Abstract
Vitamin K is a family of fat-soluble compounds including phylloquinone (vitamin K1), menaquinone (vitamin K2) and menadione (vitamin K3). Recently, it was reported that vitamin K, especially vitamins K1 and K2, exerts a variety of biological effects, and these compounds are expected to be candidates for therapeutic agents against various diseases. In this study, we investigated the anti-inflammatory effects of vitamin K3 in in vitro cultured cell experiments and in vivo animal experiments. In human embryonic kidney (HEK)293 cells, vitamin K3 inhibited the tumour necrosis factor (TNF)-α-evoked translocation of nuclear factor (NF)-κB into the nucleus, although vitamins K1 and K2 did not. Vitamin K3 also suppressed the lipopolysaccharide (LPS)-induced nuclear translocation of NF-κB and production of TNF-α in mouse macrophage RAW264·7 cells. Moreover, the addition of vitamin K3 before and after LPS administration attenuated the severity of lung injury in an animal model of acute lung injury/acute respiratory distress syndrome (ARDS), which occurs in the setting of acute severe illness complicated by systemic inflammation. In the ARDS model, vitamin K3 also suppressed the LPS-induced increase in the serum TNF-α level and inhibited the LPS-evoked nuclear translocation of NF-κB in lung tissue. Despite marked efforts, little therapeutic progress has been made, and the mortality rate of ARDS remains high. Vitamin K3 may be an effective therapeutic strategy against acute lung injury including ARDS.
Keywords: ARDS, LPS, NF-κB, TNF-α, vitamin K3
Introduction
Vitamin K is a family of fat-soluble compounds including phylloquinone (vitamin K1), menaquinone (vitamin K2) and menadione (vitamin K3) (Fig. 1). Vitamin K1, which is the best-known member of the vitamin K family, is found abundantly in many plants and algae, especially green leafy vegetables. Vitamin K2 is produced by bacteria. Vitamin K3, a synthetic analogue of vitamin K, acts as a provitamin that is converted into a vitamin in the body. Vitamin K plays important roles in physiological functions; it acts as a critical factor in blood coagulation and bone metabolism in mammals [1]. Recently, it was reported that vitamin K has anti-cancer activity, and the anti-cancer effects of vitamin K3 against hepatoma cells was found to be greater than those of vitamins K1 and K2[2]. Vitamin K3 strongly suppressed the proliferation of human colon cancer cells and induced apoptosis by inhibiting human DNA polymerase γ, which is a mitochondrial DNA polymerase, but vitamins K1 and K2 did not [1]. The inhibitory effects of vitamin K3 on angiogenesis in a rat aortic ring model were significantly stronger than those of vitamins K1 and K2[3]. From these results, vitamin K3 is a candidate as a therapeutic agent against various diseases.
Fig. 1.
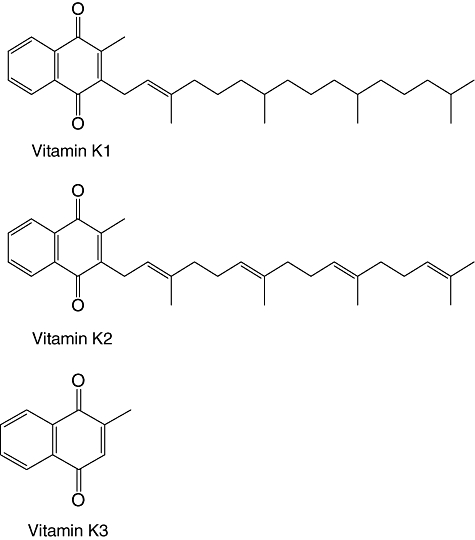
Chemical structures of vitamins K1, K2 and K3.
Acute lung injury/acute respiratory distress syndrome (ARDS) occurs in the setting of acute severe illness complicated by systemic inflammation. ARDS represents a state of excess production of inflammatory mediators from immune cells, such as cytokines, chemokines, adhesion molecules and bioactive lipid products [4]. The most common pathological condition of ARDS is sepsis. Lipopolysaccharide (LPS) released during sepsis is the major stimulus for the release of inflammatory mediators [5]. Administration of LPS to experimental animals causes the pathological condition of ongoing sepsis and concomitant ARDS-like lung injury, including polymorphonuclear neutrophil sequestration and lung oedema [6]. Despite marked efforts, little therapeutic progress has been made, and the mortality rate of ARDS remains high [7]. Therefore, the novel effective therapeutic strategy for ARDS is required.
In this study, we examined the suppressive effects of vitamin K3 on nuclear factor (NF)-κB activation and the following inflammatory responses in in vitro experiments using human embryonic kidney (HEK)293 cells and mouse macrophage RAW264·7 cells. Next, we investigated whether vitamin K3 was able to attenuate the severity of lung injury in an LPS-induced ARDS model.
Methods
Chemicals
Vitamins K1, K2 and K3 and LPS were purchased from Sigma (St Louis, MO, USA). For Western blot analysis and immunofluorescence staining, anti-NF-κB p65 antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and anti-rabbit IgG antibody was from Thermo Scientific (Kanagawa, Japan).
Cell culture
HEK293 cells and murine macrophage cell line RAW264·7 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 4·5 g of glucose per litre plus 10% fetal calf serum, 5 mM l-glutamine, 50 units/ml penicillin and 50 units/ml streptomycin and were cultured in a humidified incubator with an atmosphere of 95% air and 5% CO2 at 37°C.
