Abstract
Objective
Inflammatory processes and foam cell formation are key determinants in the initiation and progression of atherosclerosis. Electrophilic nitro-fatty acids, byproducts of nitric oxide- and nitrite-dependent redox reactions of unsaturated fatty acids, exhibit anti-inflammatory signaling actions in inflammatory and vascular cell model systems. The in vivo action of nitro-fatty acids in chronic inflammatory processes such as atherosclerosis remains to be elucidated.
Methods and Results
Herein, we demonstrate that subcutaneously administered 9- and 10-nitro-octadecenoic acid (nitro-oleic acid) potently reduced atherosclerotic lesion formation in apolipoprotein E deficient mice. Nitro-fatty acids did not modulate serum lipoprotein profiles. Immunostaining and gene expression analyses revealed that nitro-oleic acid attenuated lesion formation by suppressing tissue oxidant generation, inhibiting adhesion molecule expression and decreasing vessel wall infiltration of inflammatory cells. In addition, nitro-oleic acid reduced foam cell formation by attenuating oxidized LDL-induced phosphorylation of signal transducer and activator of transcription-1 (STAT-1), a transcription factor linked to foam cell formation in atherosclerotic plaques. Atherosclerotic lesions of nitro-oleic acid-treated animals also showed an increased content of collagen and α-smooth muscle actin, suggesting conferral of higher plaque stability.
Conclusions
These results reveal the anti-atherogenic actions of electrophilic nitro-fatty acids in a murine model of atherosclerosis.
Keywords: nitro-fatty acids, atherosclerosis, foam cells, inflammation, oxidative stress, electrophile
Nitro-fatty acid derivatives (NO2-FA) are electrophilic lipid signaling mediators generated endogenously by nitric oxide (NO) and nitrite (NO2−)-derived reactive species during oxidative stress.1 NO2-FA signal via predominantly anti-inflammatory mechanisms that inhibit neutrophil activation, platelet aggregation, and macrophage activation.2–5 NO2-FA also activate peroxisome proliferator-activated receptor-γ and induce heme oxygenase-1 expression – both critical mediators of anti-inflammatory actions in the vasculature.6–8
NO2-FA mainly exert cell signaling actions by a covalent and reversible post-translational modification (S-alkylation) of key signaling proteins, due to a kinetically rapid electrophilic reactivity.9,10 Thiol residues of proteins are highly susceptible nucleophilic targets of electrophile reaction, with S-alkylation frequently altering protein structure and function. For example, a) NO2-FA covalently bind the p65 subunit of nuclear factor kappa B (NFκB), inhibiting DNA binding activity, repressing NFκB-dependent gene expression and suppressing downstream pro-inflammatory reactions such as macrophage cytokine and nitric oxide synthase-2 expression,3 b) NO2-FA S-alkylate critical thiols of Keap-1, inducing the release of Nrf2 and activation of antioxidant response element (ARE)-dependent gene products, a reaction that in turn mitigates inflammatory responses,11 c) NO2-FA act as agonists of peroxisome proliferator-activated receptor (PPARγ) by S-alkylating PPARγ at Cys28512 and d) NO2-FA serve as robust small molecule inducers of heat shock response gene expression.13 Finally, NO2-FA inhibit the phosphorylation of the pro-inflammatory signal transducer and activator of transcription-1 (STAT-1) in LPS-stimulated macrophages.14 Notably, STAT-1 activation is closely linked to foam cell formation in murine models of atherosclerosis.15–17
Atherosclerosis is a chronic inflammatory disease, in which leukocyte activation plays an important role in atherosclerotic lesion initiation and progression.18 Since NO2-FA inhibit neutrophil function and monocyte adhesion to endothelial cells in vitro, by suppressing adhesion molecule expression and cytokine release from activated monocytes/macrophages,3 we investigated the effect of nitro-oleic acid (OA-NO2) on atherosclerotic lesion formation in apolipoprotein E deficient (apoE−/−) mice.
Herein we reveal that OA-NO2 significantly reduced atherosclerotic lesion formation by attenuating the accumulation of inflammatory cells within atherosclerotic lesions and limiting the expression of adhesion molecules and monocyte chemoattractant protein-1 (MCP-1). Moreover, OA-NO2 inhibited oxidized LDL (oxLDL) induced phosphorylation of STAT-1 both in vitro and in vivo, a reaction that minimizes lipid accumulation in macrophages and inhibits foam cell formation. These results support that electrophilic lipid oxidation and nitration products stemming from oxidative inflammatory reactions can serve to induce adaptive and anti-inflammatory actions in cardiovascular diseases.
Methods
Animals and experimental design
Male apoE−/− mice (Jackson Laboratory, 8 weeks of age) fed an atherogenic diet for 12 weeks (21% fat and 1.25% cholesterol, Harlan Teklan) were treated with OA-NO2 (8 mg/kg/day), OA (8 mg/kg/day, Nu Chek Inc.) or vehicle (polyethylene glycol/ethanol) via subcutaneously implanted osmotic mini-pumps (Alzet®, model 2004). OA-NO2 was synthesized via nitroselenation, giving an equimolar distribution of 9- and 10-nitro-octadecenoic acid regioisomers.8 All animal studies were approved by the University of Pittsburgh Institutional Animal Care and Use Committee (Approval 0709432).
Lesion analysis
Atherosclerotic lesions at the level of the aortic valve were detected by Oil Red O staining. For lesion quantification, 4 sections cut every 20 µm from one animal were analyzed (n=14 animals per treatment group). For determination of atherosclerotic plaque area in the entire aorta, aortas were dissected and stained en-face with Sudan (n=6 per group). Quantitative analysis of plaque area was performed by two blinded observers using image analysis software (MetaMorph software, MDS).
Histology, immunofluorescence and western blot analysis
Aortic root sections were stained with Trichrome, DHE (Sigma) and specific antibodies against monocytes/macrophages (MOMA-2, Serotec), VCAM (Santa Cruz), ICAM (Abcam), neutrophils (Ly6G, Abcam), α-smooth muscle actin (α-SMA, Sigma), 3-nitrotyrosine and pSTAT-1 (Tyr701-phosphorylation, Cell Signaling). Addition of PEG-SOD served as control for non-specific superoxide detection by DHE oxidation. Color threshold and planimetry was used for quantification. All quantifications were performed by using MetaMorph software (MDS), since this program is able to automatically detect positively stained areas/cells by setting specific color thresholds. For western blot analysis a specific antibody against VASP (Immunoglobe) was used.
Metabolic measurements
Plasma triacylglyceride (TAG) and cholesterol (Chol) levels were determined after a 6 hour fast using enzyme based assays (Triglyceride Assay Kit, Cayman, EnzymChrom Cholesterol Assay Kit, BioAssay Systems). Blood glucose was assessed by a TrueTrack glucometer (Home Diagnostics Inc). FPLC analysis was performed to assess lipoprotein profiles.
Quantitative real-time PCR
Aortas (n=10 per group) were dissected and RNA was extracted by TRIzol® (Invitrogen) and reverse transcribed using iScript™ cDNA Synthesis Kit (Biorad) according to the manufacturer’s instructions. Real-time PCR was performed with TaqMan Fast Universal PCR Master Mix using gene expression assays for ICAM, VCAM, CD68 and MCP-1 normalized to β2-microglobulin. Samples were run in duplicate on the StepOne detection system (Applied Biosystems).
