Abstract
Dendritic cells (DCs) are powerful antigen-presenting cells capable of maintaining peripheral tolerance. The possibility to generate tolerogenic DCs opens new therapeutic approaches in the prevention or remission of autoimmunity. There is currently no treatment inducing long-term tolerance and remission in type 1 diabetes (T1D), a disease caused by autoimmunity towards β cells. An ideal immunotherapy should inhibit the autoimmune attack, avoid systemic side effects and allow islet regeneration. Apoptotic cells – a source of autoantigens – are cleared rapidly by macrophages and DCs through an immunologically silent process that contributes to maintaining tolerance. Our aims were to prevent T1D and to evaluate the re-establishment of peripheral tolerance using autologous DCs pulsed in vitro with apoptotic bodies from β cells. Immature DCs derived from bone marrow of non-obese diabetic (NOD) mice were obtained and pulsed with antigen-specific apoptotic bodies from the β cell line NIT-1. Those DCs that phagocytosed apoptotic cells diminished the expression of co-stimulatory molecules CD40 and CD86 and reduced secretion of proinflammatory cytokines. Moreover, these cells were resistant to increase the expression of co-stimulatory molecules after lipopolysaccharide activation. The administration of these cells to NOD transgenic mice expressing interferon-β in their insulin-producing cells, a model of accelerated autoimmune diabetes, decreased diabetes incidence significantly and correlated positively with insulitis reduction. DCs pulsed with apoptotic cells that express disease-associated antigens constitutes a promising strategy to prevent T1D.
Keywords: apoptosis, autoimmunity, dendritic cells, NOD mice, tolerance
Introduction
Type 1 diabetes (T1D) results from autoimmune destruction of insulin-producing β cells in the pancreatic islets of Langerhans [1]. The prevalence of this disease, its complications and the lack of effective curative and preventive strategies necessitates a significant effort to find the means to restore the tolerance to β cells as the best way to control this disease.
To date, it has not been possible to prevent the autoimmune response to β cells in human T1D, due probably to its unknown aetiology, although it is known that self-reactive T cells that infiltrate the islets cause the destruction of these target cells. The activation of T cells that recognize β cells requires a cognate interaction between T cells and antigen-presenting cells [i.e. dendritic cells (DCs)], which are the initiators of the islet infiltration in the spontaneous model of T1D, non-obese diabetic (NOD) mice [2]. In fact, DCs have been proved to take part in the onset and propagation of the autoimmune response in T1D [3–5].
DCs generated in vitro from bone marrow (BM) in the presence of certain cytokines [e.g. granulocyte–macrophage colony-stimulating factor (GM-CSF)] possess the immunophenotypic and functional characteristics of immature DCs (iDCs) [6]. The use of iDCs, which have poor antigen presentation functions [7], could be an important tool to promote immunoregulation in autoimmunity [8]. Because the subset and functional stage of DCs appear to be critical for tolerance induction, several strategies for engineering these cells are emerging.
Apoptotic bodies are small, sealed membrane vesicles that are produced by cells while undergoing physiologically programmed cell death by apoptosis. The formation of apoptotic bodies is a mechanism for preventing the leakage of potentially toxic or immunogenic cellular contents of dying cells. It prevents inflammation or autoimmune reactions, as well as tissue destruction. Recent studies have provided new insights into the engulfment process, including how macrophages and DCs properly process apoptotic cells without inducing inflammation and maintaining cellular homeostasis [9]. Thus, the uptake of apoptotic cells will not cause maturation in DCs, and this process could induce tolerance rather than immunity [10].
Transgenic NOD mice, which express interferon (IFN)-β under the control of the insulin promoter [NOD rat insulin promoter-interferon-β (RIP-IFN-β)], develop early autoimmune diabetes with similar incidence in males and females [11], resembling the clinical presentation of T1D in humans. Here, we report a new immunotherapy protocol based on the use of iDCs loaded with β cell apoptotic bodies. This protocol clearly reduces T1D incidence and insulitis and correlates with the ability of apoptotic cells to down-modulate antigen presentation functions in DCs.
Materials and methods
Mice
NOD and NOD transgenic mice that express IFN-β in their β cells (RIP-IFN-β) were used. Transgenic NOD RIP-IFN-β mice were generated by back-crossing CD-1 RIP-IFN-β to the NOD background [11] and develop accelerated autoimmune diabetes after 3 weeks of age. For prevention experiments, 50% males and 50% females were used. Wild-type NOD mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) and only female mice were used. Mice were kept under specific pathogen-free conditions and monitored daily for diabetes assessment. The Catalan Government's guidelines for the use and care of laboratory animals were followed and the protocols were approved by our Institutional Animal Care and Use Committee.