Preparation of nuclear proteins
HEK293 cells, RAW264·7 cells and peritoneal macrophages on a six-well plate at 5 × 105 cells/well were incubated with 50 µM vitamins K1, K2 or K3 for 30 min followed by treatment with 100 ng/ml tumour necrosis factor (TNF)-α or 100 ng/ml LPS for 30 min. Preparation of nuclear proteins was performed according to the method of a previous report [8]. Briefly, the cells were washed three times with phosphate-buffered saline (PBS) and were collected with hypotonic buffer [10 mM Tris-HCl, pH 7·5, 10 mM KCl, 1·5 mM MgCl2, 0·1% nonidet P-40, 1 mM DTT, 10 µg/ml aprotinin, 10 µg/ml leupeptin, 1 mM phenylmethanesulphonylfluoride (PMSF) and 1 mM Na3VO4]. The cell lysate was incubated on ice with occasional mixing for 15 min, and the nuclei and the cytosol were separated by centrifugation at 1600 g for 10 min at 4°C. The nuclei were resuspended in hypertonic buffer [20 mM Tris-HCl, 20% glycerol, 500 mM NaCl, 1·5 mM MgCl2, 0·2 mM ethylenediamine tetraacetic acid (EDTA), 1 mM dithiothreitol (DTT), 10 µg/ml aprotinin, 10 µg/ml leupeptin, 1 mM PMSF and 1 mM Na3VO4] and incubated on ice with occasional mixing for 30 min. The nuclear proteins were obtained by centrifugation at 15 000 g for 20 min at 4°C. The protein concentration was measured using a bicinchoninic (BCA) assay kit, according to the manufacturer's protocol.
Western blot analysis
The nuclear proteins (30–50 µg protein) were boiled in a quarter-volume of sample buffer [1 M Tris-HCl, pH 7·5, 640 mM 2-mercaptoethanol, 0·2% bromphenol blue, 4% sodium dodecyl sulphate (SDS) and 20% glycerol] and were separated on 10% SDS-polyacrylamide gels. The proteins on the gels were transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with 1% skimmed milk in Tris-buffered saline Tween 20 (TBS-T) (10 mM Tris-HCl, pH 8·0. 100 mM NaCl, 0·5% Tween-20) and was probed with anti-NF-κB p65 antibody (1 : 1000) or β-actin antibody (1 : 5000) before being reacted with the corresponding horseradish peroxidase-conjugated secondary antibody (1 : 20 000). The protein/antibody complex was visualized with ChemiLumiONE (Nacalai Tesque, Kobe, Japan) and was detected using an Image Reader (LAS-3000 Imaging System, Fuji Photo Film, Tokyo, Japan).
Immunofluorescence study
HEK293 cells and RAW264·7 cells were incubated with 50 µM vitamin K3 for 30 min followed by treatment with 100 ng/ml TNF-α or 100 ng/ml LPS for 30 min. The cells were fixed with 4% paraformaldehyde for 10 min and were incubated with 0·2% saponin for 30 min. The cells were blocked with 10% normal goat serum for 30 min followed by incubation with anti-NF-κBp65 antibody (1:1000) for 60 min and the addition of Alexa546-labelled anti-rabbit IgG antibody (1:400) and Topro in PBS (1:1000). After 60 min, NF-κB p65 was visualized with a fluorescent light microscope (ZEISS, Göttingen, Germany).
DNA binding activity of NF-κBs
HEK293 cells were incubated with 50 µM vitamin K3 for 30 min followed by treatment with 100 ng/ml TNF-α for 30 min. After stimulation with TNF-α for 30 min, the nuclear proteins were prepared to evaluate the DNA binding activity of NF-κB p50 and p65. Their DNA binding activity was quantified using a commercially available NF-κB (human p50/p65) Combo Transcription Factor Assay Kit (Cayman Chemical Co., Ann Arbour, MI, USA) according to the manufacturer's protocol.
Preparation of peritoneal macrophages
All animal studies were performed according to the Kobe University Animal Experimentation Regulations. Male C57BL/6 mice were injected intraperitoneally with 100 µl of 3% thioglycollate solution. After 7 days, the mice were injected intraperitoneally with PBS, and the peritoneal cavity of the mice was washed with PBS. PBS was collected and peritoneal macrophages were separated from the PBS by centrifugation at 300 g for 5 min [9].
Measurement of TNF-α level in the cell culture medium
The RAW264·7 cells were seeded on a 12-well plate at 1 × 105 cells/well and incubated for 24 h. The cells were pretreated with vitamin K3 at various concentrations for 30 min before the addition of 100 ng/ml LPS. After stimulation with LPS for 24 h, the cell culture medium was collected to measure the TNF-α level. The TNF-α concentration was quantified using a commercially available enzyme-linked immunosorbent assay (ELISA) development system (Bay Bioscience Co. Ltd, Kobe, Japan) according to the manufacturer's protocol.
Animal experiments
C57BL/6 mice bred in-house with free access to food and water were used for all experiments. All the mice were kept under 12-h light/dark cycles and housed at 25°C. The animal experiments were designed to minimize animal suffering and to use the minimum number of animals possible to obtain a valid statistical evaluation. Male 8-week-old C57BL/6 mice were injected intraperitoneally with 100 mg/kg body weight (BW) vitamin K3 or corn oil as a vehicle control. After 30 min, the mice were injected intraperitoneally with 15 mg/kg BW LPS or saline as a vehicle control. After 1 h of 15 mg/kg BW LPS administration, the mice were killed and the blood was collected. The serum was separated from blood by centrifugation at 16 000 g for 10 min at 4°C, and the TNF-α level in serum was measured using a commercially available ELISA development system (Bay Bioscience Co. Ltd), according to the manufacturer's protocol. Before and after 30 min of 15 mg/kg BW LPS administration, the mice were injected intraperitoneally with100 mg/kg BW vitamin K3 or corn oil as a vehicle control. After 6 h of 15 mg/kg BW LPS administration, the mice were killed and their caudal lobes were removed and immediately put into 10% neutral formalin solution for fixation. The tissues were embedded in paraffin and cut into 5-µm-thick slices. Conventional haematoxylin and eosin staining (H&E ×400) was performed. Half of the lung was also used as a tissue sample. The tissue samples were homogenized in lysis buffer A (10 mM Tris-HCl, 10 mM KCl, 1·5 mM MgCl2, 0·5% Triton X-100, 10 µg/ml aprotinin, 10 µg/ml leupeptin, 1 mM PMSF, 1 mM Na3VO4 and 1·0 mM DTT). The cell lysate was incubated on ice for 10 min and centrifuged at 1600 g for 10 min at 4°C. The precipitation was resuspended in lysis buffer A, incubated on ice with 10 min and centrifuged at 1600 g for 15 min at 4°C. After this process was repeated three times, the precipitation obtained was suspended in 1 ml of lysis buffer A without Triton X-100. The homogenates were vortexed for 30 s and then centrifuged at 1600 g for 10 min at 4°C. The precipitation was resuspended in lysis buffer B (10 mM Tris-HCl, 25% glycerol, 420 mM NaCl, 1·5 mM MgCl2, 0·2 mM EDTA, 10 µg/ml aprotinin, 10 µg/ml leupeptin, 1 mM PMSF, 1 mM Na3VO4 and 1 mM DTT) and incubated on ice for 30 min with occasional mixing. The homogenates were centrifuged at 15 000 g for 20 min at 4°C, and the supernatant obtained was used as a source of nuclear proteins for Western blot analysis. Before and after 30 min of 15 mg/kg BW LPS administration, the mice were injected intraperitoneally with100 mg/kg BW vitamin K3 or corn oil as a vehicle control. After 24 h of 15 mg/kg BW LPS administration, the mice lungs were resected and lavaged. For lavage, the diaphragm was punctured, and the trachea was exposed, nicked and then cannulated with 18-gauge injection needle. The cannula was secured with 3-O surgical silk (Ethicon, Somerville, NJ, USA), and the lungs were infused with 500 µl PBS using a 1-ml syringe. In one set of animals (n = 3 per group), the lungs were lavaged three times with 1 ml of PBS. The total cell number was determined using a haemocytometer. The number of neutrophils was determined on bronchoalveolar lavage (BAL) smear slides that were stained with Diff-Quick (Sysmex International Reagents, Kobe, Japan).