Assessment of lipid accumulation in isolated peritoneal macrophages
Three days after intraperitoneal thioglycollate injection, peritoneal macrophages were harvested from OA-NO2 or vehicle treated apoE−/− mice fed a high fat diet (4 weeks of treatment, n=4 per group). Following collection, peritoneal macrophages were immediately seeded at the same density into 6 well plates with 10% FBS/DMEM and after attachment cells were fixed and stained for lipids using Oil Red O staining.
In vitro experiments
Bone marrow-derived macrophages (BMDMs) were isolated from apoE−/− mice following 4 weeks of treatment with OA-NO2 or vehicle. BMDMs were grown in 20% FBS/DMEM media (Cellgro) containing special growth factors derived from cultured CCL-1 cells (ATCC). BMDMs were cultured no longer than 25 days before use. For western blot analysis of STAT-1 and phosphorylated STAT-1 (anti-STAT-1 antibody, Tyr701, anti-pSTAT-1 antibody, Cell Signaling) BMDMs were treated with vehicle, oxLDL (100 µg/ml), OA-NO2 (500 nM, 1000 nM) or a combination of oxLDL and OA-NO2 for 2 hours in 10% FBS/DMEM. Lipopolysaccharide (LPS, 100 ng/ml) served as positive control. Densitometric analysis using ImageJ software (NIH) was applied to assess differences between the treatment groups.
Native LDL was collected with University of Pittsburgh IRB approval (PRO07110032) from healthy volunteers following a modified protocol as previously.19 In brief, after addition of diethylene triamine pentaacetic acid (DTPA) plasma density was adjusted to 1.21 g/ml using potassium bromide. After ultracentrifugation the LDL fraction was collected, concentrated and dialyzed. Purified LDL was filtered and protein concentration was determined. Following oxidation by cupric chloride, agarose gel electrophoresis revealed a relative electrophoretic mobility shift of 1.5 for oxLDL as compared to native LDL.
Superoxide production by BMDMs was determined via spectrophotometric analysis of cytochrome c reduction20 following 6 hours of incubation with LPS (20 ng/ml) alone or in combination with OA-NO2 (25 and 50 nM) in serum-free media.
Statistical analysis
Data are presented as mean ± S.E.M. Continuous variables were tested for normal distribution using the Kolmogorov-Smirnov test. Statistical analysis was performed using one-way ANOVA followed by Bonferroni post hoc test or unpaired Student’s t-test as appropriate. A value of p<0.05 was considered statistically significant. All calculations were carried out using SPSS version 15.0.
Results
OA-NO2 decreases atherosclerotic lesion formation
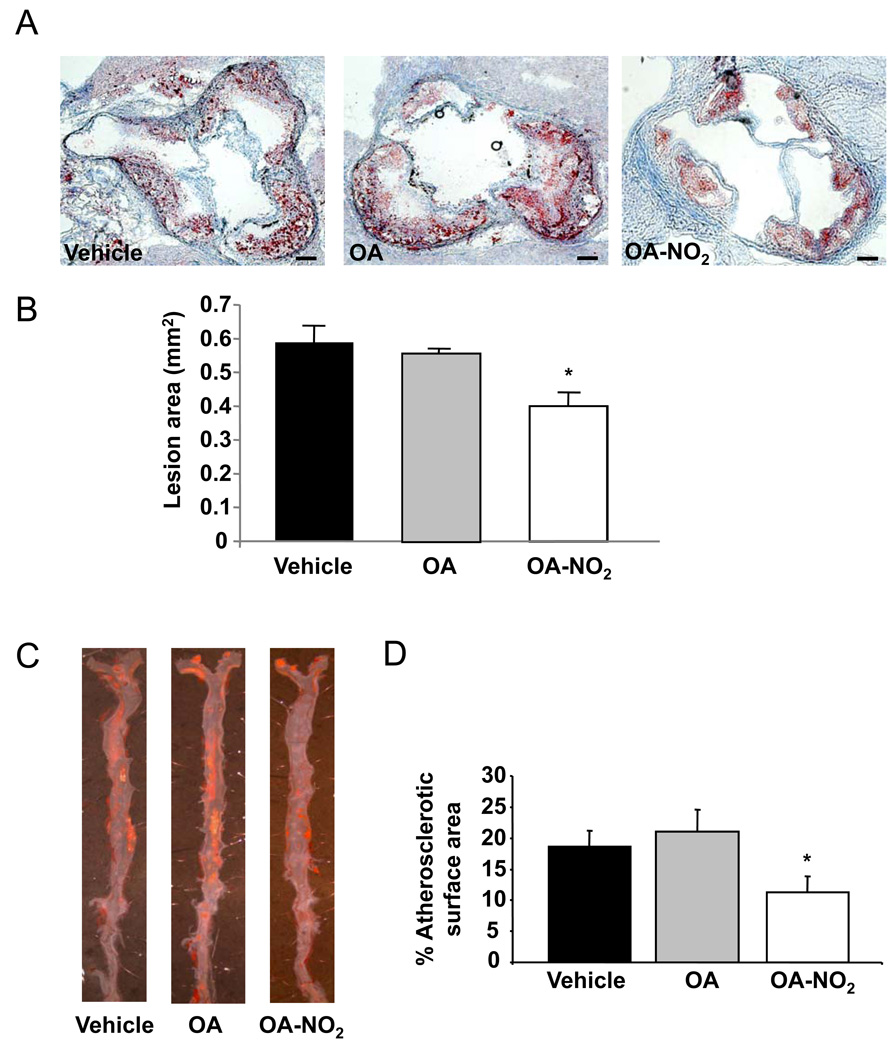
Male apoE−/− mice fed an atherogenic high-fat diet were treated with either vehicle, OA or OA-NO2 (n=14 animals per group) for 12 weeks. OA-NO2 significantly inhibited the formation of atherosclerotic lesions in the aortic root by 32%, compared with vehicle and OA (lesion area OA-NO2: 0.40±0.04 mm2; vehicle: 0.59±0.05 mm2; OA: 0.56±0.01 mm2; p=0.002, Figure 1A and B). There were no significant differences between vehicle and OA treated animal lesion areas. En-face Sudan staining of whole aortas (n=6) also revealed a significant 39% reduction of atherosclerotic plaque formation in OA-NO2 treated animals (p<0.001, Figure 1C and D).
Figure 1.
OA-NO2 inhibits atherosclerotic lesion formation. A, Representative Oil Red O stained aortic sections (Magnification 10X, scale bar indicates 100 µm). B, In apoE−/− mice (n=14 per group), fed an atherogenic diet for 12 weeks, OA-NO2 significantly reduced aortic lesion area as compared to OA and vehicle (p<0.001, *p=0.003 vs. vehicle, p=0.015 vs. OA). C, Representative en-face Sudan staining of the whole aorta. D, Quantification of atherosclerotic lesions revealing significantly less atherosclerotic area in OA-NO2 -treated mice (p<0.001, *p<0.001 vs. vehicle and OA; n=6 per group).
