Abstract
Caffeic acid phenethyl ester (CAPE), an active component in propolis, is known to have anti-tumour, anti-inflammatory and anti-oxidant properties. In this study, the effects of CAPE on the functions of primary human CD4+ T cells were evaluated in vitro. CAPE significantly suppressed interferon (IFN)-γ and interleukin (IL)-5 production and proliferation of CD4+ T cells stimulated by soluble anti-CD3 and anti-CD28 monoclonal antibodies in both healthy subjects and asthmatic patients. CAPE inhibited nuclear factor (NF)-κB activation and protein kinase B (Akt) phosphorylation, but not p38 mitogen-activated protein kinase (MAPK) phosphorylation in T cells. CAPE also induced active caspase-3 expression in CD4+ T cells; CCR4+CD4+ T cells were more sensitive to CAPE induction than CXCR3+CD4+ T cells. Together, these results indicate that CAPE inhibits cytokine production and proliferation of T cells, which might be related to the NF-κB and Akt signalling pathways, and that CCR4+CD4+ T cells are more sensitive to CAPE inhibition. This study provides a new insight into the mechanisms of CAPE for immune regulation and a rationale for the use of propolis for the treatment of allergic disorders.
Keywords: Akt, caffeic acid phenethyl ester, caspase-3, CD4+ T cells, NF-κB
Introduction
Asthma, which is characterized by chronic airways inflammation, is increasing in prevalence worldwide. After immunoglobulin E (IgE) cross-links the high-affinity IgE receptor (FcεRI), mast cells are activated and degranulated, which leads to the release of mediators such as histamine, prostaglandins, cysteinyl leukotrienes, cytokines and chemokines. This results in immediate-phase allergic reactions [1] and the recruitment of inflammatory cells, including eosinophils, dendritic cells, macrophages and T lymphocytes [2]. Eosinophils release proinflammatory mediators leading to bronchoconstriction and mucus secretion [3].
Recruitment of T helper type 2 (Th2) lymphocytes results in the late phase of the inflammatory response [4]. Th2 cells release specific cytokines, including interleukin (IL)-4, IL-5, IL-9 and IL-13, which orchestrate eosinophilic inflammation, IgE production by B lymphocytes [5], maturation and activation of mast cells and basophils [6], mucus hypersecretion [1] and airway hyperresponsiveness (AHR) [7]. IL-4 and IL-13 are also important factors involved with airway dysfunction and remodelling characterized by bronchial wall thickening, smooth muscle hypertrophy and thickening of the basement membrane [8] in response to chronic allergen exposure [9]. Airway remodelling results in a progressive decline in lung function [10]. In human studies, administration of recombinant IL-4 resulted in AHR in asthmatic subjects [11], and targeting Th2 cells or their effector functions influences disease progression in allergic asthma [12]. Thus, T cells play important roles in the late phase of asthma, especially Th2 cells.
Propolis is a natural resinous product collected by honeybees that has been reported to be effective in patients with mild to moderate asthma when administered with oral theophylline [13]. Compared with a placebo-treated group, the number of nocturnal asthma attacks decreased significantly and lung function improved for a propolis treatment group. There were also significantly lower serum levels of tumour necrosis factor (TNF)-α, intercellular adhesion molecule (ICAM)-1, IL-6, IL-8, leukotrienes and prostaglandin E2 (PGE2) after treatment for 2 months. In a murine model of asthma, propolis extracts suppressed AHR in ovalbumin (OVA)-sensitized mice. The serum levels of OVA-specific IgE and IgG1 and OVA-induced splenocyte-secreted interferon (IFN)-γ, IL-6 and IL-10 in a propolis group were significantly lower than in a control group [14].
Among various components of propolis, caffeic acid phenethyl ester (CAPE) has been one of the most extensively studied. It has been reported to have anti-tumour [15,16], anti-inflammatory [17,18], anti-oxidant [19] and anti-angiogenic [20] properties. It may also suppress eicosanoid synthesis [17]. Our previous studies showed that CAPE significantly inhibited IL-12 p40, IL-12 p70 and IL-10 protein expressions in healthy human monocyte-derived dendritic cells (MoDCs) after stimulation with lipopolysaccharide (LPS) and IL-12 p40, IL-10 and IFN-γ-inducible protein (IP)-10 after stimulation with crude mite extract [21]. CAPE inhibited IL-10 and IP-10 significantly, but not IL-12 expression in allergic patients' MoDCs stimulated by crude mite extract. These results may have been related to the nuclear factor (NF)-κB signalling pathway, as CAPE inhibited inhibitor of κBα (IκBα) phosphorylation and NF-κB activation but not mitogen-activated protein kinase (MAPK) family phosphorylation in human MoDCs.
CAPE has been reported to be a potent inhibitor of mitogen-induced T cell proliferation, lymphokine production [22], NF-κB activation [23–25] and nuclear factor of activated T cells (NFAT) dephosphorylation and transcriptional activity [25] in T cells. Because our previous studies showed that CAPE inhibited MoDCs in both healthy subjects and asthmatic patients, irrespective of T helper type 1 (Th1)- or Th2-type cytokines and chemokines, we aimed to investigate any inhibitory effects of CAPE on the functions of primary human T cells in healthy subjects and asthmatic patients, and whether these inhibitory effects were different between Th1 and Th2 cells. This study aimed to establish regulatory roles for CAPE on the functions of T cells that might provide insights into its mechanisms of action and provide a rationale for propolis administration in the management of asthma and other allergic disorders.
Materials and methods
Human subjects
Healthy adult subjects and mite-sensitized asthmatic patients were enrolled for peripheral blood sampling. This study was approved by the Research Ethics Committee of the National Taiwan University Hospital and informed consent was obtained from all subjects.
Isolation and culture of primary human CD4+ T cells
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized whole blood by Ficoll-Hypaque (GE Healthcare, Buckinghamshire, UK) density gradient centrifugation (400 g for 30 min at room temperature). The mononuclear cell layer was washed twice with sterile Hanks' solution (Sigma-Aldrich, St Louis, MO, USA). CD4+ cells were purified by positive selection using anti-CD4+ conjugated magnetic microbeads by AutoMACS (magnetic activated cell sorting) according to the manufacturer's protocol (Miltenyi Biotec, Auburn, CA, USA).