Cell lines and preparation of apoptotic cells
The NIT-1 cell line was derived from β cells from NOD/Lt mice [12] and was chosen because they express β cell-specific autoantigens. The SV-T2 cell line was obtained from embryonic fibroblasts from BALB/c mice [13] and used as control. Both cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in RPMI-1640 media containing 10% heat-inactivated fetal bovine serum (Gibco, Invitrogen, Carlsbad, CA, USA), 100 U/ml penicillin (Normon SA, Madrid, Spain), 100 µg/ml streptomycin (Laboratorio Reig Jofre, Sant Joan Despi, Spain), 2 mmol/l glutamine (Sigma, St Louis, MO, USA), 1 mmol/l sodium pyruvate (Gibco) and 25 µmol/l β-mercaptoethanol (Sigma). Apoptosis was induced by UVB irradiation (10 mJ/m2) for 45 min in 24-well plates (3 × 105 cells/well in 500 µl), followed by culture overnight at 37°C, as described previously [14]. Apoptosis was confirmed after staining with annexin V-fluorescein isothiocyanate (FITC) (BD Pharmingen, San Diego, CA, USA) and propidium iodide (BD Pharmingen) by flow cytometric [fluorescence activated cell sorter (FACS)] analysis (FACSCanto II, BD Biosciences, San Jose, CA, USA).
Dendritic cells propagation and co-culture with apoptotic bodies
Immature DCs were propagated from BM progenitors of 10- to 14-week-old female wild-type NOD mice in culture medium containing GM-CSF (Prospec, Rehovot, Israel). Briefly, BM cells were obtained from the femur and tibia, and erythrocytes were depleted by hypotonic lysis. BM cells (107) were cultured in 10 ml complete medium supplemented with 1000 U/ml GM-CSF. Cells were fed every other day by replenishment of half the volume of fresh medium and cytokine. At day 7, DCs were stained with the DC marker CD11c (BD Pharmingen) to determine the purity of the sample. Viability was assessed by annexin and propidium iodide staining. Co-culture of DCs with apoptotic bodies was performed by mixing 106 DCs with 3 × 105 apoptotic NIT-1 or SV-T2 cells (3 : 1 ratio) in 24-well plates for 2 h. A small sample was used to confirm the priming of DCs with apoptotic bodies by FACS analysis.
Sorting of DCs loaded with NIT-1 apoptotic cells
After 2 h of co-culture, DCs loaded with apoptotic bodies from NIT-1 cells were separated from unloaded DCs by sorting (FACSAria II; BD Biosciences). NIT-1 cells were pre-labelled with carboxyfluorescein succinimidyl ester (CFSE) (Molecular Probes, Invitrogen, Carlsbad, CA, USA) to allow the separation of DCs pulsed with apoptotic bodies (CD11c- and CFSE-positive) and unpulsed DCs (CD11c-positive and CFSE-negative). After sorting, both DCs loaded with NIT-1 apoptotic bodies (NITApo-loaded DCs) and unloaded DCs were cultured in 24-well plates with complete medium (105 cells/well in 500 µl) for 24 h. Co-stimulatory molecules expression and cytokine production were assessed in these subsets.
Expression of co-stimulatory molecules and cytokine production in DCs after apoptotic bodies engulfment
Expression of DCs co-stimulatory membrane molecules and major histocompatibility complex (MHC) class II were determined by FACS analysis (FACSCanto II). DCs were stained with monoclonal antibodies to mouse CD11c/phycoerythrin (PE)-cyanine (Cy)7, CD40/allophycocyanin (APC), CD86/PE (BD Pharmingen) and MHC class II/PE-Cy5 (NIMR-4; SBA, Birmingham, AL, USA). Isotype control staining was used as a control. Data were analysed using CellQuest software (BD Biosciences). To assess cytokine production, we used the cytometric bead array (CBA) system (BD Biosciences), according to the manufacturer's instructions. Culture supernatants were collected and frozen at −80°C until use. Cytokines measured were interleukin (IL)-6, IL-12 and tumour necrosis factor (TNF)-α. Data were analysed using CBA software (BD Biosciences).