OA-NO2 does not influence metabolic profile
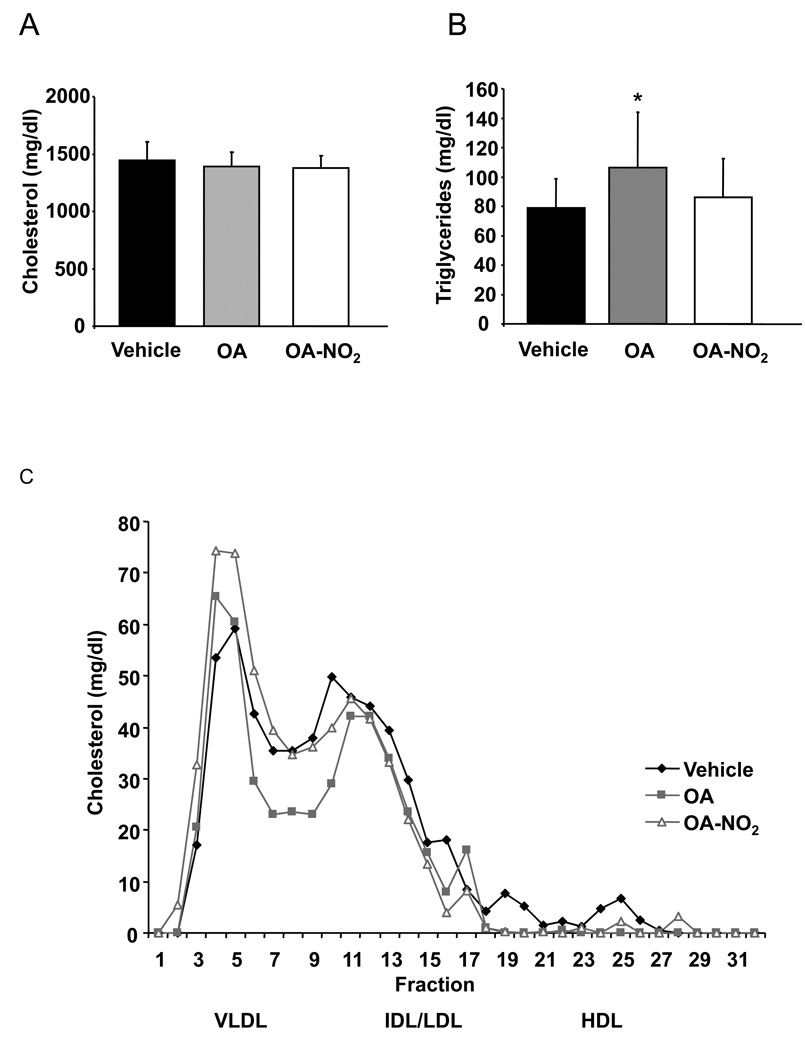
To test whether OA-NO2–induced reduction of atherosclerotic lesion formation was due to metabolic effects, fasting plasma lipid and glucose levels were determined. Total cholesterol (Chol) and total triacylglyceride (TAG) levels after 12 weeks of treatment were not affected by OA-NO2 treatment. Of note, OA significantly increased plasma TAG levels after 12 weeks, compared with vehicle and OA-NO2 (p<0.033. Figure 2A and B). FPLC analysis showed no impact of OA-NO2 or control treatments on the plasma lipoprotein profile (Figure 2C).
Figure 2.
Metabolic profile was not influenced by OA-NO2. A and B, Levels of total plasma cholesterol and triacylglycerides were not changed by OA-NO2 (p=0.03, *p=0.035 vs. vehicle). C, FPLC analysis showed no impact of any treatment on the cholesterol profile.
There were also no differences in blood glucose levels (baseline: OA-NO2: 116.3±4.6 mg/dl; vehicle: 113.6±7.4 mg/dl; OA: 115.7±6.0 mg/dl; p=0.95; 12 weeks: OA-NO2: 106.9±5.3 mg/dl; vehicle: 111.9±5.1 mg/dl; OA: 107.7±5.8 mg/dl; p=0.78), food consumption and weight gain between the three groups (weight, baseline: OA-NO2: 22.4±0.4 g; vehicle: 22.2±0.6 g; OA: 22.1±0.4 g; p=0.93; 12 weeks: OA-NO2: 30.7±0.3 g; vehicle: 30.9±0.7 g; OA: 30.3±0.4 g; p=0.68).
Anti-inflammatory and antioxidant actions of OA-NO2 inhibit atherosclerotic lesion formation
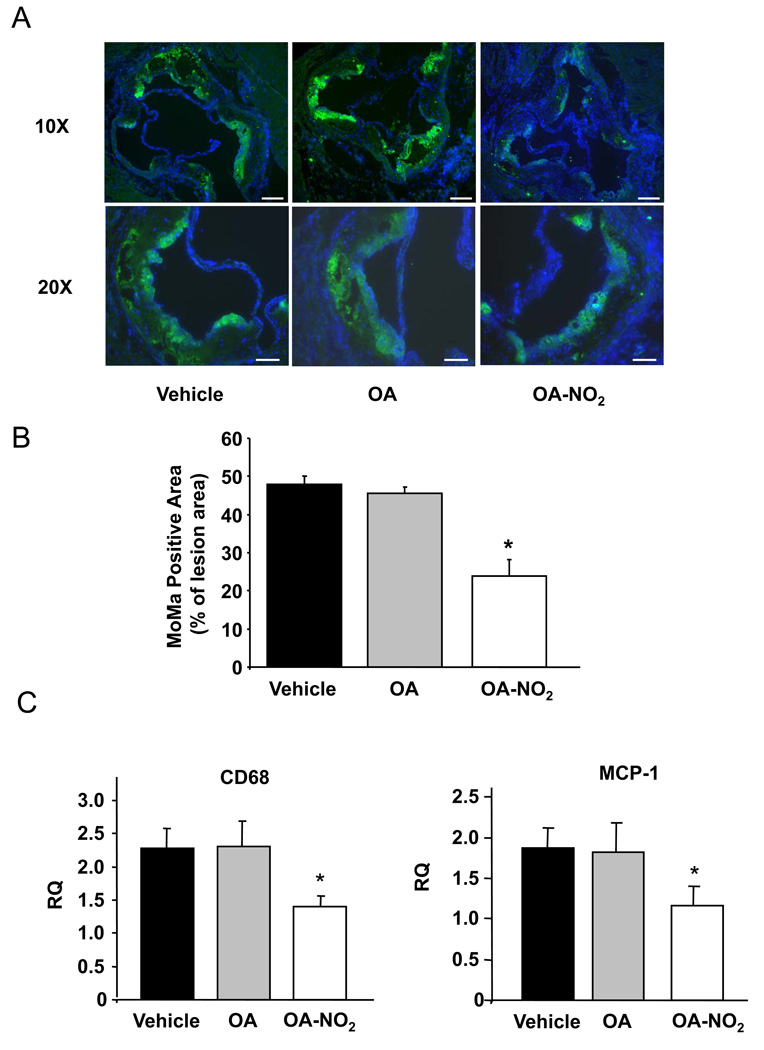
Inflammatory processes within the vessel wall mediate the initiation and progression of atherosclerosis. In this context, monocyte invasion into lesion-prone areas in the arterial wall and differentiation of monocytes into resident macrophages contributes to atherosclerotic plaque development.18,21 The vessel wall adhesion of monocytes is primarily mediated by vascular cell adhesion molecule-1 (VCAM-1).22,23 To elucidate mechanisms contributing to OA-NO2-induced decreases in atherosclerotic lesion formation, the influence of OA-NO2 on monocyte recruitment to atherosclerotic lesions was investigated. In particular, the effect of OA-NO2 on monocyte/macrophage (MOMA) accumulation in atherosclerotic plaques and the expression of VCAM-1 and MCP-1 in the aortic wall was determined. Quantitative immunofluorescent imaging revealed that the area of monocytes/macrophages within the lesion area was significantly decreased in mice treated with OA-NO2, compared with OA and vehicle treatment groups (p<0.001. Figure 3A and B). This was confirmed by quantitative real-time PCR analysis showing significantly lower expression of CD68 mRNA in the aortas of OA-NO2-treated animals (p=0.03. Figure 3C). OA-NO2 also significantly decreased levels of MCP-1 mRNA (p<0.05. Figure 3C). In concordance with previous in vitro observations,3 OA-NO2-treated animals also displayed decreased VCAM-1 expression in the aortic wall, as assessed by immunostaining and quantitative real-time PCR (Figure 4A and B).