CD4+ T cells were cultured in RPMI-1640 medium (SAFC Biosciences, Lenexa, KS, USA) containing 10% fetal calf serum (FCS), 2 mM l-glutamine, 100 U/ml penicillin, 100 U/ml streptomycin and 25 mM HEPES. Cells were stimulated with soluble anti-CD3 and anti-CD28 monoclonal antibodies (mAbs) (2 µg/ml; Immunotech, Miami, FL, USA) in the presence or absence of CAPE (10 µM; Sigma-Aldrich) in 96-well plates (Costar, Cambridge, MA, USA). CAPE was dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich); control cells received DMSO only (0·1% of culture medium, v/v). Cells were cultured in triplicate at a concentration of 106 cells/ml for 2 days (37°C/5% CO2). Tritiated thymidine (1 µCi/well; New England Nuclear, Boston, MA, USA) incorporation for 16 h was determined using a liquid scintillation counter. The doses and time-points chosen were according to the literature [21] and confirmed further in the pilot experiments (data not shown).
Isolation of naive CD4+ T cells and culture of polarized Th1 and Th2 cells
Naive CD4+ T cells from healthy adult PBMCs were isolated by negative selection using human naive CD4+ T cell biotin-cocktail antibodies and anti-biotin-conjugated magnetic microbeads by AutoMACS, according to the manufacturer's protocol (Miltenyi Biotec). The naive CD4+ T cells were cultured in 5% human AB serum of RPMI-1640 complete medium, as described above, with plate-bound anti-CD3 and anti-CD28 mAbs (1 µg/ml) for 1 day. The anti-IL-4 (100 ng/ml; BD Pharmingen, San Jose, CA, USA) and recombinant IL-12 (4 ng/ml; R&D Systems, Minneapolis, MN, USA) were then added along with recombinant IL-2 (10 ng/ml; R&D Systems) for 6 days for culturing polarized Th1 cells. The anti-IL-12 (10 µg/ml; BD Pharmingen) and recombinant IL-4 (200 ng/ml; R&D Systems) were added along with recombinant IL-2 (10 ng/ml) for 6 days for culturing polarized Th2 cells. Polarized cells were stimulated with soluble anti-CD3 and anti-CD28 mAbs (2 µg/ml) in the presence or absence of CAPE (10 µM) in 96-well plates in triplicate at a concentration of 106 cells/ml for 2 days (37°C/5% CO2). Tritiated thymidine (1 µCi/well; New England Nuclear) incorporation for 16 h was determined using a liquid scintillation counter.
Determination of cytokine levels
Concentrations of IFN-γ and IL-5 in the T cell culture supernatants were assayed using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems), according to the manufacturer's protocol.
Flow cytometry analysis
CD4+ T cells were treated with CAPE at the indicated concentrations for 48 h, and then harvested and washed with cold phosphate-buffered saline (PBS). Cells were stained with mAbs for annexin-V and 7-amino actinomycin D (7-AAD) (Becton Dickinson, San Jose, CA, USA) for 15 min at room temperature. Stained cells were analysed within 30 min using a fluorescence activated cell sorter (FACSort) cell analyser (Becton Dickinson). More than 1 × 104 cells were analysed for each sample and the data were processed using CellQuest software (Becton Dickinson). The concentration chosen (10 µM) for further studies was confirmed in pilot experiments (please see Fig. S1 in Supporting Information).
PBMCs were treated with CAPE at the indicated concentrations for 12 h, and then harvested and washed with cold buffer (PBS containing 2% FCS and 0·1% sodium azide). Cells were stained with mAbs for CD4, CXCR3 and CCR4 (Becton Dickinson) or isotype-matched controls for 30 min on ice. Cells were then fixed and permeabilized with Cytofix/Cytosper solution (BD Biosciences) and stained with anti-active caspase-3 antibody (Becton Dickinson), according to the manufacturer's protocol. Stained cells were washed and resuspended in cold buffer and analysed using a FACSort cell analyser (Becton Dickinson). More than 5 × 104 cells were analysed for each sample and the data were processed using CellQuest software (Becton Dickinson). The time-point chosen was confirmed in the pilot experiments (data not shown).
Western blotting
CD4+ T cells were pretreated with CAPE at the indicated concentrations for 2 h. Phorbol myristate acetate (PMA) (50 ng/ml; Sigma-Aldrich) and ionomycin (500 ng/ml; Sigma-Aldrich) were then added for either 10 min (37°C/5% CO2) for IκB kinase (IKK)α/IKKβ phosphorylation or 15 min for IκBα and P38 phosphorylation. Soluble anti-CD3 and anti-CD28 mAbs (2 µg/ml) were added for either 10 min (37°C/5% CO2) after CAPE pretreatment for protein kinase B (Akt) phosphorylation, 30 min for IKKα/IKKβ phosphorylation or 40 min for p38-MAPK phosphorylation, IκBα phosphorylation and degradation. Total cellular extracts were prepared using Gold lysis buffer [10% glycerol, 1% Triton X-100, 1 mM sodium orthovanadate, 1 mM ethylene glycol tetraacetic acid (EGTA), 5 mM ethylenediamine tetraacetic acid (EDTA), 10 mM sodium fluoride (NaF), 1 mM sodium pyrophosphate, 20 mM Tris-HCl, pH 7·9, 100 µM β-glycerophosphate, 137 mM NaCl, 1 mM phenylmethylsulphonyl fluoride (PMSF), 10 µg/ml aprotinin, and 10 µg/ml leupeptin] for 30 min at 4°C. Cell lysates were clarified by centrifugation at 12 000 g for 15 min at 4°C. Total protein (35 µg) was separated on 10% sodium dodecyl sulphate (SDS)-polyacrylamide minigels and transferred to Immobilon polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA). The membrane was incubated for 1 h at room temperature with 5% non-fat dry milk in PBS to block non-specific immunoglobulins, then incubated with anti-α-tubulin mAb (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-IKKα (Ser180)/IKKβ (Ser181)-P, anti-IκBα, anti-IκBα-P, anti-p38-P, anti-total p38, anti-Akt (Ser473)-P or anti-total Akt polyclonal antibodies (Cell Signaling Technology, Beverly, MA, USA). Immunodetection used the Amersham enhanced chemiluminescence plus system (GE Healthcare). The time-points chosen were confirmed in pilot experiments (data not shown). Quantification was conducted by the AlphaEase FC™ (AlphaImager 2200; Alpha Innotech, San Leandro, CA, USA). The intensity of the bands was quantitated to α-tubulin, total P38 or total Akt. The normalized intensity in the first lane was set as relative to expression level of 1.
Preparation of nuclear extracts and electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared using a NE-PER nuclear and cytoplasmic extraction reagent kit (Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer's protocol. Each nuclear extract (5 µg) was mixed with labelled, double-stranded NF-κB oligonucleotide, 5′-AGTTGAGGGGACTTTCCCAGGC-3′, and incubated at room temperature for 20 min. The incubation mixture included 1 µg of poly(dI-dC) in a binding buffer [25 mM HEPES, pH 7·9, 0·5 mM EDTA, 0·5 mM dithiothreitol (DTT), 1% Nonidet P-40, 5% glycerol, and 50 mM NaCl]. The DNA–protein complex was electrophoresed on 4·5% non-denaturing polyacrylamide gels in 0·5× Tris–borate–EDTA (TBE) buffer (0·0445 M Tris, 0·0445 M borate, 0·001 M EDTA). A double-stranded mutated oligonucleotide, 5′-AGTTGAGGCGACTTTCCCAGGC-3′, was used to examine the specificity of the binding of NF-κB to DNA. The specificity of binding was also examined by comparison with unlabelled oligonucleotide.
