Abstract
Plasmodium falciparum infection causes transient immunosuppression during the parasitaemic stage. However, the immune response during simultaneous infections with both P. vivax and P. falciparum has been investigated rarely. In particular, it is not clear whether the host's immune response to malaria will be different when infected with a single or mixed malaria species. Phenotypes of T cells from mixed P. vivax–P. falciparum (PV-PF) infection were characterized by flow cytometry, and anti-malarial antibodies in the plasma were determined by an enzyme-linked immunosorbent assay. We found the percentage of CD3+δ2+-T cell receptor (TCR) T cells in the acute-mixed PV-PF infection and single P. vivax infection three times higher than in the single P. falciparum infection. This implied that P. vivax might lead to the host immune response to the production of effector T killer cells. During the parasitaemic stage, the mixed PV-PF infection had the highest number of plasma antibodies against both P. vivax and P. falciparum. Interestingly, plasma from the group of single P. vivax or P. falciparum malaria infections had both anti-P. vivax and anti-P. falciparum antibodies. In addition, antigenic cross-reactivity of P. vivax or P. falciparum resulting in antibodies against both malaria species was shown in the supernatant of lymphocyte cultures cross-stimulated with either antigen of P. vivax or P. falciparum. The role of δ2 ± TCR T cells and the antibodies against both species during acute mixed malaria infection could have an impact on the immunity to malaria infection.
Keywords: antibody response, CD4+ T cells, CD8+ T cells, gamma delta T cells, infections
Introduction
Mixed malaria infection is common and has been reported in many parts of the world where malaria is endemic [1–5]. In regions with low malaria endemicity regions, especially in Thailand, mixed infection with Plasmodium falciparum (PF) and P. vivax (PV) is common. These co-infections can be either simultaneous or sequential. Previous studies have shown that mixed PV-PF malaria infection is less severe than the single P. falciparum infection in terms of lower frequency of anaemia, treatment failure and clinical outcomes for the patients [6]. Moreover, mixed PV-PF malaria infection is approximately a quarter as severe as single P. falciparum infection [7]. It is conceivable that interaction between the host's immunity and the two malaria species may take place during acute infection.
The immune mechanism plays an important role in resisting malaria and other infectious diseases [8]. Immunity to malaria induced by different plasmodia species may give various outcomes. Immunity to P. falciparum is still controversial. Previous study has shown immunosuppression in acute P. falciparum leading to a lower absolute number of CD3+ T cells [9], although the overall percentages of CD4+ and CD8+ T cells are not changed [9–11]. On the other hand, during acute P. vivax infection, the percentage of CD4+ but not CD8+ T cells is elevated, whereas the number of antibodies against this parasite is low [12].
γδT cells play a role in linking the innate and adaptive immunities against the broad range of parasites [13–15]. These γδT cells recognize non-peptide phosphoantigens of the microbes leading to the release of cytokines such as tumour necrosis factor (TNF)-α and interferon (IFN)-γ, and therefore exert the effector function, i.e. cytotoxicity and natural killing [16–18]. Generally, CD3+δ2+T cells predominate in the peripheral blood in response to many infectious agents such as Mycobacterium spp. [19], Pseudomonas aeroginosa and Escherichia coli[14]. In cases of P. vivax malaria, the elevation of CD3+δ2+ T cells is observed in peripheral blood but not in P. falciparum malaria infection [12]. The natural immune response against malaria in hosts during acute mixed PV-PF malaria infection has been investigated rarely. So far, only one study from Ethiopia has shown that γδT cells are increased in mixed PV-PF malaria infection and single P. falciparum infection, but not in P. vivax infection [10]. However, successful immunity to malaria required both cell-mediated and humoral immune responses. Therefore, in this study, we characterized the natural immune response during acute mixed PV-PF malaria infection in patients who live in areas of Thailand where malaria is endemic. Understanding both cell-mediated and humoral responses may disclose the roles of the host's immunity to the two malaria species.
