Abstract
This study describes the comparative analysis of two insect recombinant aminotransferases, Aedes aegypti 3-hy-droxykynurenine (3-HK) transaminase/alanine glyoxylate aminotransferase (Ae-HKT/AGT) and Drosophila melanogaster serine pyruvate aminotransferase (Dm-Spat), which share 52% identity in their amino acid sequences. Both enzymes showed AGT activity. In addition, Ae-HKT/AGT is also able to catalyze the transamination of 3-HK or kynurenine with glyoxylate, pyruvate or oxaloacetate as the amino acceptor. Kinetic analysis and other data suggest that Ae-HKT/AGT plays a critical role in mosquito tryptophan catabolism by detoxifying 3-HK and that Dm-Spat is primarily involved in glyoxylate detoxification.
Keywords: 3-Hydroxykynurenine transaminase, Alanine glyoxylate transaminase, Serine pyruvate aminotransferase, Aedes, Drosophila
1. Introduction
Oxidation of tryptophan to 3-hydroxykynurenine (3-HK) is a major pathway of tryptophan catabolism in Aedes aegypti mosquitoes, especially during larval and egg development. However, 3-HK is oxidized easily under physiological conditions, which stimulates the production of reactive oxygen species. Our previous data demonstrated that mosquitoes control the level of 3-HK by converting this chemically reactive and potentially toxic molecule to chemically stable xanthurenic acid (XA) through transaminase-mediated reactions. Accordingly, the 3-HK to XA pathway is considered an essential detoxification pathway in mosquitoes. Based on its biochemical function, the enzyme mediating the transamination of 3-HK to XA was termed 3-HK transaminase (HKT) in our previous report [1].
Recently, we isolated the A. aegypti HKT cDNA from an A. aegypti larval cDNA library. Blast search of the mosquito HKT against databases revealed that it shares 45–46% sequence identity with the mammalian alanine glyoxylate transaminase (AGT) in Gen Bank databases. Substrate specificity and kinetic analysis determined that the mosquito HKT is efficient in catalyzing the transamination of 3-HK to XA, but in addition, is also active towards alanine in the presence of glyoxylate as an amino group acceptor [2], which is the definition of AGT activity. Consequently, the mosquito HKT was termed HKT/AGT, although our data clearly in-dicate that the major biological function of this specific mosquito transaminase is the transamination of 3-HK to XA [1,2].
The biochemical characteristics of the mosquito transaminase are intriguing because no reports have discussed the 3-HK transamination activity of other AGTs and also because its primary sequence shares no apparent sequence identity with mammalian kynurenine aminotransferases (KAT) whose function is the transamination of 3-HK and kynurenine [3–5]. These results lead to a critical question: i.e. whether the AGT from other species is also active towards 3-HK and kynurenine or if only enzymes from insects have evolved such catalytic functions.
The mosquito HKT/AGT shares 52% sequence identity with a Drosophila serine pyruvate transaminase (Dm-Spat) in the database (NCB accession number AAF46168). Based on its high sequence identity with the mosquito HKT/AGT, it was speculated that the biochemical characteristics of the Dm-Spat would essentially be the same as those of the HKT/AGT and that the enzyme likely plays a similar physiological role in Drosophila tryptophan catabolism as Ae-HKT/AGT. To compare the mosquito HKT/AGT and Dm-Spat, we expressed both proteins using an insect/baculovirus expression system and screened the capability of their recombinant proteins in mediating the transamination of different amino acids in the presence of either glyoxylate, pyruvate or other commonly used α-keto acids as amino acceptors. In this report, we provide data that describe the biochemical characteristics of these two enzymes. Our results highlight the interesting phenomenon that enzymes with high sequence similarity may not have the exact same catalytic functions.
2. Materials and methods
2.1. Expression and purification of recombinant Ae-HKT/AGT and Dm-Spat
2.1.1. Recombinant transfer vector construction
The open reading frame of Ae-HKT/AGT was amplified from A. aegypti larval cDNA using a forward primer (5′-GCTCTAGATGAAATTTACCCCGC-3′) containing an XbaI restriction site (underlined), and a reverse primer (5′-GCAAGCTTAAATACGTAATCCGGATGG-3′) containing a HindIII restriction site (underlined). The Dm-Spat was amplified from Drosophila larval cDNA using a forward primer (5′-GCTCTAGATGGAGGTACCACCGCCA-3′) containing an XbaI restriction site (underlined), and a reverse primer (5′-GCAAGCTTAGATTTTCGATAGATC-3′) containing a HindIII restriction site (underlined). The amplified DNA fragments were inserted into a PCR2.1-TOPO TA cloning vector and then subcloned into a baculovirus transfer vector pBlueBac4.5 (Invitrogen) between the XbaI and HindIII restriction sites. The recombinant transfer vectors were sequenced and verified to ensure that the inserted genes were in-frame and controlled under the downstream polyhedrin promoter.