Assessment of activation ability of DCs after apoptotic bodies engulfment
The ability of DCs to respond to a maturation stimulus after engulfment of NIT-1 apoptotic bodies was determined. To that end, 105 DCs – unloaded and loaded with apoptotic bodies from NIT-1 cells – were cultured in 500 µl and exposed to 100 ng/ml lipopolysaccharide (LPS) (Sigma) during 24 h. The expression of co-stimulatory molecules CD40 and CD86 and MHC class II as well as cytokine secretion were assessed as shown above.
Type 1 diabetes prevention
NOD RIP-IFNβ mice (12–14 days old) were given a single intraperitoneal dose of 106 DCs cultured with NIT-1 apoptotic bodies (NITApo-loaded DCs group) in 150 µl saline solution. As controls, we injected two different cell preparations: (i) 106 non-pulsed DCs (unloaded DCs group); and (ii) 106 DCs pulsed with SV-T2 apoptotic cells (SVApo-loaded DCs). Cells were washed in PBS and suspended in saline solution before injection. A sham control group that received only saline solution was also included. A total of 11–26 animals per group were used. Mice were monitored daily for urine glucose using Glucocard strips (Menarini, Barcelona, Spain) during a 30-week period. Animals with glucosuria were confirmed diabetic when the blood glucose level was >300 mg/dl.
Insulitis score
The degree of islet infiltration by leucocytes (insulitis) was determined in mice at the beginning (12 days old) and at the end (30 weeks old) of the study. Briefly, pancreata from six animals for each group were snap-frozen in an isopentane/cold acetone bath. Cryosections of 5 µm were obtained at five non-overlapping levels. The sections were stained with haematoxylin and eosin (H&E), coded and analysed by two independent observers who were blinded to the experimental conditions. Each observer assessed a minimum of 40 islets per animal. Insulitis was scored as described elsewhere [11]: 0, no insulitis; 1, peri-insular; 2, mild insulitis (<25% of the infiltrated islet); 3, 25–75% of the islet infiltrated; 4, total islet infiltration.
Statistical analysis
Comparisons between groups used the unpaired t-test for unpaired data and a paired t-test for paired data after confirming normal distributions. Kaplan–Meier log-rank analysis was used for comparing incidence of diabetes between different groups. A P-value < 0·05 was considered significant.
Results
Generation of immature DCs and engulfment of apoptotic cells
Twelve hours after UVB irradiation, NIT-1 (NOD β-cell line) and SV-T2 cells showed typical morphological features of apoptosis (>75% of cells in apoptosis). DCs generated from BM progenitors cultured in GM-CSF for 7 days were >80% pure based on staining for the DC marker CD11c. Viability was always >90%. These cells, generated using GM-CSF only, were immature, as they showed low dendrite density and low co-stimulatory molecule expression. A co-culture period of 2 h was optimal to capture apoptotic cells (NIT-1 or SV-T2) by immature DCs. The subset of DCs loaded with apoptotic cells was observed by flow cytometry as being CD11c+ CFSE+. When the same experiment was performed at 4°C no double-positive cells were observed (data not shown).
DCs have reduced expressions of co-stimulatory molecules after engulfment of apoptotic cells
Expression of two co-stimulatory molecules, CD40 and CD86 and MHC class II, were assessed on the surface of BM-derived immature DCs. Two hours after co-culture of DCs with NIT-1 apoptotic bodies, loaded DCs were separated from unloaded DCs by cell sorting and cultured for 24 h. Sorted cell preparations were typically >90% pure and >90% viable. We observed significantly decreased expressions of CD40 and CD86 on the DCs that were loaded with NIT-1 apoptotic cells compared to unloaded DC cells (Fig. 1a). The median fluorescence intensity (MFI) for CD40 or CD86 was always reduced significantly on NITApo-loaded DCs when compared to unloaded DCs (P < 0·05; Fig. 1b). We found a 3·9 ± 0·9 [mean ± standard error of the mean (M ± s.e.m.)]-fold reduction for CD40 and a 16·5 ± 5·1-fold reduction for CD86 (means of nine independent experiments). As expected, no differences were found for MHC class II expression (data not shown) due to the previously described feature of the NOD mouse [15], in which BM DCs showed very low levels of MHC class II.
Fig. 1.
