Abstract
Lymphocytes play a crucial role in lung inflammation. Different interstitial lung diseases may show distinct lymphocyte activation profiles. The aim of this study was to examine the expression of a variety of activation markers on T lymphocyte subsets from blood and bronchoalveolar lavage fluid (BALF) of patients with different granulomatous interstitial lung diseases and healthy controls. Bronchoalveolar lavage cells and blood cells from 23 sarcoidosis patients, seven patients with hypersensitivity pneumonitis and 24 healthy controls were analysed. Lymphocyte activation status was determined by flow cytometry. Lymphocytes were stained with antibodies against CD3, CD4, CD8, CD25, CD28, CD69, very late antigen-1 (VLA)-1, VLA-4 and human leucocyte antigen D-related (HLA-DR). In general, CD28, CD69 and VLA-1 expression on BALF CD4+ lymphocytes and HLA-DR expression on BALF CD8+ lymphocytes was different in patients with hypersensitivity pneumonitis and sarcoidosis patients with parenchymal involvement. This BALF lymphocyte phenotype correlated with carbon monoxide diffusing lung capacity (Dlco) values across interstitial lung diseases (ILD) (r2 = 0·48, P = 0·0002). In sarcoidosis patients, CD8+CD28null blood lymphocytes correlated with lower Dlco values (r = −0·66, P = 0·004), chronic BALF lymphocyte activation phenotype (r2 = 0·65, P < 0·0001), radiographic staging (stage I versus stage II and higher, P = 0·006) and with the need for corticosteroid treatment (P = 0·001). Higher expression of CD69, VLA-1 and HLA-DR and lower expression of CD28 on BALF lymphocytes suggests prolonged stimulation and chronic lymphocyte activation in patients with ILD. In sarcoidosis, blood CD8+CD28null cells might be a new biomarker for disease severity but needs further investigation.
Keywords: activation, expression, hypersensitivity pneumonitis, sarcoidosis, T lymphocytes
Introduction
Many acute and chronic lung disorders with variable degrees of pulmonary inflammation and fibrosis are referred to collectively as interstitial lung diseases (ILD). The major abnormality in ILD is disruption of the lung parenchyma; progression of the diseases results in impaired oxygen transfer and scarring within the lung. Interstitial lung diseases are classified into four categories: (i) ILD of known association; (ii) granulomatous ILD; (iii) other and often rare ILD; and (iv) idiopathic interstitial pneumonias (IIPs). The most common ILD in the western world is sarcoidosis, with an annual incidence of 10–25 per 100 000 people in western Europe and the United States [1,2].
Sarcoidosis is a multi-system granulomatous disease characterized by an accumulation of T helper type 1 lymphocytes in the affected organs, commonly the lung. The disease course is variable. Sarcoidosis is often acute and self-limiting; in some individuals it becomes chronic, occasionally flaring up over a period of many years [3]. The majority of sarcoidosis patients undergo spontaneous remission; approximately 10–15% of the sarcoidosis cases develop parenchymal fibrosis [4].
Another common granulomatous ILD is hypersensitivity pneumonitis. Hypersensitivity pneumonitis is due to a combined type III allergic reaction with the formation of precipitins and a type IV lymphocyte reaction with a granulomatous inflammation. Hypersensitivity pneumonitis is characterized pathologically by non-caseating granulomas in the interstitium, bronchiolitis and organizing pneumonia, with or without interstitial fibrosis [5].
Most clinical manifestations of ILD are secondary to the direct effect of the accumulation of activated immune cells in involved tissues, notably T cells, macrophages or neutrophils [6,7]. Activated T lymphocytes play an important role in the pathophysiology of ILD [6]. T lymphocyte activation involves sequential expression of different, well-defined activation markers. For instance, CD25 and CD69 are expressed in vitro within 24 h after stimulation while very late antigen (VLA)-1 and VLA-4 can be detected after several days to weeks [8–10]. CD69 is an early membrane receptor expressed transiently upon lymphocyte activation and expressed selectively in chronic inflammatory infiltrates and at sites of active immune responses in vivo[11]. CD28 is a co-stimulatory molecule and expressed constitutionally by T cells. Wahlström et al. have shown differential expression of activation markers between blood and bronchoalveolar lavage fluid (BALF) lymphocytes in pulmonary sarcoidosis [12]. Highly activated T cells were found in BALF of patients, in particular CD4+ T cells that have a preferential usage of the T cell receptor (TCR) AV2S3 gene segment [13,14]. In addition, increased expression of VLA-1, VLA-4 and VLA-5 has been demonstrated on tissue-infiltrating T cells from the lungs of sarcoidosis patients, especially in patients with active disease [15,16]. Moreover, we and others have found high percentages of chronically activated BALF CD103+ intra-epithelial lymphocytes (IEL) in fibrotic lung diseases [8,17,18]. However, a detailed comparison of lymphocyte activation profiles between different ILD has not yet been made.
In order to characterize more clearly the lymphocyte phenotype in sarcoidosis and hypersensitivity pneumonitis we examined the expression of a comprehensive set of activation markers on T lymphocyte subsets obtained by bronchoalveolar lavage and in peripheral blood. Correlations of T lymphocyte marker profiles with lung function parameters and radiographic staging were analysed to understand the relation between lymphocyte activation phenotype and disease severity.