Materials and methods
Sample collection
Blood samples were collected in 20 µl of heparin from 17 acutely mixed PV-PF malaria-infected individuals, 63 P. vivax- and 63 P. falciparum-infected individuals at malaria clinics in Mae Sot, Tak province, Thailand; 50 malaria naive volunteers from non-malaria endemic areas were recruited as naive controls. Diagnosis of malaria infection was based on the examination of Giemsa-stained thick and thin blood films. Recruitment criteria were: age ≥ 15 years; body temperature ≤ 40°C; systolic blood pressure ≥ 90 mm; haematocrit ≥ 25%. Clinical data are shown in Table 1. This study was approved by the Committee on Human Rights Related to Human Experimentation, Mahidol University, Bangkok. Informed consent was obtained from each individual before blood samples were taken.
Table 1.
Information and clinical data* of mixed Plasmodium vivax–P. falciparum (PV-PF), single P. vivax, single P. falciparum infections and naive controls
| Naive controls | Mixed species | P. vivax | P. falciparum | |
|---|---|---|---|---|
| No. of patients | 50 | 17 | 63 | 63 |
| Age (year)* | 39 ± 5·1 | 27 ± 9 | 31 ± 13 | 28 ± 8·2 |
| Male | 45 | 14 | 51 | 54 |
| Female | 5 | 3 | 12 | 9 |
| Temperature (°C)* | 36·5 ± 0·5 | 38·5 ± 0·8 | 37·3 ± 0·8 | 38 ± 1·3 |
| Parasitaemia (%)* | 0 | 1·8 ± 2† | 0·2 ± 0·2 | 1·9 ± 2·1 |
| 0·07 ± 0·1‡ | ||||
| Haematocrit (%)* | 42 ± 5·3 | 37·6 ± 7·3 | 40 ± 4·2 | 41 ± 8·5 |
Mean ± standard deviation.
P. falciparum;
P. vivax.
Preparation of peripheral blood mononuclear cells (PBMCs)
PBMCs were separated from the collected blood by gradient centrifugation at 800 g for 20 min using Lymphoprep™ (AXIS-Shield PoC AS, Oslo, Norway). PBMCs were washed twice with RPMI-1640 by centrifugation at 800 g for 10 min and resuspended in RPMI-1640 containing 10% fetal calf serum (FCS). The viability of the PBMCs was determined by trypan blue exclusion dye. PBMCs (107 cells/ml) diluted in Cell banker® (Nihon Zenuaku Kohgyo, Japan) were stored in liquid nitrogen until further analysis.
Antigen preparation
White blood cells were depleted from P. vivax-infected blood by filtering through a Plasmodiper® (Whatman, Maidstone, UK). The red blood cells were washed twice with RPMI-1640 by centrifugation at 1190 g for 5 min. The parasites were cultured at 5% haematocrit in McCoy's medium (Gibco, Carlsbad, CA. USA) supplemented with 25% human antibody serum for 24–30 h in 5% CO2 until a mature schizont of P. vivax appeared [20]. P. falciparum culture was performed as described previously [21] in RPMI-1640 medium supplemented with 10% human serum until a mature schizont of P. falciparum appeared. P. vivax and P. falciparum parasites were separated by centrifugation on 60% Percoll®. The infected red blood cell (iRBCs) pellets were pulsed for 40 s on ice at 150 watts and stored at –70°C to be used in a lymphocyte stimulation assay and enzyme-linked immunosorbent assay (ELISA). The protein concentration of the P. vivax schizont extract (PvSE), and P. falciparum schizont extract (PfSE) was determined by a Bradford assay (Bio-Rad, Hercules, CA, USA). Uninfected red blood cells (uRBC) were processed similarly and used as control protein.
Antigenic cross-stimulation of lymphocytes
To investigate the antigenic cross-reactivity between P. vivax and P. falciparum parasites. PBMCs from a single P. vivax or P. falciparum infection were stimulated with PfSE and PvSE. The PBMCs were cultured at (2 × 105 cells/100 µl/well) in a HEPES-buffer RPMI-1640 supplemented with 10% FCS. The PvSE, PfSE and uRBC at 10 µg/100 µl and 100 µg/100 µl were added to each well. The cell quality control of the PBMCs was tested with a medium containing 10 µg/100 µl of phytohaemagglutinin (PHA) and incubated at 37°C in 5% CO2 for 5 days. The culture supernatant was stored at –20°C for further investigation of cross-reactivity.