2.1.2. Recombinant baculovirus isolation
Recombinant Ae-HKT/AGT and Dm-Spat pBlueBac4.5 transfer vectors were cotransfected with linearized Bac-N-Blue™ (AcMNPV, Autographa californica multiple nuclear polyhedrosis virus) viral DNA in the presence of InsectinPlus™ insect cell-specific liposomes to Spodoptera frugiperda (Sf9) insect cells (Invitrogen). The recombinant baculoviruses were purified by the plaque assay procedure. The titer of the high-titer viral stock (HTS) and the time-course at a multiplicity of infection (MOI) of 6 for both recombinant baculoviruses were established according to the manufacturer’s instructions.
2.1.3. Expression and purification
Sf9 cells were cultured in spinner flasks in TNM-FH medium containing 10% fetal bovine serum (Gibco BRL). HTS of pure recombinant Ae-HKT/AGT or recombinant Dm-Spat virus with an MOI of 6 was inoculated into the culture at a cell density of 2.5×106 cells/ml. Sf9 cells were harvested 3 days post inoculation by centrifugation (800×g for 15 min at 4°C). The pellet was washed twice with phosphate-buffered saline, and dissolved in 20 mM phosphate buffer, pH 7.0, containing 40 µM pyridoxal-5′-phosphate (PLP) and 1 mM phenylmethylsulfonyl fluoride. After sonication in ice for 1 min, the cell lysate was centrifuged at 20 000×g for 20 min at 4°C and the supernatant was used as the starting material for the purification of recombinant Ae-HKT/AGT or Dm-Spat.
Recombinant Ae-HKT/AGT was sequentially purified using DEAE Sepharose chromatography, phenyl Sepharose and hydroxyapatite chromatography (Bio-Rad). The Dm-Spat was purified by phenyl Sepharose and hydroxyapatite chromatography. The molecular mass and the purity of the proteins were assessed with a Superdex 200 HR gel filtration column (1×30 cm, Pharmacia) and also by SDS–PAGE analysis.
2.2. Biochemical characterization
2.2.1. KAT and HKT activity assays
The assay was based on previously described methods [2]. Briefly, a reaction mixture of 50 µl, containing either 10 mM l-kynurenine or 10 mM 3-HK, 5 mM amino acceptor, 40 µM PLP and 1 µg protein sample, was prepared using 200 mM phosphate buffer, pH 7.0. The mixture was incubated for 5 min at 50°C and the reaction was stopped by adding an equal volume of 0.8 M formic acid. Supernatant of the reaction mixture, obtained by centrifugation of the reaction mixture at 15 000×g at 4°C for 10 min, was analyzed by high-performance liquid chromatography (HPLC) with UV detection at 330 nm or 340 nm for KA or XA, respectively.
2.2.2. Other aminotransferase assays
The assay mixture contained, unless otherwise specified, 20 mM different amino acids, 7.5 mM different α-keto acids, 40 µM PLP, 200 mM potassium phosphate buffer, pH 7.0, and 1 µg enzyme in a total volume of 50 µl. The mixture was incubated for 5 min at 50°C. The product was quantitated based on the detection of o-phthaldialdehyde thiol (OPT)–amino acid product conjugate by HPLC with electrochemical detection after their corresponding reaction mixtures were derivatized by OPT reagent [2,6,7].
2.2.3. Kinetic analysis
The kinetic parameters of the recombinant enzymes towards the amino acid or α-keto acid were calculated by fitting the experimental data to the Michaelis–Menten equation using the Enzyme Kinetics Module (SPSS Science). Protein was assayed by a Bio-Rad protein assay kit using bovine serum albumin as a standard.
