project 7-2004-E).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham GE. Ovarian and adrenal contribution to peripheral androgens during menstrual cycle. Journal of Clinical Endocrinology & Metabolism. 1974;39:340–346. doi: 10.1210/jcem-39-2-340. [DOI] [PubMed] [Google Scholar]
- Adair LS, Kuzawa CW, Borja J. Maternal energy stores and diet composition during pregnancy program adolescent blood pressure. Circulation. 2001;104:1034–9. doi: 10.1161/hc3401.095037. [DOI] [PubMed] [Google Scholar]
- Adair LS, Popkin BM, Guilkey DK. The duration of breast-feeding: How is it affected by biological, sociodemographic, health sector, and food-industry factors. Demography. 1993;30:63–80. [PubMed] [Google Scholar]
- Alder EM, Cook A, Davidson D, West C, Bancroft J. Hormones, mood, and sexuality in lactating women. British Journal of Psychiatry. 1986;148:74–79. doi: 10.1192/bjp.148.1.74. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Bancroft J, Wu FCW. The effects of exogenous testosterone on sexuality and mood of normal men. Journal of Clinical Endocrinology and Metabolism. 1992;75:1503–1507. doi: 10.1210/jcem.75.6.1464655. [DOI] [PubMed] [Google Scholar]
- Berg SJ, Wynne-Edwards KE. Changes in testosterone, cortisol, and estradiol levels in men becoming fathers. Mayo Clinic Proceedings. 2001;76:582–592. doi: 10.4065/76.6.582. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, Chen XH, Yarasheski KE, Magliano L, Dzekov C, Dzekov J, Bross R, Phillips J, Sinha-Hikim I, Shen RQ, Storer TW. Testosterone dose-response relationships in healthy young men. American Journal of Physiology-Endocrinology and Metabolism. 2001;281:E1172–E1181. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- Booth A, Dabbs JM. Testosterone and men’s marriages. Social Forces. 1993;72:463–477. [Google Scholar]
- Braunstein GD, Sundwall DA, Katz M, Shifren JL, Buster JE, Simon JA, Bachman G, Aguirre OA, Lucas JD, Rodenberg C, Buch A, Watts NB. Safety and efficacy of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Arch Intern Med. 2005;165:1582–1589. doi: 10.1001/archinte.165.14.1582. [DOI] [PubMed] [Google Scholar]
- Buster JE, Kingsberg SA, Aguirre O, Brovm C, Breaux JG, Buch A, Rodenberg CA, Wekselman K, Casson P. Testosterone patch for low sexual desire in surgically menopausal women: A randomized trial. Obstetrics and Gynecology. 2005;105:944–952. doi: 10.1097/01.AOG.0000158103.27672.0d. [DOI] [PubMed] [Google Scholar]
- Dabbs JM. Salivary testosterone measurements: Reliability across hours, days, and weeks. Physiology & Behavior. 1990;48:83–86. doi: 10.1016/0031-9384(90)90265-6. [DOI] [PubMed] [Google Scholar]
- Dada OA, Laditan AAO. Circulating hormonal levels during prolonged lactational amenorrhea. Clinica Chimica Acta. 1982;123:287–292. doi: 10.1016/0009-8981(82)90173-5. [DOI] [PubMed] [Google Scholar]
- Davis SR, Davison SL, Donath S, Bell RJ. Circulating androgen levels and self- reported sexual function in women. JAMA. 2005;294:91–96. doi: 10.1001/jama.294.1.91. [DOI] [PubMed] [Google Scholar]
- Davis SR, Moreau M, Kroll R, Bouchard C, Panay N, Gass M, Braunstein GD, Hirschberg AL, Rodenberg C, Pack S, Koch H, Moufarege A, Studd J. Testosterone for low libido in postmenopausal women not taking estrogen. New England Journal of Medicine. 2008;359:2005–2017. doi: 10.1056/NEJMoa0707302. [DOI] [PubMed] [Google Scholar]
- ECD, Early Child Development survey (ECD) Office of Population Studies Foundation. University of San Carlos; Cebu City, Philippines: unpublished data. [Google Scholar]