Flow cytometric analysis
Phenotyping of T cells was performed by three-colour flow cytometry (FACScan; Becton Dickinson, Oxford, UK). PBMCs (105 cells) were stained with the following three-colour combinations of fluorescent dye conjugated with antibodies that were specific to T cell surface markers: fluorescein isothiocyanate (FITC)-conjugated antibody to CD4, R-phycoerythrin (R-PE)-conjugated antibody to CD3 and R-PE-cyanine 5 (R-PE-Cy5) conjugated antibody to CD8; and FITC-conjugated antibody to δ2+ TCR T cells and R-PE antibody to CD3 (Caltag, Burlingame, CA, USA) for 30 min at 4°C. After staining and washing, the cells were fixed with 1% paraformaldehyde in PBS pH 7·4 and analysed using CellQuest software (Becton Dickinson, San Jose, CA, USA).
Determination of antibodies against parasite antigen of P. vivax and P. falciparum antigen
Two methods were used to determine anti-malarial antibodies.
Enzyme-linked immunosorbent assay
Fifty ml of 10 µg/ml (PBS, pH 7·4) PvSE and PfSE were incubated overnight at 4°C in a 96-well polystyrene immunoplate (Corning, NY, USA). The immunoplate was blocked with 100 µl/well of blocking buffer (0·5% boiled casein in PBS and 0·05% Tween 20) for 2 h at room temperature. After three washings, 50 µl of serum (1 : 100 dilution in PBS pH 7·4) were added into duplicate wells and incubated overnight at 4°C. After washing with 0·05% Tween 20 in PBS, pH 7·4, 50 µl of horseradish peroxidase-conjugated goat anti-human immunoglobulin G (IgG) (Caltag) and 50 µl of 2,2′-azino-di-(3-ethylbenzthiaoline sulphonic acid) containing 50% hydrogen peroxide (Kirkepaard & Perry Laboratories, Gaithersburg, MD, USA) were added sequentially to each well and incubated for 1 h at room temperature. Enzyme activity was measured by microplate reader, Wallac Victor (Perkin Elmer, Jügesheim, Germany) at 405 nm. The levels of anti-P. falciparum and anti-P. vivax antibodies were expressed as a ratio increase in a median optical density (OD) compared to naive controls.
Immunofluorescence assay (IFA)
The pellets of infected red blood cells by P. vivax and P. falciparum parasites were spotted, dried and fixed on the multi-well slides. The cultured supernatant from PvSE-stimulated lymphocytes and PfSE-stimulated lymphocytes was added to the multi-well slides and incubated in a humidified box at room temperature for 1·5 h. The slides were then washed three times for 5 min. The multi-well slides were then incubated with goat anti-human IgG conjugated to FITC (Serotec, Oxford, UK). The slides were washed three times and mounted with a coverslip using 50% glycerol in PBS and then analysed with a fluorescence microscope.
Statistical analysis
The phenotypes of lymphocyte were analysed using the spss program (version 11·5; SPSS Inc., Chicago, IL, USA). To compare the phenotype of T cells in different groups, these data were log-transformed in order to obtain a normal distribution. Statistical significance was determined by one-way analysis of variance (anova). Non-parametric analysis (two independent samples) and Mann–Whitney U-test were used to determine the significance levels of anti-P. falciparum and anti-P. vivax antibodies. The results were considered significant at P < 0·05.
Results
Phenotypes of T cells
Flow cytometry was used to identify subsets of T cells, including CD4+, CD8+ and CD3+δ2+ (Fig. 1). The median percentage of CD3+δ2+ T cells was significantly higher in acute mixed PV-PF malaria infection (7·4%) compared with acute P. falciparum infection (2·3%, P < 0·01) and naive controls (1·8%, P < 0·03). There was no difference in CD3+δ2+T cells between acute P. vivax and the mixed malaria infections (7·4% versus 5·9%, P > 0·05). The median percentage of CD4+ and CD8+ T cells in the mixed malaria infection (36·7% and 24·6%, respectively) was similar to that of the single P. vivax (29·2% and 25·5%, P > 0·05) and P. falciparum infection (44·8% and 23·5%, P > 0·05) and these levels were not different from the naive controls (44·7% and 24·0%, P > 0·05).
Fig. 1.