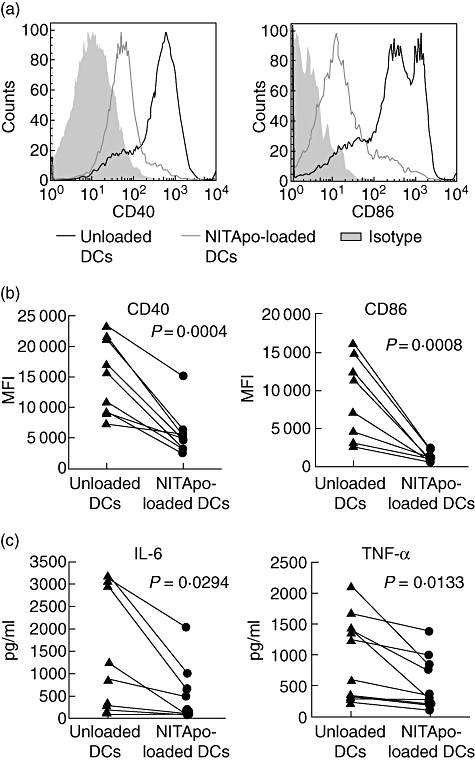
CD40 and CD86 expression and cytokine secretion on NIT-1 apoptotic bodies (NITApo)-loaded DCs and unloaded dendritic cells (DCs) in basal conditions. (a) Representative histograms of CD40 and CD86 expressions for NITApo-loaded DCs (thin line) and unloaded DCs (thick line). Grey areas are isotype-matched controls. (b) Median fluorescence intensity (MFI) of unloaded DCs and NITApo-loaded DCs for CD40 and CD86. Results are from nine independent experiments (each line represents an individual experiment). Comparisons between groups showed significant differences (paired t-test, P < 0·05). (c) Interleukin (IL)-6 and tumour necrosis factor (TNF)-α secretion (pg/ml) by NITApo-loaded DCs and unloaded DCs. Results are from 10 independent experiments (each line represents an individual experiment). Comparisons between groups showed significant differences (paired t-test, P < 0·05).
DCs loaded with apoptotic cells show reduced IL-6 and TNF-α secretion
Both unloaded and NITApo-loaded DCs obtained after cell sorting were cultured for 24 h to allow cytokine secretion. The results showed significant reductions of IL-6 and TNF-α secretion by NITApo-loaded DCs when compared to unloaded DCs (P < 0·05; Fig. 1c). We found a 5·7 ± 2·7-fold reduction for IL-6 and 2·3 ± 0·5-fold reduction for TNF-α (means of 10 independent experiments). We found very little IL-12 secretion, as has been described in BM DCs of NOD mice [15].
Activation with LPS results in the maintenance of a low co-stimulatory phenotype in DCs loaded with apoptotic cells
LPS was used as inflammatory stimulus to determine the maturation ability of dendritic cells. Loaded or unloaded sorted DCs obtained from the co-culture with NIT-1 apoptotic bodies were stimulated with LPS during 24 h. Figure 2 shows the subtraction between LPS-stimulated DCs and unstimulated DCs (Δ) for each parameter analysed. After LPS exposure, NITApo-loaded DCs were significantly more resistant to increase the expression of co-stimulatory molecules CD40 and CD86 than unloaded DCs (P < 0·05; Fig. 2a). However, the secretion of IL-6 and TNF-α after LPS stimulation was detected equally in both NITApo-loaded and unloaded DCs (Fig. 2b).
Fig. 2.
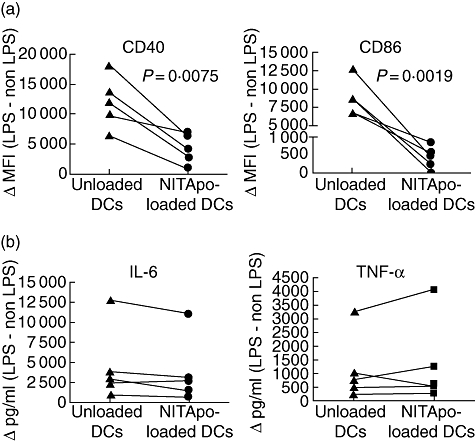
Effect of stimulation with lipopolysaccharide (LPS) on CD40 and CD86 expression and cytokine secretion on NIT-1 apoptotic bodies (NITApo)-loaded dendritic cells (DCs) and unloaded DCs. (a) Paired data for CD40 and CD86 expression of unloaded DCs and NITApo-loaded DCs after LPS stimulation (100 ng/ml). Results are expressed as the subtraction between median fluorescence intensity (MFI) of stimulated cells and the MFI of unstimulated cells (Δ MFI). Data are from five independent experiments (each line represents an individual experiment). Comparisons between groups showed significant differences (paired t-test, P < 0·05). (b) Interleukin (IL)-6 and tumour necrosis factor (TNF)-α secretion (pg/ml) by NITApo-loaded DCs and unloaded DCs after LPS stimulation. Results are expressed as the subtraction between cytokine secretion of stimulated and unstimulated cells (Δ pg/ml). Data are from five independent experiments (each line represents an individual experiment).
