Abstract
Toll-like receptors (TLRs), which are a family of pattern recognition receptors (PRRs), are involved critically in the generation and regulation of innate immunity as well as initiation of subsequent adaptive immune responses. However, recent research results showed that different subsets of T cells express certain types of TLRs during development and activation stages. Importantly, TLRs participate in the direct regulation of adaptive immune response, possibly as co-stimulatory molecules. In this review we summarize recent studies about the novel regulation of TLRs on the homeostasis and immunity of different T cell subtypes including CD4+CD25+T regulatory cells (Treg) and interleukin (IL)-17-producing CD4+T cells (T helper type 17). The direct involvement of TLRs in T cell-mediated immunity prompted us to reconsider the role of TLRs in the occurrence of autoimmune diseases, infectious diseases and graft rejection. The important effects of TLRs in T cell-intrinsic components also prompt us to explore novel vaccine adjuvants for modifying desired immune responses in an efficient way.
Keywords: immune response, regulatory T cells, Th17, Toll-like receptor (TLR)
Introduction
Toll-like receptors (TLRs) are germline-encoded pathogen recognition receptors expressed most prominently on or in antigen-presenting cells (APCs) such as macrophages and dendritic cells (DCs) [1]. TLR-2, -4, -5 and -11 are expressed on the cell surface while TLR-3, -7, -8 and -9 locate in endosomal compartments. They detect a broad range of pathogen-associated molecular patterns (PAMPs) to recognize different microbial as a means to distinguish ‘non-self’ from ‘self’, and in some cases they also recognize endogenous ligands, which are considered damage-associated molecular patterns (DAMPs) [2,3]. For example, TLR-4 can be activated by lipopolysaccharide (LPS) from Gram-negative bacteria, heat shock proteins and the anti-cancer drug taxol [4]. TLR-2 can be activated by the yeast cell wall component zymosan and lipoteichoic acid from Gram-positive bacteria. TLR-3 is activated by double-stranded RNAs from viruses, and TLR-9 recognizes cytosine-guanine dinucleotide (CpG) DNA motifs present in viruses and bacteria [5]. It is well known that activation of TLRs on APCs initiates a cascade of intracellular signalling events, resulting ultimately in enhancing antigen presentation, the production and release of inflammatory cytokines and up-regulation of adhesion and co-stimulatory molecules on the cell surface of APCs as well as priming the adaptive immune system [6–8] (Fig. 1). However, recent studies have shown that T cells also express certain types of TLRs [9,10]. TLRs can function as co-stimulatory receptors that complement T cell receptor (TCR)-induced signals to enhance effector T cell proliferation, survival and cytokine production [11]. TLRs could thus be involved in the modulation of the adaptive immunity, including regulatory T cell (Treg)-mediated immune suppression and the induction of different subtypes of effector T cells, particularly interleukin (IL)-17-producing cell [T helper type 17 (Th17)] differentiation in autoimmune diseases and other immune response processes [9]. In this review we summarize mainly recent advances about the novel mechanisms of TLRs for the homeostasis and function of different T cell subtypes.
Fig. 1.
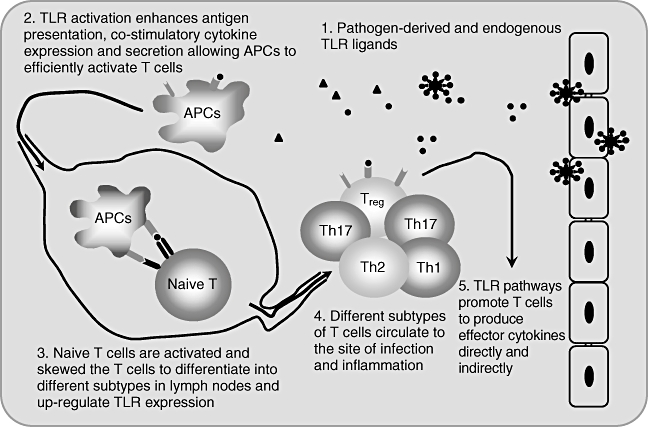
Modulation of innate and adaptive immunity through Toll-like receptor (TLR) signalling. A brief summary for the role of TLRs in triggering innate and adaptive immunity via multiple approaches.
Innate immunity initiated by TLRs
Engagement of pattern recognition receptors (PRRs) with their microbial ligands induces specific downstream signalling events, and thereby provides immediate first-line protection of the host from invading pathogens. This is mediated by a number of components of innate systems, including activation of the complement pathway, phagocytosis of microbes, the release of direct anti-microbial mediators and production of cytokines and chemokines that, collectively, instruct mechanisms to combat infection [12]. Several PRRs have been characterized in a number of different hosts, such as pathogen-resistance proteins in plants [13,14], the Drosophila Toll protein [14,15] and TLRs in Caenorhabditis elegans and mammals [15,16]. During the last decade, many microbial motifs sensed by TLRs and their impact on the induction of first-line host responses have been demonstrated [9,16–18].