Materials and methods
Subjects
Sarcoidosis patients
Twenty-three patients presenting to our department because of symptomatic sarcoidosis with >15% lymphocytes in bronchoalveolar lavage were included in this study [median age, 42 (range: 24–65); male/female, 16/7]. The diagnosis of sarcoidosis was established on the basis of clinical findings and histological evidence of non-caseating epithelioid granulomas and after exclusion of other known causes of granulomatosis in accordance with the consensus of the American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders (ATS/ERS/WASOG) statement on sarcoidosis [19]. In six patients, the diagnosis was made without biopsy proof because these patients presented with the classic symptoms of Löfgren's syndrome, namely fever, erythema nodosum, arthralgia and bilateral hilar lymphadenopathy. All patients with Löfgren's syndrome were confirmed as tuberculosis (TB)-negative. All patients were steroid naive at the time of inclusion into the study. Pulmonary disease severity at presentation was evaluated by chest radiography. In brief, this comprises five stages: stage 0, normal, stage I, bilateral hilar lymphadenopathy (BHL), stage II, BHL and parenchymal infiltration, stage III, parenchymal infiltration without BHL and stage IV, irreversible fibrosis with loss of lung volume. Distribution of chest radiographic stages at presentation showed that 14 patients presented with stage I (including six patients diagnosed with Löfgren's syndrome), four patients presented with stage II, four patients presented with stage III and one patient presented with stage IV. For further analysis, patients were divided into a group presented without parenchymal involvement (radiographic stage I, n = 14) and a group presented with parenchymal involvement (radiographic stage II and higher, n = 9).
Of 21 patients, data of corticosteroid treatment within 2 years after presentation were available. Seven patients received no medication while 14 patients needed corticosteroids within 2 years after presentation (Table 4) [20]. The clinical and demographic data of the study subjects are summarized in Table 1.
Table 4.
Parameters of disease severity and percentages of CD8+CD28null cells in sarcoidosis patients.
| Patient | Chest X-ray | Dlco (% pred.) | Corticosteroid therapy (<2 years) | BAL T lymphocyte phenotype (SOR) | Peripheral blood CD8+CD28null cells (%) |
|---|---|---|---|---|---|
| ILD50 | I | 100 | No | 35·5 | 3·9 |
| ILD51 | I | 99 | No | 14·5 | 6·6 |
| ILD31 | I | 94 | No | 28 | 8·3 |
| ILD39, Löfgren | I | ND | No | 27·5 | 13·3 |
| ILD20 | I | 81 | No | 35 | 13·9 |
| ILD17, Löfgren | I | 75 | No | 20 | 18·6 |
| ILD37 | I | ND | No | 56 | 18·8 |
| ILD22, Löfgren | I | 105 | No | 73 | 24·1 |
| ILD14 | I | ND | Unknown | 66 | 31·7 |
| ILD09 | I | ND | No | 60 | 32·7 |
| ILD08 | II | 90 | No | 66 | 33·5 |
| ILD13 | IV | 84 | No | 88 | 46·0 |
| ILD11, Löfgren | I | 70 | No | 59 | 52·7 |
| ILD47 | III | 89 | P | 62·5 | 54·7 |
| ILD25, Löfgren | I | ND | No | 49 | 60·1 |
| ILD24 | III | 82 | P + M | 79 | 63·4 |
| ILD26, Löfgren | I | 75 | No | 60 | 73·8 |
| ILD10 | II | 66 | P + I | 68 | 76·4 |
| ILD32 | II | 44 | P + M | 92 | 78·7 |
| ILD27 | III | 79 | P | 94 | 80·7 |
| ILD15 | II | 62 | P + M | 91 | 86·8 |
| ILD42 | I | 85 | P + M + I | 92 | 90·0 |
Dlco: carbon monoxide diffusing lung capacity; ILD: interstitial lung diseases; I: infliximab; M: methatroxate; ND: not determined; P: prednisone/prednisolone; SOR: sum of ranks.
Table 1.
Characteristics of the study population.
| SARC | HP | HC | |
|---|---|---|---|
| Characteristics | (n = 23) | (n = 7) | (n = 24) |
| Age,† year | 41·2 (24–65) | 60·3 (41–75) | 30·0 (20–64) |
| Male/female gender | 16/7 | 4/3 | 9/15 |
| Smoker/non-smoker | 1/19 | 1/5 | 7/17 |
| Lung function parameters† | |||
| FEV1 % pred (n = 18/6/21) | 93 (59–115)* | 87 (52–142)* | 107 (79–130) |
| FVC % pred (n = 18/6/21) | 97 (65–117)* | 108 (56–134)** | 110 (81–135) |
| Dlco % pred (n = 18/6) | 82 (44–105)*** | 48 (24–83) | ND |
| Chest radiographic stages I/II/III/IV | 14/4/4/1 | ||
| Löfgren's syndrome | 6 | ||
| Organ involvementठ| |||
| Pulmonary involvement | 100 (17) | ||
| Extrapulmonary involvement | 35 (6) | ||
| Kidney | 24 (4) | ||
| Skin | 12 (2) | ||
| Extrathoracic lymph node | 6 (1) | ||
| Neurological | 6 (1) | ||
| Eyes | 6 (1) | ||
| Parotid/salivary | 6 (1) | ||
| Muscles | 6 (1) | ||
| Bone/joints | 6 (1) |
Different from healthy controls (HC), P < 0·001;
different from HC, P < 0·01;
sarcoidosis (SARC) versus hypersensitivity pneumonitis (HP), P = 0·0007.
Values are given as the mean (range).
Values are given as % (no.).
Based on the ACCESS assessment instrument [29]. Organ involvement data are from non-Löfgren's sarcoidosis patients (n = 17). Dlco: carbon monoxide diffusing lung capacity; FVC: forced expiratory volume; ND: not determined.
Hypersensitivity pneumonitis patients
Seven patients presenting to our department because of symptomatic hypersensitivity pneumonitis were included in this study [median age, 64 (range: 41–75); male/female, four/three]. All patients experienced respiratory symptoms including dyspnoea or cough, with or without systemic symptoms such as fever and arthralgia after exposure to birds. Additionally, they all had precipitating antibodies to birds and a lymphocytic alveolitis, i.e. >15% lymphocytes in BALF. None of the patients received corticosteroids, nor had they within the previous 3 months.
Healthy controls
Twenty-four healthy subjects were included in this study [mean age, 30 (range 20–64); male/female, 9/15]. All healthy controls had normal chest radiography, normal lung function [forced expiratory volume in 1 s ((FEV1), forced vital capacity (FVC)] and none had any sign of pulmonary disease.
Verbal and written consent was obtained from all subjects, and authorization was given by the Ethics Committee of the St Antonius Hospital, Nieuwegein.