Statistical analysis
All data are expressed as means ± standard error (s.e.) of at least three independent determinations for each experiment. Statistical significance was analysed using the Student's t-test, and a level of probability of 0·05 was used as the criterion for significance.
Results
Inhibitory effects of vitamin K3 on the TNF-α-induced activation of NF-κBs in HEK293 cells
First, we investigated whether vitamin K3 was able to inhibit the nuclear translocation of NF-κB p65 and the binding of NF-κBs to DNA by TNF-α stimulation in HEK293 cells. TNF-α, an inflammatory cytokine, activates the NF-κB signalling pathway via its binding to the TNF-α receptor (TNFR) and initiates inflammatory responses, resulting in various inflammatory diseases [10]. In Western blot analysis (Fig. 2a) and the immunofluorescence study (Fig. 2b), 100 ng/ml TNF-α caused the translocation of NF-κB p65 into the nucleus, and 50 µM vitamin K3 inhibited the 100 ng/ml TNF-α-caused nuclear translocation. Vitamin K3 at 50 µM also reduced significantly the DNA binding activity of NF-κB p50 and p65 in HEK293 cells stimulated with 100 ng/ml TNF-α (Fig. 2c). Vitamins K1 and K2, which belong to the vitamin K family, were reported to have anti-inflammatory effects [11,12]. Therefore, the inhibitory effect of vitamins K1 and K2 on the NF-κB p65 nuclear translocation was compared with that of vitamin K3 (Fig. 2d). Under our experimental conditions, vitamins K1 and K2 were not able to inhibit the nuclear translocation of NF-κB p65, unlike vitamin K3.
Fig. 2.
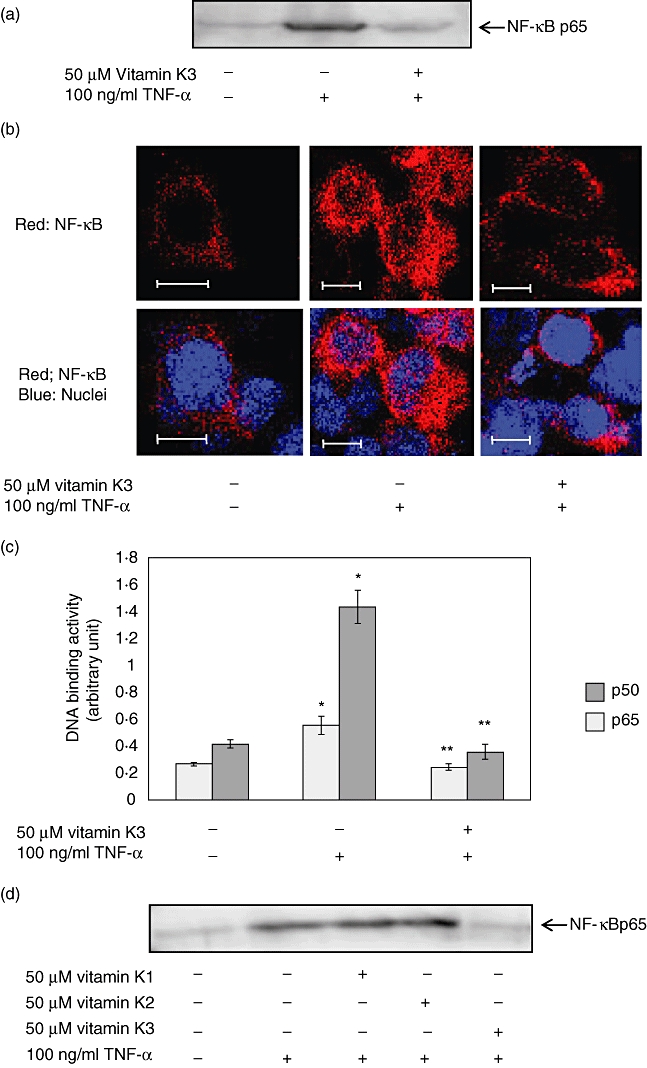
Inhibitory effects of vitamin K3 on the tumour necrosis factor (TNF)-α-evoked nuclear factor (NF)-κB activation in human embryonic kidney HEK293 cells. HEK293 cells were incubated with 50 µM vitamin K3 or dimethylsulphoxide (DMSO) as a vehicle control for 30 min, followed by treatment with 100 ng/ml TNF-α for 30 min. (a) The nuclear proteins were prepared from the cells and were subjected to Western blot analysis for evaluation of the nuclear translocation of NF-κB p65. (b) The cells were fixed in 4% paraformaldehyde, and the nuclear translocation of NF-κB p65 was assessed by immunofluorescence microscopy (red: NF-κB; blue: nuclei). Scale bar, 10 µm. Typical pictures are shown from at least triplicate determinations. (c) The nuclear proteins were prepared from the cells, and the DNA binding activity of NF-κB p50 and p65 were evaluated. Light grey bar: NF-κB p50; dark grey bar: NF-κB p65. Each data value is expressed as mean ± standard error of duplicates of three experiments. The single asterisk indicates the presence of significant differences between negative control and TNF-α alone (P < 0·05), and the double asterisks indicate the presence of significant differences between TNF-α alone and vitamin K3 plus TNF-α (P < 0·05). (d) HEK293 cells were pretreated with 50 µM vitamins K1, K2 and K3 or DMSO as a vehicle control for 30 min followed by the addition of 100 ng/ml TNF-α. After 30 min the nuclear proteins were prepared, and NF-κB p65 in the nucleus was detected by Western blot analysis. Typical pictures are shown from at least triplicate determinations.