TLRs represent a major innate pathway through which pathogens induce DC maturation and acquisition of immunostimulatory functions. TLR signal transduction is initiated usually by the recruitment of one or more adaptor proteins [18–20], which include myeloid differentiation primary response protein 88 (MyD88), MyD88-adaptor-like [Mal, also referred to as Toll/IL-1 receptor (TIR) domain-containing adaptor protein (TIRAP)], TIR domain-containing adaptor protein inducing interferon (IFN)-β (TRIF, also known as TICAM1) and TRIF-related adaptor molecule (TRAM; also known as TICAM2) [21,22]. These adaptors associate with the cytoplasmic domains of TLRs through homophilic interactions between TIR domains present in each TLR. All TLR family members use the MyD88 adaptor, except TLR-3, which recruits TRIF [23]. TLR-4 is the only family member that activates both MyD88-dependent and TRIF-dependent signal transduction pathways [24]. The structural or conformational changes that facilitate adaptor binding remain poorly defined, although it seems likely that increased proximity between the cytoplasmic domains of TLRs creates a binding interface for the relevant TIR domain-containing adaptors. Although the signalling events downstream of MyD88 and TRIF differ, the outcome of each pathway is conceptually similar: nuclear factor-κB, interferon-regulatory factors (IRFs) and other more general transcription factors are activated [16,22,25]. In certain cases differential activation of IRF family members leads to distinct transcriptional responses.
TLRs bridge the innate immunity and adaptive immunity
Efficient immune responses depend upon a close interaction between the innate and adaptive immune systems. The innate immune system not only reacts promptly to microbial infection or environmental insult, but also instructs APCs to activate and secrete cytokines in order to polarize T cells towards an appropriate effector phenotype [26]. Only mature DCs will be able, through appropriate antigen presentation, to stimulate naive T cells such that they differentiate into effector T cells. The types of effector T cells that evolve from the naive cells are influenced greatly by the pattern of cytokines induced by the TLR engagement. Apparently, in addition to presenting antigens to naive T cells in an appropriate major histocompatibility complex (MHC) context, the range of co-stimulatory signals delivered to T cells by APCs is determined, if not all, at least partially, by TLR ligation.
TLRs serve as an important link between the innate and adaptive immune responses [27]. Different types of DCs selectively express cytokines, co-receptors and several other polarizing signals that promote the development of Th1, Th2, CD4+CD25+ Treg cells or the recently defined Th17 lineage, respectively [28,29]. In this context, selected TLR ligands can be used alone or in combination as potential vaccine adjuvants to elicit the most appropriate immune response in humans or mice. The majority of known TLRs mediate the development of Th1-promoting DCs (type 1 DCs), whereas most of the PRRs mediate Th2-inducing DCs (type 2 DCs) [30,31]. DCs stimulated directly or indirectly by PRRs from pathogens mature into a specific form and are able to activate a single specific immune response that is appropriate for the elimination of the pathogen [32]. In this regard, DCs determine the nature of the foreign antigen and the intensity and phenotype of immune response generated. The development of different subtypes of effector T cell differentiation, a Th1, Th2 or Th17 immune response, is dependent upon the physical interaction between the activated status of the DCs and the naive T cells [8,33] (Fig. 1). It will not be discussed in this review.
It is worth mentioning that in addition to its importance in infectious diseases, TLRs also participate in inflammation and immune responses that are driven by self-, allo- or xeno-antigens [18,34,35]. TLR signalling has been demonstrated to be involved in the immune recognition of allo- or xenografts and the occurrence of autoimmunity [35,36]. This observation is supported strongly by the expression of TLRs on almost all immune cells and the identification of their endogenously expressing ligands by mammalian cells [9,37–39].
TLRs on effector T cells as co-stimulatory molecules
TLRs are expressed widely in many types of immune cells, including DCs, T cells, neutrophils, eosinophils, mast cells, macrophages, monocytes and epithelial cells [1,40,41]. Interestingly, TLR expression is related to the functional status of different subtype T cells. TLR-3, -6, -7 and -9 have been reported to be expressed on CD4+ T cells [42]. Naive CD4+ T cells do not express significant levels of mRNA and intracellular proteins of TLR-2 and TLR-4. Only few CD3+ T cells express TLR-1, -2 or -4 on the cell surface when they have not been activated [43]. However, activated/memory T cells express appreciable levels of cell surface TLR-2 and TLR-4 [34,42]. TCR stimulation by cross-linked anti-CD3 monoclonal antibody (mAb) induces cell surface expression of TLR-2 and TLR-4 on naive human and murine CD4+ T cells [34,44]. By contrast, TCR stimulation down-modulates significantly surface TLR-5 expression on human CD4+ T cells [45] (Table 1). TLR expression on T cells may be regulated by TCR signalling, which needs further investigation in the future. These data thus offer the possibility that pathogens, via their PAMPs, may contribute directly to the perpetuation and activation of T cells.
Table 1.
Toll-like receptor (TLR) expression and direct regulation on T cells.
| TLRs | Expression on T cells | Location of TLRs | PAMPs recognized by TLRs | Direct regulation on T cells |
|---|---|---|---|---|
| TLR-1/2 | Very few expression on naive T cells, up-regulate expression on activated or memory T cells | Plasma membrane (cell surface) | Triacyl lipopeptides (bacteria and mycobacteria) | Abrogate or reverse the suppressive function of Treg |
| TLR-2 | Very few expression on naive T cells, up-regulate expression on activated or memory T cells | Plasma membrane (cell surface) | Peptidoglycan (Gram-positive bacteria) | Enhance the suppressive function of Treg |
| TLR-3 | Expression | Endosome | ssRNA virus (WNV), dsRNAvirus (reovirus), RSV, MCMV | |
| TLR-4 | Very few expression on naive T cells, up-regulate expression on activated or memory T cells | Plasma membrane (cell surface) | LPS (Gram-negative bacteria) | Enhance the suppressive function of Treg |
| TLR-5 | Expression | Plasma membrane (cell surface) | Flagellin (flagellated bacteria) | Enhance the suppressive function of Treg |
| TLR-6 | Expression | Diacyl lipopeptides (mycoplasma), LTA (Streptococcus), zymosan saccharomyces) | ||
| TLR-7 | Expression | Endosome | ssRNA viruses (VSV, influenza virus) | Abrogate or reverse the suppressive function of Treg |
| TLR-8 | Expression | Endosome | ssRNA from RNA virus | Abrogate or reverse the suppressive function of Treg |
| TLR-9 | Expression | Endosome | dsDNA viruses (HSV, MCMV), CpG motifs from bacteria and viruses, haemozoin (plasmodium) | Abrogate or reverse the suppressive function of Treg |
CpG, cytosine-guanine dinucleotide; LPS, lipopolysaccharide; LTA, lipoteichoic acid; MCMV, murine cytomegalovirus; PAMP, pathogen-associated molecular patterns; RSV, respiratory syncytial virus; Treg, regulatory T cell; VSV, vesicular stomatitis virus; WNV, West Nile virus.