Administration of DCs pulsed with NIT-1 apoptotic cells prevents T1D in NOD RIP-IFN-β mice
To assess the efficacy of DCs pulsed with NIT-1 apoptotic cells for preventing T1D, we treated NOD RIP-IFN-β mice during the pre-diabetic period (12–14 days old). At this early age, which was chosen for preventive immunotherapy prior to diabetes onset, pups are still unweaned but the treatments were safe and well tolerated, as the mice showed the appearance and behaviour similar to that of untreated mice. As expected, animals from the sham control group developed diabetes at 3 weeks of age and with an incidence of 65·4% (17 of 26 animals) (Fig. 3). The treatment with DCs pulsed with apoptotic NIT-1 cells (NITApo-loaded DCs group) resulted in a significant reduction of disease incidence (21%, four of 19 animals; P < 0·05) when compared to the sham group, to unloaded DCs group (57%, eight of 14 animals) and, remarkably, to the SVApo-loaded DCs group (54%, six of 11 animals) that did not cause any preventive effect.
Fig. 3.
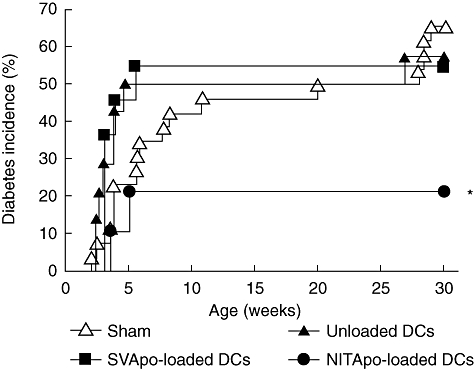
Immunotherapy using dendritic cells (DCs) pulsed with NIT-1 apoptotic bodies (NITApo) decreases type 1 diabetic (T1D) incidence. Cumulative incidence (percentage) of diabetes in non-obese diabetic (NOD) rat insulin promoter-interferon-β (RIP-IFN-β) mice treated with NITApo-loaded DCs (black circles, n = 19), SV-T2 apoptotic cells (SVApo)-loaded DCs (black squares, n = 11), unpulsed DCs (black triangles, n = 14) and in the control group that received saline solution (white triangles, n = 26). *Significant differences (P < 0·05) were found when NITApo-loaded DCs group versus the other three groups were compared, using Kaplan–Meier log-rank analysis.
Insulitis is reduced in mice treated with DC pulsed with apoptotic bodies
Insulitis was scored for six non-diabetic animals from each group at the end of the follow-up period (30 weeks old) to determine any effects of the treatment on islet leucocytic infiltration, a feature of T1D autoimmunity in NOD mice. As expected, animals in the sham group showed high insulitis scores (2·33 ± 0·12), which were the same as those we reported previously for NOD RIP-IFN-β mice [11] (Fig. 4a). Cell therapy with NITApo-loaded DCs reduced the insulitis scores significantly (1·27 ± 0·28; P < 0·05) when compared to the sham group, to the unloaded DCs group and to the SVApo-loaded DCs group. Insulitis was also scored at the beginning of the study (12 days of age) when the treatment was given, showing a weak peri-insulitis (0·65 ± 0·02). Mice treated with NITApo-loaded DCs slightly increased the degree of insulitis in relation to mice at 12 days of age (twofold), although the differences were not statistically significant. On the other hand, the insulitis score was increased significantly in control groups when compared to mice at the beginning of the treatment (3·4-fold). Moreover, analysis of the percentage of islets classified in each of the five infiltration categories showed that in mice treated with NITApo-loaded DCs most of the islets remained free of insulitis or with peri-insulitis (similar to mice at 12 days of age), whereas in the other groups most of the islets were scored with a severe degree of insulitis (Fig. 4b). Thus, the insulitis degree observed after antigen-specific immunotherapy correlated well with the prevention of the disease (R2 = 0·9654; P < 0·05; Fig. 4c).
Fig. 4.