Inhibitory effects of vitamin K3 on the LPS-evoked NF-κB p65 nuclear translocation and TNF-α production in RAW 264·7 cells
LPS, a membrane constituent of Gram-negative bacteria and a bacterial endotoxin, is a potent stimulator of inflammatory responses. LPS also induces the NF-κB activation via Toll-like receptor 4 and causes the following production of various cytokines [13]. Therefore, we investigated whether vitamin K3 was able to suppress the LPS-induced production of TNF-α in RAW264·7 cells (Fig. 3a). LPS at the concentration of 100 ng/ml produced 5769 pg/ml TNF-α, and 50 µM vitamin K3 decreased the LPS-evoked TNF-α production to 232 pg/ml. Next, the inhibitory effects of vitamin K3 on the LPS-caused NF-κB p65 nuclear translocation were evaluated in RAW264·7 cells. In Western blot analysis (Fig. 3b) and the immunofluorescence study (Fig. 3c), vitamin K3 suppressed completely the LPS-induced nuclear translocation of NF-κB p65.
Fig. 3.
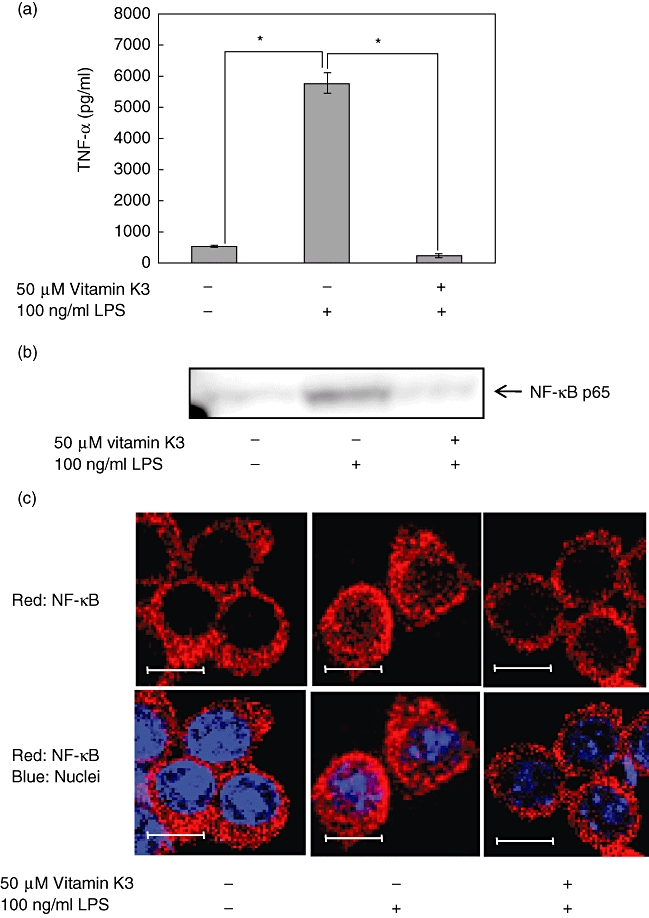
Inhibitory effects of vitamin K3 on the lipopolysaccharide (LPS)-induced tumour necrosis factor (TNF)-α production and nuclear factor (NF)-κB nuclear translocation in RAW264·7 cells. (a) RAW264·7 cells were pretreated with 50 µM vitamin K3 or DMSO as a vehicle control for 30 min and then treated with 100 ng/ml LPS for 24 h, and the TNF-α concentration in the cell medium was measured by enzyme-linked immunosorbent assay. Each data value is expressed as mean ± standard error of duplicates of three experiments. The presence of significant differences is indicated by asterisks (P < 0·05). (b) RAW264·7 cells were incubated with 50 µM vitamin K3 or dimethylsulphoxide (DMSO) as a vehicle control for 30 min, followed by treatment with 100 ng/ml LPS for 30 min. The nuclear proteins were prepared from the cells and were subjected to Western blot analysis for evaluation of the nuclear translocation of NF-κB p65. Typical pictures are shown from at least triplicate determinations. (c) RAW264·7 cells were treated as described in Fig. 3b, and the nuclear translocation of NF-κB p65 was assessed by immunofluorescence microscopy (red; NF-κB; blue: nuclei). Typical pictures are shown from at least triplicate determinations. Scale bar, 10 µm.
Inhibitory effects of vitamin K3 on the LPS-evoked NF-κB nuclear translocation and TNF-α production in peritoneal macrophages
To confirm the anti-inflammatory effects of vitamin K3in vivo, peritoneal macrophages were prepared from C57BL/6 mice, and the dose-dependent suppressive effect of vitamin K3 on the TNF-α production was evaluated in peritoneal macrophages (Fig. 4a). As a result, 25 and 50 µM vitamin K3 inhibited significantly the 100 ng/ml LPS-induced TNF-α production, although 5 µM vitamin K3 had no effect. In Western blot analysis, it was also confirmed that vitamin K3 could suppress the LPS-induced nuclear translocation of NF-κB p65 in peritoneal macrophages (Fig. 4b).
Fig. 4.