- Edwards DA, Wetzel K, Wyner DR. Intercollegiate soccer: Saliva cortisol and testosterone are elevated during competition, and testosterone is related to status and social connectedness with teammates. Physiology & Behavior. 2006;87:135–143. doi: 10.1016/j.physbeh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Elias M. Serum cortisol, testosterone, and testosterone binding globulin responses to competitive fighting in human males. Aggressive Behavior. 1981;7:215–224. [Google Scholar]
- FNRI. Food and nutrition research institute: food composition tables recommended for use in the Philippines. Manila: Food and Nutrition Research Institute of the Philippines; 1997. [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation - Plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychological Science. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Fite JE, French JA, Patera KJ, Hopkins EC, Rukstalis M, Ross CN. Elevated urinary testosterone excretion and decreased maternal caregiving effort in marmosets when conception occurs during the period of infant dependence. Hormones and Behavior. 2005;47:39–48. doi: 10.1016/j.yhbeh.2004.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, Ruble D, Krieger H, Wong PY. Hormonal and experiential correlates of maternal responsiveness during pregnancy and the puerperium in human mothers. Hormones and Behavior. 1997a;31:145–158. doi: 10.1006/hbeh.1997.1376. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Steiner M, Corter C. Cortisol, hedonics, and maternal responsiveness in human mothers. Hormones and Behavior. 1997b;32:85–98. doi: 10.1006/hbeh.1997.1407. [DOI] [PubMed] [Google Scholar]
- Flinn MV, Baewald C, Decker S, England B. Evolutionary functions of neuroendocrine response to social environment. Behav Brain Sci. 1998;21:372–374. [Google Scholar]
- Gloria CK. Who needs divorce in the Philippines? Mindanao Law Journal. 2007;1:18–28. [Google Scholar]
- Goymann W, Landys MM, Wingfield JC. Distinguishing seasonal androgen responses from male-male androgen responsiveness--revisiting the challenge hypothesis. Hormones and Behavior. 2007;51:463–476. doi: 10.1016/j.yhbeh.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Grant VJ, France JT. Dominance and testosterone in women. Biological Psychology. 2001;58:41–47. doi: 10.1016/s0301-0511(01)00100-4. [DOI] [PubMed] [Google Scholar]
- Gray PB. Marriage, parenting, and testosterone variation among Kenyan Swahili men. American Journal of Physical Anthropology. 2003;122:279–286. doi: 10.1002/ajpa.10293. [DOI] [PubMed] [Google Scholar]
- Gray PB, Campbell BC. Human male testosterone, pair bonding and fatherhood. In: Ellison PT, Gray PB, editors. Endocrinology of social relationships. Harvard University Press; Cambridge, Mass: 2009. pp. 270–293. [Google Scholar]
- Gray PB, Campbell BC, Marlowe FW, Lipson SF, Ellison PT. Social variables predict between-subject but not day-to-day variation in the testosterone of US men. Psychoneuroendocrinology. 2004;29:1153–1162. doi: 10.1016/j.psyneuen.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Gray PB, Kahlenberg SM, Barrett ES, Lipson SF, Ellison PT. Marriage and fatherhood are associated with lower testosterone in males. Evolution and Human Behavior. 2002;23:193–201. [Google Scholar]
- Gray PB, Yang C-FJ, Pope HG. Fathers have lower salivary testosterone levels than unmarried men and married non-fathers in Beijing, China. Proceedings of the Royal Society B-Biological Sciences. 2006;273:333–339. doi: 10.1098/rspb.2005.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco T, Graham CA, Bancroft J, Tanner A, Doll HA. The effects of oral contraceptives on androgen levels and their relevance to premenstrual mood and sexual interest: a comparison of two triphasic formulations containing norgestimate and either 35 or 25 μg of ethinyl estradiol. Contraception. 2007;76:8–17. doi: 10.1016/j.contraception.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Guay AT. Decreased testosterone in regularly menstruating women with decreased libido: a clinical observation. Journal of Sex & Marital Therapy. 2001;27:513–519. doi: 10.1080/713846816. [DOI] [PubMed] [Google Scholar]