At least some TLRs may function as a co-stimulatory receptor for antigen-specific T cell responses and participate in the maintenance of T cell memory [46–48]. It has been shown that ligands for TLR-2, -3, -4, -5 and -9 enhance the proliferation and/or biological functions of conventional effector T cells [44,46,48–51]. Co-stimulation of CD4+ T effector cells with anti-CD3 mAb and TLR-5 ligand flagellin results in enhanced proliferation and production of IL-2 at levels equivalent to those achieved by co-stimulation with CD28 [52,53]. CpG-containing oligodeoxynucleotides (CpG-ODN) can co-stimulate primary T cells in the absence of APCs [54]. In the presence of the TCR signal, CpG-ODN induces IL-2 production, IL-2R expression and thus T cell proliferation. Furthermore, CpG-co-stimulated T cells differentiate into cytolytic T lymphocytes in vitro[54]. Naive human T cells express high levels of cell-surface TLR-2 after activation by anti-TCR antibody and interferon (IFN)-α. Activated cells produce more cytokines in response to the TLR-2 ligand, bacterial lipopeptide [44]. Furthermore, memory human CD4+CD45RO+ T cells express TLR-2 constitutively and produce IFN-γ in response to bacterial lipopeptide [44]. Co-stimulation of antigen-activated murine CD8+ T cells with the lipopeptide Pam3CysSK4 (Pam), a TLR-1/2 ligand, enhances the proliferation, survival and effector functions of these cells [54]. TLR-2 engagement on CD8+ T cells reduces significantly their need for co-stimulatory signals delivered usually by mature APCs [39]. Importantly, human T cells were also reported to respond similarly to the endogenous ligand HSP60 through TLR-2, although these results could reflect potential contamination of commercially available HSP60 with bacterial TLR-2 ligands [55]. T cells responding to endogenous TLR ligands is intriguing, because it opens the possibility that DAMPs may potentially support T cell responses at sites of damaging tissue. It should be noted that TLR ligands do not induce functional responses in T cells in the absence of concurrent TCR stimulation [11], indicating that TLR-induced signals in T cells are strictly co-stimulatory, which may be important for preventing TLR signal-mediated non-specific T cell activation.
On the other hand, LPS treatment results in increased adhesion of mouse and human T cells to fibronectin and inhibited chemotaxis [56]. Thus, in addition to functioning as potential co-stimulatory molecules, TLRs may also play a role in controlling T cell trafficking.
Direct modulation of CD4+CD25+ Treg cell bio-function by TLRs
Naturally occurring and antigen-induced CD4+CD25+ Treg cells have been studied extensively in mice and humans. Depletion of the naturally occurring subset of CD4+CD25+ Treg cells results in various types of autoimmune diseases [57,58]. TLR ligands modulate CD4+CD25+ Treg cell responses indirectly by promoting inflammatory cytokine production in APCs, which can inhibit the suppressive capacity of CD4+CD25+ Treg cells [59]. However, some TLRs are expressed on CD4+CD25+ Treg cells. It has been reported that naive CD4+CD25+ Treg cells express TLR-4, -5, -7 and -8 selectively, whereas TLR-1, -2, -3 and -6 appear to be expressed more broadly on CD4+ T cells, but not confined to CD4+CD25+ Treg cells [10]. The distinct expression pattern of TLRs on CD4+CD25+ Treg cells supports the potential involvement of these TLRs in the direct regulation of CD4+CD25+ Treg cells [9,60]. It has been shown that ligands for TLR-2, -5 and -8 modulate the proliferation and suppressive functions of CD4+CD25+ Treg cells.
TLR-2−/− mice, unlike TLR-4−/− mice, contain significantly lower levels of CD4+CD25+ Treg cells than control mice [9,55,61]. Administration of TLR-2 ligands to wild-type mice results in significantly increased CD4+CD25+ Treg cell numbers [42,62]. In the presence of a TLR-2 agonist, such as the synthetic bacterial lipoprotein Pam3Cys-SK4, CD4+CD25+ Treg cells expand markedly, but their immunosuppressive function is abrogated temporarily [34,61]. However, engagement of TLR-2 does not reverse the suppressor function of mouse CD4+CD25+ Treg cells, but promotes their survival via induction of Bcl-x(L) [63]. It is also reported that signals through TLR-2 can enhance the suppressive function of Treg cells as well as forkhead box protein 3 (FoxP3) expression [55].
Exposure of CD4+CD25+ Treg cells to the TLR-4 ligand LPS induces up-regulation of several activation markers and enhances their survival or proliferation [10,55]. The proliferative response does not require APCs and is augmented by TCR triggering and IL-2 stimulation. Most importantly, LPS treatment increases the immunosuppressive ability of CD4+CD25+ Treg cells by 10-fold. Moreover, LPS-activated CD4+CD25+ Treg cells can control efficiently the occurrence of naive CD4+ T effector cell-mediated diseases [64,65]. Others failed to observe effects of LPS on CD4+CD25+ Treg cells, indicating that LPS-induced signalling on CD4+CD25+ Treg cells is still controversial.