Figure 3.
Monocyte and macrophage accumulation in atherosclerotic plaques was reduced by OA-NO2. A, Representative immunostained aortic sections (Magnification 10X and 20X, scale bar indicates 100 µm; blue=nuclei, green=MOMA). B, Quantification of MOMA-positive area relative to lesion area. OA-NO2 significantly reduced MOMA-positive area (p<0.001,*p<0.001 vs. vehicle and vs. OA). C, Real-time PCR showed a significant reduction in CD68 and MCP-1 expression in the aortic wall (p<0.05, *p<0.05 vs. vehicle and vs. OA).
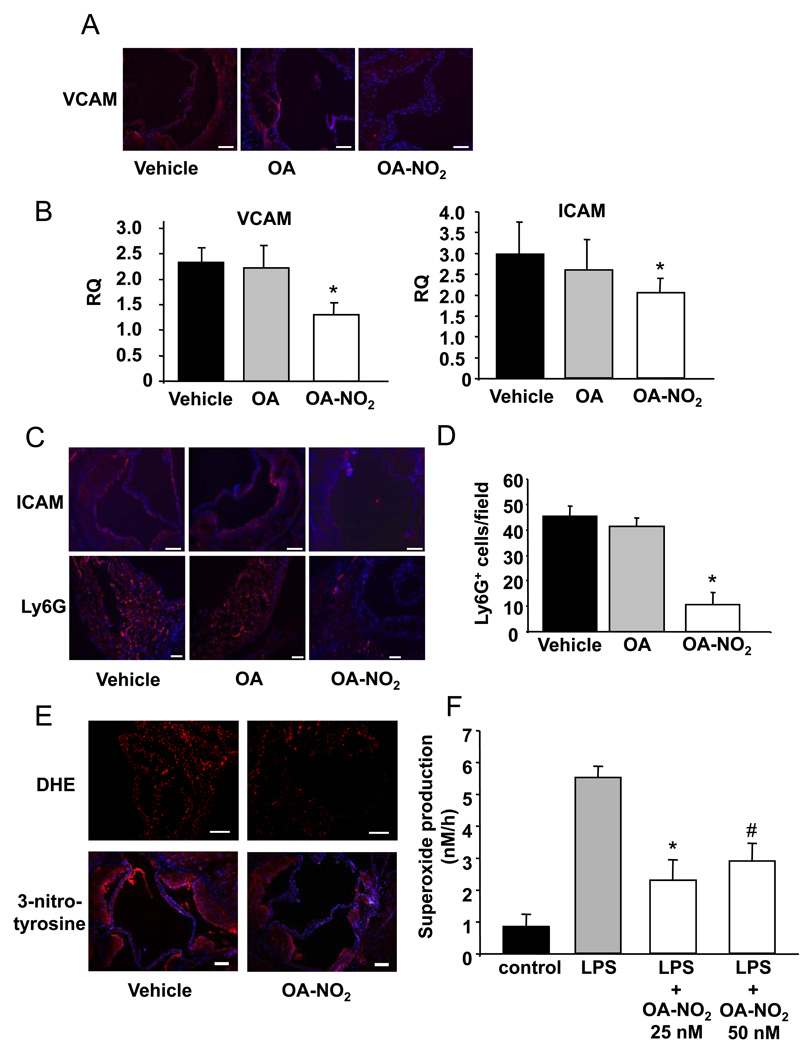
Figure 4.
Vascular and intracellular adhesion molecules were down-regulated by OA-NO2. A, Representative immunostained aortic sections using specific antibodies against VCAM (Magnification 20X, scale bar indicates 100 µm; blue=nuclei, red=VCAM). B, VCAM and ICAM mRNA expression in the aortic wall was lower in OA-NO2-treated animals (p<0.05, *p<0.05 vs. vehicle and vs. OA). C, Representative immunostained aortic sections using ICAM and Ly6G antibodies showed less ICAM expression and neutrophil infiltration in OA-NO2-treated animals (Magnification 20X, scale bar indicates 100 µm, blue = nuclei, red = ICAM/neutrophils). D, Densitometric quantification of Ly6G positive cells per field of view (p<0.001, *p<0.001 vs. vehicle and vs. OA). E, Representative immunostaining for DHE (Magnification 20X, scale bar indicates 100 µm) and 3-nitrotyrosine (Magnification 10X, scale bar indicates 100 µm, blue=nuclei, red=3-nitrotyrosine). F, Ex vivo LPS-induced superoxide production by BMDMs isolated from apoE−/− mice with and without OA-NO2 treatment (p=0.004, *p=0.003 vs. LPS, #p=0.07 vs. LPS).
The invasion of neutrophils into the arterial wall is facilitated by intercellular adhesion molecule (ICAM)-assisted trafficking, an event that also contributes to atherosclerotic plaque initiation and progression.18,24 Immunofluorescent analysis and quantitative real-time PCR showed decreased ICAM expression in the aortic wall (Figure 4B and C) in concert with a reduction in neutrophil accumulation in atherosclerotic lesions of OA-NO2 treated animals (Figure 4C and D). In aggregate, these findings reinforce the precept that the anti-inflammatory signaling actions of OA-NO2 mediate a reduction in atherosclerotic lesion formation and development.
DHE and 3-nitrotyrosine staining were used to reflect the extents of generation of superoxide and both peroxynitrite and heme peroxidase-catalyzed protein nitration reactions occurring in aortic root sections (Figure 4E). These indices revealed that OA-NO2-dependent signaling actions ultimately led to the attenuation of the generation of reactive oxygen species and downstream nitration reactions, events that may also contribute to the net anti-atherosclerotic actions of nitro-fatty acid derivatives. This OA-NO2-mediated response, observed in vivo, was also reflected by supporting in vitro studies. LPS-stimulated BMDMs, isolated from apoE−/− mice and treated with OA-NO2, displayed attenuated rates of superoxide generation (Figure 4F, p<0.004). Furthermore, the assessment of the expression of VASP and phosphorylated VASP in aortic tissue indicated a trend towards greater extents of NO bioavailability in mice treated with OA-NO2 (p=0.08), however endothelial function was not assessed directly in this study.