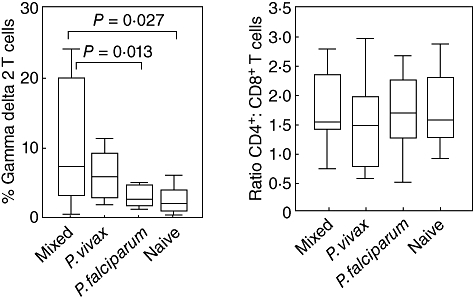
Phenotyping of T cells in mixed Plasmodium vivax–P. falciparum (PV-PF), P. vivax, P. falciparum malaria infection and malaria naive controls determined by flow cytometry. Data are shown as interquartile ranges (box plots), maximum and minimum (upper–lower line) and bars indicate median. (a) CD3+δ2+ T cells; (b) CD4+ : CD8+ T cells.
Antibodies to P. falciparum and P. vivax protein extracts
Antibodies to the parasite protein extracts in the plasma were determined and are shown in Fig. 2. Both mixed malaria and single malaria infections had antibodies against P. vivax and P. falciparum antigens. Interestingly, the mixed infection had the highest plasma levels of antibodies to both P. vivax and P. falciparum antigens [13-fold (P > 0·05) and 24-fold (P < 0·04), respectively] compared with those of the naive controls. The single P. falciparum group had an increased ratio of antibodies to the P. vivax (sixfold, P > 0·05) and P. falciparum infection (10-fold, P > 0·05). The single P. vivax group had a sixfold increase of anti-P. vivax and anti-P. falciparum antibodies compared with the naive control. Of the three malaria infection groups, anti-P. falciparum antibodies were significantly higher in the mixed PV-PF malaria infection (P < 0·04) compared with each single malaria infection group, as shown in Fig. 2b. Antibodies specific to P. vivax protein extracts were low in all groups, as shown in Fig. 2a.
Fig. 2.
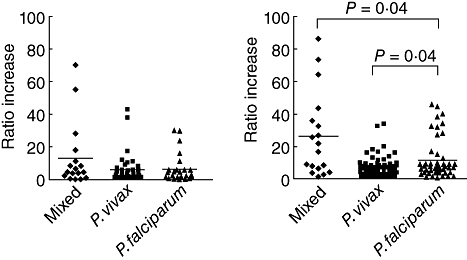
Antibodies to Plasmodium vivax (PV) and P. falciparum (PF) in mixed PV-PF, P. vivax, P. falciparum malaria infection, and malaria naive controls determined by enzyme-linked immunosorbant assay. Data are shown in median ratio and bars indicate median of the ratios. (a) Anti-P. vivax antibodies; (b) anti-P. falciparum antibodies.
Cross-reactivity of antibodies to P. vivax and P. falciparum parasites
The plasma antibodies of the single malaria infections, either P. vivax or P. falciparum, showed reactivity with the other malaria species regardless of the species that had currently caused the infection. Interestingly, the supernatant collected from the PvSE- or PfSE-stimulated lymphocytes of the single P. falciparum infection produced both anti-P. vivax and anti-P. falciparum antibodies. Similarly, the supernatant collected from the PfSE- or PvSE-stimulated lymphocytes of the single P. vivax infection also produced both anti-P. vivax and anti-P. falciparum antibodies. The antibodies showed strongly positive with mature stages of both parasites as determined by the IFA (data not shown). The supernatant collected from the malaria PvSE- or PfSE-stimulated lymphocytes of the malaria naives showed no reactivity with any parasite.
Discussion
The objective of this study was to characterize the profiles of T cells and the response of antibodies to blood stage antigens of both P. vivax and P. falciparum during acute mixed-malaria infection. Our study gave novel evidence of the natural immune response of γδT cells against mixed malaria infection, were modulated by P. vivax and their role in the reduction of malaria severity caused by P. falciparum. The percentage of CD3+δ2+T cells were increased significantly in acute mixed and single P. vivax infections compared with these of the naive controls, whereas only a low level of these cells was found in acute P. falciparum patients. The CD4 : CD8 ratio did not show any difference between malaria patient groups, which was in line with previous studies [9–11]. These results suggested the role of CD3+δ2+T cells in the development of the cell-mediated immunity during mixed malaria infection.