TLR-5 ligand flagellin plays a critical role in regulating mucosal immune responses [45,66]. Both human CD4+CD25+ Treg cells and CD4+CD25- T cells express TLR-5 at levels comparable to those on monocytes and DCs [66]. Co-stimulation with flagellin does not break the hyporesponsiveness of CD4+CD25+ Treg cells but, rather, increases their immunosuppressive capacity potently and enhances FoxP3 expression [45]. It is reported that TLR-7 signalling enhances the suppressor function of CD4+CD25+ Treg cells by sensitizing CD4+CD25+ Treg cells to IL-2-induced activation [67]. TLR-8 could directly reverse the immunosuppressive function of CD4+CD25+ Treg cells [68]. It has been reported that CpG-A and poly(G10) oligonucleotides could directly reverse the immunosuppressive function of CD4+CD25+ Treg cells in the absence of DCs, but the exact functional ingredients were not identified in that study [69]. Interestingly, when TLR-8 and MyD88 were knocked down using a RNA interference method, the response of CD4+CD25+ Treg cells to poly(G) oligonucleotides was abolished [68]. Accordingly, TLR-8 was expressed consistently by naturally occurring as well as induced CD4+CD25+ Treg cells [70]. These results support the hypothesis that the TLR-8–MyD88 signalling pathway controls directly the immunosuppressive function of CD4+CD25+ Treg cells without the involvement of APCs.
The TLR-9 ligand CpG-ODN synergizes with anti-CD3 mAb to induce proliferation of both rat CD4+CD25- and CD4+CD25+ Treg cells [71]. Surprisingly, TLR-9 ligand abrogates partially the suppressive activity mediated by CD4+CD25+ Treg cells, which is attributable partially to the direct effect of TLR-9 ligand on effector T cells which are rendered more resistant to the suppression exerted by CD4+CD25+ Treg cells [71]. Thus, TLR-9 ligand may increase the host's adaptive immunity rapidly by expanding effector T cells and also by attenuating the immunosuppressive activity mediated by CD4+CD25+ Treg cells [71].
Although relevant studies are limited and somewhat controversial, TLR-2, -8 or -9 ligations abrogate or reverse the immunosuppressive function of CD4+CD25+ Treg cells, whereas TLR-2, -4 or -5 ligations enhance CD4+CD25+ Treg cell-mediated immunosuppressive capacity (Fig. 2). Nevertheless, these findings provide important evidence that CD4+CD25+ Treg cells respond directly to proinflammatory bacterial products or endogenous ligands via TLRs, a mechanism that is likely to contribute to the control of inflammatory responses. It should be recognized that, once TLR ligands are removed, CD4+CD25+ Treg cells fully regain their immunosuppressive phenotypes and function [34,42]. Thus it is hypothesized that, during immune response, TLR ligands can regulate T cell-mediated immune responses directly by multiple approaches, possibly including: (a) enhancing effector T cell functions and clonal expansion through increased proliferation, survival and cytokine production and (b) by expanding the CD4+CD25+ Treg cell population with a transient loss of immunosuppressive function in the early response stage, but these expanded CD4+CD25+ Treg cells will regain their immunosuppressive capacity to regulate the expanded effector T cells following clearance of the TLR ligands at the late stage of immune response.
Fig. 2.
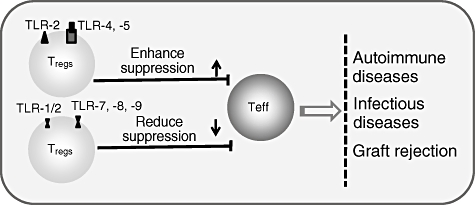
Effects of Toll-like receptor (TLR) agonist proposed to modulate directly the function of naturally occurring CD4+CD25+ T regulatory (Treg) cells upon direct interaction of the TLR ligand with the TLRs expressing on/in CD4+CD25+ T cells. Pretreatment of CD4+CD25+ Treg cells with TLR-2, -4 and -5 agonists enhance the immunosuppressive activity; in contrast, TLR-1/2, -8 and -9 agonists abrogate the suppressive activity of CD4+CD25+ Treg cells. Teff: effector T cells.
Modulating the differentiation and function of Th17 cells by TLRs
Activation of naive T cells and their subsequent differentiation into specific types of effector T cells are dependent upon TLR-mediated MHC and co-stimulatory molecule induction, and cytokine production by APCs. The cytokine IL-12 is known to drive IFN-γ-producing Th1 cells, whereas IL-6, IL-23, IL-21, IL-1 and transforming growth factor (TGF)-β have been shown to promote Th17 cells [72–76]. TGF-β at low doses does not directly promote Th17 cell differentiation, but instead acts indirectly by blocking expression of the transcription factors signal transducer and activator of transcription-4 (STAT)-4 and GATA-binding protein-3 (GATA-3), thus preventing Th1 and Th2 cell differentiation, the subsets of which suppress Th17 differentiation [77].