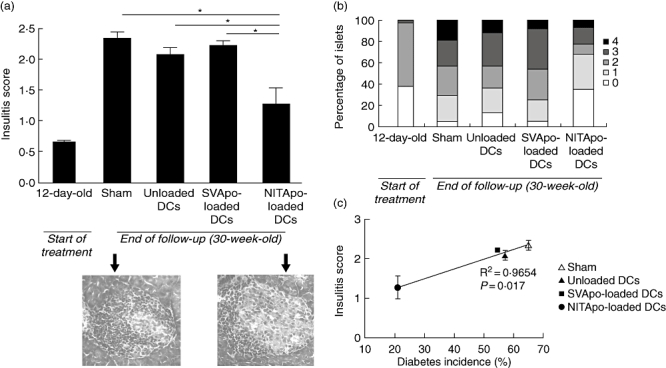
Effect of the treatment with dendritic cells (DCs) pulsed with NIT-1 apoptotic bodies (NITApo) on insulitis in non-obese diabetic (NOD) rat insulin promoter-interferon-β (RIP-IFN-β) mice. (a) Upper panel: insulitis score at the beginning of the treatment (12-day-old; narrow bar) and at the end of the follow-up (30-week-old; wide bars) in different groups. Pancreata from six animals/group were analysed. Results are expressed as mean (M) ± standard error of the mean (s.e.m.). *Significant differences (P < 0·05) versus control groups (unpaired t-test). Lower panel: representative images of islets from haematoxylin and eosin-pancreatic cryostat sections from sham and NITApo-loaded DC mice. (b) Percentage of islets classified in each of the five infiltration categories at the beginning of the treatment (12-day-old; narrow bar) and at the end of the follow-up (30-week-old; wide bars) in different groups. (c) Significant correlation between diabetes incidence (%) and insulitis score (M ± s.e.m.) in different treatment groups (R2 = 0·9654, P < 0·05).
Discussion
An ideal preventive immunotherapy for T1D should inhibit autoimmune attack against β cells while avoiding systemic side effects, and permit the regeneration of damaged tissue by specific mechanisms. As has been demonstrated previously, BM-derived iDCs generated with GM-CSF decrease experimental T1D incidence [16]. Here, we show for the first time that iDCs, when loaded with apoptotic islet cells, constitute potent immunomodulatory cells resulting in a very low incidence of T1D in NOD RIP-IFN-β mice. This model, established in our laboratory [11], develops autoimmune diabetes at an early age, and was used in this study due to its resemblance with T1D in children and young adults in terms of age at onset and its similar incidence in males and females. Moreover, the success of this treatment in NOD RIP-IFN-β mice, a more rigorous model to test immunotherapies than standard NOD mice due to the intrinsic inflammatory environment of the islets, may encourage the use of customized DCs to achieve tolerance. The presently reported prevention of T1D in NOD RIP-IFN-β correlates with a significant reduction of insulitis, and a decrease of co-stimulatory signals and proinflammatory cytokines secretion in iDCs.
As a cell dies, the immune system is alerted by several mechanisms, the processes of which are relevant to host defence and disease pathogenesis [17]. Apoptotic cells, an important source of autoantigens, are captured by DCs and macrophages. The physiological uptake of β-apoptotic bodies resulting from cell death by DCs promulgates a preventive effect in T1D [18]. In fact, a decrease in diabetes incidence in NOD mice after transfusion of UVB-apoptotic NIT-1 cells was reported [19], indicating that apoptotic cells have an immunomodulatory role.
We have demonstrated synergy in T1D prevention combining iDCs and specific apoptotic bodies. The T1D prevention reported here was achieved using DCs loaded with apoptotic β cells, but not with apoptotic bodies from other cell types or unloaded DCs, suggesting that the antigenic specificity of the apoptotic bodies is a key factor in the process of selective tolerance induction. The putative autoantigen in T1D is still unknown, but many proteins have been identified as target autoantigens. Among other β cell autoantigens, NIT-1 cells express insulin and glutamic acid decarboxylase (GAD). NOD mice treated with immature BM-derived DCs pulsed with a mixture of three islet antigen-derived peptides [16] showed a decreased T1D incidence. The synergy between DCs and specific-apoptotic bodies has been demonstrated recently to be a promising strategy in the prevention of chronic rejection [20] by inducing allograft tolerance. The administration of apoptotic cells to DCs rather than peptides highlights selectivity for the re-establishment of immunological tolerance.