Statistics
Results are given as means ± standard error of the mean (s.e.m.). Experimental group comparisons used Student's t test. P-values < 0·05 were considered significant.
Results
CAPE inhibits cytokine production and proliferation of human CD4+ T cells in both healthy subjects and asthmatic patients
To evaluate the effects of CAPE on human CD4+ T cells, we detected the cytokine secretion (Th1 IFN-γ and Th2 IL-5) and lymphoproliferation of T cells. The IFN-γ and IL-5 levels in the supernatants of human CD4+ T cell cultures were analysed by ELISA (Fig. 1). CAPE inhibited IFN-γ (P = 0·0080) and IL-5 production (P = 0·0003) significantly after T cell stimulation with soluble anti-CD3 and anti-CD28 mAbs in healthy subjects, and inhibited IFN-γ (P = 0·0358) and IL-5 production (P = 0·0144) significantly in mite-sensitized asthmatic patients. The lymphoproliferative response (Fig. 2) was also inhibited for CAPE-treated CD4+ T cells stimulated by soluble anti-CD3 and anti-CD28 mAbs in healthy subjects and asthmatic patients (P = 0·0011 and 0·0316, respectively).
Fig. 1.
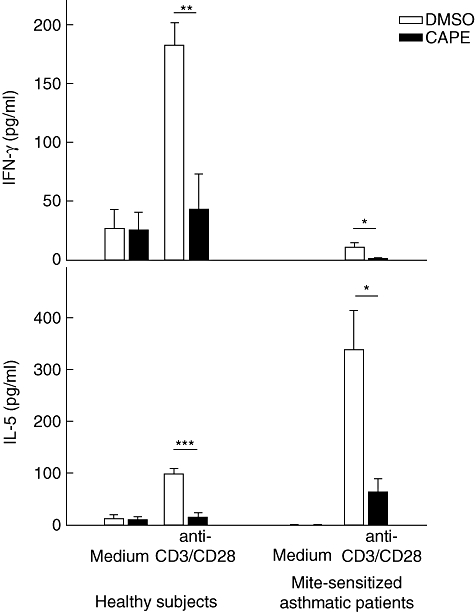
Caffeic acid phenethyl ester (CAPE) inhibits interferon (IFN)-γ and interleukin (IL)-5 production by human CD4+ T cells. CD4+ T cells (106 cells/ml) were cultured for 48 h in the absence or presence of CAPE (10 µM) with soluble anti-CD3 and anti-CD28 monoclonal antibodies (2 µg/ml) stimulation. Cytokine levels in the culture supernatants for six healthy subjects and four asthmatic patients were measured by enzyme-linked immunosorbent assay. *P < 0·05; **P < 0·01; ***P < 0·001.
Fig. 2.
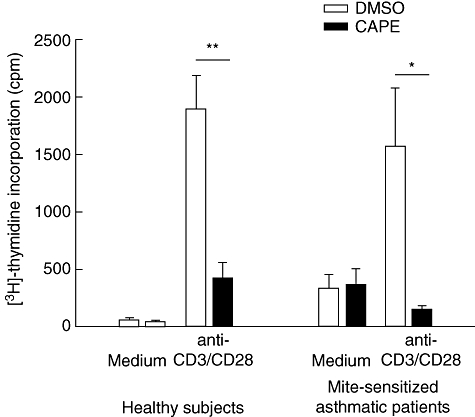
Caffeic acid phenethyl ester (CAPE) inhibits proliferation of human CD4+ T cells. CD4+ T cells (106 cells/ml) were cultured for 48 h in the absence or presence of CAPE (10 µM) with soluble anti-CD3 and anti-CD28 monoclonal antibodies (2 µg/ml) stimulation. Tritiated thymidine [3H] incorporation for six healthy subjects and four asthmatic patients was measured by liquid scintillation counter and expressed as mean counts per minute (cpm). *P < 0·05; **P < 0·01.
CAPE inhibits IKK complex and IκBα phosphorylation in human CD4+ T cells in both healthy subjects and asthmatic patients
CAPE has been reported to be a potent inhibitor of NF-κB. Thus, we investigated if CAPE could inhibit the NF-κB signalling pathway in CD4+ T cells. Primary human CD4+ T cells from healthy subjects were pretreated with CAPE at the indicated concentrations for 2 h, and then stimulated with PMA and ionomycin for either 10 min for IKK complex phosphorylation or 15 min for IκBα phosphorylation. CAPE inhibited cytosolic IKKα/IKKβ and IκBα phosphorylation in a dose-dependent manner (Fig. 3a). In case of different activation between PMA, ionomycin and anti-CD3, anti-CD28 mAbs, and to check the inihibitory effects of CAPE in asthmatic patients, primary human CD4+ T cells from patients pretreated as above were stimulated by soluble anti-CD3 and anti-CD28 mAbs for 30 min for IKK complex phosphorylation or for 40 min for IκBα phosphorylation and degradation. CAPE also inhibited cytosolic IKKα/IKKβ and IκBα phosphorylation and IκBα degradation in a dose-dependent manner (Fig. 3b).
Fig. 3.