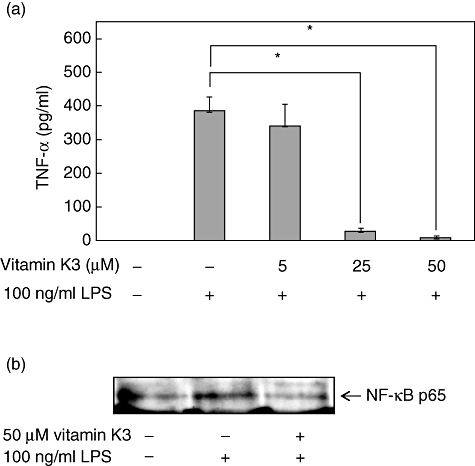
Inhibitory effects of vitamin K3 on the lipopolysaccharide (LPS)-induced tumour necrosis factor (TNF)-α production and nuclear factor (NF)-κB p65 nuclear translocation in peritoneal macrophages. (a) Peritoneal macrophages were prepared from C57BL/6 mice and were incubated with 5, 10 or 50 µM vitamin K3 or dimethylsulphoxide (DMSO) as a vehicle control for 30 min. The cells were then treated with 100 ng/ml LPS for 24 h, and the TNF-α concentration in the cell medium was measured by enzyme-linked immunosorbent assay. Each data value is expressed as mean ± standard error of duplicates of three experiments. The presence of significant differences is indicated by asterisks (P < 0·05). (b) Peritoneal macrophages were incubated with 50 µM vitamin K3 or DMSO as a vehicle control for 30 min, followed by treatment with 100 ng/ml LPS for 30 min. The nuclear proteins were prepared from the cells and were subjected to Western blot analysis for evaluation of the nuclear translocation of NF-κB p65. Typical pictures are shown from at least triplicate determinations.
Anti-inflammatory effects of vitamin K3 in the ARDS model
Administration of high dose LPS causes ARDS, which occurs in the setting of acute severe illness complicated by systemic inflammation [6]. Therefore the mice were injected intraperitoneally with 15 mg/kg BW LPS, and the mouse model of ARDS was developed. To evaluate the suppressive effects of vitamin K3 in the LPS-induced ARDS mouse model, the mice were injected intraperitoneally with 15 mg/kg BW LPS 30 min after 100 mg/kg BW vitamin K3 administration. Administration of 15 mg/kg BW LPS led to a significant increase in the serum TNF-α concentration to 2828 pg/ml, and vitamin K3 reduced significantly the serum TNF-α level (Fig. 5a). Next, we investigated whether vitamin K3 was able to attenuate the severity of lung injury in the ARDS model (Fig. 5b). In mice treated with 15 mg/kg BW LPS, lung injury including intra-alveolar haemorrhaging, interstitial oedema, alveolar collapse and massive inflammatory cells infiltration was confirmed by H&E staining using light microscopy. In the group that was treated with 100 mg/kg BW vitamin K3 and 15 mg/kg BW LPS, the disruption of lung tissue was attenuated in comparison with that of LPS alone. The suppressive effects of vitamin K3 in the ARDS model were also examined quantitatively. The total cell count in the BAL fluid was increased approximately 25-fold 24 h after administration of 15 mg/kg BW LPS, and the number of neutrophils in BAL was also increased significantly. Treatment of 100 mg/kg BW vitamin K3 reduced significantly the LPS-evoked increases in the total cell and neutrophil counts in the BAL fluid (Fig. 5c,d). To elucidate the mechanism underlying the attenuation of LPS-evoked lung injury by vitamin K3, the inhibitory effect of vitamin K3 on the nuclear translocation of NF-κB p65 in lung tissue was examined by Western blot analysis. As shown in Fig. 5e, vitamin K3 suppressed the LPS-induced nuclear translocation of NF-κB p65.
Fig. 5.
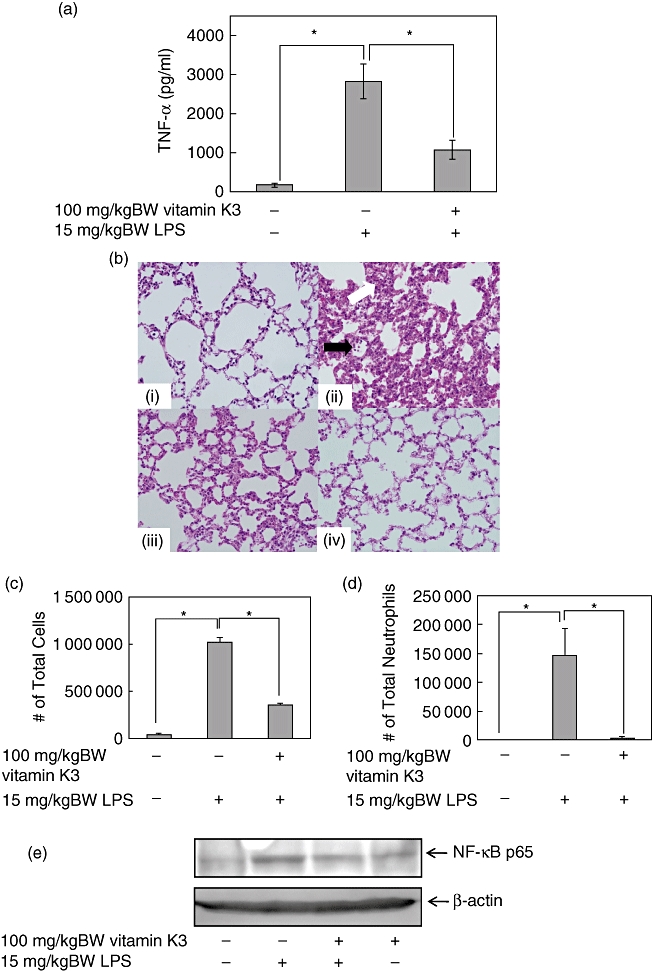
Suppressive effects of vitamin K3 in the lipopolysaccharide (LPS)-induced acute respiratory distress syndrome (ARDS) mouse model. Male C57BL/6 mice were injected intraperitoneally with 100 mg/kg body weight (BW) vitamin K3 or corn oil as vehicle control. After 30 min, the mice were injected intraperitoneally with 15 mg/kg BW LPS or saline as a vehicle control. (a) After 1 h, the mice were killed, and the serum tumour necrosis factor (TNF)-α concentration was measured by enzyme-linked immunosorbent assay. Each data value is expressed as mean ± standard error (s.e.), n = 3. The presence of significant differences is indicated by asterisks (P < 0·05). (b) After 6 h, the mice were killed, and the lung tissue was collected. The lung tissue was stained with haematoxylin and eosin (H&E ×400), and histopathological examination of the lung tissue was performed. Typical pictures are shown from at least triplicate determinations. (i) Negative control group; (ii) LPS group; (iii) vitamin K3+LPS group; (iv) vitamin K3 group. The white arrow indicates oedematous change in the alveolar wall and the swelling of alveolar epithelial cells, and the black arrow indicates massive polymorphonuclear infiltration. (c,d) After 24 h the mice were killed, and total cell (c) and neutrophil (d) counts in the broncoalveolar lavage (BAL) fluid were performed. Each data value is expressed as mean ± s.e., n = 3. The presence of significant differences is indicated by asterisks (P < 0·05). (e) After 6 h the mice were killed and the lung tissue was collected. Nuclear proteins were prepared from lung tissue and were subjected to Western blot analysis to evaluate the nuclear translocation of NF-κB p65, and the β-actin protein was detected as the internal standard. Typical pictures are shown from at least triplicate determinations.
