Abstract
Obesity is associated with increased cardiovascular risk. Although short-term weight loss improves vascular endothelial function, longer term outcomes have not been widely investigated. We examined brachial artery endothelium-dependent vasodilation and metabolic parameters in 29 severely obese subjects who lost ≥10% body weight (age 45 ± 13 years; BMI 48 ± 9 kg/m2) at baseline and after 12 months of dietary and/or surgical intervention. We compared these parameters to 14 obese individuals (age 49 ± 11 years; BMI 39 ± 7 kg/m2) who failed to lose weight. For the entire group, mean brachial artery flow-mediated dilation (FMD) was impaired at 6.7 ± 4.1%. Following sustained weight loss, FMD increased significantly from 6.8 ± 4.2 to 10.0 ± 4.7%, but remained blunted in patients without weight decline from 6.5 ± 4.0 to 5.7 ± 4.1%, P = 0.013 by ANOVA. Endothelium-independent, nitroglycerin-mediated dilation (NMD) was unaltered. BMI fell by 13 ± 7 kg/m2 following successful weight intervention and was associated with reduced total and low-density lipoprotein cholesterol, glucose, hemoglobin A1c, and high-sensitivity C-reactive protein (CRP). Vascular improvement correlated most strongly with glucose levels (r = −0.51, P = 0.002) and was independent of weight change. In this cohort of severely obese subjects, sustained weight loss at 1 year improved vascular function and metabolic parameters. The findings suggest that reversal of endothelial dysfunction and restoration of arterial homeostasis could potentially reduce cardiovascular risk. The results also demonstrate that metabolic changes in association with weight loss are stronger determinants of vascular phenotype than degree of weight reduction.
INTRODUCTION
The risk of premature atherosclerosis and cardiovascular events rises with increasing obesity and excess adiposity has been linked to increased all-cause mortality (1-3). Current estimates show that one in four ischemic heart disease events are attributable to excess weight (3). Weight loss reduces myo-cardial infarction risk while improving long-term survival (4), although underlying therapeutic mechanisms remain largely unknown. The vascular endothelium plays a central role in the regulation of arterial tone, inflammation, and thrombosis. Endothelial function is impaired in obesity and represents the earliest stage of clinical atherosclerosis. Abnormalities in endothelial function worsen with increased weight burden owing to several mechanisms associated with excess fat mass including impaired glucose tolerance, insulin resistance, metabolic dysregulation, adipocytokine release, and systemic inflammation that play a key role in the evolution and clinical expression of cardiovascular disease (5,6).
Although endothelial dysfunction is a strong predictor of cardiovascular events, restoring arterial homeostasis reduces vascular risk (7). Our group and others have demonstrated that short-term weight loss improves endothelial function within weeks via mechanisms that relate more closely to metabolic changes than degree or mode of weight intervention (8-11). Whether the beneficial vascular effect represents a transient phenotypic shift in relation to acute metabolic changes or a sustained physiologic adaptation that could favorably modulate heart disease risk remains unknown. The purpose of this study was to examine the effect of long-term (≥1 year) sustained weight loss on endothelial function and to identify determinants of vascular phenotype in association with weight alteration in a group of severely obese individuals.
METHODS AND PROCEDURES
Subjects
We enrolled consecutive obese subjects with BMI ≥30 kg/m2 seeking weight loss at the Nutrition and Weight Management Center at Boston Medical Center (Boston, MA). Subjects in this clinic receive comprehensive weight loss treatment incorporating tailored dietary, behavioral, medical, and surgical intervention. Patients with unstable cardio vascular syndromes including heart failure, unstable angina, recent myocardial infarction or stroke (≤3 months), pregnancy, or malignancy were excluded. Each subject made a visit at baseline and ≥12 months after the start of weight loss intervention and vascular studies were conducted during a baseline weight stable period. Weight reduction treatments included dietary intervention including a standard low-fat American Heart Association diet or bariatric surgery as clinically indicated based on National Heart, Lung, and Blood Institute guidelines (12). Low-carbohydrate Atkins-type diets were not prescribed. Exercise was encouraged per standard of care in the weight management clinic, but specific exercise regimens were not assigned as part of the study protocol. Eligible surgically managed patients with BMI ≥35 kg/m2 with comorbidities or ≥40 kg/m2 underwent either laparoscopic Roux-en-Y gastric bypass or laparoscopic banding. All subjects provided informed consent and the study was approved by the Boston Medical Center Institutional Review Board.