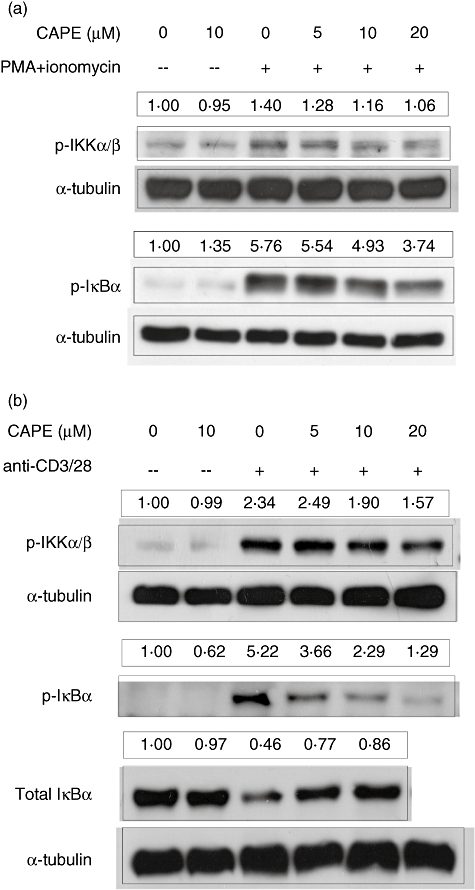
Caffeic acid phenethyl ester (CAPE) inhibits the phosphorylation of inhibitor of κB (IκB) kinase (IKK)α/IKKβ and IκBα. (a) Human CD4+ T cells from healthy subjects were pretreated with CAPE at the indicated concentrations for 2 h, and then stimulated with phorbol myristate acetate (PMA) (50 ng/ml) and ionomycin (500 ng/ml). Cell lysates were collected for the levels of phosphorylation of the IKK complex after 10 min or phosphorylation of IκBα after 15 min, which were assayed by Western blotting with anti-phosphorylated IKKα (ser180)/IKKβ (ser181) and anti-phosphorylated IκBα polyclonal antibodies. (b) Human CD4+ T cells from mite-sensitized asthmatic patients were pretreated with CAPE at the indicated concentrations for 2 h, and then stimulated with soluble anti-CD3 and anti-CD28 monoclonal antibodies (2 µg/ml). Cell lysates were collected for the levels of phosphorylation of the IKK complex after 30 min or phosphorylation and degradation of IκBα after 40 min. Anti-α-tubulin mAb was used as an internal control. The quantification results were normalized with the first lane set as relative expression level of 1. Representative results of one of at least three independent experiments are shown.
CAPE inhibits NF-κB activation in human CD4+ T cells
To monitor any inhibitory effects of CAPE on NF-κB translocation into the nucleus, CD4+ T cells from healthy subjects pretreated as described above were cultured with PMA and ionomycin for 45 min. Nuclear extracts were analysed for NF-κB DNA binding by EMSA. CAPE inhibited NF-κB translocation and activation in a dose-dependent manner (Fig. 4). The binding of NF-κB was specific and could be blocked competitively by unlabelled NF-κB oligonucleotide.
Fig. 4.
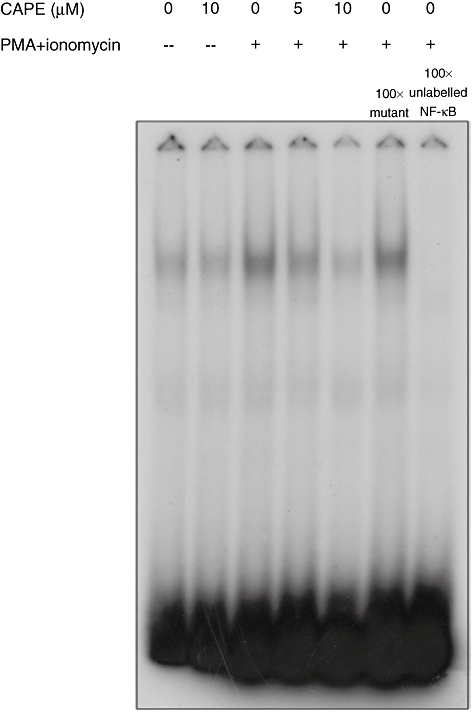
Caffeic acid phenethyl ester (CAPE) inhibits nuclear factor (NF)-κB activation. Human CD4+ T cells from healthy subjects were pretreated with CAPE at the indicated concentrations for 2 h, and then stimulated with phorbol myristate acetate (PMA) (50 ng/ml) and ionomycin (500 ng/ml) for 45 min. Nuclear fractions were analysed for NF-κB binding activity by electrophoretic mobility shift assay. To assess the specificity of the binding, 100-fold excess of cold NF-κB probe or a mutant probe was added to the PMA and ionomycin conditions. Representative results of one of at least three independent experiments are shown.
CAPE does not inhibit the phosphorylation of p38 MAPK in human CD4+ T cells
P38 MAPK is another inflammatory signalling pathway in which we were interested. The levels of p38 MAPK phosphorylation in CD4+ T cells treated as described above (PMA and ionomycin stimulation for 15 min) were analysed by Western blot analysis. CAPE did not inhibit the phosphorylation of p38 MAPK (Fig. 5a). Primary human CD4+ T cells from asthmatic patients pretreated as above were stimulated by soluble anti-CD3 and anti-CD28 mAbs for 40 min for p38 MAPK phosphorylation; CAPE did not inhibit the phosphorylation of p38 MAPK (Fig. 5a) either.
Fig. 5.
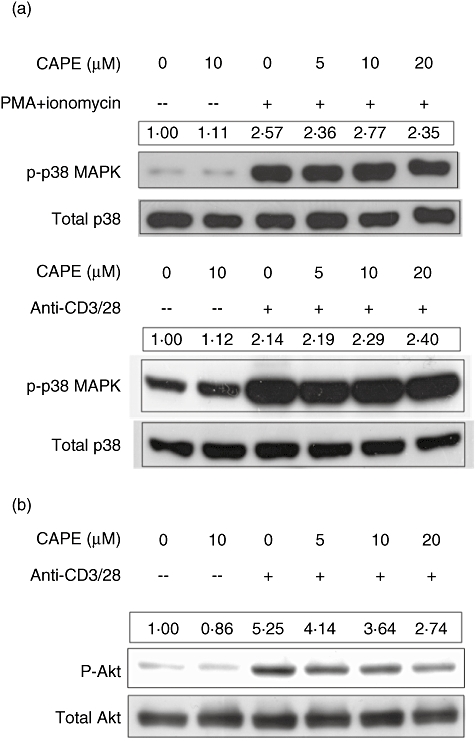
Caffeic acid phenethyl ester (CAPE) inhibits the phosphorylation of protein kinase B (Akt) but not p38 mitogen-activated protein kinase (MAPK). (a) Human CD4+ T cells from healthy subjects were pretreated with CAPE at the indicated concentration for 2 h, and then stimulated with phorbol myristate acetate (PMA) (50 ng/ml) and ionomycin (500 ng/ml) for 15 min. Human CD4+ T cells from mite-sensitized asthmatic patients were pretreated with CAPE at the indicated concentrations for 2 h, and then stimulated with soluble anti-CD3 and anti-CD28 monoclonal antibodies (2 µg/ml) for 40 min. Cell lysates were collected and the levels of phosphorylation of p38 were assayed by Western blotting with anti-phosphorylated p38 polyclonal antibody; anti-total p38 polyclonal antibody was used as an internal control. (b) Human CD4+ T cells from healthy subjects were pretreated with CAPE at the indicated concentrations for 2 h, and then stimulated with soluble anti-CD3 and anti-CD28 mAbs (2 µg/ml) for 10 min. Cell lysates were collected and the levels of phosphorylation of Akt were assayed by Western blotting with anti-phosphorylated Akt (Ser473) polyclonal antibody; anti-total Akt polyclonal antibody was used for an internal control. The quantification results were normalized with the first lane set as relative expression level of 1. Representative results of one of at least three independent experiments are shown.