Because it was found that vitamin K3 could exert the suppressive effects in the ARDS model (Fig. 5), the therapeutic effects of vitamin K3 in the ARDS model are also expected. To evaluate the therapeutic effects of vitamin K3 in the LPS-induced ARDS mouse model, the mice were injected intraperitoneally with 100 mg/kg BW vitamin K3 30 min after 15 mg/kg BW LPS administration. First, we investigated whether the post-treatment of vitamin K3 was able to attenuate the severity of lung injury in the LPS-induced ARDS model. In the group treated with 15 mg/kg BW LPS and 100 mg/kg BW vitamin K3, the disruption of lung tissue was attenuated in comparison with the group of LPS alone (Fig. 6a). In addition, 100 mg/kg BW vitamin K3 could reduce significantly the LPS-caused increases in both the total cell and neutrophil counts in the BAL fluid (Fig. 6b,c).
Fig. 6.
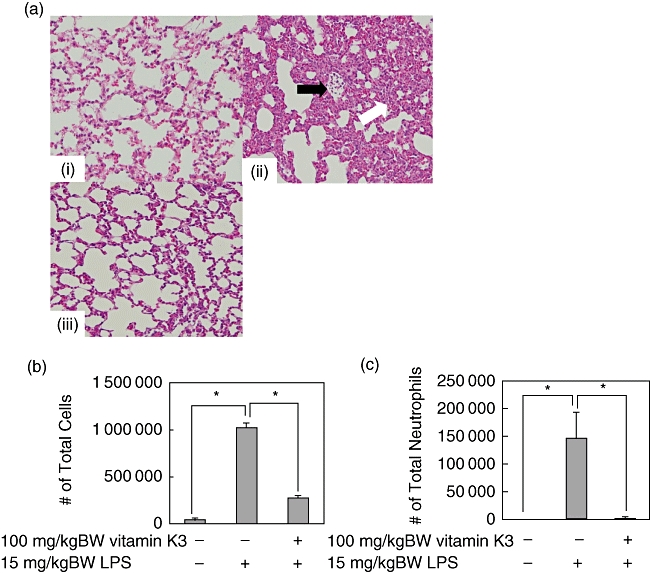
Therapeutic effects of vitamin K3 in the lipopolysaccharide (LPS)-induced acute respiratory distress syndrome (ARDS) mouse model. Male C57BL/6 mice were injected intraperitoneally with 15 mg/kg body weight (BW) LPS or saline as a vehicle control. After 30 min, the mice were injected intraperitoneally with 100 mg/kg BW vitamin K3 or corn oil as vehicle control. (a) After 6 h the mice were killed, and the lung tissue was collected. The lung tissue was stained with haematoxylin & eosin (H&E ×400), and histopathological examination of the lung tissue was performed. Typical pictures are shown from at least triplicate determinations. (i) Negative control group; (ii) LPS group; (iii) LPS+vitamin K3 group. The white arrow indicates oedematous change in the alveolar wall and the swelling of alveolar epithelial cells, and the black arrow indicates massive polymorphonuclear infiltration. (b,c). After 24 h the mice were killed, and total cell (b) and neutrophil (c) counts in the bronchoalveolar lavage (BAL) fluid were performed. Each data value is expressed as mean ± standard error, n = 3. The presence of significant differences is indicated by asterisks (P < 0·05).
Discussion
Vitamin K, an essential nutrient, has an important role in normal blood coagulation systems via post-translational modification, and acts as a co-factor of various plasma proteins such as prothrombin, factor VII, factor IX and factor X [14]. Recently, various chemopreventive activities of vitamin K were also reported. In a rat paw oedema model, vitamin K1 and the seven carbon aliphatic carboxylic acid catabolic products from vitamin K were more potent than either phenylbutazone or Saridone® (a mixed analgesic preparation) in ameliorating inflammation and pain [15,16]. Menaquinone-4, a vitamin K2 congener, was reported to be capable of modulating osteoblast proliferation [17], alkaline phosphatase activity [17] and cyclooxygenase activity [18]. In a chronic antigen-induced rabbit model of arthritis, the oral administration of vitamin K3 was effective in inhibiting synovitis [19]. Thus, vitamin K is a possible candidate as a chemopreventive agent against various diseases.