- Harper SE. Exploring the role of Filipino fathers: paternal behaviors and child outcomes. Journal of Family Issues. 2010;31:66–89. [Google Scholar]
- Harris JA, Rushton JP, Hampson E, Jackson DN. Salivary testosterone and self-report aggressive and pro-social personality characteristics in men and women. Aggressive Behavior. 1996;22:321–331. [Google Scholar]
- Hohlagschwandtner M, Husslein P, Klier C, Ulm B. Correlation between serum testosterone levels and peripartal mood states. Acta Obstetricia Et Gynecologica Scandinavica. 2001;80:326–326. doi: 10.1034/j.1600-0412.2001.080004326.x. [DOI] [PubMed] [Google Scholar]
- Ketterson ED, Nolan V, Sandell M. Testosterone in females: Mediator of adaptive traits, constraint on sexual dimorphism, or both? American Naturalist. 2005;166:S85–S98. doi: 10.1086/444602. [DOI] [PubMed] [Google Scholar]
- Kivlighan KT, Granger DA, Booth A. Gender differences in testosterone and cortisol response to competition. Psychoneuroendocrinology. 2005;30:58–71. doi: 10.1016/j.psyneuen.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Kuzawa C, Gettler LT, Muller MN, McDade TW, Feranil AB. Fatherhood, pairbonding, and testosterone in the Philippines. Hormones and Behavior. 2009;56:429–435. doi: 10.1016/j.yhbeh.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzawa CW, Adair LS. Lipid profiles in adolescent Filipinos: relation to birth weight and maternal energy status during pregnancy. American Journal of Clinical Nutrition. 2003;77:960–966. doi: 10.1093/ajcn/77.4.960. [DOI] [PubMed] [Google Scholar]
- Liwag MEC, Dela Cruz A, Macapagal ME. How we raise our daughters and sons: child rearing and gender socialization in the Philippines. Philipp J Psychol. 1998;31:1–46. [Google Scholar]
- Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Human Kinetics Books; Champaign, IL: 1988. [Google Scholar]
- Mank JE. The evolution of sexually selected traits and antagonistic androgen expression in actinopterygiian fishes. American Naturalist. 2007;169:142–149. doi: 10.1086/510103. [DOI] [PubMed] [Google Scholar]
- Mazur A, Booth A, Dabbs JM. Testosterone and chess competition. Social Psychology Quarterly. 1992;55:70–77. [Google Scholar]
- Mazur A, Susman EJ, Edelbrock S. Sex difference in testosterone response to a video game contest. Evolution and Human Behavior. 1997;18:317–326. [Google Scholar]
- Mazur A, Michalek J. Marriage, divorce, and male testosterone. Social Forces. 1998;77:315–330. [Google Scholar]
- McNeilly AS. Prolactin and the control of gonadotropin secretion in the female. Journal of Reproduction and Fertility. 1980;58:537–549. doi: 10.1530/jrf.0.0580537. [DOI] [PubMed] [Google Scholar]
- Medina B. The Filipino family. University of the Philippines Press; Quezon City, Philippines: 2001. [Google Scholar]
- Moller AP, Garamszegi LZ, Gil D, Hurtrez-Bousses S, Eens M. Correlated evolution of male and female testosterone profiles in birds and its consequences. Behavioral Ecology and Sociobiology. 2005;58:534–544. [Google Scholar]
- Muller MN, Marlowe FW, Bugumba R, Ellison PT. Testosterone and paternal care in East African foragers and pastoralists. Proceedings of the Royal Society B-Biological Sciences. 2009;276:347–354. doi: 10.1098/rspb.2008.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler RD, Graham CE, Gosselin RE, Collins DC. Serum levels of gonadotropins and gonadal steroids, including testosterone, during the menstrual cycle of the chimpanzee (Pan troglodytes) American Journal of Primatology. 1985;9:273–284. doi: 10.1002/ajp.1350090404. [DOI] [PubMed] [Google Scholar]