Researchers have investigated recently the hypothesis that the cytokines secreted by human peripheral blood mononuclear cells (PBMCs), in response to a subset of TLR ligands, would influence Th17 polarization. Through comprehensive screening they confirmed that a subset of TLR agonists induces a panel of proinflammatory cytokines that combine to promote robust secretion of IL-17 upon activation of human naive CD4+ T cells in vitro[78]. Conditioned medium from PBMCs stimulated with TLR-4 or TLR-8/7 agonists, but not from those stimulated with TLR-2/1, -3 or -9 agonists, evoked robust secretion of IL-17 by T helper cells, independent of co-culture with APCs [79]. This indicates that ligation of a subset of TLRs generates proinflammatory cytokines that co-ordinate to potentiate human Th17 differentiation. In addition, the synergy between TLR-4 and TLR-7/8 in controlling the sequential production of regulatory and proinflammatory cytokines by naive CD4+ T cells was detected [78]. The observed polymorphism in DC responses to such TLR-mediated stimuli could explain differences in the susceptibility to infectious pathogens or autoimmune diseases within the human population. Furthermore, using agonists specific for TLR-7 (i.e. Imiquimod, Gardiquimod) or TLR-8 (ssPolyU), together with LPS, confirmed that a significant synergy in cytokine induction is observed consistently after joint engagement of TLR-4 plus TLR-7 and/or TLR-8 [80,81]. However, the TLR-7, which is not present in DCs under normal conditions, is up-regulated dramatically in selected donors after stimulation by LPS, in agreement with a previous study [78,80]. Thus, the observed polymorphism between high and low DC responders is due probably to differences in TLR-7/8 up-regulation following TLR-4 stimulation, suggesting that a threshold stimulation of TLR-7 and/or TLR-8 is required to activate the joint secretion of multiple cytokines by DCs. Taken together, TLR-3, -4, -7 and -8 are required in the induction of Th17 cell differentiation and subsequent biological effects, but the role of TLR-9 is controversial, which urgently needs to be illustrated (Fig. 3).
Fig. 3.
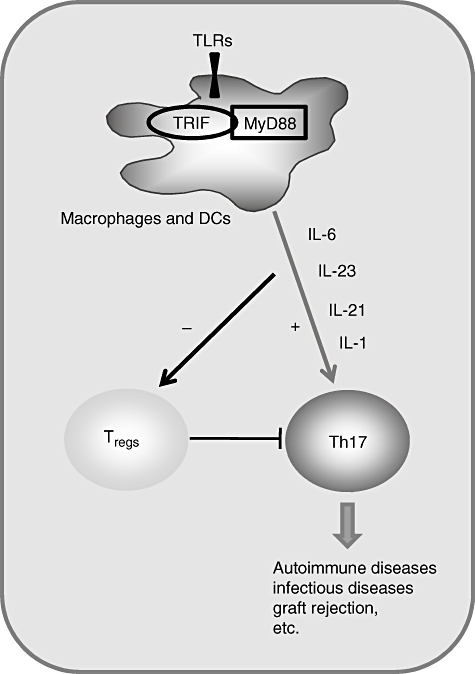
Antigen-presenting cells (APCs) [macrophages and/or dendritic cells (DCs)] to induce T helper type 17 (Th17) or CD4+CD25+ T regulatory (Treg) responses through TLR signalling. Signalling via TLR-3, -4, -7 or -8 involved in triggering of APCs promotes induction of proinflammatory cytokines including interleukin (IL)-6, IL-1, IL-21 and IL-23 and thus mediate the Th17 response, and also inhibits the CD4+CD25+ Treg cell activity.
In mice, coincidental activation of complement and several TLRs (TLR-3, -4, -7, -8 and -9) led to the synergistic production of serum factors that promote Th17 differentiation from anti-CD3/CD28 or antigen-stimulated T cells [82] (Fig. 3). Although multiple TLR-triggered cytokines were regulated by complement, Th17 cell-promoting activity in the serum was correlated with IL-6 induction, and antibody neutralization of IL-6 abrogated the complement effect [82]. These data establish a link between complement/TLR interaction and Th17 cell differentiation, and provide new insight into the mechanism of action of complement and TLR signalling in autoimmunity.
Although CD4+ T cells are considered to be the major source of IL-17, especially in autoimmune diseases, recent studies have indicated that other T cell subpopulations such as CD8+ T cells, natural killer (NK) T cells and γδ T cells can also produce IL-17 [74,83]. It is reported that CCR6+ IL-17-producing γδ T cells, but not other γδ T cells, express TLR-1 and TLR-2, but not TLR-4 [84,85]. Ligands that target these pathogen recognition receptors can cause the selective expansion of IL-17+γδ T cells and functional consequences, such as neutrophil recruitment [86]. Studies have shown that γδ T cells activated by IL-1β and IL-23 are an important source of innate IL-17 and IL-21 and may act in an amplification loop for IL-17 production by Th17 cells [74,86].
However, no studies on the direct effects of TLRs in Th17 cells have yet been reported. Investigations on the direct involvement of TLRs in Th17 cells are vitally required in the near future.
Closing remarks and prospectives
It has long been recognized that TLR ligands play an important indirect role in promoting T cell-mediated responses via their effects on innate immune cells, including up-regulating antigen presentation, co-stimulatory molecule expressions and inflammatory cytokine productions. It has become increasingly clear that TLR ligands can also act directly on T cells, possibly as co-stimulatory molecules. In general, TLRs enhance effector T responses including cytokine production, proliferation and survival, while expanding the CD4+CD25+ Treg cell population with a transient loss of immunosuppressive function. The molecular mechanisms for the TLR-mediated function in T cells and the direct effect of TLRs on Th17 cells need to be addressed in the future. More attention should be paid to the significance of the direct role of TLRs in T cells as, significantly, it will help us to understand fully the biological function of so-called innate receptors and develop more powerful adjuvants for controlling cellular immunity on purpose.