In this study, we found that vitamin K3 was able to attenuate the lung injury and inhibit NF-κB activation in the ARDS mouse model (Figs 5 and 6). The pathogenesis of ARDS is complex and involves multiple signal transduction processes. Particular attention has been given to the NF-κB signalling, which is required for expression of various cytokines and the following inflammatory responses [20]. The degree of NF-κB activation was reported to increase in patients with sepsis or acute lung injury [21–24], and the nuclear accumulation of NF-κB p65 was observed in alveolar macrophages from patients with acute lung injury caused by severe infection, in contrast to alveolar macrophages from control patients [21,22]. The increased nuclear levels of NF-κB p65 were also confirmed in peripheral blood mononuclear cells (PBMCs) from patients with sepsis [23,24]. In addition, it appears that acute lung injury patients who do not survive exert greater and more prolonged NF-κB activation than the surviving patients [23,24]. These results suggest that NF-κB activation is central to the development of pulmonary inflammation and acute lung injury. In cell culture experiments using HEK293 cells and RAW264·7 cells, vitamin K3 was able to inhibit the NF-κB p65 activation induced by both TNF-α stimulation (Fig. 2a,b) and LPS stimulation (Fig. 3b,c). Vitamin K3 was also able to reduce the DNA binding activity of NF-κBs in HEK293 cells (Fig. 2c). TNF-α activates the NF-κB signalling pathway via the binding of TNF-α to TNFR [10]. On the other hand, LPS-evoked NF-κB activation begins with the binding of LPS to Toll-like receptor 4 [13]. Thus, the NF-κB signalling pathway has multiple first steps. Therefore, the pathway common to both TNF-α and LPS-dependent NF-κB signalling pathways may be the action site of vitamin K3. A recent study revealed that vitamin K2 inhibited cytokine-induced NF-κB activation through suppression of IκB kinase (IKK) activity [25]. IKK is associated with both TNF-α and LPS-dependent NF-κB signalling pathways, and vitamin K3 may also be able to inhibit NF-κB activation via suppressing the IKK activity. Anti-inflammatory activity was also reported to be linked to anti-oxidative activity [26], and the reactive oxygen species (ROS) and redox pathways can modulate the NF-κB signalling [27]. Vitamin K3 is the effective regulator for ROS generation and the redox system. In fact, vitamin K3 inhibited the tumour cell growth through oxidative stress via redox cycling of quinine to produce ROS [28,29]. Therefore, there is a possibility that vitamin K3 exerted the inhibitory effects on the NF-κB signalling through acting to the ROS and/or redox pathways.
Under our experimental conditions, vitamin K3 inhibited NF-κB activation more potently than vitamins K1 or K2 (Fig. 2d), although vitamins K1 and K2 were reported to have anti-inflammatory activity [11] and the suppressive effects of vitamin K2 against the NF-κB activity were observed [26]. These results mean that our data were different from the previous reports. In a previous study, the 24-h treatment of vitamin K2 led to suppression of NF-κB activation in hepatoma cells [24]. In our experiments, vitamin K2 could not inhibit the NF-κB p65 nuclear translocation 1 h after the treatment in HEK293 cells (Fig. 2d). These results suggest that vitamin K2 itself may have slight anti-inflammatory effects and its metabolized form may be a strong anti-inflammatory agent.
In conclusion, our data suggest that vitamin K3 can attenuate NF-κB-dependent inflammatory responses and related inflammatory diseases, such as ARDS. Little therapeutic progress has been made, although it was reported that some compounds, such as liquorice flavonoids, SB203580 p38MAPK specific inhibitor and Gardenia-awetu compounds, were able to attenuate acute lung injury/ARDS [30–32], and the mortality rate of ARDS remains high. Therefore, usage of vitamin K3 may be an effective therapeutic strategy against acute lung injury/ARDS.
Acknowledgments
This work was supported, in part, by a grant for the Global COE Program ‘Global Center of Excellence for Education and Research on Signal Transduction Medicine in the Coming Generation’ from the Ministry of Education, Culture, Sports, Science and Technology of Japan (M. Y.). This work was also supported by a grant for the Education Program for Specialized Clinician in the Support Program for Improving Graduate School Education from the Ministry of Education, Culture, Sports, Science and Technology of Japan (S. T.).
Disclosure
All authors declare there is no conflict of interest.
References
- 1.Sasaki R, Suzuki Y, Yonezawa Y, et al. DNA polymerase gamma inhibition by vitamin K3 induces mitochondria-mediated cytotoxicity in human cancer cells. Cancer Sci. 2008;99:1040–8. doi: 10.1111/j.1349-7006.2008.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishikawa Y, Carr BI, Wang M, et al. Growth inhibition of hepatoma cells induced by vitamin K and its analogs. J Biol Chem. 1995;270:304–10. doi: 10.1074/jbc.270.47.28304. [DOI] [PubMed] [Google Scholar]
- 3.Matsubara K, Kayashima T, Mori M, Yoshida H, Mizushina Y. Inhibitory effects of vitamin K3 on DNA polymerase and angiogenesis. Int J Mol Med. 2007;22:381–7. [PubMed] [Google Scholar]
- 4.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;31:660–1. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 5.Pittet JF, Mackersie RC, Martin TR, Matthay MA. Biological markers of acute lung injury: prognostic and pathogenetic significance. Am J Respir Crit Care Med. 1997;155:1187–205. doi: 10.1164/ajrccm.155.4.9105054. [DOI] [PubMed] [Google Scholar]
- 6.Yoshinari D, Takeyoshi I, Koibuchi Y, et al. Effects of a dual inhibitor of tumor necrosis factor-alpha and interleukin-1 on lipopolysaccharide-induced lung injury in rats: involvement of the p38 mitogen-activated protein kinase pathway. Crit Care Med. 2001;29:628–34. doi: 10.1097/00003246-200103000-00029. [DOI] [PubMed] [Google Scholar]
- 7.Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and timing of death in patients with ARDS. Chest. 2005;128:525–32. doi: 10.1378/chest.128.2.525. [DOI] [PubMed] [Google Scholar]
- 8.Nishiumi S, Yamamoto N, Kodoi R, Fukuda I, Yoshida K, Ashida H. Antagonistic and agonistic effects of indigoids on the transformation of an aryl hydrocarbon receptor. Arch Biochem Biophys. 2008;470:187–99. doi: 10.1016/j.abb.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 9.Kreckler LM, Gizewski E, Wan TC, Auchampach JA. Adenosine suppresses LPS-induced TNF-α production by murine macrophages through a protein kinase A- and Epac-independent signaling pathway. J Pharmacol Exp Ther. 2009 doi: 10.1124/jpet.109.157651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–56. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 11.Reddi K, Henderson B, Meghji S, et al. Interleukin 6 production by lipopolysaccharide-stimulated human fibroblasts is potently inhibited by naphthoquinone (vitamin K) compounds. Cytokine. 1995;7:287–90. doi: 10.1006/cyto.1995.0034. [DOI] [PubMed] [Google Scholar]
- 12.Ohsaki Y, Shirakawa H, Hiwatashi K, Furukawa Y, Mizutani T, Komai M. Vitamin K suppresses lipopolysaccharide-induced inflammation in the rat. Biosci Biotechnol Biochem. 2006;70:926–32. doi: 10.1271/bbb.70.926. [DOI] [PubMed] [Google Scholar]
- 13.Kopp E, Medzhitov R. Recognition of microbial infection by Toll-like receptors. Curr Opin Immunol. 2003;15:396–401. doi: 10.1016/s0952-7915(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 14.Cranenburg EC, Schurgers LJ, Vermeer C, Vitamin K. The coagulation vitamin that became omnipotent. Thromb Haemost. 2007;98:120–5. [PubMed] [Google Scholar]
- 15.Hanck A, Weiser H. Physiological and pharmacological effects of vitamin K. Int J Vitam Nutr Res. 1983;24:155–70. [PubMed] [Google Scholar]
- 16.Weiser H, Kormann A. Pharmacological properties of vitamin K1 and analogues: antidote functions, analgesic and antiphlogistic properties. Unpublished results presented as poster at the 17th Steenbock Symposium June 1987, Current Advances in Vitamin K Research, University of Wiscosin, Madison, USA, 1988.