3. Results
3.1. Protein expression and purification
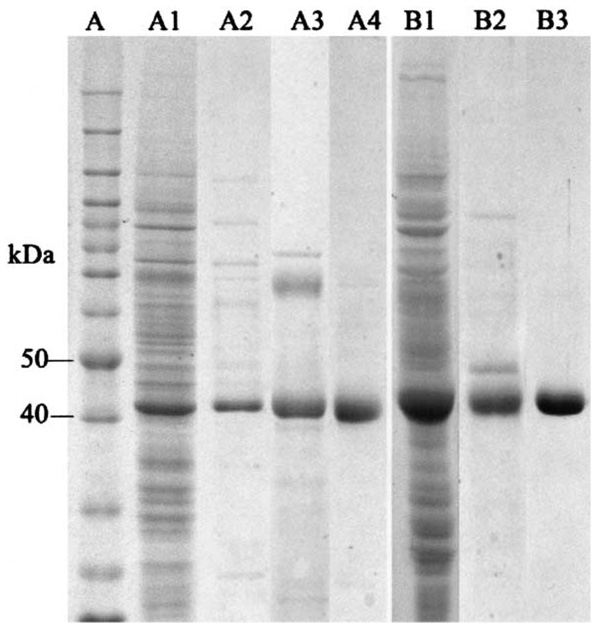
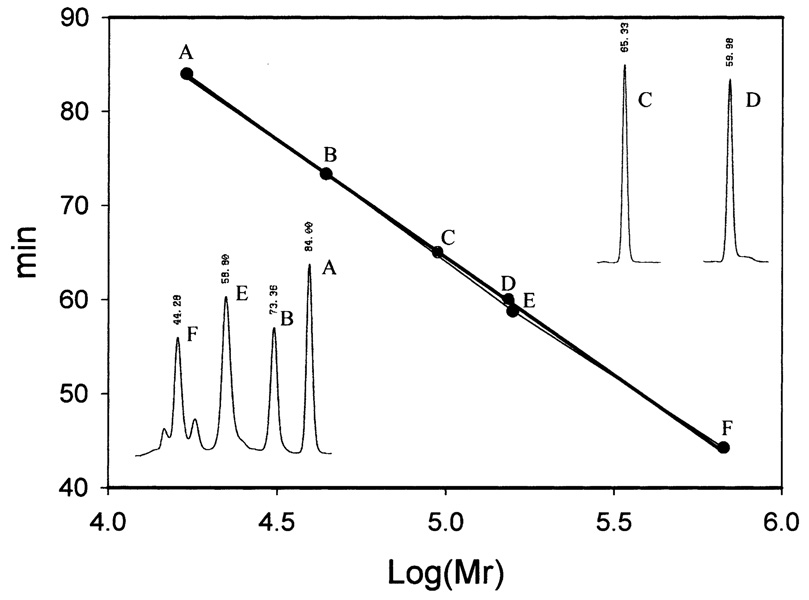
When soluble protein samples from Sf9 cells, collected at the third day after viral inoculation, were analyzed by SDS–PAGE, a major protein band with a relative molecular mass (Mr) of 42 000−43 000 was observed in the Ae-HKT/AGT recombinant virus-infected cells (Fig. 1A1) and a major band with a Mr of 43 000−44 000 was observed in Dm-Spat recombinant virus-infected cells (Fig. 1B1). The Mr of these two major proteins closely matches the expected Mr for Ae-HKT/AGT (43 239) and Dm-Spat (43 800), respectively. Purification of the recombinant Ae-HKT/AGT was achieved by DEAE Sepharose (Fig. 1A2), phenyl Sepharose (Fig. 1A3) and hydroxyapatite chromatographies (Fig. 1A4). Isolation of Dm-Spat was accomplished by phenyl Sepharose (Fig. 1B2) and hydroxyapatite chromatographies (Fig. 1B3). Analysis of the purified recombinant proteins using a Superdex 200 HR gel filtration column showed a single peak for Ae-HKT/AGT with a calculated Mr of 151.7 K, and a single peak for Dm-Spat with a calculated molecular mass of 92.6 K, which suggests that Ae-HKT/AGT is present as a trimer or tetramer and that Dm-Spat is likely a dimer under non-denaturing conditions (Fig. 2).
Fig. 1.
Recombinant Ae-HKT/AGT and Dm-Spat expression and purification. Soluble proteins were obtained as described in Section 2 and subjected to SDS–PAGE analysis. Lanes (left side) illustrate protein profiles of crude soluble proteins from cells infected with the Ae-HKT/AGT recombinant virus (A1), the remaining proteins after DEAE Sepharose (A2), phenyl Sepharose (A3) and hydroxyapatite (A4) chromatographic separations, respectively. Lanes (right side) represent soluble protein from cells infected with the Dm-Spat recombinant virus (B1), the remaining protein after phenyl Sepharose (B2) and hydroxyapatite (B3) chromatographic separations, respectively. Lane A shows the protein molecular mass standards.
Fig. 2.