Vascular studies
Noninvasive vascular ultrasound studies of brachial artery vasomotor function were performed in a temperature-controlled room with subjects resting supine in a fasting state. All vasoactive medications were held 24 h before ultrasound examination. Brachial artery vasoreactivity was examined by trained sonographers blinded to all clinical data using a noninvasive, standardized method of ultrasound imaging as previously described, using a Toshiba Powervision 6000 system (Toshiba Medical USA, Tustin, CA) (13). Flow-mediated dilation (FMD) and nitroglycerin-mediated dilation (NMD) of the brachial artery were examined as measures of endothelium-dependent and -independent dilation, respectively. Brachial artery FMD responses were measured following a 5-min cuff occlusion in an upper arm position above the antecubital crease. Pulsed-Doppler flow velocity signals at baseline and after cuff deflation quantified measures of reactive hyperemia. Sublingual nitroglycerin (0.4 mg) was omitted if the subject declined or had a history of migraines, systolic blood pressure <100 mm Hg, previous adverse reaction to nitrates, or used phosphodiesterase type-5 medications. Specific soft tissue landmarks for each digitized frame were identified to ensure that the exact same arterial segment was imaged in serial follow-up studies. An investigator blinded to clinical information performed all offline analyses of digitized end-diastolic images. FMD was expressed as relative change (%) or absolute change (mm) in arterial diameter.
Anthropometric, hemodynamic, and metabolic measures
During each visit, weight, height, heart rate, blood pressure, and BMI were recorded for each subject by trained personnel. Weight was measured using a calibrated scale (Ohaus, Pine Brook, NJ). All biochemical analyses were performed by the Boston Medical Center Clinical Chemistry Laboratory, quantified from blood samples collected in a fasted state. Self-report of regular exercise three times or more per week was recorded at baseline. Metabolic syndrome was defined according to ATP III criteria (14).
Statistical analysis
Subjects were categorized dichotomously as having achieved weight loss (≥10% decline from initial body weight, n = 29) or unsuccessful weight intervention (no weight loss or gain, n = 14). Independent t-tests were used to compare baseline clinical differences between the two groups for continuous variables and χ2 or Fisher’s exact test for categorical variables. Paired comparisons of categorical variables were examined using the McNemar exact test. Repeated-measures ANOVA was employed to examine the effect of weight intervention on group differences in clinical variables. Examined variables included age, gender, BMI, blood pressure, lipids, glucose, glycosylated hemoglobin A1c, FMD, NMD, high-sensitivity C-reactive protein (CRP), diabetes, hypertension, smoking status, and medications. Correlations between vascular parameters and clinical or biochemical data were examined using linear regression analysis. Univariate correlates of vascular function (P < 0.05) were entered into a stepwise multiple regression analysis to identify independent predictors of change in FMD. Based on our previously published data (9), a sample size of 43 subjects provided 80% power to detect a mean change in FMD of 3.9 ± 4.0%, and 90% power to detect a 4.5 ± 4.0% group difference in FMD. With regard to correlation analyses, 43 subjects provided 80% power to detect an R2-value of 0.22, and 90% power to detect an R2-value of 0.275, based on two-sided tests at the 0.05 significance level. All data are presented as mean ± s.d., unless indicated otherwise. Analyses were completed using SPSS for Windows, version 16 (SPSS, Chicago, IL).