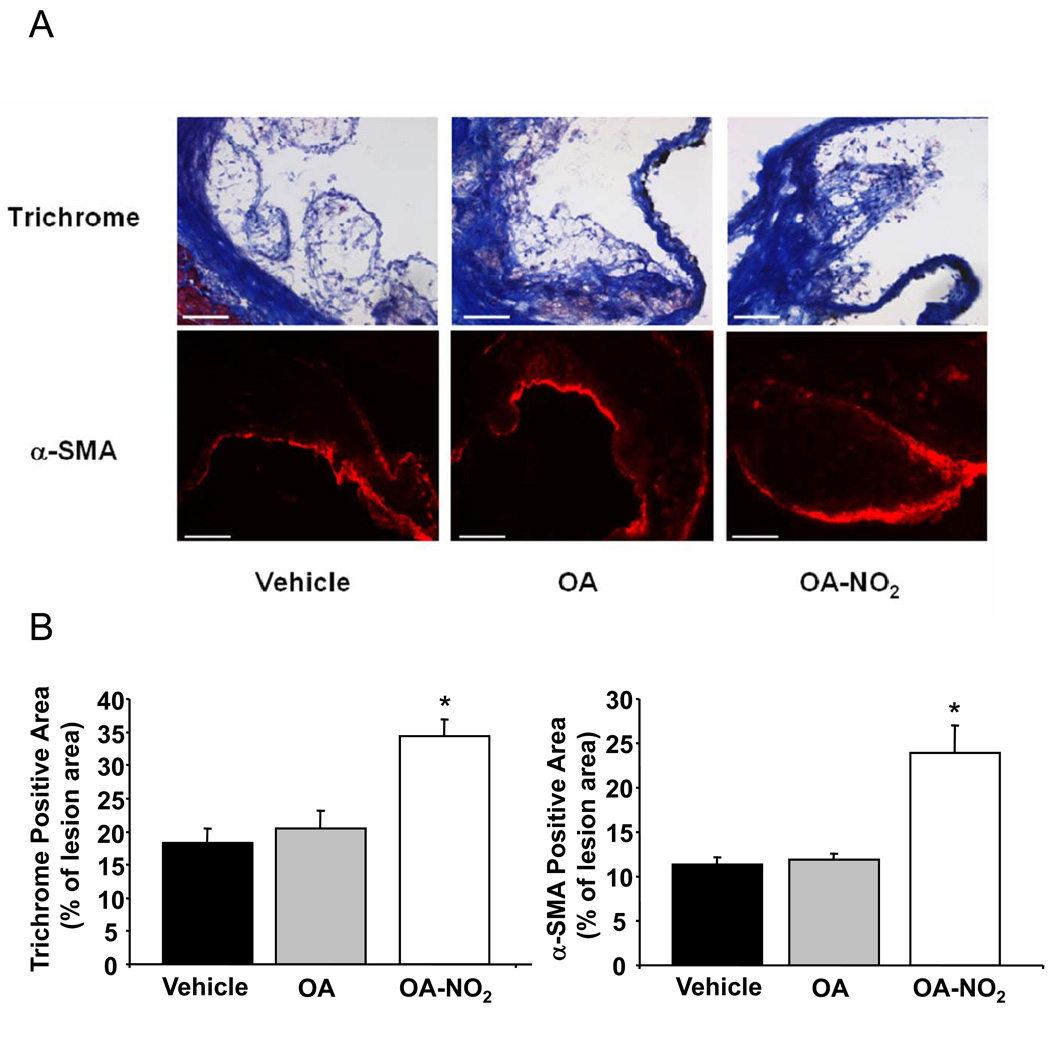
In addition to inhibiting plaque formation, OA-NO2 also increased plaque stability, a clinically-significant event that is attributed in part to extents of collagen synthesis by smooth muscle cells.25 Trichrome staining revealed that the collagen content of atherosclerotic lesions in animals treated with OA-NO2 was significantly greater. OA-NO2 treatment also resulted in an increased content of α-SMA within individual plaques (Figure 5A and B).
Figure 5.
OA-NO2 increased plaque stability. A, Representative aortic sections stained by Trichrome and a α-SMA specific antibody (Magnification 20X, scale bar indicates 100 µm). B, Quantification revealed a significantly larger collagen and α-SMA positive area in animals treated with OA-NO2 (p<0.001, *<0.001 vs. vehicle and vs. OA).
Foam cell formation is inhibited by OA-NO2 in vitro and in vivo
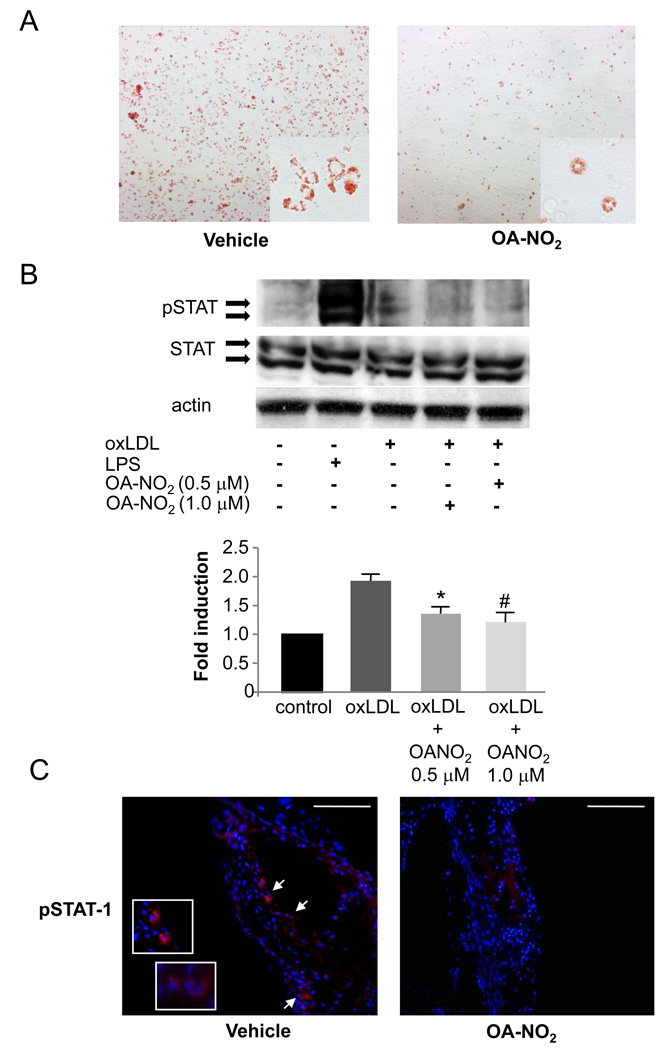
The transformation of invading macrophages into foam cells by oxLDL uptake and the subsequent activation-induced expression and secretion of multiple inflammatory mediators is a key aspect of atherogenesis. To determine whether OA-NO2 influences foam cell formation and function, beyond inhibition of monocyte/macrophage infiltration, peritoneal and BMDMs isolated from apoE−/− mice were studied. Peritoneal macrophages from OA-NO2-treated mice showed markedly reduced intracellular lipid accumulation as compared to controls. (Figure 6A). To further elucidate the mechanism of reduced foam cell formation, the impact of OA-NO2 on oxLDL-induced phosphorylation of STAT-1 was determined. Western blot analysis, confirmed by densitometry, showed that OA-NO2 decreased phosphorylation of STAT-1 in BMDMs (Figure 6B, p=0.001). Immunofluorescent analysis also revealed that phosphorylation of STAT-1 was reduced in atherosclerotic plaques of OA-NO2 treated mice (Figure 6C).