Pulmonary function tests
Pulmonary function tests were performed at presentation. FVC, FEV1 and carbon monoxide diffusing lung capacity (Dlco) were used to assess the presence of lung function impairment at presentation of the disease. All lung function parameters are expressed as percentage of predicted values. FVC and FEV1 were calculated from volumes in litres and adjusted to BTPS (body temperature, ambient pressure, saturated with water vapour).
Bronchoalveolar lavage
BAL was performed during flexible fibreoptic bronchoscopy at the time of the diagnosis according to the standardized and validated procedure described previously [18]. The procedure involved detailed explanation to the patient, premedication (0·5 mg atropine subcutaneously, 20 mg codeine per os) and local anaesthesia of the larynx and lower airways (0·5% tetracaine in the oropharynx, 8 cc 0·5% tetracaine in lower airways). Transcutaneous oxygen saturation was monitored continuously by oximeter with a finger probe. BAL was performed, preferably in the right middle lobe, with four 50-ml aliquots of sterile isotonic saline solution (37°C). The aspirated lavage fluid from the first 50-ml aliquot was kept apart and excluded from further analysis. The BALF recovered from the three subsequent aliquots was collected in a siliconized specimen trap and kept on ice. BALF was filtered through nylon gauze and centrifuged (10 min at 400 g at 4°C).
The cell pellet was washed twice, counted and resuspended in minimal essential medium/RPMI-1640 (Gibco, Grand Island, NY, USA), supplemented with 0·5% bovine serum albumin (Organon Teknika, Boxtel, The Netherlands). Cells were counted in a Bürker chamber. Cell viability was determined by trypan blue exclusion. Smears for cell differentiation were prepared by cytocentrifugation (Shandon, Runcorn, UK). Cell differentiation was performed on cytospinslide after staining with May–Grünwald–Giemsa (Merck, Darmstadt, Germany); at least 2 × 500 cells were counted.
Flow cytometry
Fifty µl of ethylenediamine tetraacetic acid (EDTA) blood and 50 µl BALF (at least 500 000 cells) were prepared for four-colour flow cytometry. BALF and blood samples were incubated for 15 min at room temperature with 10 µl of the following monoclonal antibodies (mAbs): anti-CD3 fluorescein isothiocyanate (FITC), anti-CD4 peridinin chlorophyll–cyanine (PerCP-Cy)5·5 and anti-CD8 allophycocyanin combined with either anti-CD25 phycoerythrin (PE), anti-CD28 PE, anti-CD69 PE, anti-VLA-1 PE, anti-VLA-4 PE or anti-human leucocyte antigen D-related (HLA-DR) PE, all from BD Biosciences (Alphen aan den Rijn, The Netherlands). Immunofluorescence was measured by flow cytometry (FACSCalibur; Becton Dickinson, Alphen aan den Rijn, The Netherlands). Lymphocytes were defined based on forward-scatter/side-scatter (FSC/SSC) characteristics and expression of the activation markers was evaluated on CD4+ and CD8+ T cells in BALF and PB from healthy controls (HC) and ILD patients (Fig. 1). Isotype-matched negative control antibodies stained fewer than 1% of CD4+ and CD8+ BALF and PB cells, and were used to set markers to delineate positive and negative cells. At least 10 000 lymphocyte events were analysed with FlowJo software (Tree Star, Inc., Ashland, OR, USA).
Fig. 1.
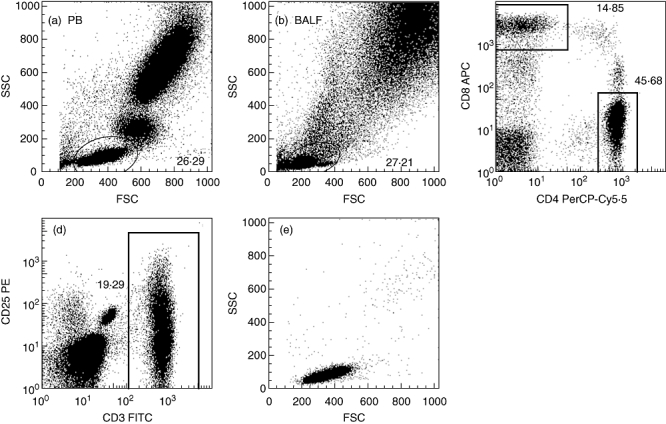
Gating strategy. A representative side-scatter versus forward-scatter plot and lymphocyte gate is shown in peripheral blood (PB) and bronchoalveolar lavage fluid (BALF) samples. Expression of activation markers was analysed on CD4+ and CD8+ cells within the lymphocyte gate (a,b,c). Back gating of gated cell populations double-stained for T cell receptor (TCR) marker CD3 and an activation marker into the side scatter–forward scatter (SSC–FSC) plot showed that the gated cells fell within the SSC–FSC lymphocyte gate, hence no cells were omitted using the original lymphocyte gate (d,e).
Statistics
Data are expressed as median ± range or as stated otherwise. Multiple groups were compared performing Kruskal–Wallis with Dunn's post-hoc test. The Mann–Whitney U-test was used to compare BALF and PB cell populations. Correlations between different variables were determined using Spearman's rank coefficient. To analyse correlations between an expression profile of multiple activation markers and other parameters, individual values of the different activation markers were ranked and the sum of ranks (SOR) was correlated to lung function or CD8+CD28null cells.
Fisher's exact test was used to test the prognostic value of CD8+CD28null cells for the need for medication. Considering multiple comparisons, a P-value of less than 0·01 was regarded as significant. Statistical evaluation of our data was performed using spss version 15·0 (SPSS Inc., Chicago, IL, USA) and Graphpad Prism version 5 (Graphpad Software, Inc., San Diego, CA, USA) software packages.
Results
Total and differential cell counts for BALF, PB and CD4/CD8 ratios are presented in Table 2.
Table 2.