Dendritic cells are critical for the initiation of immune responses, as well as for maintenance of tolerance to self [21]. A link between innate with adaptive immunity via maturing dendritic cells depends upon co-stimulation during the maturation process [22], which also affects the decision between either tolerance or autoimmunity. Tolerogenic DCs are iDCs with reduced allostimulatory capacities. We have demonstrated that DCs that engulf NIT-1 apoptotic bodies have reductions of co-stimulatory molecules that could explain, in part, the re-establishment of peripheral tolerance. This protective function of DCs may be due to antigenic presentation in a tolerogenic manner. In other studies, a decreased expression of co-stimulatory molecules in DCs was associated with a reduced incidence of diabetes [23] or delayed disease onset in NOD mice [24].
We have shown that these DCs are tolerogenic to the extent that they are resistant to CD40 and CD86 expression induction after LPS stimulation. However, the ability to secrete proinflammatory cytokines (IL-6 and TNF-α) is not affected, suggesting that these cells can take part in an anti-inflammatory response but, due to the lack of co-stimulatory signals, do not induce a complete antigen presentation process.
The mechanisms by which DCs pulsed with NIT-1 apoptotic cells suppress disease in the transgenic mouse model are under study. This immunotherapy may induce anergy/apoptosis in autoreactive T cells and stimulate regulatory T cell populations, avoiding the progression of leucocyte infiltration in the islets and the consequent destruction of β cells.
We are well aware that the prevention and cure of T1D in animal models is easier than in humans. Many experiments in recent years have shown significant receptiveness of the NOD mouse to interventions that prevent or reverse the disease [25]. For example, treatment of newly diabetic NOD mice with a monoclonal antibody to CD3 resulted in permanent reversal of the disease [26]. In fact, immunomodulation, in conjunction with Freund's adjuvant, arrests an active diabetic process in the mouse [27].
For clinical use, immunotherapy must be easy, low cost and provide a rational application. The advantages of the treatment presented here include: (i) treatment with autologous cells that would not require immunosuppressants; (ii) the feasibility to obtain tolerogenic DCs in the laboratory; and (iii) the possibility of several doses, if long-term tolerance is not achieved with a single course of DCs. Ideally, this immunotherapy, in combination with islet transplantation or stem cells for β cell regeneration, could re-establish peripheral tolerance in order to avoid T1D recurrence. In this sense, a possible source of human antigen-specific apoptotic bodies could be fresh islets from organ donors. However, the enzymatic digestion and the long process to purify pancreatic islets can be detrimental to the subsequent obtaining of apoptotic bodies. For that reason, a better source of β cell apoptotic bodies could, in future, be autologous β cells derived from adult stem cells [28].
Our study demonstrates the preventive effects of immunization with antigen-specific loaded DCs in the experimental model of T1D, suggesting that this form of therapy could be of paramount importance for the design of future treatments in human T1D.
Acknowledgments
We thank Marco Fernández (Germans Trias i Pujol Research Institute) for flow cytometry support. This work was supported by the Fondo de Investigaciones Sanitarias of the Spanish National Institute of Health (04/1686 and 06/0465). R. P. was supported by the Instituto de Salud Carlos III of the Spanish National Institute of Health (FIS05/00418). M. V. P. and F. E. B. are co-funded by the stabilization programme of biomedical researchers and R. A. by the programme of research technicians of the Instituto de Salud Carlos III and Direcció d'Estrategia i Coordinacio, Health Department of the Catalan Government.
Disclosure
None.