- 17.Akedo Y, Hosoi T, Ikegami A, et al. Orimo H vitamin K2 modulates proliferation and function of osteoblastic cells in vivo. Biochem Biophys Res Comm. 1992;187:814–20. doi: 10.1016/0006-291x(92)91269-v. [DOI] [PubMed] [Google Scholar]
- 18.Koshihara Y, Hoshi K, Shiraki M. Vitamin K2 (menatetranone) inhibits prostaglandin synthesis in cultured human osteoblast-like periosteal cells by inhibiting prostaglandin H synthase activity. Biochem Pharmacol. 1993;46:1355–62. doi: 10.1016/0006-2952(93)90099-i. [DOI] [PubMed] [Google Scholar]
- 19.Pitsillides AA, Blake SM, Glynn LE, Bitensky L, Chayen J. Amelioration by menadione of the experimentally chronic immune arthritis in the rabbit. Cell Biochem Funct. 1990;8:221–6. doi: 10.1002/cbf.290080406. [DOI] [PubMed] [Google Scholar]
- 20.Kurosaki T. Checks and balances on developing B cells. Nat Immunol. 2003;4:13–15. doi: 10.1038/ni0103-13. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz MD, Moore EE, Moore FA, et al. Nuclear factor-kappa B is activated in alveolar macrophages from patients with acute respiratory distress syndrome. Crit Care Med. 1996;24:1285–92. doi: 10.1097/00003246-199608000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Moine P, McIntyre R, Schwartz MD, et al. NF-kappaB regulatory mechanisms in alveolar macrophages from patients with acute respiratory distress syndrome. Shock. 2000;13:85–91. doi: 10.1097/00024382-200013020-00001. [DOI] [PubMed] [Google Scholar]
- 23.Arnalich F, Garcia-Palomero E, López J, et al. Predictive value of nuclear factor kappaB activity and plasma cytokine levels in patients with sepsis. Infect Immun. 2000;68:1942–5. doi: 10.1128/iai.68.4.1942-1945.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Böhrer H, Qiu F, Zimmermann T, et al. Role of NFkappaB in the mortality of sepsis. J Clin Invest. 1997;100:972–85. doi: 10.1172/JCI119648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozaki I, Zhang H, Mizuta T, et al. Menatetreoen, a vitamin K2 analogue, inhibits hepatocellular carcinoma cell growth by suppressing cycling D1 expression through inhibition of nuclear factor kB activation. Clin Cancer Res. 2007;13:2236–45. doi: 10.1158/1078-0432.CCR-06-2308. [DOI] [PubMed] [Google Scholar]
- 26.Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006;72:1439–52. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Bode A, Dong Z. Apoptosis induction by arsenic: mechanisms of actions and possible clinical applications for treating therapy-resistant cancers. J Drug Resist. 2000;3:21–9. doi: 10.1054/drup.2000.0114. [DOI] [PubMed] [Google Scholar]
- 28.Gant T, Rao DN, Mason RP, Cohen GM. Redox cycling and sulphydryl arylation; their relative importance in the mechanism of quinone cytotoxicity to isolated hepatocytes. Chem Biol Interact. 1988;65:157–73. doi: 10.1016/0009-2797(88)90052-x. [DOI] [PubMed] [Google Scholar]
- 29.Sun JS, Tsuang YH, Huang WC, Chen LT, Hang YS, Lu FJ. Menadione-induced cytotoxicity to rat osteoblasts. Cell Mol Life Sci. 1997;53:967–76. doi: 10.1007/s000180050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu S, Feng G, Wang GL, Liu GJ. p38MAPK inhibition attenuates LPS-induced acute lung injury involvement of NF-kappaB pathway. Eur J Pharmacol. 2008;14:159–65. doi: 10.1016/j.ejphar.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Xie YC, Dong XW, Wu XM, Yan XF, Xie QM. Inhibitory effects of flavonoids extracted from licorice on lipopolysaccharide-induced acute pulmonary inflammation in mice. Int Immunopharmacol. 2009;9:194–200. doi: 10.1016/j.intimp.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Yan D, Li LJ, Du WB, et al. Effects of Gardenia-aweto compound by different extraction method on antagonizing acute respiratory distress syndrome. Zhongguo Zhong Yao Za Zhi. 2005;30:700–3. [PubMed] [Google Scholar]