Total and differential cell counts for bronchoalveolar lavage fluid (BALF) and peripheral blood (PB) from patients and controls.
| SARC | HP | HC | |
|---|---|---|---|
| Characteristics | (n = 23) | (n = 7) | (n = 24) |
| Total BALF cells (×104/ml) | 31·1 (9·9–65·0)* | 26·2 (5·1–102·9)‡ | 10·1 (4·3–16·3) |
| Cell types: | |||
| Alveolar macrophages % | 65·2 (13·4–84·6)* | 67·6 (8·5–79·7)* | 87·1 (56·3–96·8) |
| (×104/ml) | 16·0 (6·8–34·5)* | 9·8 (4·0–21·2) | 8·2 (3·9–15·8) |
| Lymphocytes % | 47·0 (15·0–83·0)* | 37·0 (31·0–80·0)* | 9·6 (2·3–39·9) |
| (×104/ml) | 11·2 (1·5–54·0)* | 9·9 (1·8–82·3)* | 0·9 (0·3–5·4) |
| Neutrophils % | 1·1 (0·1–2·4)‡ | 1·9 (0·5–4·7) | 1·6 (0·1–8·1) |
| (×104/ml) | 0·3 (0·02–1·0) | 0·8 (0·07–2·0) | 0·2 (0·0–0·8) |
| Eosinophils % | 0·5 (0·1–4·1) | 0·7 (0·1–3·0) | 0·3 (0·0–4·1) |
| (×104/ml) | 0·1 (0·01–0·7)† | 0·3 (0·01–0·9)† | 0·03 (0·0–0·4) |
| BALF CD4/CD8 cell ratio | 3·6 (0·5–25·2)* | 0·8 (0·6–6·3) | 1·1 (0·2–11·0) |
| Total PB cells (×106/ml) | 5·6 (3·2–14·4)§ | 7·7 (6·5–11·2) | 5·8 (3·2–9·0)§ |
| Lymphocytes % | 23·0 (11·0–44·0)* | 25·0 (20·0–36·0) | 33·5 (20·6–49·9) |
| (×106/ml) | 1·3 (0·4–2·5) | 1·8 (1·4–3·2)¶ | 1·9 (1·3–2·8)¶ |
| PB CD4/CD8 cell ratio | 1·2 (0·4–3·3)‡ | 2·0 (0·6–4·0) | 1·8 (0·9–5·8) |
Significantly different from healthy controls (HC):
P < 0·001;
P < 0·01;
P < 0·05. Significantly different from hypersensitivity pneumonitis (HP):
P < 0·05. Significantly different from sarcoidosis (SARC);
P < 0·01. Numbers are presented as median (range). BALF: bronchoalveolar lavage fluid.
Expression of activation markers on lymphocytes
BALF versus PB
In BALF, highly activated T lymphocyte subsets were found both in ILD patients and in healthy controls. CD69, VLA-1 and HLA-DR expression was higher on CD4+ and CD8+ BALF cells compared to corresponding PB lymphocytes (P < 0·0001). VLA-4 was increased only on BALF CD4+ cells (P < 0·0001). CD4+CD25+ cells were lower in BALF versus PB in sarcoidosis (P < 0·0001). In contrast, in HC CD25+ cells were higher in CD8+ BALF cells (P < 0·0001). Finally, CD28 expression was decreased on BALF versus PB lymphocyte subsets in HC (P < 0·0001).
Expression of activation markers on BALF lymphocytes
ILD patients and controls
Comparisons of BALF cells between groups showed significantly higher expression of CD25 [interleukin (IL)-2 receptor alpha chain] on BALF CD4+ and CD8+ cells in HC versus sarcoidosis. Moreover, the co-stimulatory molecule CD28 was expressed by a lower percentage of BALF CD4+ lymphocytes in hypersensitivity pneumonitis versus HC and sarcoidosis. Increased expression of CD69 and VLA-1 was found on BALF CD4+ lymphocytes in hypersensitivity pneumonitis versus HC and sarcoidosis. HLA-DR expression was increased in hypersensitivity pneumonitis compared to sarcoidosis on BALF CD8+ lymphocytes (Table 3).
Table 3.
Expression of activation markers on bronchoalveolar lavage fluid (BALF) and peripheral blood (PB) CD4+ and CD8+ lymphocytes from interstitial lung disease (ILD) patients and healthy controls.
| SARC (n = 23) | HP (n = 7) | HC (n = 24) | SARC (n = 23) | HP (n = 7) | HC (n = 24) | |
|---|---|---|---|---|---|---|
| Markers | Bronchoalveolar lavage cells | Peripheral blood cells | ||||
| CD4+ lymphocytes | ||||||
| CD25 | 17·6 (7·5–39·5) | 19·7 (15·6–75·4) | 48·8 (28·5–75·0)* | 52·4 (22·7–74·9) | 58·6 (44·0–63·5) | 44·4 (30·8–81·6) |
| CD28 | 97·4 (73·9–99·9)† | 57·3 (38·9–93·1) | 94·4 (80·4–99·4)† | 99·4 (73·6–100) | 95·9 (90·5–99·8) | 99·9 (80·5–100) |
| CD69 | 69·8 (37·1–94·8)† | 89·4 (80·5–95·7) | 66·7 (44·8–79·3)† | 2·7 (0·3–13·8) | 1·2 (0·8–4·8) | 2·2 (1·2–16·5) |
| VLA-1 | 25·6 (7·7–71·5)† | 55·9 (35·5–69·4) | 27·9 (11·3–54·2)† | 5·5 (1·8–17·2) | 5·0 (3·1–12·5) | 2·9 (0·6–17·5) |
| VLA-4 | 99·2 (97·3–99·8) | 99·6 (97·2–99·9) | ND | 86·7 (66·7–93·5) | 86·2 (78·8–89·1) | ND |
| HLA-DR | 93·4 (84·5–98·7) | 93·9 (91·7–97·4) | ND | 19·0 (7·2–64·7) | 15·6 (10·6–21·4) | ND |
| CD8+ lymphocytes | ||||||
| CD25 | 6·1 (1·7–20·5) | 7·5 (5·3–46·1) | 19·3 (7·8–40·3)* | 8·4 (1·3–26·1) | 11·8 (4·4–22·2) | 7·1 (3·5–29·5) |
| CD28 | 57·0 (27·5–85·2) | 24·1 (8·9–76·7) | 64·2 (39·3–80·1) | 60·2 (10·0–96·2) | 69·5 (19·3–100) | 87·6 (62·3–96·4)* |
| CD69 | 86·4 (37·0–97·3) | 97·3 (79·8–98·8) | 85·1 (75·0–94·2) | 5·1 (0–21·2) | 1·0 (0·3–3·3)* | 2·9 (1·6–23·1) |
| VLA-1 | 75·6 (28·3–96·3) | 94·9 (68·9–97·2) | 80·5 (53·6–96·0) | 5·1 (0·6–36·8) | 5·4 (1·8–10·3) | 3·3 (0·9–29·8) |
| VLA-4 | 99·7 (97·2–100) | 99·9 (99·2–100) | ND | 98·4 (93·6–100) | 99·0 (98·8–99·4) | ND |
| HLA-DR | 80·4 (61·3–98·8)† | 96·4 (89·0–99·1) | ND | 35·3 (9·9–91·8) | 43·4 (22·7–64·0) | ND |
Significant difference (P < 0·01), compared to sarcoidosis (SARC).