Acknowledgments
The authors wish to thank Dr Zeqing Niu for his kind review of the manuscript. This work was supported by grants from the National Natural Science Foundation of China for Key Programs (C30630060 to Y. Z.), the National Natural Science Foundation of China for General Program (C30972685 to G. L.), the grant from the Ministry of Science and Technology of China (2010CB945300) and the National Natural Science Foundation of China for Young Scientists (C30600567 to G. L.).
Disclosure
The authors have no financial conflict of interest.
References
- 1.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21:317–37. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 3.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 5.Panter G, Kuznik A, Jerala R. Therapeutic applications of nucleic acids as ligands for Toll-like receptors. Curr Opin Mol Ther. 2009;11:133–45. [PubMed] [Google Scholar]
- 6.Werling D, Jann OC, Offord V, Glass EJ, Coffey TJ. Variation matters: TLR structure and species-specific pathogen recognition. Trends Immunol. 2009;30:124–30. doi: 10.1016/j.it.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–8. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 8.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Adv Exp Med Biol. 2005;560:11–18. doi: 10.1007/0-387-24180-9_2. [DOI] [PubMed] [Google Scholar]
- 9.Liu G, Zhao Y. Toll-like receptors and immune regulation: their direct and indirect modulation on regulatory CD4+ CD25+ T cells. Immunology. 2007;122:149–56. doi: 10.1111/j.1365-2567.2007.02651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express Toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–11. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cottalorda A, Mercier BC, Mbitikon-Kobo FM, et al. TLR2 engagement on memory CD8(+) T cells improves their cytokine-mediated proliferation and IFN-gamma secretion in the absence of Ag. Eur J Immunol. 2009;39:2673–81. doi: 10.1002/eji.200939627. [DOI] [PubMed] [Google Scholar]
- 12.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–79. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zipfel C. Early molecular events in PAMP-triggered immunity. Curr Opin Plant Biol. 2009;12:414–20. doi: 10.1016/j.pbi.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 15.Bowie AG, Unterholzner L. Viral evasion and subversion of pattern-recognition receptor signalling. Nat Rev Immunol. 2008;8:911–22. doi: 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivashkiv LB. A signal-switch hypothesis for cross-regulation of cytokine and TLR signalling pathways. Nat Rev Immunol. 2008;8:816–22. doi: 10.1038/nri2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang LY, Dumontelle JL, Zolodz M, Deora A, Mozier NM, Golding B. Use of Toll-like receptors (TLR) assays to detect and identify microbial contaminants in biological products. J Clin Microbiol. 2009 doi: 10.1128/JCM.00373-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu G, Wu Y, Gong S, Zhao Y. Toll-like receptors and graft rejection. Transpl Immunol. 2006;16:25–31. doi: 10.1016/j.trim.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Baccala R, Gonzalez-Quintial R, Lawson BR, et al. Sensors of the innate immune system: their mode of action. Nat Rev Rheumatol. 2009;5:448–56. doi: 10.1038/nrrheum.2009.136. [DOI] [PubMed] [Google Scholar]
- 20.McAleer JP, Vella AT. Understanding how lipopolysaccharide impacts CD4 T-cell immunity. Crit Rev Immunol. 2008;28:281–99. doi: 10.1615/critrevimmunol.v28.i4.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Kooyk Y. C-type lectins on dendritic cells: key modulators for the induction of immune responses. Biochem Soc Trans. 2008;36:1478–81. doi: 10.1042/BST0361478. [DOI] [PubMed] [Google Scholar]
- 22.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–42. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang M, Jin W, Sun SC. Peli1 facilitates TRIF-dependent Toll-like receptor signaling and proinflammatory cytokine production. Nat Immunol. 2009;10:1089–95. doi: 10.1038/ni.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marta M, Meier UC, Lobell A. Regulation of autoimmune encephalomyelitis by Toll-like receptors. Autoimmun Rev. 2009;8:506–9. doi: 10.1016/j.autrev.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Nikoopour E, Schwartz JA, Singh B. Therapeutic benefits of regulating inflammation in autoimmunity. Inflamm Allergy Drug Targets. 2008;7:203–10. doi: 10.2174/187152808785748155. [DOI] [PubMed] [Google Scholar]
- 26.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 27.de Souza AL, Seguro AC. Two centuries of meningococcal infection: from Vieusseux to the cellular and molecular basis of disease. J Med Microbiol. 2008;57:1313–21. doi: 10.1099/jmm.0.47599-0. [DOI] [PubMed] [Google Scholar]
- 28.Pulko V, Liu X, Krco CJ, et al. TLR3-stimulated dendritic cells up-regulate B7-H1 expression and influence the magnitude of CD8 T cell responses to tumor vaccination. J Immunol. 2009;183:3634–41. doi: 10.4049/jimmunol.0900974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stary G, Klein I, Kohlhofer S, et al. Plasmacytoid dendritic cells express TRAIL and induce CD4+ T cell apoptosis in HIV-1 viremic patients. Blood. 2009;114:3723–4. doi: 10.1182/blood-2009-04-217927. [DOI] [PubMed] [Google Scholar]
- 30.Allam JP, Bieber T, Novak N. Dendritic cells as potential targets for mucosal immunotherapy. Curr Opin Allergy Clin Immunol. 2009 doi: 10.1097/ACI.0b013e32833239a9. [DOI] [PubMed] [Google Scholar]