Relative molecular mass (Mr) of native Ae-HKT/AGT and Dm-Spat. The Mr of native Ae-HKT/AGT and Dm-AGT was assessed by gel filtration chromatography on a Pharmacia Superdex 200 HR gel filtration column (1×30 cm) with thyroglobulin (670 000), γ-globulin (158 000), ovalbumin (44 000) and myoglobin (17 000) (Bio-Rad) as molecular mass standards. The mobile phase consisted of 20 mM phosphate buffer (pH 7.0) containing 150 mM NaCl at a flow rate of 0.2 ml per minute. Under the applied chromatographic conditions, the retention times of thyroglobulin (F), γ-globulin (E), Ae-HKT/AGT (D), Dm-Spat (C), ovalbumin (B), and myoglobin (A) were 44.3, 58.8, 60.0, 65.3, 77.4 and 84.0 min, respectively.
3.2. Biochemical characterization
3.2.1. Substrate specificity
Both recombinant proteins were tested for AGT activity using alanine and glyoxylate as an amino donor and acceptor, respectively, and displayed a fairly high AGT activity. Ae-HKT/AGT was active towards both kynurenine and 3-HK. However, under the same assay conditions, Dm-Spat showed no detectable activity towards either kynurenine or 3-HK. The ability of these two enzymes to catalyze the transamination of other amino acids in the presence of glyoxylate or pyruvate was determined and their relative activities towards those amino acids were expressed as the percent of their activity towards alanine in the presence of glyoxylate (see Table 1). Both recombinant proteins were capable of catalyzing the transamination of amino-butyrate, glycine, methionine, phenylalanine, serine, and tryptophan in the presence of glyoxylate or pyruvate. The Spat activity of Dm-Spat was only 20% of its AGT activity under identical assay conditions, which suggests that Dm-Spat functions as an AGT.
Table 1.
Transamination activity of Dm-Spat and Ae-HKT/AGT towards amino acids with glyoxylate as an amino acceptor
| Keto acids → | Glyoxylate | Pyruvate | ||
|---|---|---|---|---|
| Amino acid ↓ | Dm | Ae | Dm | Ae |
| Alanine | 100 | 100 | – | – |
| Amino-butyrate | 18 | 4.7 | 24 | 18.1 |
| Amino-adipate | ND | ND | ND | ND |
| Arginine | ND | ND | 1.1 | 2.8 |
| Asparagine | ND | ND | ND | ND |
| Aspartate | ND | ND | ND | ND |
| Cysteine | ND | ND | 2.1 | 27.8 |
| Glutamate | ND | ND | ND | ND |
| Glutamine | ND | 3.8 | ND | 12.5 |
| Glycine | – | – | 1.6 | 38.9 |
| Histidine | 4.1 | 3.8 | 5.9 | ND |
| Isoleucine | ND | ND | ND | ND |
| Leucine | ND | ND | 1.1 | ND |
| Lysine | ND | ND | ND | ND |
| Methionine | 7.2 | 3.8 | 5.9 | 8.3 |
| Phenylalanine | 16.5 | 9.4 | 26.4 | 36.1 |
| Serine | 18 | 4.2 | 20 | 16.7 |
| Threonine | ND | ND | ND | ND |
| Tryptophan | 1 | 0.7 | 1.1 | 5.6 |
| Tyrosine | 1.5 | ND | 1.1 | ND |
| Valine | ND | ND | ND | ND |
Purified recombinant Dm-Spat or Ae-HKT/AGT was incubated with each of the 21 amino acids in the presence of glyoxylate or py-ruvate as described in Section 2 and the activity was quantitated by the amount of glycine or alanine produced in the reaction mixture. Data are expressed as a percentage of the activity of that with alanine as the amino donor, glyoxylate as amino acceptor and are the means of two separate experiments. Under the applied conditions, the specific activities of recombinant Ae-HKT/AGT and Dm-Spat towards alanine were 22 and 74 µmol/min/mg protein, respectively. ND: not detectable.
The recombinant Ae-HKT/AGT was able to catalyze the transamination of both 3-HK and kynurenine in the presence of several different common biological amino group acceptors, and among them, oxaloacetate, glyoxylate and pyruvate were efficient amino acceptors for the enzyme (Table 2). In contrast, Dm-Spat showed no activity towards either 3-HK or kynurenine under identical assay conditions (not shown).
Table 2.