Significant difference (P < 0·01), compared to hypersensitivity pneumonitis (HP). Numbers are expressed as median (range). HLA-DR: human leucocyte antigen D-related; ND, not determined; VLA: very late antigen.
Sarcoidosis clinical phenotypes
Analysis of different clinical sarcoidosis phenotypes showed a significantly higher proportion of BALF CD4+ lymphocytes expressing VLA-1 [35·9 (14·6–71·5) versus 16·4 (7·7–34·2), P < 0·01] and more BALF CD8+ cells expressing HLA-DR [93·0 (77·0–98·8) versus 77·3 (61·3–95·6), P < 0·01] in patients with parenchymal involvement (radiographic stage II and higher) versus patients without parenchymal involvement (radiographic stage I).
Figure 2 illustrates a flow cytometric analysis of the chronicly activated BALF lymphocytes from one HC, sarcoidosis stage I, sarcoidosis stage IV and hypersensitivity pneumonitis subject.
Fig. 2.
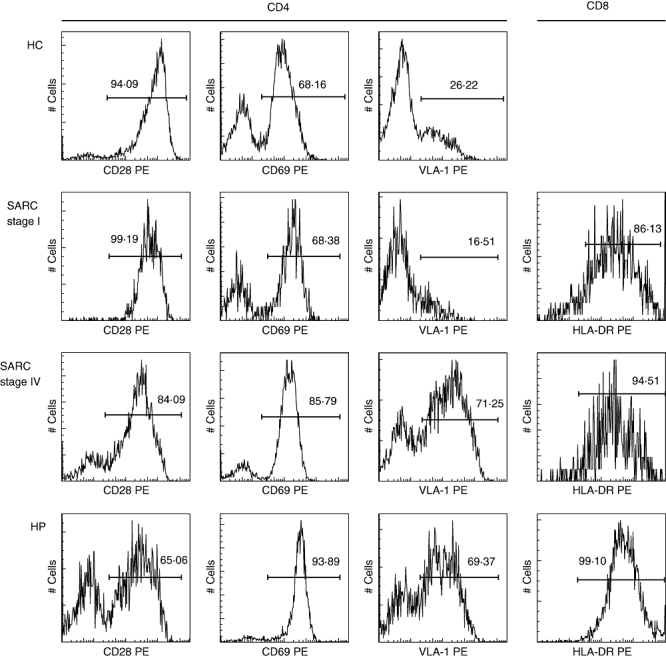
Fluorescence histograms presenting CD28, CD69 and very late antigen (VLA)-1 expression on bronchoalveolar lavage fluid CD4+ cells and human leucocyte antigen D-related (HLA-DR) expression on BALF CD8+ cells from one representative healthy control (HC), sarcoidosis (SARC) stage I, SARC stage IV and hypersensitivity pneumonitis (HP) subject.
To analyse a combination of BALF CD4+ and CD8+ lymphocyte phenotypes, individual values of the different activation markers were ranked and the sum of ranks was compared between ILD. Analysis of variance (anova) revealed the highest sum for hypersensitivity pneumonitis and the lowest sum for sarcoidosis patients without parenchymal involvement (radiographic stage I) (P < 0·0001) (Fig. 3).
Fig. 3.
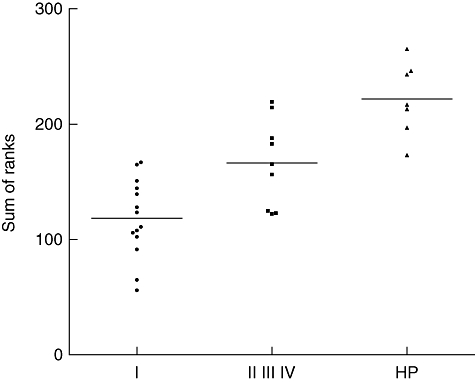
Scatter diagram illustrating the sum of ranks of CD25, CD28, CD69, very late antigen (VLA)-1 and human leucocyte antigen D-related (HLA-DR) expression on bronchoalveolar lavage fluid (BALF) CD4+ lymphocytes and BALF CD8+ lymphocytes in sarcoidosis patients without parenchymal involvement (radiographic stage I), sarcoidosis patients with parenchymal involvement (radiographic stage II and higher) and patients with hypersensitivity pneumonitis (HP). VLA-4 expression was 100% in both BALF lymphocyte subsets in all patients, hence VLA-4 expression was excluded from sum of rank analysis (all subjects would have been assigned the same rank number, i.e. 1).
Analysis of absolute cell numbers revealed no differences between groups (data not shown). Separation of Löfgren's patients from the stage I group did not reveal significant differences between Löfgren's patients and sarcoidosis patients, with radiographic stage I for all comparisons (data not shown). No major differences were found between sexes or smokers and non-smokers for all comparisons in patients and controls. Moreover, multivariate regression analysis of normally distributed markers, controlled for age as a covariate and gender as fixed factor, did not alter the results significantly (data not shown).