- NSO. National Statistics Office (NSO) Philippines and ORC Macro, National Demographic and Health Survey 2003. Calverton, Maryland: NSO and ORC Macro; 2004. [Google Scholar]
- Oliveira T, Gouveia MJ, Oliveira RF. Testosterone responsiveness to winning and losing experiences in female soccer players. Psychoneuroendocrinology. 2009 doi: 10.1016/j.psyneuen.2009.02.006. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- Salvador A, Suay F, Gonzalez-Bono E, Serrano MA. Anticipatory cortisol, testosterone and psychological responses to judo competition in young men. Psychoneuroendocrinology. 2003;28:364–375. doi: 10.1016/s0306-4530(02)00028-8. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Likos A. Gender differences in the validity of testosterone measured in saliva by immunoassay. Hormones and Behavior. 2002;42:62–69. doi: 10.1006/hbeh.2002.1798. [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I, Arver S, Beall G, Shen RQ, Guerrero M, Sattler F, Shikuma C, Nelson JC, Landgren BM, Mazer NA, Bhasin S. The use of a sensitive equilibrium dialysis method for the measurement of free testosterone levels in healthy, cycling women and in human immunodeficiency virus-infected women. Journal of Clinical Endocrinology & Metabolism. 1998;83:1312–1318. doi: 10.1210/jcem.83.4.4718. [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I, Roth SM, Lee MI, Bhasin S. Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. American Journal of Physiology-Endocrinology and Metabolism. 2003;285:E197–E205. doi: 10.1152/ajpendo.00370.2002. [DOI] [PubMed] [Google Scholar]
- Storey AE, Walsh CJ, Quinton RL, Wynne-Edwards KE. Hormonal correlates of paternal responsiveness in new and expectant fathers. Evolution and Human Behavior. 2000;21:79–95. doi: 10.1016/s1090-5138(99)00042-2. [DOI] [PubMed] [Google Scholar]
- Valeggia C, Ellison PT. Interactions between metabolic and reproductive functions in the resumption of postpartum fecundity. American Journal of Human Biology. 2009;21:559–566. doi: 10.1002/ajhb.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Anders SM, Hamilton LD, Schmidt N, Watson NV. Associations between testosterone secretion and sexual activity in women. Hormones and Behavior. 2007a;51:477–482. doi: 10.1016/j.yhbeh.2007.01.003. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Hamilton LD, Watson NV. Multiple partners are associated with higher testosterone in North American men and women. Hormones and Behavior. 2007b;51:454–459. doi: 10.1016/j.yhbeh.2007.01.002. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Watson NV. Relationship status and testosterone in North American heterosexual and non-heterosexual men and women: Cross-sectional and longitudinal data. Psychoneuroendocrinology. 2006;31:715–723. doi: 10.1016/j.psyneuen.2006.01.008. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Watson NV. Testosterone levels in women and men who are single, in long-distance relationships, or same-city relationships. Hormones and Behavior. 2007;51:286–291. doi: 10.1016/j.yhbeh.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Wang C, Swerdloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunningham G, Matsumoto AM, Weber T, Berman N. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. Journal of Clinical Endocrinology and Metabolism. 2000;85:2839–2853. doi: 10.1210/jcem.85.8.6747. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, Ball GF. The challenge hypothesis- theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. American Naturalist. 1990;136:829–846. [Google Scholar]
- Wynne-Edwards KE. Hormonal changes in mammalian fathers. Hormones and Behavior. 2001;40:139–145. doi: 10.1006/hbeh.2001.1699. [DOI] [PubMed] [Google Scholar]