Cosubstrate specificity of Ae-HKT/AGT
| Keto acid | 3-HK | Kynurenne |
|---|---|---|
| Pyruvate | 100 | 100 |
| Glyoxylate | 123 | 78 |
| p-Hydroxypyruvate | 3 | 2 |
| Indo-3-pyruvate | 1 | 2 |
| α-Ketoadipate | ND | ND |
| α-Ketobutyrate | 22 | 26 |
| α-Ketoglutarate | ND | ND |
| α-Ketovalerate | 5 | 7 |
| α-Keto-γ-methiolbutyrate | 12 | 17 |
| Methy-oxobutanoic acid | ND | ND |
| Oxaloacetate | 177 | 92 |
| Phenylpyruvate | 33 | 42 |
Purified recombinant Ae-HKT/AGT was incubated in the presence of 3-HK or kynurenine and different amino group acceptors (α-keto acids) as described in Section 2. Data are expressed as a percentage of the activity of that with pyruvate as the amino acceptor and are the means of two separate experiments. Under the applied conditions, the specific activities of recombinant Ae-HKT/AGT towards 3-HK and kynurenine were 38 and 17 µmol/min/mg protein, respectively. ND: no detectable activity.
3.2.2. Kinetic parameters of AGT
Dm-Spat had a much higher Vmax towards alanine (170 µmol/min/mg) and glyoxylate (197 µmol/min/mg) than that of Ae-HKT/AGT to alanine and glyoxylate (24 and 30 µmol/min/mg, respectively). However, Ae-HKT/AGT showed a much higher affinity to alanine (Km = 18 mM) and glyoxylate (Km = 1.6 mM) than that of Dm-Spat to alanine (Km = 149 mM) and glyoxylate (Km =64 mM). Although Dm-Spat has a much greater specific activity towards glyoxylate and alanine, catalytically it is less efficient than mosquito HKT/AGT towards these two compounds (Table 3). The kinetic parameters of Dm-Spat to serine and pyruvate were studied for comparison. The low affinity of Dm-Spat to serine (Km = 256 mM) and pyruvate (Km = 51 mM) makes it much less efficient as a Spat, because the kcat/Km towards serine (13.3 min−1 mM−1) is much smaller than the kcat/Km towards alanine (106 min−1 mM−1).
Table 3.
Kinetic parameters of Dm-Spat and Ae-HKT/AGT towards alanine and glyoxylate
| Substrates | Enzyme | Km (mM) | Vmax (µmol/min/mg) | kcat (min−1) | kcat/Km (min−1 mM−1) |
|---|---|---|---|---|---|
| Glyoxylate | Dm | 64 ± 11 | 170 ± 14 | 6800 ± 560 | 106 ± 28 |
| Ae | 1.6 ± 0.6 | 24 ± 3 | 960 ± 120 | 600 ± 349 | |
| Alanine | Dm | 149 ± 44 | 197 ± 30 | 7880 ± 1200 | 53 ± 26 |
| Ae | 18 ± 2 | 30 ± 1.5 | 1200 ± 60 | 67 ± 11 |
The activities were measured as described in Section 2. The glyoxylate concentrations were 0.3–10 mM in the presence of 40 mM alanine, and the alanine concentrations were 6.3–30 mM in the presence of 7.5 mM glyoxylate for the determination of kinetic parameters of Ae-HKT/AGT to glyoxylate and alanine. The glyoxylate concentrations were 6.3–100 mM in the presence of 160 mM alanine, and the alanine concentrations were 5–160 mM in the presence of 100 mM glyoxylate for the determination of kinetic parameters of Dm-Spat to glyoxylate and alanine. The kinetic parameters of the two enzymes to substrates were calculated by fitting the experimental data to the Michaelis–Menten equation using the Enzyme Kinetics Module (SPSS Science).
3.2.3. Kinetic parameters of HKT
Among the three amino group acceptors, Ae-HKT/AGT showed the highest affinity to glyoxylate (Km = 2.2 and 1.7 mM), using either 3-HK or kynurenine as the amino group donor (Table 4), and was more active towards 3-HK than towards kynurenine based on results of kinetic analysis (Table 4).
Table 4.