Correlation between BALF T lymphocyte phenotype and lung function tests in ILD
Next, we investigated whether the presence of activated T lymphocytes in BALF correlated with lung function parameters in ILD. Dlco is the best descriptor for lung function and decreases with increasing parenchymal involvement. The combination of CD28, CD69 and VLA-1 expression on BALF CD4+ lymphocytes and CD28 and HLA-DR expression on BALF CD8+ lymphocytes showed a positive correlation with Dlco values, superior to the individual significant correlations (r2 = 0·48, P = 0·0002).
Expression of activation markers on PB lymphocytes
ILD patients and controls
In peripheral blood, minor differences were found between sarcoidosis, hypersensitivity pneumonitis and HC comparing the expression of the activation markers on CD4+ cells and CD8+ cells (Table 3). Notably, the percentage of CD28+CD8+ cells was lower in both ILD compared to healthy controls, in particular in sarcoidosis patients (Table 3).
Correlation between blood CD8+CD28null cells and parameters of disease severity in sarcoidosis
In sarcoidosis patients, significant correlations were found between the percentage of PB CD8+CD28null cells (Fig. 4) and Dlco values (r = −0·66, P = 0·004; 95% CI −0·87 to −0·26) (Fig. 5). Moreover, the percentage of blood CD8+CD28null lymphocytes was increased in sarcoidosis patients with parenchymal abnormalities, compared to sarcoidosis patients without parenchymal abnormalities (P = 0·006) (Fig. 5, inlay) and correlated strongly with BALF CD4+CD28+, CD4+VLA-1+, CD8+CD25+, CD8+VLA-1+ and CD8+HLA-DR+ lymphocytes (sum of ranks, r2 = 0·65, P < 0·0001) (Fig. 6). In addition, the proportion of blood CD8+CD28null cells revealed prognostic value for the need for corticosteroid treatment within 2 years after presentation (Fig. 7). Seven of 10 patients with higher percentages of CD8+CD28null cells (>50%) needed corticosteroid treatment within 2 years after presentation compared to none of the 11 patients with lower CD8+CD28null percentages (<50%) (P = 0·001). Individual CD8+CD28null levels and values of disease severity parameters are summarized in Table 4. Finally, the presence of blood CD8+CD28null cells in sarcoidosis did not correlate with extrapulmonary organ involvement (data not shown).
Fig. 4.
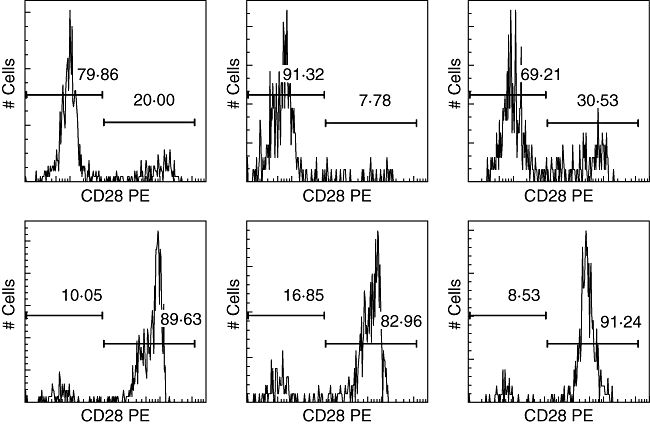
Fluorescence histograms showing blood CD8+CD28null and CD8+CD28+ cell populations in sarcoidosis patients (upper row) and healthy controls (lower row).
Fig. 5.
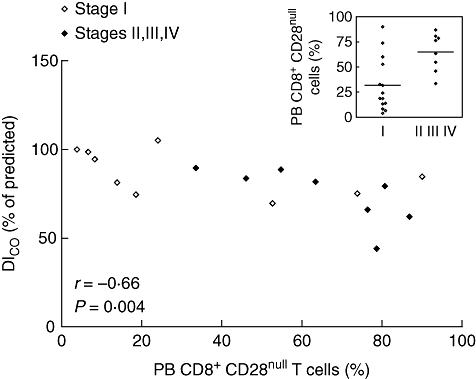
Scatter diagram illustrating the correlation between the percentage of peripheral blood (PB) CD8+CD28null T cells and carbon monoxide diffusing lung capacity (Dlco) in 17 sarcoidosis patients. Inlay presents the percentage of PB CD8+CD28null T cells between sarcoidosis patients without parenchymal involvement, radiographic stage I (n = 14) and with parenchymal involvement, radiographic stage II and higher (n = 9) at presentation.
Fig. 6.
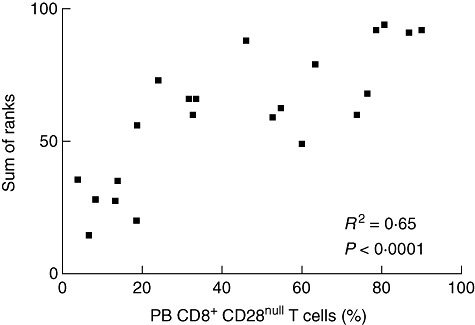
Scatter diagram illustrating the correlation between the percentage of peripheral blood (PB) CD8+CD28null T cells and the sum of ranks of the individual significant correlations with the percentage of bronchoalveolar lavage fluid (BALF) CD4+CD28+, CD4+ very late antigen (VLA-1)+, CD8+CD25+, CD8+VLA-1+ and CD8+ human leucocyte antigen D-related (HLA-DR)+ lymphocytes from sarcoidosis patients.
Fig. 7.