The γδT cells inhibit development of the pre-erythrocytic stage of P. yoelii in a mouse model lacking αβ T cells [22], and the α-galactosyl-ceramide (a-GalCer)-activated natural killer (NK) T cells protect mice from P. yoelii infection [23]. Moreover, γδT cells, NK, NK T cells and macrophages harboured in the liver where the pre-erythrocytic stage of malaria are presented may boost the immunity to malaria infection [24] more effectively than those malaria having only the erythrocytic stages.
The γδT cells control the expansion of P. chabaudi in a mouse model [25] and inhibit growth of P. falciparum parasites [26] through the release of granulysin [8]. The elevation of CD3+δ2+ T cells in acute P. vivax infection conceivably plays a similar role to that in P. falciparum infection. Interestingly, a greater elevation of the CD3+δ2+ T cells was shown in the mixed malaria infection and the single P. vivax infection compared with that of the P. falciparum infection. By contrast, a previous study in Ethiopia showed that there was no difference in CD3+δ2+ T cells in mixed PV-PF malaria infection compared with single P. vivax or P. falciparum infection. However, the CD3+δ1+ T cells, having a killer effector function, were more numerous in the single P. falciparum and mixed PV-PF malaria infections [10].
Our data showed no correlation between the percentage of CD3+δ2+ T cells and the number of P. vivax parasites or the number of the P. falciparum parasites. Nevertheless, the lower number of P. falciparum parasites in the mixed malaria infection, as opposed to that in the single P. falciparum infection, suggested that P. vivax might activate the effector killer function of the CD3+δ2+ T cells resulting in the inhibition of P. falciparum growth.
When P. vivax was co-infected with P. falciparum in the malaria infection, patients had a higher fever than those with single P. falciparum or single P. vivax infections [27]. High temperature is shown to kill P. falciparum parasites in in vitro studies [28,29]. In addition, serum from a P. vivax-infected donor during paroxysm inhibits maturation of P. falciparum schizonts [30]. Together, these data support the notion that a mechanism by which P. vivax controls the expansion of P. falciparum in mixed malaria infection could be via the induction and persistency of high fever in patients, particularly the high systemic temperature in the organs where P. falciparum sequestered. Therefore, if this can be established, the vaccine development imperative against P. falciparum infection and severity may derive from the combination of P. falciparum antigen and the antigen candidate from P. vivax.
Antibodies to malaria, although short-lived, are the primary mechanisms of defence against parasitic infection [31]. Induction and maintenance of anti-malarial antibodies requires repetitive infections [32,33]. Evidence such as the existence of the asymptomatic parasitaemic individual confirms development of immunity against malaria [34,35]. Recently, a study has shown that the incidence of severe malaria in patients with mixed PV-PF malaria infection is 4·2 times less than that in P. falciparum infection alone [7].
In this study we have shown that the anti-P. falciparum and anti-P. vivax antibody levels in the mixed malaria infection were higher than those of the single P. vivax or P. falciparum infections. Our data provide a new basis to support previous findings that, on one hand, development of a cross-immune reactivity between P. vivax and P. falciparum during acute mixed infection could be due to the activation of a pool of memory T cells having specificities to both P. vivax and P. falciparum antigens. These cells co-existed in the residents of the endemic areas where there is regular exposure to malaria parasites.
On the other hand, the antigenic cross-stimulation by P. vivax antigens sharing common epitopes with P. falciparum[36] results in the cell-mediated and antibody responses at high levels against P. falciparum during the acute phase of infection. Further investigations in the different geographical endemic areas are needed to verify the two categories. To stratify this finding further, we performed an in vitro T cell stimulation assay using PvSE and PfSE antigens derived from P. vivax and P. falciparum, respectively, to stimulate the PBMCs from acute single P. vivax or single P. falciparum infections with the two antigens. Both anti-P. vivax and anti-P. falciparum antibodies, tested by the IFA, were found in the supernatant collected from these assays, regardless of the parasite species which caused the infection. This suggested that the patients had developed antigen-specific memory T cells against both P. vivax and P. falciparum parasites which were activated upon re-exposure to either P. vivax or P. falciparum antigens. Supporting evidence from a study in Thailand shows the antibody cross-reactivity from a single P. vivax-infected patient against both the schizont extract of P. falciparum parasite and the PfMSP119 parasite protein [30]. In addition, the cross-reactivity between anti-PvMSP5 and anti-PfMSP5 antibodies was observed in single P. vivax or single P. falciparum infections [37]. Our findings were also supported by the evidence from an epidemiological observation and cross-sectional study [2], during a wet season, which showed that the dominant parasite is P. falciparum whereas P. vivax dominates during the dry season. Overall immunity, effector T cells and anti-malaria antibodies to malaria among the residents of endemic areas would be strengthened by the existence of P. vivax.