Kinetic parameters of Ae-HKT/AGT
| Transaminase activity | Substrate | Km (mM) | kcat (min−1) | kcat/Km (min−1 mM−1) |
|---|---|---|---|---|
| 3-HK/glyoxylate | 3-HK | 2.6 ± 0.6 | 2050 ± 320 | 789 ± 322 |
| Glyoxylate | 2.2 ± 0.6 | 2640 ± 440 | 1200 ± 570 | |
| 3-HK/oxaloacetate | 3-HK | 5.4 ± 1.1 | 2560 ± 232 | 474 ± 146 |
| Oxaloacetate | 7.5 ± 3 | 4280 ± 960 | 571 ± 424 | |
| 3-HK/pyruvate | 3-HK | 3 ± 0.4 | 1760 ± 80 | 587 ± 107 |
| Pyruvate | 4 ± 1 | 1400 ± 200 | 350 ± 147 | |
| Kynurenine/glyoxylate | Kynurenine | 3.7 ± 0.2 | 720 ± 224 | 195 ± 71 |
| Glyoxylate | 1.7 ± 0.8 | 1040 ± 268 | 612 ± 572 | |
| Kynurenine/oxaloacetate | Kynurenine | 9.7 ± 2.6 | 1400 ± 560 | 144 ± 104 |
| Oxaloacetate | 3 ± 0.7 | 920 ± 108 | 307 ± 134 | |
| Kynurenine/pyruvate | Kynurenine | 6 ± 0.4 | 1040 ± 40 | 173 ± 18 |
| Pyruvate | 3.8 ± 0.7 | 840 ± 72 | 221 ± 62 |
The activities were measured as described in Section 2. The Km and Vmax for amino acceptors were derived by using varying concentrations of individual amino acceptors from 0.16 to 10 mM final concentration in the presence of 10 mM 3-HK or kynurenine, and the Km and Vmax for 3-HK or kynurenine were determined by using varying concentrations of 3-HK or kynurenine from 0.2 to 15 mM in the presence of 7.5 mM each of the three amino acceptors. The parameters were calculated by fitting the experimental data to the Michaelis–Menten equation using the Enzyme Kinetics Module (SPSS Science).
3.2.4. Effect of pH and temperature on Ae-HKT/AGT or Dm-Spat activity
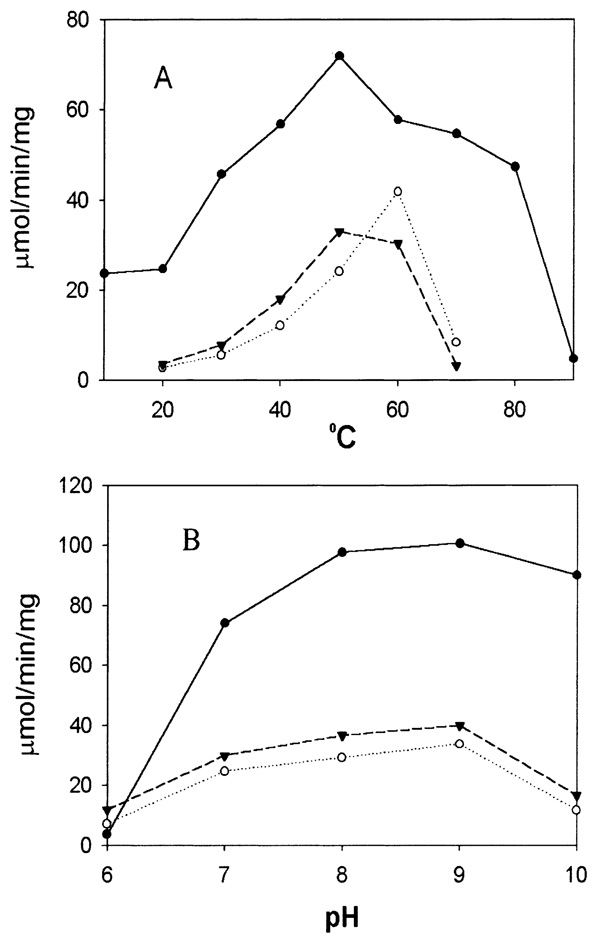
Both the pH and temperature of the reaction mixture greatly affected enzyme activity towards 3-HK and alanine in the presence of glyoxylate. Among the temperature points tested, Ae-HKT/AGT showed the highest activity at around 55°C for 3-HK and 60°C for alanine and Dm-Spat showed a high activity towards alanine at 30–80°C (Fig. 3A). Optimal pH for the two enzymes was 7–10, and the best pH was 9.0 for activity towards alanine and 3-HK (Fig. 3B).
Fig. 3.