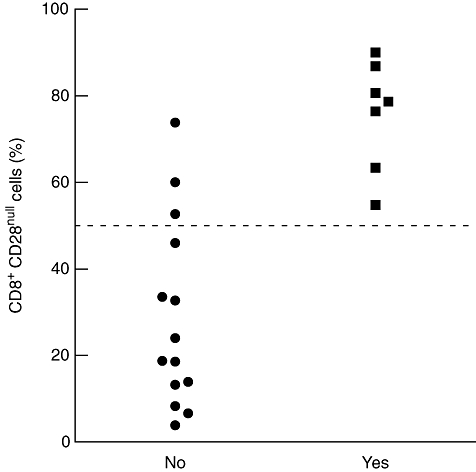
Scatter plot of the percentage of peripheral blood CD8+CD28null T cells in sarcoidosis patients (n = 21) at presentation and the need for corticosteroid treatment within 2 years after presentation. The dotted line indicates 50% level. Ten patients had CD8+CD28null values above 50%. Of these patients, seven needed medication. None of the patients with CD8+CD28null values lower than 50% received corticosteroids within 2 years after presentation. Fisher's exact test: P = 0·001.
Discussion
This study showed compartmentalization of activated lymphocytes in the lungs of patients with different granulomatous interstitial lung diseases and healthy subjects. Early (CD69) as well as late (HLA-DR, VLA-1 and VLA-4) activation markers were found in a much higher frequency on BALF lymphocyte subsets than on blood lymphocyte subsets in all groups. In sarcoidosis and hypersensitivity pneumonitis patients, increased expression of CD69, VLA-1 and decreased expression of CD28 on BALF CD4+ cells and increased expression of HLA-DR on BALF CD8+ cells correlated with the extent of parenchymal involvement and decreased Dlco values. Corresponding peripheral blood lymphocytes in ILD patients hardly expressed an activated phenotype, a finding which is consistent with other studies [12,14,16]. However, we found a remarkable loss of CD28 expression on PB CD8+ T cells, in particular, in sarcoidosis patients. Interestingly, in sarcoidosis, the increased proportion of peripheral blood CD8+CD28null T cells correlated significantly with Dlco values, radiographic staging and with BALF lymphocytes with a chronically activated phenotype (sum of ranks). Consistent with the observed correlations between blood CD8+CD28null cells and parameters of disease severity, the proportion of CD8+CD28null cells at presentation had prognostic value for the need for corticosteroid medication within 2 years after presentation. Loss of CD28 expression on T cells from sarcoidosis patients has been described, but concerned mainly expansion of CD4+CD28null cells [12,14,21].
The loss of CD28 is associated with chronic inflammation upon exposure to inflammatory cytokines and in the context of chronic antigenic exposure [22,23]. CD8+CD28null cells are major histocompatibility complex (MHC) class I-restricted and function in an antigen-dependent manner. They might exert their immunosuppressive effect by rendering antigen presenting cells unable to initiate and support T helper cell activation and growth [24]. On the other hand, CD8+CD28null cells might exert their cytotoxic T lymphocyte (CTL) effector cell activity by production of granzymes, perforins, tumour necrosis factor (TNF)-α and interferon (IFN)-γ[25,26]. Loss of CD28 may not merely indicate chronic inflammation, but is also the result of ageing of the host. CD8+ cells show a higher susceptibility to age-dependent functional and phenotypic changes than CD4+ cells [27] and the percentage of PB CD8+CD28null cells has been shown to correlate positively with age and with a decrease in CD4+/CD8+ ratio [28]. However, univariate analysis of variance revealed no effect of age on the relationship between CD8+CD28null cells and Dlco values, radiological stages or BALF lymphocyte phenotype in sarcoidosis (data not shown). The functional significance of circulating CD8+CD28null cells in sarcoidosis remains to be determined. The correlations with indicators of disease severity suggest that measuring the percentage of PB CD8+CD28null cells might be used as a marker for the amount of lung parenchymal infiltration in sarcoidosis. This hypothesis undoubtedly needs to be validated in a larger patient cohort, including follow-up measurements.
BALF cells from patients with hypersensitivity pneumonitis showed the most chronic activated phenotype compared to controls and compared to sarcoidosis patients. Higher expression of early (CD69) and late (VLA-1, HLA-DR) activation markers and lower expression of co-stimulatory molecule CD28 was found on BALF lymphocytes. This suggests continuous lymphocyte activation, consistent with prolonged local antigen exposure in hypersensitivity pneumonitis.
In conclusion, a specific phenotype of chronically activated BALF lymphocyte subsets was found in sarcoidosis and hypersensitivity pneumonitis patients and correlated with the extent of parenchymal involvement and lower lung function. In sarcoidosis, the blood CD8+CD28null subset may be a new biomarker for disease severity. Further research is necessary to confirm this hypothesis and to understand its role in sarcoidosis disease progression.
Acknowledgments
The authors thank Mrs A. van Heugten-Roeling (Department of Medical Immunology and Microbiology, St Antonius Hospital) for expert technical assistance.
Disclosure
None.