CAPE inhibits Akt phosphorylation in human CD4+ T cells
To determine the effect of CAPE on the activation of the Akt pathway, which promotes cell proliferation and survival, CD4+ T cells pretreated with CAPE at the indicated concentrations for 2 h were stimulated with soluble anti-CD3 and anti-CD28 mAbs for 10 min. The levels of Akt phosphorylation were analysed by Western blot analysis. CAPE inhibited Akt phosphorylation in a dose-dependent manner (Fig. 5b).
CAPE induces the expression of active caspase-3 in human CD4+ T cells
Because CAPE could inhibit the cytokine production and proliferation of CD4+ T cells, we were interested in its effects on different types of CD4+ T cells. We used surface markers of CXCR3 and CCR4 representative of Th1-like and Th2-like cells. The expressions of active caspase-3 for CXCR3+CD4+ and CCR4+CD4+ T cells were analysed by intracellular staining and flow cytometry (Fig. 6). CAPE induced the expression of active caspase-3 in CCR4+CD4+ T cells significantly, as only 10 µM CAPE was required (P = 0·0127, 0·0025, 0·0003, 0·0002, respectively, in 10, 20, 50 and 100 µM compared to controls). However, CAPE could induce the expression of active caspase-3 in CXCR3+CD4+ T cells only at a much higher (50 µM) concentration (P = 0·0087, < 0·0001, respectively, in 50 and 100 µM). The active caspase-3 expressions in CCR4+CD4+ T cells were significantly higher than for CXCR3+CD4+ T cells at 10, 20 and 50 µM CAPE (P = 0·0080, 0·0024, 0·0366, respectively).
Fig. 6.
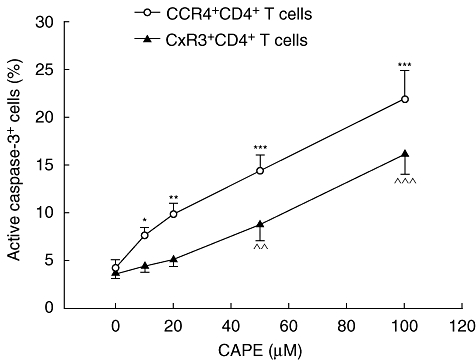
Caffeic acid phenethyl ester (CAPE) induces active caspase-3 expression in human CD4+ T cells. Peripheral blood mononuclear cells (PBMCs) (106 cells/ml) were cultured for 12 h at the indicated concentrations of CAPE (0–100 µM). Cells were stained with monoclonal antibodies for CD4, CCR4, CXCR3 and active caspase-3 and then analysed by flow cytometry. Results are means ± standard error of the mean of six separate experiments. *P < 0·05; **,^^P < 0·01; ***,^^^P < 0·001 compared to controls.
Discussion
The primary human CD4+ T cells play an important role in the pathogenesis of asthma and allergic disease, and the aim of this study was to elucidate the effects of CAPE on primary human CD4+ T cells in healthy subjects and asthmatic patients to determine whether CAPE is a promising candidate for treatment of asthma and allergic disease. First, cytokine production and proliferation were assessed using soluble anti-CD3 and anti-CD28 mAbs for stimulation to mimic complete T cell activation by antigen, which requires three signals: the T cell receptor (TCR)–CD3 complex, CD28–B7 interaction (accessory cell co-stimulation) and autocrine IL-2/IL-2 receptor interaction [26]. As expected, after soluble anti-CD3 and anti-CD28 stimulation, primary human CD4+ T cells produced large amounts of IFN-γ (a Th1 cytokine) and IL-5 (a Th2 cytokine) and had increased proliferation compared to non-stimulated T cells (medium only) (Figs 1 and 2). CAPE inhibited IFN-γ and IL-5 production and proliferation significantly in both healthy subjects and mite-sensitized asthmatic patients. Inhibition of cytokine production has also been noted for stimulated PBMCs (IL-2, IL-4, IL-12) [22] and stimulated T cells (IL-2) [25]. This inhibition was not excluded to either Th1 or Th2 cytokines, which we also observed in a previous study of the effects of CAPE on human MoDCs [21].
Secondly, we attempted to identify possible mechanisms for the inhibitory effects of CAPE on cytokine production and proliferation by analysing signalling pathways by Western blot. The first pathway of interest is the transcription factor NF-κB, which is crucial for numerous cellular processes such as inflammation, immune regulation, cell proliferation and apoptosis, and may be responsible for increased expressions of many inflammatory genes in asthma [27]. In the resting state, NF-κB is sequestered in the cytoplasm by its tight associations with inhibitory proteins, IκBs [28]. Upon cell stimulation, a phosphorylated IKK complex can phosphorylate IκBα, which is subsequently ubiquitinated and degraded via the proteasomic pathway. NF-κB is then released and translocates into the nucleus where it activates the transcription of target genes, such as for cytokines, chemokines, adhesion molecules and inhibitors of apoptosis [28,29].
CAPE is a potent inhibitor of NF-κB activation [23–25], although different inhibitory results of CAPE on the NF-κB signalling pathway have been noted. The direct inhibition of NF-κB nuclear translocation [23,25] and an indirect inhibition of IκBα phosphorylation [25] have been noted in T cells. However, CAPE inhibited IκB degradation in monocytic cells, but not astroglial cells, in which activation of IKK was suppressed [30]. NF-κB DNA binding was inhibited by CAPE, but not IκBα phosphorylation in receptor activator of nuclear factor κB ligand (RANKL)-stimulated bone marrow derived-macrophages (BMMs) [31]. It appears that for different cells and, possibly, different concentrations of CAPE (10–100 µM), different inhibitory mechanisms are involved.
Our previous study of MoDCs showed that CAPE inhibited IκBα phosphorylation, subsequent IκBα degradation and further NF-κB activation and nuclear translocation [21]. The present study showed that CAPE inhibited IKKα/IKKβ phosphorylation, IκBα phosphorylation and further NF-κB activation and nuclear translocation. The inhibitory effect was noted in both healthy subjects and asthmatic patients under PMA and ionomycin or soluble anti-CD3 and anti-CD28 mAbs stimulation. These inhibitory effects on the NF-κB signalling pathway could have resulted in the inhibition of cytokine production and proliferation.
Another possible pathway is p38 MAPK, which could enhance cytokine expressions for both Th1 and Th2 cells. For Th2 cells, activation of p38 MAPK is necessary for IL-10 and Th2 cytokine expressions, while for Th1 cells, p38 MAPK is involved with IL-12-induced IFN-γ production [32]. Our present study showed that CAPE could not inhibit p38 MAPK signalling under either PMA and ionomycin or soluble anti-CD3 and anti-CD28 mAbs stimulation. Thus, the inhibition of cytokine production in T cells was not related to p38 MAPK signalling. Similarly, CAPE did not affect MAPK signalling [including p38 MAPK, c-Jun N-terminal kinases (JNK) and extracellular signal-regulated kinases (ERK) phosphorylation] either in RANKL-stimulated BMMs [31] or LPS-stimulated MoDCs [21].