- 31.Liu YJ. TSLP in epithelial cell and dendritic cell cross talk. Adv Immunol. 2009;101:1–25. doi: 10.1016/S0065-2776(08)01001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dulay AT, Buhimschi CS, Zhao G, et al. Soluble TLR2 is present in human amniotic fluid and modulates the intraamniotic inflammatory response to infection. J Immunol. 2009;182:7244–53. doi: 10.4049/jimmunol.0803517. [DOI] [PubMed] [Google Scholar]
- 33.van Vliet SJ, den Dunnen J, Gringhuis SI, Geijtenbeek TB, van Kooyk Y. Innate signaling and regulation of dendritic cell immunity. Curr Opin Immunol. 2007;19:435–40. doi: 10.1016/j.coi.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc Natl Acad Sci USA. 2006;103:7048–53. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrade CF, Waddell TK, Keshavjee S, Liu M. Innate immunity and organ transplantation: the potential role of Toll-like receptors. Am J Transplant. 2005;5:969–75. doi: 10.1111/j.1600-6143.2005.00829.x. [DOI] [PubMed] [Google Scholar]
- 36.Bjornvold M, Munthe-Kaas MC, Egeland T, et al. A TLR2 polymorphism is associated with type 1 diabetes and allergic asthma. Genes Immun. 2009;10:181–7. doi: 10.1038/gene.2008.100. [DOI] [PubMed] [Google Scholar]
- 37.Curtin JF, Liu N, Candolfi M, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009:6. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang QQ, Sobkoviak R, Jockheck-Clark AR, et al. Heat shock protein 96 is elevated in rheumatoid arthritis and activates macrophages primarily via TLR2 signaling. J Immunol. 2009;182:4965–73. doi: 10.4049/jimmunol.0801563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mercier BC, Cottalorda A, Coupet CA, Marvel J, Bonnefoy-Berard N. TLR2 engagement on CD8 T cells enables generation of functional memory cells in response to a suboptimal TCR signal. J Immunol. 2009;182:1860–7. doi: 10.4049/jimmunol.0801167. [DOI] [PubMed] [Google Scholar]
- 40.Beutler B. Microbe sensing, positive feedback loops, and the pathogenesis of inflammatory diseases. Immunol Rev. 2009;227:248–63. doi: 10.1111/j.1600-065X.2008.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beutler BA. TLRs and innate immunity. Blood. 2009;113:1399–407. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sutmuller RP, den Brok MH, Kramer M, et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116:485–94. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Cutting edge: diminished T cell TLR expression and function modulates the immune response in human filarial infection. J Immunol. 2006;176:3885–9. doi: 10.4049/jimmunol.176.7.3885. [DOI] [PubMed] [Google Scholar]
- 44.Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci USA. 2004;101:3029–34. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crellin NK, Garcia RV, Hadisfar O, Allan SE, Steiner TS, Levings MK. Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4+CD25+ T regulatory cells. J Immunol. 2005;175:8051–9. doi: 10.4049/jimmunol.175.12.8051. [DOI] [PubMed] [Google Scholar]
- 46.Caron G, Duluc D, Fremaux I, et al. Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J Immunol. 2005;175:1551–7. doi: 10.4049/jimmunol.175.3.1551. [DOI] [PubMed] [Google Scholar]
- 47.Gelman AE, LaRosa DF, Zhang J, et al. The adaptor molecule MyD88 activates PI-3 kinase signaling in CD4+ T cells and enables CpG oligodeoxynucleotide-mediated costimulation. Immunity. 2006;25:783–93. doi: 10.1016/j.immuni.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol. 2004;172:6065–73. doi: 10.4049/jimmunol.172.10.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Komai-Koma M, Gilchrist DS, Xu D. Direct recognition of LPS by human but not murine CD8+ T cells via TLR4 complex. Eur J Immunol. 2009;39:1564–72. doi: 10.1002/eji.200838866. [DOI] [PubMed] [Google Scholar]
- 50.Tabiasco J, Devevre E, Rufer N, et al. Human effector CD8+ T lymphocytes express TLR3 as a functional coreceptor. J Immunol. 2006;177:8708–13. doi: 10.4049/jimmunol.177.12.8708. [DOI] [PubMed] [Google Scholar]
- 51.Sobek V, Birkner N, Falk I, et al. Direct Toll-like receptor 2 mediated co-stimulation of T cells in the mouse system as a basis for chronic inflammatory joint disease. Arthritis Res Ther. 2004;6:R433–46. doi: 10.1186/ar1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCarron M, Reen DJ. Activated human neonatal CD8+ T cells are subject to immunomodulation by direct TLR2 or TLR5 stimulation. J Immunol. 2009;182:55–62. doi: 10.4049/jimmunol.182.1.55. [DOI] [PubMed] [Google Scholar]
- 53.Simone R, Floriani A, Saverino D. Stimulation of human CD4 T lymphocytes via TLR3, TLR5 and TLR7/8 up-regulates expression of costimulatory and modulates proliferation. Open Microbiol J. 2009;3:1–8. doi: 10.2174/1874285800903010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bendigs S, Salzer U, Lipford GB, Wagner H, Heeg K. CpG-oligodeoxynucleotides co-stimulate primary T cells in the absence of antigen-presenting cells. Eur J Immunol. 1999;29:1209–18. doi: 10.1002/(SICI)1521-4141(199904)29:04<1209::AID-IMMU1209>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 55.Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116:2022–32. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Zanin-Zhorov A, Tal-Lapidot G, Cahalon L, et al. Cutting edge: T cells respond to lipopolysaccharide innately via TLR4 signaling. J Immunol. 2007;179:41–4. doi: 10.4049/jimmunol.179.1.41. [DOI] [PubMed] [Google Scholar]
- 57.Walker LS. Regulatory T cells overturned: the effectors fight back. Immunology. 2009;126:466–74. doi: 10.1111/j.1365-2567.2009.03053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qu Y, Zhao Y. Regulatory CD4(+)CD25(+) T-cells are controlled by multiple pathways at multiple levels. Int Rev Immunol. 2007;26:145–60. doi: 10.1080/08830180701365917. [DOI] [PubMed] [Google Scholar]