RESULTS
A total of 43 subjects (age 46 ± 12 years, BMI 45 ± 9 kg/m2) completed the 12-month study. Forty-two percent of subjects were of minority demographic based on National Institutes of Health definitions. Baseline clinical characteristics of subjects with successful weight loss (n = 29) as compared to the weight stable group (n = 14) are displayed in Table 1. Initial total body weight and BMI were higher in the weight loss group otherwise there were no baseline differences in age, gender, metabolic status, vascular function, plasma inflammatory markers, physical activity, or medications. As expected, none of the weight stable subjects were bariatric patients whereas 59% (n = 17) of individuals in the weight loss group had undergone surgical intervention. The bariatric subjects had a higher baseline weight as compared to the nonsurgical weight loss group (144 ± 22 kg vs. 121 ± 18 kg, P = 0.008). Otherwise there were no statistically significant differences in the baseline parameters listed in Table 1.
Table 1. Baseline characteristics.
| Weight loss |
No weight loss |
||
|---|---|---|---|
| Variable | (n = 29) | (n = 14) | P value |
| Age (years) | 45 ± 13 | 49 ± 11 | 0.290 |
| Women | 76% | 71% | 1.000 |
| Minority | 41% | 43% | 0.927 |
| Weight (kg) | 134 ± 23 | 107 ± 29 | 0.002 |
| BMI (kg/m2) | 48 ± 9 | 39 ± 7 | 0.001 |
| Systolic BP (mm Hg) | 131 ± 14 | 129 ± 12 | 0.560 |
| Diastolic BP (mm Hg) | 73 ± 10 | 75 ± 8 | 0.547 |
| Baseline diameter (mm) | 4.3 ± 0.7 | 4.3 ± 0.8 | 0.949 |
| FMD (%) | 6.8 ± 4.2 | 6.5 ± 4.0 | 0.847 |
| FMD (mm) | 0.28 ± 0.16 | 0.26 ± 0.14 | 0.734 |
| NMD (%) | 11.5 ± 7.3 | 7.6 ± 6.6 | 0.245 |
| Glucose (mg/dl) | 116 ± 42 | 100 ± 17 | 0.107 |
| HbA1c (%) | 6.5 ± 1.7 | 5.7 ± 0.6 | 0.243 |
| Total cholesterol (mg/dl) | 192 ± 34 | 179 ± 27 | 0.233 |
| HDL (mg/dl) | 53 ± 22 | 53 ± 22 | 0.985 |
| LDL (mg/dl) | 109 ± 31 | 104 ± 19 | 0.606 |
| Triglyceride (mg/dl) | 154 ± 97 | 112 ± 58 | 0.165 |
| hs-CRP (mg/l) | 11.5 ± 6.5 | 7.7 ± 8.4 | 0.240 |
| Family history CAD | 24% | 21% | 1.000 |
| Current smoker | 14% | 0% | 0.286 |
| Diabetes mellitus | 31% | 14% | 0.291 |
| Hypertension | 55% | 50% | 0.750 |
| Metabolic syndrome | 45% | 63% | 0.447 |
| Regular exercisea | 52% | 57% | 0.803 |
| ACEI or ARB use | 31% | 29% | 1.000 |
| Hypoglycemic use | 21% | 14% | 1.000 |
| Insulin use | 3% | 7% | 1.000 |
| Lipid lowering use | 24% | 50% | 0.090 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; CAD, coronary artery disease; FMD, fow-mediated dilation; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; NMD, nitroglycerin-mediated dilation.
Sessions per week.
One-year follow-up data for all subjects are displayed in Table 2. Weight reduction was associated with significant improvements in plasma glucose, hemoglobin A1c, total and low-density lipoprotein cholesterol, and high-sensitivity CRP. Although endothelium-dependent FMD was impaired in both groups at baseline, weight loss was associated with a significant increase in FMD from 6.8 ± 4.2 to 10.0 ± 4.7% but remained blunted in patients with no weight decline from 6.5 ± 4.0 to 5.7 ± 4.1%, P = 0.013 by repeated measures ANOVA (Figure 1). Endothelium-independent, NMD remained unchanged in both groups (Table 2). Among subjects with successful weight loss, there was no differential effect of surgical vs. dietary intervention on %FMD (P = 0.784) or NMD (P = 0.406), although surgical intervention produced greater weight loss (P < 0.001), and lower high-sensitivity CRP (P = 0.02) and glucose (P = 0.03) than medical intervention, there were no other statistically significant differences in the parameters listed in Table 2. Clinical treatment with angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and hypoglycemic agents decreased significantly at 12 months in the weight loss group (P < 0.05) with also a strong trend for reduced lipid-lowering treatment (P = 0.06). In contrast, pharmacological therapy was unchanged in control subjects (P = NS).