Effect of pH and temperature on enzyme activity. Assay conditions were the same as described in Section 2, except that glyoxylate was used as the amino acceptor and potassium phosphate buffer was used at pH 6–8, borate buffer at pH 9, and carbonate buffer at pH 10. The effect of temperature on transamination activity was determined by incubation of the corresponding substrate preparations at 10°C, 20°C, 30°C, 40°C, 50°C, 60°C, 70°C, 80°C or 90°C for 5 min prior to the addition of the enzyme and continuously incubated for 5 min following enzyme addition. Panels A and B show the activities of recombinant Ae-HKT/AGT and Dm-Spat under different temperatures and pHs, respectively. The lines with closed circles, closed triangles and open circles represent the specific activities of Dm-Spat towards alanine, Ae-HKT/AGT towards 3-HK, and Ae-HKT/AGT towards alanine, respectively.
4. Discussion
Dm-Spat shares the highest sequence identity to Ae-HKT/AGT among the available protein sequences; accordingly, it was presumed that the biochemical characteristics of the Dm-Spat would likely be similar to those of Ae-HKT/AGT and that the enzyme would play a similar physiological role in tryptophan catabolism in Drosophila. However, comparative analysis revealed that the biochemical characteristics of these two enzymes are quite diffierent. The substrate specificities and kinetic parameters of the recombinant Ae-HKT/AGT are similar to our previously purified enzyme from A. aegypti larvae, which provides conclusive evidence demonstrating that the isolated clone in our previous report encodes Ae-HKT/AGT [2]. The kinetic data suggest that the mosquito enzyme is more efficient as a HKT than as an AGT and, based on this study and previous reports [1,2], the enzyme is considered to be responsible for the detoxification of 3-HK produced during tryptophan oxidation. In contrast, Dm-Spat functions more like an AGT than as a Spat and has no HKT activity. These data suggest that Dm-Spat functions as an AGT and that the enzyme may play a role in preventing the accumulation of glyoxylate in Drosophila.
AGT was suggested to be involved in the gluconeogenesis or detoxification of glyoxylate, a toxic plant metabolite, in vertebrates [8–10]. The importance of hepatic AGT in some mammals is clearly shown by the autosomal recessive disorder of glyoxylate metabolism primary hyperoxaluria type 1, a potentially lethal condition in which AGT deficiency leads to excessive oxalate synthesis and excretion and the deposition of insoluble calcium oxalate in the kidney [11].
AGT has been studied in a few mammalian species, but there has been no discussion about the enzyme’s function in insects. The function of AGT, gluconeogenesis or detoxification, in vertebrates depends on its subcellular localization. When the enzyme is localized in mitochondria, AGT is involved primarily in gluconeogenesis; when localized in peroxisomes, it detoxifies glyoxylate, a toxic plant metabolite [8,10]. The localization of AGT in mitochondria and peroxisomes is determined by the N-terminal and C-terminal signal peptide of the enzyme, respectively. The C-terminal tripeptide of AGT is considered to be responsible for directing the enzyme into peroxisomes [9]. For example, the C-terminus of Ser-Arg-Ile of Arabidopsis thaliana AGT has been proved to be a peroxisomal signal peptide [12]. Dm-Spat, which has Ser-Lys-Ile at its C-terminus, is also predicted to be a peroxisomal protein (FlyBase report in http://flybase.bio.indiana.edu/.bin/fbidq.html?FBgn0014031). The C-terminal residues (Leu-Arg-Ile) of Ae-HKT/AGT resemble to the known peroxisomal targeting sequences [12], suggesting its possible localization in peroxisomes, but it requires extensive experiments for verification. The functions of peroxisomes, in general, are related to detoxification; therefore, the possible localization of Ae-HKT/AGT and Dm-Spat in peroxisomes favors the proposed role for them in detoxifying 3-HK and glyoxylate in mosquito and Drosophila, respectively.