The other possible pathway is Akt, which is pivotal in promoting cell growth and survival and preventing apoptosis through numerous downstream effectors [33–36]. We found that CAPE inhibited Akt phosphorylation in a dose-dependent manner, which contributed partly to the inhibition of proliferation. The same inhibitory effect was also noted for transforming growth factor (TGF)-β-stimulated A549 cells [37], but CAPE could induce Akt phosphorylation in skeletal muscle cells [38], which was the contrary effect.
Chemokine receptors are expressed differentially on polarized Th cells [39]. In particular, CCR5 and CXCR3 predominate on Th1 cells, whereas CCR3, CCR4 and CCR8 are found primarily on Th2 cells [40,41]. The CCR4-specific ligands thymus- and activation-regulated chemokine (TARC or CCL17) and macrophage-derived chemokine (MDC or CCL22) are highly up-regulated on airway epithelial cells upon allergen challenge, which attracts CCR4+ T cells to the asthmatic bronchi [42,43], and antibodies directed against TARC and MDC have been effective in animal models of asthma [44].
Although in the present study the inhibitory effects of CAPE on cytokine production by T cells was not different between Th1 and Th2 cytokines, we were curious about possible different effects of CAPE on Th1 and Th2 cells, as Th2 cells play an important role in allergic disease. We used cell surface expressions of CXCR3 and CCR4 on CD4+ T cells to represent the Th1-like and Th2-like subsets. Although CCR4 is also expressed on regulatory T cells [45], there are far fewer regulatory T cells in the peripheral blood than Th2 cells.
We investigated active caspase-3 expression, a key component for apoptosis that is expressed highly in lymphocytes [46], by intracellular staining, as the activation/cleavage of caspase-3 has been noted for CAPE-treated U937 cells [47] and human leukaemic HL-60 cells [48]. We found that CCR4+CD4+ T cells expressed active caspase-3 in low concentrations of CAPE, which indicates that CCR4+CD4+ T cells were more vulnerable to CAPE-induced apoptosis than CXCR3+CD4+ T cells. Thus, CAPE could decrease the number of CCR4+CD4+ T cells, which would subsequently decrease further recruitment to the asthmatic airway and the continuation of inflammatory processes. Annexin-V and 7-AAD staining, which represents the unspecific apoptotic marker [49], showed that apoptosis of T cells was increased under a high concentration of CAPE (100 µM) but not the working concentration (10 µM), compared to the non-CAPE-treated T cells (Fig. S1). Also, the apoptosis of CAPE-treated T cells (10 µM) and non-CAPE-treated T cells were not significantly different with either the stimulation or not of anti-CD3/CD28 mAbs (Fig. S2). Therefore, the inhibitory effect of CAPE in cytokine production and proliferation of T cells in the present study was not mainly the consequence of apoptosis of T cells. However, annexin-V staining cannot be performed with the staining of surface markers, thus whether the apoptosis was noted more prominently in Th2 cells cannot be evaluated through annexin-V staining. In addition, CXCR3 and CCR4 expressions do not reflect Th1 and Th2 cells completely. We had cultured the naive CD4+ T cells with anti-IL-4 and IL-12 or anti-IL-12 and IL-4 for polarizing Th1 or Th2 cells. The cytokine production (Fig. S3) and lymphoproliferation (Fig. S4) under soluble anti-CD3 and anti-CD28 mAbs stimulation was inhibited by CAPE without preference of polarized Th1 or Th2 cells. The active caspase-3 expression (Fig. S5) was higher in polarized Th2 cells than in polarized Th1 cells, which supported our speculation. However, the cultured polarized Th1 and Th2 cells in vitro may not represent the tissue Th1 and Th2 cells in vivo. Therefore, additional methods to isolate Th1 and Th2 cells for cytokine, lymphoproliferation, active caspase-3 and annexin-V staining will be necessary for future studies.
In conclusion, our results show that CAPE inhibits IFN-γ and IL-5 secretion and proliferation significantly by primary human CD4+ T cells from healthy subjects and mite-sensitized asthmatic patients. The mechanism for inhibition is thought to be related to NF-κB and Akt signalling (Fig. 7), but not the p38 MAPK pathway. In addition, CCR4+CD4+ T cells were more sensitive than CXCR3+CD4+ T cells to CAPE-induced active caspase-3 expression at low concentrations of CAPE. Although the inhibitory effects of CAPE on the NF-κB signalling pathway in T cells were reported in the literature, the study of CAPE on primary human CD4+ T cells from asthmatic patients has not been reported previously. In addition, the effects of CAPE on p38 MAPK and Akt pathways and active caspase-3 expression have not been reported previously in T cells. The findings of this study provide new information and evidence for the inhibitory effects of CAPE on human CD4+ T cells in both healthy subjects and asthmatic patients, which are relevant to the pathogenesis of allergic airway diseases, including asthma and allergic rhinitis. Further, it is possible to suggest that CAPE and propolis might be useful for the management of allergic disorders, because CAPE inhibited cytokine production and proliferation in T cells and induced the apoptosis of CCR4+CD4+ T cells, which would result in decreased downstream inflammatory processes.
Fig. 7.
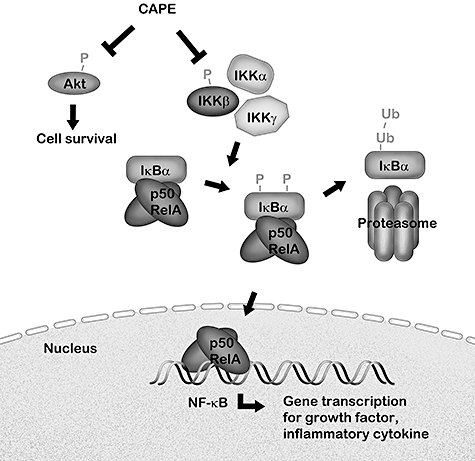
The schematic model of inhibitory effects of CAPE. CAPE inhibits κB (IκB) kinase (IKKα)/IKKβ phosphorylation, then IκBα phosphorylation, degradation and further nuclear factor (NF)-κB activation and nuclear translocation, which enhances the transcription of inflammatory cytokines, chemokines, growth factor, adhesion molecules and inhibitors of apoptosis. CAPE also inhibits protein kinase B (Akt) phosphorylation, which promotes cell growth and survival. The inhibitory effect of CAPE results in the inhibition of cytokine production and proliferation in primary human CD4+ T cells.