Table 2. Absolute changes in variables: 1-year follow-up.
| Weight loss |
No weight loss |
||
|---|---|---|---|
| Variable | (n = 29) | (n = 14) | P value |
| Weight (kg) | −37 ± 20 | 7 ± 7 | <0.0001 |
| BMI (kg/m2) | −13 ± 7 | 3 ± 2 | <0.0001 |
| Systolic BP (mm Hg) | −3 ± 17 | 1 ± 19 | 0.523 |
| Diastolic BP (mm Hg) | 0 ± 13 | −1 ± 9 | 0.832 |
| Baseline diameter (mm) | −0.23 ± 0.38 | 0.04 ± 0.57 | 0.074 |
| FMD (%) | 3.3 ± 5.3 | −0.8 ± 3.4 | 0.013 |
| FMD (mm) | 0.11 ± 0.19 | −0.03 ± 0.12 | 0.022 |
| NMD (%) | 1.4 ± 6.6 | −8.0 ± 1.2 | 0.069 |
| Glucose (mg/dl) | −26 ± 36 | 13 ± 17 | 0.006 |
| HbA1c | −0.8 ± 1.1 | 0.3 ± 0.3 | 0.039 |
| Total cholesterol (mg/dl) | −12 ± 30 | 24 ± 30 | 0.006 |
| HDL (mg/dl) | 2 ± 32 | −1 ± 6 | 0.802 |
| LDL (mg/dl) | −9 ± 30 | 19 ± 31 | 0.032 |
| Triglyceride (mg/dl) | −30 ± 87 | 26 ± 52 | 0.093 |
| hs-CRP (mg/l) | −6.3 ± 5.7 | −1.4 ± 3.9 | 0.049 |
BP, blood pressure; FMD, fow-mediated dilation; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; NMD, nitroglycerin-mediated dilation.
Figure 1.
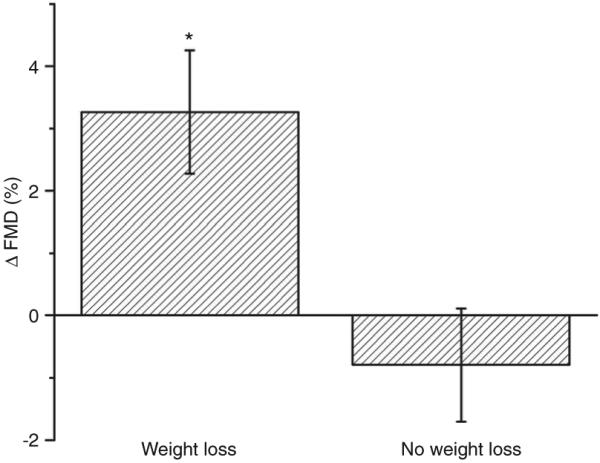
Absolute improvement in FMD(%) following 1 year of sustained weight loss vs. no weight loss (*P = 0.013, by repeated measures ANOVA). Data are presented as mean ± s.e.m. FMD, flow-mediated dilation.