Figure 6.
OA-NO2 reduced foam cell formation in vivo and inhibited phosphorylation of STAT-1 in vitro as well as in vivo. A, Representative Oil Red O stained peritoneal macrophages harvested from apoE−/− mice after 4 weeks of treatment. OA-NO2 reduced intracellular lipid accumulation. B, OA-NO2 decreased oxLDL-induced phosphorylation of STAT-1 in BMDMs. One representative blot is shown. Densitometric analysis of 3 independent experiments (p=0.001, *p=0.02 and #p=0.006 vs. oxLDL). C, Phosphorylation of STAT-1 was also reduced in vivo as shown by immunofluorescence. Arrows indicate pSTAT-1 positive cells (Magnification 20X and 60X, scale bar indicates 100 µm; blue=nuclei, red=pSTAT-1).
Discussion
The extended exposure of apoE−/− mice to low concentrations of an electrophilic nitro-fatty acid, OA-NO2, markedly reduced atherosclerotic lesion formation and enhanced expression of proteins associated with plaque stability. The anti-atherosclerotic actions of OA-NO2 were linked with decreased adhesion molecule expression, vessel wall deposition of inflammatory cells and oxidative stress. Finally, OA-NO2 treatment further diminished foam cell formation by inhibition of oxLDL-induced STAT-1 phosphorylation. Atherosclerosis has long been associated with excess vascular production of reactive oxygen species and oxides of nitrogen.26,27 Secondary nitric oxide- and nitrite-derived nitrating species stemming from this inflammatory milieu nitrates not only protein tyrosine residues, but also unsaturated fatty acids, yielding electrophilic products react rapidly and reversibly with thiols of critical signaling mediators.9,28 Inflammatory conditions that have been modeled in vitro and studied in vivo reveal increased nitration of fatty acids.1 Acceleration of nitration reactions can also be promoted by the hydrophobic milieu of cell membranes and lipoproteins, due to greater local concentrations of reactants such as oxygen and nitric oxide.29,30 Since the second order rate constants for thiol reaction of the endogenously formed nitro-fatty acid derivatives oleic acid and linoleic acid are similar,9 OA-NO2 was selected for extended administration in vivo via osmotic mini-pump due to greater stability and resistance of the monounsaturated oleic acid to secondary oxidation reactions. Due to this kinetically rapid and reversible electrophilic reaction with thiols, NO2-FA readily modify biological targets post-translationally.9,10,28 To date, multiple electrophile-sensitive transcriptional regulatory factors that mediate defense against or resolution of inflammation have been identified as targets of NO2-FA.3,11 Several transcription factors which regulate adaptive and anti-inflammatory signaling reactions possess functionally significant electrophile-reactive amino acids that are critical for DNA binding and gene expression. These highly conserved signaling mediators evolved to facilitate tissue responses to oxidizing and electrophilic species generated by inflammatory and metabolic stress.31
Formerly regarded as a “lipid accumulating disease” it is now widely accepted that inflammatory processes play a critical role in all phases of atherosclerosis. Following cell adhesion molecule-mediated recruitment and vessel wall infiltration, leukocytes propagate the formation of inflammatory stimuli that in turn instigate a cascade of reactions ultimately responsible for the development of atherosclerotic plaques.15,32,33 In the present study, OA-NO2 potently inhibited the expression of cell adhesion molecules. There was also reduced monocyte/macrophage and neutrophil accumulation in the atherosclerotic lesions of animals treated with OA-NO2. These findings are concordant with previous in vitro results, where NO2-FA decreased endothelial VCAM expression and inhibited monocyte adhesion to endothelial cell monolayers.3 The current data also reinforce the concept that NO2-FA inhibit monocyte/macrophage activation, since there was a significant reduction in MCP-1 expression in OA-NO2-treated animals. In addition to these anti-inflammatory properties, OA-NO2 displayed indirect antioxidant effects in vivo, also potentially contributing to the observed anti-atherosclerotic actions of OA-NO2. Current data do not indicate direct free radical or oxidant scavenging properties of nitro-fatty acids, beyond the initial and quantitatively small consumption of nitrogen dioxide (˙NO2) that would occur during unsaturated fatty acid nitration.1 Once formed, nitroalkene derivatives of fatty acids convey indirect, but potentially highly potent, antioxidant effects via their signaling actions as electrophiles.34 Cell and tissue responses that have been defined for nitro-fatty acids could result in apparent anti-oxidant effects including receptor-dependent (PPARγ), transcription factor-mediated (NFκB, Nrf2, heat shock factor) and enzyme catalytic (xanthine oxidoreductase) modulatory actions of these electrophilic species.1,13,34,35
In addition to a marked reduction in atherosclerotic lesion formation, the present data also suggest that NO2-FA can have an impact on plaque stability, since there was an increased collagen and α-SMA content - surrogates for plaque stability. The inhibition of collagen synthesis and/or increased catabolism of extracellular matrix by metalloproteinases are factors that lead to plaque instability and can result in plaque rupture and consequent acute occlusion of the vessel.27,36–39 Our data reveal that these processes are inhibited by NO2-FA administration.
OA-NO2 also further influenced foam cell formation, beyond inhibiting inflammatory cell invasion and activation. During atherogenesis, macrophages ingest and accumulate oxLDL by different receptor-dependent mechanisms, ultimately transforming into lipid-rich foam cells, a process under stringent regulation by STAT-1-mediated signaling reactions.17 Of note, the Janus kinase-STAT pathway has also been identified as a critical mediator of interferon-γ-dependent signaling, which propagates initiation and progression of atherosclerotic plaque formation. This in turn induces the further release of pro-atherogenic chemokines such as MCP-1 and stimulates the apoptosis of plaque-associated macrophages, in turn enhancing the progression of lesion formation.16 Previous in vitro studies revealed that nitro-fatty acids, and not native fatty acid controls, potently inhibited LPS-induced STAT-1 phosphorylation. For this reason, the effect of OA-NO2 on oxLDL-induced STAT-1 phosphorylation was evaluated.14 Both in vivo and in vitro-based studies revealed that peritoneal macrophages isolated from OA-NO2 treated animals displayed less intracellular lipid accumulation and that the oxLDL-induced phosphorylation of STAT-1 was suppressed by OA-NO2. These findings indicates that OA-NO2 can in part limit foam cell formation by interfering with STAT-1 activation, with this effect ultimately being translated into a marked reduction in atherosclerotic lesion formation.