In summary, our results indicate the possibility of P. vivax suppressing P. falciparum parasites, because P. vivax induces CD3+δ2+T cells which are effector T killer cells. P. vivax infection also elevates anti-P. falciparum antibodies during the acute phase, and induces a very high fever. These findings suggest that the interaction between the host and P. vivax parasites could offer protection as demonstrated in the mixed PV-PF malaria infection. However, further clinical and experimental research is needed in order to verify these assumptions. Furthermore, in single P. vivax or P. falciparum infection, similar levels of T helper type 1 (Th1)/Th2 cytokine responses are shown [38]. Clarification of such responses to mixed malaria infection in man, i.e. conversion between Th1- and Th2-type responses, are of interest and require further investigation.
Acknowledgments
We thank all staff at the Mae Sot and Mae Kasa Malaria Clinics, Tak province, the staff of the Department of Entomology, AFRIMS, Bangkok, and the staff of the Malaria Research and Training Center at Prabuthabath, Saraburi, Thailand, for the collection of specimens. We also thank J. Opasnawakun and S. Lerdwana for technical assistances, Dr W. Pan-ngum for statistical analysis, and Mr P. Whalley (ASEAN Institute for Health Development) for the language revision. This work was supported by the Royal Golden Jubilee Programme (5MMU48AH1), the Thailand Research Fund (BRG498009), the Thailand Research Fund-Senior Research Scholar Award, the Commission on Higher Education (CHE-RES-PD) and the FIC, NIH (D43-TW006571).
Disclosure
None.
References
- 1.Black J, Hommel M, Snounou G, Pinder M. Mixed infections with Plasmodium falciparum and P. malariae and fever in malaria. Lancet. 1994;343:1095. doi: 10.1016/s0140-6736(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 2.Maitland K, Williams TN, Newbold CI. Plasmodium vivax and P. falciparum: biological interactions and the possibility of cross-species immunity. Parasitol Today. 1997;13:227–31. doi: 10.1016/s0169-4758(97)01061-2. [DOI] [PubMed] [Google Scholar]
- 3.Mason DP, McKenzie FE. Blood-stage dynamics and clinical implications of mixed Plasmodium vivax–Plasmodium falciparum infections. Am J Trop Med Hyg. 1999;61:367–74. doi: 10.4269/ajtmh.1999.61.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayxay M, Pukrittayakamee S, Newton PN, White NJ. Mixed-species malaria infections in humans. Trends Parasitol. 2004;20:233–40. doi: 10.1016/j.pt.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Snounou G, White NJ. The co-existence of Plasmodium: sidelights from falciparum and vivax malaria in Thailand. Trends Parasitol. 2004;20:333–9. doi: 10.1016/j.pt.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Price RN, Simpson JA, Nosten F, et al. Factors contributing to anemia after uncomplicated falciparum malaria. Am J Trop Med Hyg. 2001;65:614–22. doi: 10.4269/ajtmh.2001.65.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luxemburger C, Ricci F, Nosten F, Raimond D, Bathet S, White NJ. The epidemiology of severe malaria in an area of low transmission in Thailand. Tran R Soc Trop Med Hyg. 1997;91:256–62. doi: 10.1016/s0035-9203(97)90066-3. [DOI] [PubMed] [Google Scholar]
- 8.Farouk SE, Mincheva-Nilsson L, Krensky AM, Dieli F, Troye-Blomberg M. Gamma delta T cells inhibit in vitro growth of the asexual blood stages of Plasmodium falciparum by a granule exocytosis-dependent cytotoxic pathway that requires granulysin. Eur J Immunol. 2004;34:2248–56. doi: 10.1002/eji.200424861. [DOI] [PubMed] [Google Scholar]