Among the protein sequences (including putative protein sequences) from Drosophila genomic database, Dm-Spat shares the highest sequence identity with Ae-HKT/AGT. Accordingly, it is reasonable to consider that Dm-Spat is the counterpart of Ae-HKT/AGT. Both mosquito and Drosophila belong to Diptera and are closely related in terms of evolution. Consequently, the major difference in the biochemical functions of these two enzymes raises a critical question: why has a similar enzyme from these two species different physiological functions. The difference might be explained by the dissimilarity in their food resources. Drosophila, although more primitive than vertebrate animals, is similar to herbivores in terms of their food resources. Therefore, a mechanism that prevents accumulation of oxalate from glyoxylate intermediate likely is necessary for Drosophila. The biochemical and kinetic parameters of the enzyme support its possible role in glyoxylate detoxification. In contrast to Drosophila, the driving force for the biochemical and molecular evolution of Ae-HKT/AGT likely results from the adaptation of mosquitoes to their living environment. Mosquito larvae and pupae are aquatic and their food resources include mainly microorganims, such as bacteria and protozoa. During adult stages, females obtain bloodmeal for egg development and also for food supply. It is obvious that food sources for mosquitoes are protein-rich. In mosquitoes, a considerable portion of tryptophan obtained from food supplies is oxidized to 3-HK [1]. Because mosquitoes do not have kynureninase like mammals and cannot dispose of 3-HK through hydrolysis and subsequent oxidations like mammals [2], they have to deal with 3-HK in a different manner and transamination of the chemically reactive 3-HK to the relatively chemically stable XA has been proved to be the mechanism through which mosquitoes control the 3-HK from over-accumulation during larval and egg development [1,2]. In contrast to mosquitoes, Drosophila get their food from plant resources that are rich in glyoxylate; therefore, a mechanism preventing the accumulation of oxalate via glyoxylate intermediate might be more important in Drosophila. Based on the biochemical characteristics of Ae-HKT/AGT and Dm-Spat and the physiological requirements in tryptophan and glyoxylate metabolism in mosquitoes and Drosophila, it is reasonable to propose that Ae-HKT/AGT plays a critical role in mosquito tryptophan catabolism and that Dm-Spat is responsible for preventing glyoxylate accumulation in Drosophila, which reflects their different physiological requirements during the adaptation to their living environment.
Acknowledgements
This work was supported by National Institutes of Health Grant (AI 44399).
Abbreviations
- Ae
Aedes aegypti
- AGT
alanine glyoxylate transaminase
- Dm
Drosophila melanogaster
- 3-HK
3-hydroxy-dl-kynurenine
- HKT
3-hydroxykynurenine transaminase
- HTS
high-titer viral stock
- KAT
kynurenine aminotransferase
- MOI
multiplicity of infection
- PLP
pyridoxal-5′-phosphate
- Spat
serine pyruvate amino-transferase
- XA
xanthurenic acid
References
- 1.Li J, Li G. Insect. Biochem. Mol. Biol. 1997;27:859–867. doi: 10.1016/s0965-1748(97)00068-4. [DOI] [PubMed] [Google Scholar]
- 2.Han Q, Fang J, Li J. J. Biol. Chem. 2002;277:15781–15787. doi: 10.1074/jbc.M201202200. [DOI] [PubMed] [Google Scholar]
- 3.Buchli R, Alberati-Giani D, Malherbe P, Kohler C, Broger C, Cesura AM. J. Biol. Chem. 1995;270:29330–29335. doi: 10.1074/jbc.270.49.29330. [DOI] [PubMed] [Google Scholar]
- 4.Mosca M, Cozzi L, Breton J, Avanzi N, Toma S, Okuno E, Schwarcz R, Speciale C, Magagnin S, Mostardini M, Benatti L. Adv. Exp. Med. Biol. 1996;398:449–454. doi: 10.1007/978-1-4613-0381-7_70. [DOI] [PubMed] [Google Scholar]
- 5.Okuno E, Du F, Ishikawa T, Tsujimoto M, Nakamura M, Schwarcz R, Kido R. Brain Res. 1990;534:37–44. doi: 10.1016/0006-8993(90)90109-o. [DOI] [PubMed] [Google Scholar]
- 6.Antoine FR, Wei CI, Littell RC, Marshall MR. J. Agric. Food Chem. 1999;47:5100–5107. doi: 10.1021/jf990032+. [DOI] [PubMed] [Google Scholar]
- 7.Han Q, Fang J, Li J. Biochem. J. 2001;360:617–623. doi: 10.1042/0264-6021:3600617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danpure CJ. Bioessays. 1997;19:317–326. doi: 10.1002/bies.950190409. [DOI] [PubMed] [Google Scholar]
- 9.Holbrook JD, Danpure CJ. J. Biol. Chem. 2002;277:2336–2344. doi: 10.1074/jbc.M107047200. [DOI] [PubMed] [Google Scholar]
- 10.Watts RW. Adv. Enzyme Regul. 1992;32:309–327. doi: 10.1016/0065-2571(92)90024-t. [DOI] [PubMed] [Google Scholar]
- 11.Danpure CJ, Jennings PR, Leiper JM, Lumb MJ, Oatey PB. Ann. N.Y. Acad. Sci. 1996;804:477–490. doi: 10.1111/j.1749-6632.1996.tb18638.x. [DOI] [PubMed] [Google Scholar]
- 12.Liepman AH, Olsen LJ. Plant J. 2001;25:487–498. doi: 10.1046/j.1365-313x.2001.00961.x. [DOI] [PubMed] [Google Scholar]