Our study of serum lipid profiles and blood glucose levels did not provide any evidence that NO2-FA influenced the metabolic profile of apoE−/− mice, affirming the concept that the protective anti-atherosclerotic actions of NO2-FA were due to their pluripotent anti-inflammatory signaling properties.
In conclusion, the present study reveals that in vivo administration of nM levels of electrophilic NO2-FA potently inhibit atherosclerotic lesion formation and induce plaque stability in a murine model of atherosclerosis, by targeting several pathways involved in atherogenesis. Since anti-atherogenic and cardioprotective dietary omega-6 and omega-3 polyunsaturated fatty acids40 can also be endogenously oxidized and nitrated to electrophilic derivatives such as α,β-unsaturated ketone and NO2-containing derivatives, it is intriguing to speculate that these redox-derived byproducts and their downstream signaling actions contribute to the salutary cardiovascular actions of polyunsaturated fatty acids. Since human atherosclerosis is primarily a consequence of vessel wall inflammation, NO2-FA administration may thus represent a useful therapeutic strategy for avoiding or treating atherosclerosis.
Acknowledgements
a) Acknowledgement
We thank Chen Shan Chen, Franca Golin-Bisello and Hartwig Wieboldt for expert technical assistance.
b) Sources of Funding
This work was supported by the Deutsche Forschungsgemeinschaft (Ru-1472/1–1 to T.K.R.), Deutsche Herzstiftung (to V. R.), Academy of Science of the Czech Republic (M200040908 to M. P.), the American Diabetes Association (to A. H.) and the National Institutes of Health (HL58115 and HL64937 to B.A.F. and HL089466 to A.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
c) Disclosure
Dr. Freeman has financial interest in Complexa, Inc.
References
- 1.Freeman BA, Baker PR, Schopfer FJ, Woodcock SR, Napolitano A, d'Ischia M. Nitro-fatty acid formation and signaling. J Biol Chem. 2008;283:15515–15519. doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferreira AM, Ferrari M, Trostchansky A, Batthyany C, Souza JM, Alvarez MN, López GV, Baker PR, Schopfer FJ, O'Donnell V, Freeman BA, Rubbo H. Macrophage activation induces formation of the anti-inflammatory lipid cholesteryl-nitrolinoleate. Biochem J. 2008;417:223–234. doi: 10.1042/BJ20080701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui T, Schopfer FJ, Zhang J, Chen K, Ichikawa T, Baker PR, Batthyany C, Chacko BK, Feng X, Patel RP, Agarwal A, Freeman BA, Chen YE. Nitrated fatty acids: Endogenous anti-inflammatory signaling mediators. J Biol Chem. 2006;281:35686–35698. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coles B, Bloodsworth A, Clark SR, Lewis MJ, Cross AR, Freeman BA, O'Donnell VB. Nitrolinoleate Inhibits Superoxide Generation, Degranulation, and Integrin Expression by Human Neutrophils: Novel Antiinflammatory Properties of Nitric Oxide-Derived Reactive Species in Vascular Cells. Circulation Research. 2002;91:375–381. doi: 10.1161/01.res.0000032114.68919.ef. [DOI] [PubMed] [Google Scholar]
- 5.Coles B, Bloodsworth A, Eiserich JP, Coffey MJ, McLoughlin RM, Giddings JC, Lewis MJ, Haslam RJ, Freeman BA, O'Donnell VB. Nitrolinoleate inhibits platelet activation by attenuating calcium mobilization and inducing phosphorylation of vasodilator-stimulated phosphoprotein through elevation of cAMP. J Biol Chem. 2002;277:5832–5840. doi: 10.1074/jbc.M105209200. [DOI] [PubMed] [Google Scholar]
- 6.Cole MP, Rudolph TK, Khoo NK, Motanya UN, Golin-Bisello F, Wertz JW, Schopfer FJ, Rudolph V, Woodcock SR, Bolisetty S, Ali MS, Zhang J, Chen YE, Agarwal A, Freeman BA, Bauer PM. Nitro-fatty acid inhibition of neointima formation after endoluminal vessel injury. Circulation Research. 2009;105:965–972. doi: 10.1161/CIRCRESAHA.109.199075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker PR, Lin Y, Schopfer FJ, Woodcock SR, Groeger AL, Batthyany C, Sweeney S, Long MH, Iles KE, Baker LM, Branchaud BP, Chen YE, Freeman BA. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J Biol Chem. 2005;280:42464–42475. doi: 10.1074/jbc.M504212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schopfer FJ, Lin Y, Baker PR, Cui T, Garcia-Barrio M, Zhang J, Chen K, Chen YE, Freeman BA. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand. Proc Natl Acad Sci U S A. 2005;102:2340–2345. doi: 10.1073/pnas.0408384102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker LM, Baker PR, Golin-Bisello F, Schopfer FJ, Fink M, Woodcock SR, Branchaud BP, Radi R, Freeman BA. Nitro-fatty acid reaction with glutathione and cysteine. Kinetic analysis of thiol alkylation by a Michael addition reaction. J Biol Chem. 2007;282:31085–31093. doi: 10.1074/jbc.M704085200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batthyany C, Schopfer FJ, Baker PR, Durán R, Baker LM, Huang Y, Cerveñansky C, Branchaud BP, Freeman BA. Reversible post-translational modification of proteins by nitrated fatty acids in vivo. J Biol Chem. 2006;281:20450–20463. doi: 10.1074/jbc.M602814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villacorta L, Zhang J, Garcia-Barrio MT, Chen XL, Freeman BA, Chen YE, Cui T. Nitro-linoleic acid inhibits vascular smooth muscle cell proliferation via the Keap1/Nrf2 signaling pathway. Am J Physiol Heart Circ Physiol. 2007;293:H770–H776. doi: 10.1152/ajpheart.00261.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Zhang J, Schopfer FJ, Martynowski D, Garcia-Barrio MT, Kovach A, Suino-Powell K, Baker PR, Freeman BA, Chen YE, Xu EH. Molecular recognition of nitrated fatty acids by PPAR gamma. Nat Struct Mol Biol. 2008;15:865–867. doi: 10.1038/nsmb.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kansanen E, Jyrkkänen HK, Volger OL, Leinonen H, Annukka MK, Häkkinen SK, Woodcock SR, Schopfer FJ, Horrevoets AJ, Herttuala SY, Freeman BA, Levonen AL. Nrf2-dependent and -independent responses to nitro-fatty acids in human endothelial cells: identification of heat shock response as the major pathway activated by nitro-oleic acid. J Biol Chem. 2009;284:33233–33241. doi: 10.1074/jbc.M109.064873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichikawa T, Zhang J, Chen K, Liu Y, Schopfer FJ, Baker PR, Freeman BA, Chen YE, Cui T. Nitroalkenes suppress lipopolysaccharide-induced signal transducer and activator of transcription signaling in macrophages: a critical role of mitogen-activated protein kinase phosphatase 1. Endocrinology. 2008;149:4086–4094. doi: 10.1210/en.2007-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim WS, Timmins JM, Seimon TA, Sadler A, Kolodgie FD, Virmani R, Tabas I. Signal transducer and activator of transcription-1 is critical for apoptosis in macrophages subjected to endoplasmic reticulum stress in vitro and in advanced atherosclerotic lesions in vivo. Circulation. 2008;117:940–951. doi: 10.1161/CIRCULATIONAHA.107.711275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koga M, Kai H, Yasukawa H, Yamamoto T, Kawai Y, Kato S, Kusaba K, Kai M, Egashira K, Kataoka Y, Imaizumi T. Inhibition of progression and stabilization of plaques by postnatal interferon-gamma function blocking in ApoE-knockout mice. Circulation Research. 2007;101:348–356. doi: 10.1161/CIRCRESAHA.106.147256. [DOI] [PubMed] [Google Scholar]