- 9.Kassa D, Petros B, Mesele T, Hailu E, Wolday D. Characterization of peripheral blood lymphocyte subsets in patients with acute Plasmodium falciparum and P. vivax malaria infections at Wonji Sugar Estate, Ethiopia. Clin Vaccine Immunol. 2006;13:376–9. doi: 10.1128/CVI.13.3.376-379.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Worku S, Bjorkman A, Troye-Blomberg M, Jemaneh L, Farnert A, Christensson B. Lymphocyte activation and subset redistribution in the peripheral blood in acute malaria illness: distinct gamma delta+ T cell patterns in Plasmodium falciparum and P. vivax infections. Clin Exp Immunol. 1997;108:34–41. doi: 10.1046/j.1365-2249.1997.d01-981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lisse IM, Aaby P, Whittle H, Knudsen K. A community study of T lymphocyte subsets and malaria parasitaemia. Tran R Soc Trop Med Hyg. 1994;88:709–10. doi: 10.1016/0035-9203(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 12.Jangpatarapongsa K, Sirichaisinthop J, Sattabongkot J, et al. Memory T cells protect against Plasmodium vivax infection. Microbes Infect. 2006;8:680–6. doi: 10.1016/j.micinf.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Kabelitz D, Marischen L, Oberg HH, Holtmeier W, Wesch D. Epithelial defence by gamma delta T cells. Int Arch Allergy Imm. 2005;137:73–81. doi: 10.1159/000085107. [DOI] [PubMed] [Google Scholar]
- 14.Kersten CM, McCluskey RT, Boyle LA, Kurnick JT. Escherichia coli and Pseudomonas aeruginosa induce expansion of V delta 2 cells in adult peripheral blood, but of V delta 1 cells in cord blood. J Immunol. 1996;157:1613–19. [PubMed] [Google Scholar]
- 15.Ferrick DA, Schrenzel MD, Mulvania T, Hsieh B, Ferlin WG, Lepper H. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature. 1995;373:255–7. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 16.Garcia VE, Jullien D, Song M, et al. IL-15 enhances the response of human gamma delta T cells to nonpeptide [correction of nonpetide] microbial antigens. J Immunol. 1998;160:4322–9. [PubMed] [Google Scholar]
- 17.Tsukaguchi K, de Lange B, Boom WH. Differential regulation of IFN-gamma, TNF-alpha, and IL-10 production by CD4(+) alpha beta TCR+ T cells and vdelta2(+) gammadelta T cells in response to monocytes infected with Mycobacterium tuberculosis-H37Ra. Cell Immunol. 1999;194:12–20. doi: 10.1006/cimm.1999.1497. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Das H, Kamath A, Bukowski JF. Human V gamma 2V delta 2 T cells produce IFN-gamma and TNF-alpha with an on/off/on cycling pattern in response to live bacterial products. J Immunol. 2001;167:6195–201. doi: 10.4049/jimmunol.167.11.6195. [DOI] [PubMed] [Google Scholar]
- 19.Dieli F, Troye-Blomberg M, Ivanyi J, et al. Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vgamma9/Vdelta2 T lymphocytes. J Infect Dis. 2001;184:1082–5. doi: 10.1086/323600. [DOI] [PubMed] [Google Scholar]
- 20.Udomsangpetch R, Somsri S, Panichakul T, et al. Short-term in vitro culture of field isolates of Plasmodium vivax using umbilical cord blood. Parasitol Int. 2007;56:65–9. doi: 10.1016/j.parint.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Cranmer SL, Magowan C, Liang J, Coppel RL, Cooke BM. An alternative to serum for cultivation of Plasmodium falciparum in vitro. Tran R Soc Trop Med Hyg. 1997;91:363–5. doi: 10.1016/s0035-9203(97)90110-3. [DOI] [PubMed] [Google Scholar]
- 22.Tsuji M, Mombaerts P, Lefrancois L, Nussenzweig RS, Zavala F, Tonegawa S. Gamma delta T cells contribute to immunity against the liver stages of malaria in alpha beta T-cell-deficient mice. Proc Natl Acad Sci USA. 1994;91:345–9. doi: 10.1073/pnas.91.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Aseguinolaza G, de Oliveira C, Tomaska M, et al. alpha-galactosylceramide-activated Valpha 14 natural killer T cells mediate protection against murine malaria. Proc Natl Acad Sci USA. 2000;97:8461–6. doi: 10.1073/pnas.97.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmieg J, Gonzalez-Aseguinolaza G, Tsuji M. The role of natural killer T cells and other T cell subsets against infection by the pre-erythrocytic stages of malaria parasites. Microbes Infect. 2003;5:499–506. doi: 10.1016/s1286-4579(03)00072-8. [DOI] [PubMed] [Google Scholar]