References
- 1.Henke CE, Henke G, Elveback LR, Beard CM, Ballard DJ, Kurland LT. The epidemiology of sarcoidosis in Rochester, Minnesota: a population-based study of incidence and survival. Am J Epidemiol. 1986;123:840–5. doi: 10.1093/oxfordjournals.aje.a114313. [DOI] [PubMed] [Google Scholar]
- 2.Hillerdal G, Nou E, Osterman K, Schmekel B. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984;130:29–32. doi: 10.1164/arrd.1984.130.1.29. [DOI] [PubMed] [Google Scholar]
- 3.Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med. 1997;336:1224–34. doi: 10.1056/NEJM199704243361706. [DOI] [PubMed] [Google Scholar]
- 4.Crystal RG, Bitterman PB, Rennard SI, Hance AJ, Keogh BA. Interstitial lung diseases of unknown cause. Disorders characterized by chronic inflammation of the lower respiratory tract. N Engl J Med. 1984;310:235–44. doi: 10.1056/NEJM198401263100406. [DOI] [PubMed] [Google Scholar]
- 5.Bourke SJ, Dalphin JC, Boyd G, McSharry C, Baldwin CI, Calvert JE. Hypersensitivity pneumonitis: current concepts. Eur Respir J Suppl. 2001;32:81s–92s. [PubMed] [Google Scholar]
- 6.Keogh BA, Crystal RG. Alveolitis: the key to the interstitial lung disorders. Thorax. 1982;37:1–10. doi: 10.1136/thx.37.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semenzato G, Adami F, Maschio N, Agostini C. Immune mechanisms in interstitial lung diseases. Allergy. 2000;55:1103–20. doi: 10.1034/j.1398-9995.2000.00127.x. [DOI] [PubMed] [Google Scholar]
- 8.Braun RK, Foerster M, Grahmann PR, Haefner D, Workalemahu G, Kroegel C. Phenotypic and molecular characterization of CD103+ CD4+ T cells in bronchoalveolar lavage from patients with interstitial lung diseases. Cytometry B Clin Cytom. 2003;54:19–27. doi: 10.1002/cyto.b.10021. [DOI] [PubMed] [Google Scholar]
- 9.Hemler ME, Jacobson JG, Brenner MB, Mann D, Strominger JL. VLA-1: a T cell surface antigen which defines a novel late stage of human T cell activation. Eur J Immunol. 1985;15:502–8. doi: 10.1002/eji.1830150515. [DOI] [PubMed] [Google Scholar]
- 10.Testi R, Phillips JH, Lanier LL. T cell activation via Leu-23 (CD69) J Immunol. 1989;143:1123–8. [PubMed] [Google Scholar]
- 11.Sancho D, Gomez M, Sanchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26:136–40. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Wahlstrom J, Berlin M, Skold CM, Wigzell H, Eklund A, Grunewald J. Phenotypic analysis of lymphocytes and monocytes/macrophages in peripheral blood and bronchoalveolar lavage fluid from patients with pulmonary sarcoidosis. Thorax. 1999;54:339–46. doi: 10.1136/thx.54.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grunewald J, Olerup O, Persson U, Ohrn MB, Wigzell H, Eklund A. T-cell receptor variable region gene usage by CD4+ and CD8+ T cells in bronchoalveolar lavage fluid and peripheral blood of sarcoidosis patients. Proc Natl Acad Sci USA. 1994;91:4965–9. doi: 10.1073/pnas.91.11.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katchar K, Wahlstrom J, Eklund A, Grunewald J. Highly activated T-cell receptor AV2S3(+) CD4(+) lung T-cell expansions in pulmonary sarcoidosis. Am J Respir Crit Care Med. 2001;163:1540–5. doi: 10.1164/ajrccm.163.7.2005028. [DOI] [PubMed] [Google Scholar]
- 15.Berlin M, Lundahl J, Skold CM, Grunewald J, Eklund A. The lymphocytic alveolitis in sarcoidosis is associated with increased amounts of soluble and cell-bound adhesion molecules in bronchoalveolar lavage fluid and serum. J Intern Med. 1998;244:333–40. doi: 10.1046/j.1365-2796.1998.00378.x. [DOI] [PubMed] [Google Scholar]
- 16.Saltini C, Hemler ME, Crystal RG. T lymphocytes compartmentalized on the epithelial surface of the lower respiratory tract express the very late activation antigen complex VLA-1. Clin Immunol Immunopathol. 1988;46:221–33. doi: 10.1016/0090-1229(88)90185-7. [DOI] [PubMed] [Google Scholar]
- 17.Lohmeyer J, Friedrich J, Grimminger F, et al. Expression of mucosa-related integrin alphaEbeta7 on alveolar T cells in interstitial lung diseases. Clin Exp Immunol. 1999;116:340–6. doi: 10.1046/j.1365-2249.1999.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heron M, Slieker WA, Zanen P, et al. Evaluation of CD103 as a cellular marker for the diagnosis of pulmonary sarcoidosis. Clin Immunol. 2008;126:338–44. doi: 10.1016/j.clim.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149–73. [PubMed] [Google Scholar]
- 20.Grutters JC, van den Bosch JM. Corticosteroid treatment in sarcoidosis. Eur Respir J. 2006;28:627–36. doi: 10.1183/09031936.06.00105805. [DOI] [PubMed] [Google Scholar]
- 21.Roberts SD, Kohli LL, Wood KL, Wilkes DS, Knox KS. CD4+CD28-T cells are expanded in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2005;22:13–9. [PubMed] [Google Scholar]
- 22.Bryl E, Vallejo AN, Weyand CM, Goronzy JJ. Down-regulation of CD28 expression by TNF-alpha. J Immunol. 2001;167:3231–8. doi: 10.4049/jimmunol.167.6.3231. [DOI] [PubMed] [Google Scholar]
- 23.Vallejo AN, Weyand CM, Goronzy JJ. T-cell senescence: a culprit of immune abnormalities in chronic inflammation and persistent infection. Trends Mol Med. 2004;10:119–24. doi: 10.1016/j.molmed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Cortesini R, LeMaoult J, Ciubotariu R, Cortesini NS. CD8+ Immunol Rev. 2001;182:201–6. doi: 10.1034/j.1600-065x.2001.1820116.x. [DOI] [PubMed] [Google Scholar]
- 25.Baars PA, Ribeiro Do Couto LM, Leusen JH, et al. Cytolytic mechanisms and expression of activation-regulating receptors on effector-type CD8+CD45RA+ J Immunol. 2000;165:1910–7. doi: 10.4049/jimmunol.165.4.1910. [DOI] [PubMed] [Google Scholar]
- 26.Hamann D, Baars PA, Rep MH, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–18. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Czesnikiewicz-Guzik M, Lee WW, Cui D, et al. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–18. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Effros RB, Boucher N, Porter V, et al. Decline in CD28+ T cells in centenarians and in long-term T cell cultures: a possible cause for both in vivo and in vitro immunosenescence. Exp Gerontol. 1994;29:601–9. doi: 10.1016/0531-5565(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 29.Judson MA, Baughman RP, Teirstein AS, Terrin ML, Yeager H., Jr. Defining organ involvement in sarcoidosis: the ACCESS proposed instrument. ACCESS Research Group. A case control etiologic study of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:75–86. [PubMed] [Google Scholar]