- 17.Agrawal S, Febbraio M, Podrez E, Cathcart MK, Stark GR, Chisolm GM. Signal transducer and activator of transcription 1 is required for optimal foam cell formation and atherosclerotic lesion development. Circulation. 2007;115:2939–2947. doi: 10.1161/CIRCULATIONAHA.107.696922. [DOI] [PubMed] [Google Scholar]
- 18.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 19.Vieira OV, Laranjinha JA, Madeira VM, Almeida LM. Rapid isolation of low density lipoproteins in a concentrated fraction free from water-soluble plasma antioxidants. J Lipid Res. 1996;37:2715–2721. [PubMed] [Google Scholar]
- 20.Chou WC, Jie C, Kenedy AA, Jones RJ, Trush MA, Dang CV. Role of NADPH oxidase in arsenic-induced reactive oxygen species formation and cytotoxicity in myeloid leukemia cells. Proc Natl Acad Sci U S A. 2004;101:4578–4583. doi: 10.1073/pnas.0306687101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansson GK. Immunological control mechanisms in plaque formation. Basic Research in Cardiology. 1994;89:41–46. doi: 10.1007/978-3-642-85660-0_4. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Cybulsky MI, Gimbrone MA, Jr, Libby P. Inducible expression of vascular cell adhesion molecule-1 by vascular smooth muscle cells in vitro and within rabbit atheroma. The American Journal of Pathology. 1993;143:1551–1559. [PMC free article] [PubMed] [Google Scholar]
- 23.Iiyama K, Hajra L, Iiyama M, Li H, DiChiara M, Medoff BD, Cybulsky MI. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circulation Research. 1999;85:199–207. doi: 10.1161/01.res.85.2.199. [DOI] [PubMed] [Google Scholar]
- 24.van Leeuwen M, Gijbels MJ, Duijvestijn A, Smook M, van de Gaar MJ, Heeringa P, de Winther MP, Tervaert JW. Accumulation of myeloperoxidase-positive neutrophils in atherosclerotic lesions in LDLR−/− mice. Arterioscler Thromb Vasc Biol. 2008;28:84–89. doi: 10.1161/ATVBAHA.107.154807. [DOI] [PubMed] [Google Scholar]
- 25.Halvorsen B, Otterdal K, Dahl TB, Skjelland M, Gullestad L, Øie E, Aukrust P. Atherosclerotic Plaque Stability—What Determines the Fate of a Plaque? Progress in Cardiovascular Diseases. 2008;51:183–194. doi: 10.1016/j.pcad.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 26.White CR, Brock TA, Chang LY, Crapo J, Briscoe P, Ku D, Bradley WA, Gianturco SH, Gore J, Freeman BA. Superoxide and peroxynitrite in atherosclerosis. Proceedings of the National Academy of Sciences. 1994;91:1044–1048. doi: 10.1073/pnas.91.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beckmann JS, Ye YZ, Anderson PG, Chen J, Accavitti MA, Tarpey MM, White CR. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- 28.Rudolph V, Schopfer FJ, Khoo NK, Rudolph TK, Cole MP, Woodcock SR, Bonacci G, Groeger AL, Golin-Bisello F, Chen CS, Baker PR, Freeman BA. Nitro-fatty acid metabolome: saturation, desaturation, beta-oxidation, and protein adduction. J Biol Chem. 2009;284:1461–1473. doi: 10.1074/jbc.M802298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nadtochiy SM, Baker PR, Freeman BA, Brookes PS. Mitochondrial nitroalkene formation and mild uncoupling in ischaemic preconditioning: implications for cardioprotection. Cardiovasc Res. 2009;82:333–340. doi: 10.1093/cvr/cvn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Donnell VB, Eiserich JP, Chumley PH, Jablonsky MJ, Krishna NR, Kirk M, Barnes S, Darley-Usmar VM, Freeman BA. Nitration of unsaturated fatty acids by nitric oxide-derived reactive nitrogen species peroxynitrite, nitrous acid, nitrogen dioxide, and nitroniumion. Chem Res Toxicol. 1999;12:83–92. doi: 10.1021/tx980207u. [DOI] [PubMed] [Google Scholar]
- 31.Kaplowitz N, Fernández-Checa JC, Kannan R, Garcia-Ruiz C, Ookhtens M, Yi JR. GSH transporters: molecular characterization and role in GSH homeostasis. Biol Chem Hoppe Seyler. 1996;377:267–273. doi: 10.1515/bchm3.1996.377.5.267. [DOI] [PubMed] [Google Scholar]
- 32.Ross R. Atherosclerosis-an inflammatory disease. New England Journal of Medicine. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 33.Zernecke A, Weber C. Inflammatory mediators in atherosclerotic vascular disease. Basic Research in Cardiology. 2005;100:93–101. doi: 10.1007/s00395-005-0511-6. [DOI] [PubMed] [Google Scholar]
- 34.Rudolph TK, Freeman BA. Transduction of redox signaling by electrophile-protein reactions. Sci Signal. 2009;2:re7. doi: 10.1126/scisignal.290re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelley EE, Batthyany CI, Hundley NJ, Woodcock SR, Bonacci G, Del Rio JM, Schopfer FJ, Lancaster JR, Jr, Freeman BA, Tarpey MM. Nitro-oleic acid: A novel and irreversible inhibitor of xanthine oxidoreductase. J Biol Chem. 2008;283:36176–36184. doi: 10.1074/jbc.M802402200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 2006;116:59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah PK. Inflammation and plaque vulnerability. Cardiovasc Drugs Ther. 2009;23:31–40. doi: 10.1007/s10557-008-6147-2. [DOI] [PubMed] [Google Scholar]
- 38.Molloy KJ, Thompson MM, Jones JL, Schwalbe EC, Bell PR, Naylor AR, Loftus IM. Unstable carotid plaques exhibit raised matrix metalloproteinase-8 activity. Circulation. 2004;110:337–343. doi: 10.1161/01.CIR.0000135588.65188.14. [DOI] [PubMed] [Google Scholar]
- 39.Orbe J, Rodríguez JA, Arias R, Belzunce M, Nespereira B, Pérez-Ilzarbe M, Roncal C, Páramo JA. Antioxidant vitamins increase the collagen content and reduce MMP-1 in a porcine model of atherosclerosis: implications for plaque stabilization. Atherosclerosis. 2003;167:45–53. doi: 10.1016/s0021-9150(02)00392-1. [DOI] [PubMed] [Google Scholar]
- 40.Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Mantini L, Marfisi RM, Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa F. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105 doi: 10.1161/01.cir.0000014682.14181.f2. 18971903. [DOI] [PubMed] [Google Scholar]