- 59.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 60.Rahman AH, Taylor DK, Turka LA. The contribution of direct TLR signaling to T cell responses. Immunol Res. 2009;45:25–36. doi: 10.1007/s12026-009-8113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grauer OM, Molling JW, Bennink E, et al. TLR ligands in the local treatment of established intracerebral murine gliomas. J Immunol. 2008;181:6720–9. doi: 10.4049/jimmunol.181.10.6720. [DOI] [PubMed] [Google Scholar]
- 62.Loures FV, Pina A, Felonato M, Calich VL. TLR2 is a negative regulator of Th17 cells and tissue pathology in a pulmonary model of fungal infection. J Immunol. 2009;183:1279–90. doi: 10.4049/jimmunol.0801599. [DOI] [PubMed] [Google Scholar]
- 63.Chen Q, Davidson TS, Huter EN, Shevach EM. Engagement of TLR2 does not reverse the suppressor function of mouse regulatory T cells, but promotes their survival. J Immunol. 2009;183:4458–66. doi: 10.4049/jimmunol.0901465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci USA. 2007;104:19446–51. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Medvedev AE, Sabroe I, Hasday JD, Vogel SN. Tolerance to microbial TLR ligands: molecular mechanisms and relevance to disease. J Endotoxin Res. 2006;12:133–50. doi: 10.1179/096805106X102255. [DOI] [PubMed] [Google Scholar]
- 66.Rehli M. Of mice and men: species variations of Toll-like receptor expression. Trends Immunol. 2002;23:375–8. doi: 10.1016/s1471-4906(02)02259-7. [DOI] [PubMed] [Google Scholar]
- 67.Forward NA, Furlong SJ, Yang Y, Lin TJ, Hoskin DW. Signaling through TLR7 enhances the immunosuppressive activity of murine CD4+CD25+ T regulatory cells. J Leukoc Biol. 2009 doi: 10.1189/jlb.0908559. [DOI] [PubMed] [Google Scholar]
- 68.Peng G, Guo Z, Kiniwa Y, et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–4. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 69.Wang HY, Lee DA, Peng G, et al. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107–18. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 70.Heil F, Hemmi H, Hochrein H, et al. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science. 2004;303:1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 71.Chiffoleau E, Heslan JM, Heslan M, Louvet C, Condamine T, Cuturi MC. TLR9 ligand enhances proliferation of rat CD4+ T cell and modulates suppressive activity mediated by CD4+ CD25+ T cell. Int Immunol. 2007;19:193–201. doi: 10.1093/intimm/dxl136. [DOI] [PubMed] [Google Scholar]
- 72.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 73.Nurieva RI, Dong C. Keeping autoimmunity in check: how to control a Th17 cell controller. Immunity. 2008;29:841–3. doi: 10.1016/j.immuni.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 74.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–41. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 75.Chung Y, Chang SH, Martinez GJ, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–87. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bi Y, Liu G, Yang R. Th17 cell induction and immune regulatory effects. J Cell Physiol. 2007;211:273–8. doi: 10.1002/jcp.20973. [DOI] [PubMed] [Google Scholar]
- 77.Aggarwal M, Villuendas R, Gomez G, et al. TCL1A expression delineates biological and clinical variability in B-cell lymphoma. Mod Pathol. 2009;22:206–15. doi: 10.1038/modpathol.2008.148. [DOI] [PubMed] [Google Scholar]
- 78.Lombardi V, Van Overtvelt L, Horiot S, Moingeon P. Human dendritic cells stimulated via TLR7 and/or TLR8 induce the sequential production of IL-10, IFN-gamma, and IL-17A by naive CD4+ T cells. J Immunol. 2009;182:3372–9. doi: 10.4049/jimmunol.0801969. [DOI] [PubMed] [Google Scholar]
- 79.Kattah MG, Wong MT, Yocum MD, Utz PJ. Cytokines secreted in response to Toll-like receptor ligand stimulation modulate differentiation of human Th17 cells. Arthritis Rheum. 2008;58:1619–29. doi: 10.1002/art.23497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang X, Jin J, Tang Y, Speer D, Sujkowska D, Markovic-Plese S. IFN-beta1a inhibits the secretion of Th17-polarizing cytokines in human dendritic cells via TLR7 up-regulation. J Immunol. 2009;182:3928–36. doi: 10.4049/jimmunol.0802226. [DOI] [PubMed] [Google Scholar]
- 81.Wang LF, Chiu HC, Hsu CJ, Liu CY, Hsueh YH, Miaw SC. Epicutaneous sensitization with a protein antigen induces Th17 cells. J Dermatol Sci. 2009;54:192–7. doi: 10.1016/j.jdermsci.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 82.Fang C, Zhang X, Miwa T, Song WC. Complement promotes the development of inflammatory T-helper 17 cells through synergistic interaction with Toll-like receptor signaling and interleukin-6 production. Blood. 2009;114:1005–15. doi: 10.1182/blood-2009-01-198283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rachitskaya AV, Hansen AM, Horai R, et al. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008;180:5167–71. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Girardi M. Immunosurveillance and immunoregulation by gammadelta T cells. J Invest Dermatol. 2006;126:25–31. doi: 10.1038/sj.jid.5700003. [DOI] [PubMed] [Google Scholar]
- 85.Schwacha MG, Daniel T. Up-regulation of cell surface Toll-like receptors on circulating gammadelta T-cells following burn injury. Cytokine. 2008;44:328–34. doi: 10.1016/j.cyto.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–30. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]