- 25.Seixas EM, Langhorne J. gamma delta T cells contribute to control of chronic parasitemia in Plasmodium chabaudi infections in mice. J Immunol. 1999;162:2837–41. [PubMed] [Google Scholar]
- 26.Elloso MM, van der Heyde HC, vande Waa JA, Manning DD, Weidanz WP. Inhibition of Plasmodium falciparum in vitro by human gamma delta T cells. J Immunol. 1994;153:1187–94. [PubMed] [Google Scholar]
- 27.McKenzie FE, Smith DL, O'Meara WP, et al. Fever in patients with mixed-species malaria. Clin Infect Dis. 2006;42:1713–18. doi: 10.1086/504330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwiatkowski D. Febrile temperatures can synchronize the growth of Plasmodium falciparum in vitro. J Exp Med. 1989;169:357–61. doi: 10.1084/jem.169.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long HY, Lell B, Dietz K, Kremsner PG. Plasmodium falciparum: in vitro growth inhibition by febrile temperatures. Parasitol Res. 2001;87:553–5. doi: 10.1007/s004360100374. [DOI] [PubMed] [Google Scholar]
- 30.Nagao Y, Kimura-Sato M, Chavalitshewinkoon-Petmitr P, et al. Suppression of Plasmodium falciparum by serum collected from a case of Plasmodium vivax infection. Malar J. 2008;7:113. doi: 10.1186/1475-2875-7-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wipasa J, Elliott S, Xu H, Good MF. Immunity to asexual blood stage malaria and vaccine approaches. Immunol Cell Biol. 2002;80:401–14. doi: 10.1046/j.1440-1711.2002.01107.x. [DOI] [PubMed] [Google Scholar]
- 32.Alves FP, Durlacher RR, Menezes MJ, Krieger H, Silva LH, Camargo EP. High prevalence of asymptomatic Plasmodium vivax and Plasmodium falciparum infections in native Amazonian populations. Am J Trop Med Hyg. 2002;66:641–8. doi: 10.4269/ajtmh.2002.66.641. [DOI] [PubMed] [Google Scholar]
- 33.Ladeia-Andrade S, Ferreira MU, de Carvalho ME, Curado I, Coura JR. Age-dependent acquisition of protective immunity to malaria in riverine populations of the Amazon Basin of Brazil. Am J Trop Med Hyg. 2009;80:452–9. [PubMed] [Google Scholar]
- 34.Kinyanjui SM, Mwangi T, Bull PC, Newbold CI, Marsh K. Protection against clinical malaria by heterologous immunoglobulin G antibodies against malaria-infected erythrocyte variant surface antigens requires interaction with asymptomatic infections. J Infect Dis. 2004;190:1527–33. doi: 10.1086/424675. [DOI] [PubMed] [Google Scholar]
- 35.Osier FH, Fegan G, Polley SD, et al. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun. 2008;76:2240–8. doi: 10.1128/IAI.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Black CG, Barnwell JW, Huber CS, Galinski MR, Coppel RL. The Plasmodium vivax homologues of merozoite surface proteins 4 and 5 from Plasmodium falciparum are expressed at different locations in the merozoite. Mol Biochem Parasitol. 2002;120:215–24. doi: 10.1016/s0166-6851(01)00458-3. [DOI] [PubMed] [Google Scholar]
- 37.Woodberry T, Minigo G, Piera KA, et al. Antibodies to Plasmodium falciparum and Plasmodium vivax merozoite surface protein 5 in Indonesia: species-specific and cross-reactive responses. J Infect Dis. 2008;198:134–42. doi: 10.1086/588711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernandes AA, Carvalho LJ, Zanini GM, et al. Similar cytokine responses and degrees of anemia in patients with Plasmodium falciparum and Plasmodium vivax infections in the Brazilian Amazon region. Clin Vaccine Immunol. 2008;15:650–8. doi: 10.1128/CVI.00475-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