References
- 1.Bach JF, Chatenoud L. Tolerance to islet autoantigens in type 1 diabetes. Annu Rev Immunol. 2001;19:131–61. doi: 10.1146/annurev.immunol.19.1.131. [DOI] [PubMed] [Google Scholar]
- 2.Durant S, Alves V, Coulaud J, Homo-Delarche F. Nonobese diabetic (NOD) mouse dendritic cells stimulate insulin secretion by prediabetic islets. Autoimmunity. 2002;35:449–55. doi: 10.1080/0891693021000040575. [DOI] [PubMed] [Google Scholar]
- 3.Vasquez AC, Feili-Hariri M, Tan RJ, Morel PA. Qualitative and quantitative abnormalities in splenic dendritic cell populations in NOD mice. Clin Exp Immunol. 2004;135:209–18. doi: 10.1111/j.1365-2249.2003.02359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Clare-Salzler MJ, Brooks J, Chai A, Van Herle K, Anderson C. Prevention of diabetes in nonobese diabetic mice by dendritic cell transfer. J Clin Invest. 1992;90:741–8. doi: 10.1172/JCI115946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lutz MB, Suri RM, Niimi M, et al. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur J Immunol. 2000;30:1813–22. doi: 10.1002/1521-4141(200007)30:7<1813::AID-IMMU1813>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo J, Clare-Salzler MJ. Dendritic cell subsets and type 1 diabetes: focus upon DC-based therapy. Autoimmun Rev. 2006;5:419–23. doi: 10.1016/j.autrev.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–74. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 10.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–16. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alba A, Puertas MC, Carrillo J, et al. IFN beta accelerates autoimmune type 1 diabetes in nonobese diabetic mice and breaks the tolerance to beta cells in nondiabetes-prone mice. J Immunol. 2004;173:6667–75. doi: 10.4049/jimmunol.173.11.6667. [DOI] [PubMed] [Google Scholar]
- 12.Hamaguchi K, Gaskins HR, Leiter EH. NIT-1, a pancreatic beta-cell line established from a transgenic NOD/Lt mouse. Diabetes. 1991;40:842–9. doi: 10.2337/diab.40.7.842. [DOI] [PubMed] [Google Scholar]
- 13.Aaronson SA, Todaro G. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968;72:141–8. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- 14.Morelli AE, Larregina AT, Shufesky WJ, et al. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood. 2003;10:611–20. doi: 10.1182/blood-2002-06-1769. [DOI] [PubMed] [Google Scholar]
- 15.Strid J, Lopes L, Marcinkiewicz J Petrovska L, Nowak B, Chain BM, Lund T. A defect in bone marrow derived dendritic cell maturation in the nonobese diabetic mouse. Clin Exp Immunol. 2001;123:375–81. doi: 10.1046/j.1365-2249.2001.01473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feili-Hariri M, Dong X, Alber SM, Watkins SC, Salter RD, Morel PA. Immunotherapy of NOD mice with bone marrow-derived dendritic cells. Diabetes. 1999;48:2300–8. doi: 10.2337/diabetes.48.12.2300. [DOI] [PubMed] [Google Scholar]
- 17.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–89. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hugues S, Mougneau E, Ferlin W, et al. Tolerance to islet antigens and prevention from diabetes induced by limited apoptosis of pancreatic ß cells. Immunity. 2002;16:169–81. doi: 10.1016/s1074-7613(02)00273-x. [DOI] [PubMed] [Google Scholar]
- 19.Xia CQ, Peng R, Qiu Y, Annamalai M, Gordon D, Clare-Salzler MJ. Transfusion of apoptotic beta-cells induces immune tolerance to beta-cell antigens and prevents type 1 diabetes in NOD mice. Diabetes. 2007;56:2116–23. doi: 10.2337/db06-0825. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Shufesky WJ, Montecalvo A, Divito SJ, Larregina AT, Morelli AE. In situ-targeting of dendritic cells with donor-derived apoptotic cells restrains indirect allorecognition and ameliorates allograft vasculopathy. PLoS ONE. 2009;4:e4940. doi: 10.1371/journal.pone.0004940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohnmacht C, Pullner A, King SB, et al. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous autoimmunity. J Exp Med. 2009;206:549–59. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–18. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma L, Qian S, Liang X, et al. Prevention of diabetes in NOD mice by administration of dendritic cells deficient in nuclear transcription factor-kappaB activity. Diabetes. 2003;52:1976–85. doi: 10.2337/diabetes.52.8.1976. [DOI] [PubMed] [Google Scholar]
- 24.Machen J, Harnaha J, Lakomy R, Styche A, Trucco M, Giannoukakis N. Antisense oligonucleotides down-regulating costimulation confer diabetes-preventive properties to nonobese diabetic mouse dendritic cells. J Immunol. 2004;173:4331–41. doi: 10.4049/jimmunol.173.7.4331. [DOI] [PubMed] [Google Scholar]
- 25.Shoda LK, Young DL, Ramanujanm S, et al. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity. 2005;23:115–26. doi: 10.1016/j.immuni.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Chatenoud L, Primo J, Bach JF. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol. 1997;158:2947–54. [PubMed] [Google Scholar]
- 27.Ulaeto D, Lacy PE, Kipnis DM, Kanagawa O, Unanue ERA. T-cell dormant state in the autoimmune process of nonobese diabetic mice treated with complete Freund's adjuvant. Proc Natl Acad Sci USA. 1992;89:3927–31. doi: 10.1073/pnas.89.9.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vija L, Farge D, Gautier J, et al. Mesenchymal stem cells: stem cell therapy perspectives for type 1 diabetes. Diabetes Metab. 2009;35:85–93. doi: 10.1016/j.diabet.2008.10.003. [DOI] [PubMed] [Google Scholar]