Acknowledgments
This study was supported by grants from the National Science Council, Republic of China (NSC 96-2314-B-002-047). This study was supported in part by the Department of Medical Research in NTUH.
Disclosure
All authors declare that they have no competing financial or other interest in relation to their work.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. The apoptotic effect of caffeic acid phenethyl ester (CAPE) on human CD4+ T cells. CD4+ T cells (106 cells/ml) were cultured for 48 h in the indicated concentrations of CAPE (0–100 μM). The percentage of annexin-V+ and 7-amino actinomycin D (7-AAD)+ cells were measured by flow cytometry.
Fig. S2. The apoptotic effect of caffeic acid phenethyl ester (CAPE) on stimulated human CD4+ T cells. CD4+ T cells (106 cells/ml) were cultured for 48 h in the absence or presence of CAPE (10 μM) with soluble anti-CD3 and anti-CD28 monoclonal antibodies (2 μg/ml) stimulation. The percentage of annexin-V+ and 7-amino actinomycin D (7-AAD)+ cells from four asthmatic patients were measured by flow cytometry.
Fig. S3. Caffeic acid phenethyl ester (CAPE) inhibits interferon (IFN)-γ and interleukin (IL)-5 production by polarized T helper type 1 (Th1) and Th2 cells. Polarized helper T cells (106 cells/ml) were cultured for 48 h in the absence or presence of CAPE (10 μM) with soluble anti-CD3 and anti-CD28 monoclonal antibodies (2 μg/ml) stimulation. Cytokine levels in the culture supernatants were measured by enzyme-linked immunosorbent assay. **P < 0·01.
Fig. S4. Caffeic acid phenethyl ester (CAPE) inhibits proliferation of polarized T helper type 1 (Th) and Th2 cells. Polarized helper T cells (106 cells/ml) were cultured for 48 h in the absence or presence of CAPE (10 μM) with soluble anti-CD3 and anti-CD28 monoclonal antibodies (2 μg/ml) stimulation. Tritiated thymidine [3H] incorporation was measured by liquid scintillation counter and expressed as mean counts per minute (cpm). **P < 0·01; ***P < 0·001.
Fig. S5. Caffeic acid phenethyl ester (CAPE) induces active caspase-3 expression in polarized T helper type 1 (Th1) and Th2 cells. Polarized helper T cells (106 cells/ml) were cultured for 12 h at the indicated concentrations of CAPE (0–100 μM). Cells were stained with monoclonal antibodies for CD4 and active caspase-3 and then analysed by flow cytometry. Results are means ± standard error of the mean of two separate experiments. *P < 0·05; **P < 0·01 compared to controls.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Larche M. Immunoregulation by targeting T cells in the treatment of allergy and asthma. Curr Opin Immunol. 2006;18:745–50. doi: 10.1016/j.coi.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Williams CM, Galli SJ. The diverse potential effector and immunoregulatory roles of mast cells in allergic disease. J Allergy Clin Immunol. 2000;105:847–59. doi: 10.1067/mai.2000.106485. [DOI] [PubMed] [Google Scholar]
- 3.Gleich GJ, Flavahan NA, Fujisawa T, Vanhoutte PM. The eosinophil as a mediator of damage to respiratory epithelium: a model for bronchial hyperreactivity. J Allergy Clin Immunol. 1988;81:776–81. doi: 10.1016/0091-6749(88)90931-1. [DOI] [PubMed] [Google Scholar]
- 4.Bochner BS, Busse WW. Allergy and asthma. J Allergy Clin Immunol. 2005;115:953–9. doi: 10.1016/j.jaci.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 5.Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol. 2003;111:450–63. doi: 10.1067/mai.2003.169. [DOI] [PubMed] [Google Scholar]
- 6.Kay AB. The role of T lymphocytes in asthma. Chem Immunol Allergy. 2006;91:59–75. doi: 10.1159/000090230. [DOI] [PubMed] [Google Scholar]
- 7.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 8.Roche WR, Beasley R, Williams JH, Holgate ST. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989;1:520–4. doi: 10.1016/s0140-6736(89)90067-6. [DOI] [PubMed] [Google Scholar]
- 9.Leigh R, Ellis R, Wattie JN, et al. Type 2 cytokines in the pathogenesis of sustained airway dysfunction and airway remodeling in mice. Am J Respir Crit Care Med. 2004;169:860–7. doi: 10.1164/rccm.200305-706OC. [DOI] [PubMed] [Google Scholar]
- 10.Haahtela T, Jarvinen M, Kava T, et al. Effects of reducing or discontinuing inhaled budesonide in patients with mild asthma. N Engl J Med. 1994;331:700–5. doi: 10.1056/NEJM199409153311103. [DOI] [PubMed] [Google Scholar]
- 11.Shi HZ, Deng JM, Xu H, et al. Effect of inhaled interleukin-4 on airway hyperreactivity in asthmatics. Am J Respir Crit Care Med. 1998;157:1818–21. doi: 10.1164/ajrccm.157.6.9710023. [DOI] [PubMed] [Google Scholar]
- 12.Epstein MM. Targeting memory Th2 cells for the treatment of allergic asthma. Pharmacol Ther. 2006;109:107–36. doi: 10.1016/j.pharmthera.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Khayyal MT, el-Ghazaly MA, el-Khatib AS, et al. A clinical pharmacological study of the potential beneficial effects of a propolis food product as an adjuvant in asthmatic patients. Fundam Clin Pharmacol. 2003;17:93–102. doi: 10.1046/j.1472-8206.2003.00117.x. [DOI] [PubMed] [Google Scholar]
- 14.Sy LB, Wu YL, Chiang BL, Wang YH, Wu WM. Propolis extracts exhibit an immunoregulatory activity in an OVA-sensitized airway inflammatory animal model. Int Immunopharmacol. 2006;6:1053–60. doi: 10.1016/j.intimp.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Chiao C, Carothers AM, Grunberger D, Solomon G, Preston GA, Barrett JC. Apoptosis and altered redox state induced by caffeic acid phenethyl ester (CAPE) in transformed rat fibroblast cells. Cancer Res. 1995;55:3576–83. [PubMed] [Google Scholar]
- 16.Huang MT, Ma W, Yen P, et al. Inhibitory effects of caffeic acid phenethyl ester (CAPE) on 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion in mouse skin and the synthesis of DNA, RNA and protein in HeLa cells. Carcinogenesis. 1996;17:761–5. doi: 10.1093/carcin/17.4.761. [DOI] [PubMed] [Google Scholar]
- 17.Mirzoeva OK, Calder PC. The effect of propolis and its components on eicosanoid production during the inflammatory response. Prostaglandins Leukot Essent Fatty Acids. 1996;55:441–9. doi: 10.1016/s0952-3278(96)90129-5. [DOI] [PubMed] [Google Scholar]
- 18.Michaluart P, Masferrer JL, Carothers AM, et al. Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Res. 1999;59:2347–52. [PubMed] [Google Scholar]
- 19.Ahn MR, Kumazawa S, Hamasaka T, Bang KS, Nakayama T. Antioxidant activity and constituents of propolis collected in various areas of Korea. J Agric Food Chem. 2004;52:7286–92. doi: 10.1021/jf048726s. [DOI] [PubMed] [Google Scholar]
- 20.Liao HF, Chen YY, Liu JJ, et al. Inhibitory effect of caffeic acid phenethyl ester on angiogenesis, tumor invasion, and metastasis. J Agric Food Chem. 2003;51:7907–12. doi: 10.1021/jf034729d. [DOI] [PubMed] [Google Scholar]
- 21.Wang LC, Lin YL, Liang YC, et al. The effect of caffeic acid phenethyl ester on the functions of human monocyte-derived dendritic cells. BMC Immunol. 2009;10:39. doi: 10.1186/1471-2172-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ansorge S, Reinhold D, Lendeckel U. Propolis and some of its constituents down-regulate DNA synthesis and inflammatory cytokine production but induce TGF-beta1 production of human immune cells. Z Naturforsch [C] 2003;58:580–9. doi: 10.1515/znc-2003-7-823. [DOI] [PubMed] [Google Scholar]
- 23.Natarajan K, Singh S, Burke TR, Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci USA. 1996;93:9090–5. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orban Z, Mitsiades N, Burke TR, Jr, Tsokos M, Chrousos GP. Caffeic acid phenethyl ester induces leukocyte apoptosis, modulates nuclear factor-kappa B and suppresses acute inflammation. Neuroimmunomodulation. 2000;7:99–105. doi: 10.1159/000026427. [DOI] [PubMed] [Google Scholar]
- 25.Marquez N, Sancho R, Macho A, Calzado MA, Fiebich BL, Munoz E. Caffeic acid phenethyl ester inhibits T-cell activation by targeting both nuclear factor of activated T-cells and NF-kappaB transcription factors. J Pharmacol Exp Ther. 2004;308:993–1001. doi: 10.1124/jpet.103.060673. [DOI] [PubMed] [Google Scholar]
- 26.Crabtree GR, Clipstone NA. Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu Rev Biochem. 1994;63:1045–83. doi: 10.1146/annurev.bi.63.070194.005145. [DOI] [PubMed] [Google Scholar]
- 27.Hart LA, Krishnan VL, Adcock IM, Barnes PJ, Chung KF. Activation and localization of transcription factor, nuclear factor-kappaB, in asthma. Am J Respir Crit Care Med. 1998;158:1585–92. doi: 10.1164/ajrccm.158.5.9706116. [DOI] [PubMed] [Google Scholar]
- 28.Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–8. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Choi K, Choi C. Differential regulation of c-Jun N-terminal kinase and NF-kappaB pathway by caffeic acid phenethyl ester in astroglial and monocytic cells. J Neurochem. 2008;105:557–64. doi: 10.1111/j.1471-4159.2007.05193.x. [DOI] [PubMed] [Google Scholar]
- 31.Ha J, Choi HS, Lee Y, Lee ZH, Kim HH. Caffeic acid phenethyl ester inhibits osteoclastogenesis by suppressing NF kappaB and downregulating NFATc1 and c-Fos. Int Immunopharmacol. 2009;9:774–80. doi: 10.1016/j.intimp.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Dodeller F, Schulze-Koops H. The p38 mitogen-activated protein kinase signaling cascade in CD4 T cells. Arthritis Res Ther. 2006;8:205. doi: 10.1186/ar1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan CB, Ye K. PIKE GTPase are phosphoinositide-3-kinase enhancers, suppressing programmed cell death. J Cell Mol Med. 2007;11:39–53. doi: 10.1111/j.1582-4934.2007.00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 35.Brennan P, Babbage JW, Burgering BM, Groner B, Reif K, Cantrell DA. Phosphatidylinositol 3-kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity. 1997;7:679–89. doi: 10.1016/s1074-7613(00)80388-x. [DOI] [PubMed] [Google Scholar]
- 36.Burgering BM, Kops GJ. Cell cycle and death control: long live Forkheads. Trends Biochem Sci. 2002;27:352–60. doi: 10.1016/s0968-0004(02)02113-8. [DOI] [PubMed] [Google Scholar]
- 37.Shigeoka Y, Igishi T, Matsumoto S, et al. Sulindac sulfide and caffeic acid phenethyl ester suppress the motility of lung adenocarcinoma cells promoted by transforming growth factor-beta through Akt inhibition. J Cancer Res Clin Oncol. 2004;130:146–52. doi: 10.1007/s00432-003-0520-0. [DOI] [PubMed] [Google Scholar]
- 38.Lee ES, Lee JO, Lee SK, et al. Caffeic acid phenethyl ester accumulates beta-catenin through GSK-3beta and participates in proliferation through mTOR in C2C12 cells. Life Sci. 2009;84:755–9. doi: 10.1016/j.lfs.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 40.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–83. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viola A, Contento RL, Molon B. T cells and their partners: the chemokine dating agency. Trends Immunol. 2006;27:421–7. doi: 10.1016/j.it.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Panina-Bordignon P, Papi A, Mariani M, et al. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J Clin Invest. 2001;107:1357–64. doi: 10.1172/JCI12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kallinich T, Schmidt S, Hamelmann E, et al. Chemokine-receptor expression on T cells in lung compartments of challenged asthmatic patients. Clin Exp Allergy. 2005;35:26–33. doi: 10.1111/j.1365-2222.2004.02132.x. [DOI] [PubMed] [Google Scholar]
- 44.Medina-Tato DA, Watson ML, Ward SG. Leukocyte navigation mechanisms as targets in airway diseases. Drug Discov Today. 2006;11:866–79. doi: 10.1016/j.drudis.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Iellem A, Mariani M, Lang R, et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194:847–53. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin UH, Song KH, Motomura M, et al. Caffeic acid phenethyl ester induces mitochondria-mediated apoptosis in human myeloid leukemia U937 cells. Mol Cell Biochem. 2008;310:43–8. doi: 10.1007/s11010-007-9663-7. [DOI] [PubMed] [Google Scholar]
- 48.Chen YJ, Shiao MS, Hsu ML, Tsai TH, Wang SY. Effect of caffeic acid phenethyl ester, an antioxidant from propolis, on inducing apoptosis in human leukemic HL-60 cells. J Agric Food Chem. 2001;49:5615–19. doi: 10.1021/jf0107252. [DOI] [PubMed] [Google Scholar]
- 49.Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331–71. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