To identify longitudinal determinants of vascular function, correlation analyses were performed between all measured clinical variables and change in brachial artery FMD. The significant univariate predictors for ΔFMD were Δglucose (r = −0.51, P = 0.002, Figure 2), and ΔBMI (r = −0.37, P = 0.014; Figure 3). When these two univariate correlates were entered into a stepwise multiple linear regression analysis, Δglucose remained a significant independent predictor of endothelial function (β ± s.e. = −0.072 ± 0.021, P = 0.002). Further, Δglucose correlated significantly with ΔCRP (r = 0.419, P = 0.047). Subgroup analysis excluding diabetic subjects reduced study power and did not show a significant relationship between ΔFMD and Δglucose.
Figure 2.
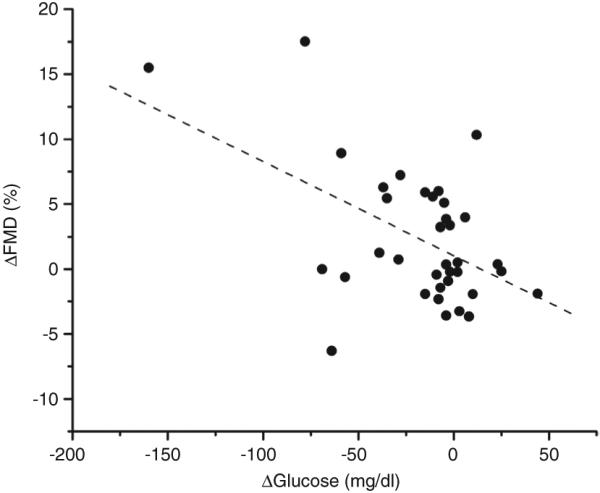
Univariate correlation examining the relationship between ΔFMD (%) and Δglucose (mg/dl) (r = −0.509, P = 0.002). FMD, flow-mediated dilation.
Figure 3.
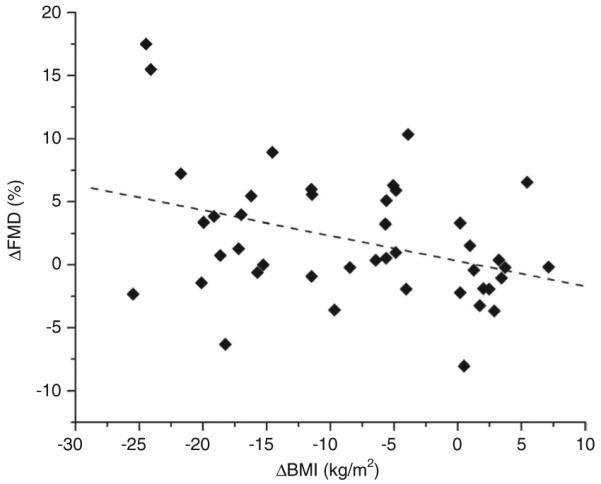
Univariate correlation examining the relationship between ΔFMD (%) and ΔBMI (kg/m2) (r = −0.372, P = 0.014). FMD, flow-mediated dilation.
DISCUSSION
In this 12-month prospective study, we demonstrated that sustained weight loss, regardless of intervention method, improved brachial artery endothelium-dependent vasodilation while failure to reduce weight was associated with persistent vascular impairment. Weight loss was associated with significant improvement in metabolic parameters including glycemic status, lipid profile, and CRP among which the strongest correlate of improved vasoreactivity was fasting glucose. The results extend findings from short-term studies demonstrating that a dysfunctional endothelial phenotype associated with obesity can be reversed with sustained intervention that may reduce cardiovascular risk.
Before short-term studies have demonstrated a beneficial effect of weight loss on endothelium-dependent FMD. Weight reduction over 6–24 weeks ranging from 10 to 25% weight change significantly improved arterial FMD that primarily tracked changes in glucose status (9,10). In studies that examined microvascular dilation, 8–17% BMI decrease improved acetylcholine-mediated forearm blood flow that correlated with cholesterol lowering and insulin sensitivity (8,11). Although these heterogeneous studies differed in subject population and treatment, a shared feature of all these short-term investigations was the consistent finding that metabolic changes were stronger determinants of vascular improvement than degree of weight reduction. We extend this conceptual paradigm in the present study by illustrating that increased brachial FMD following an average 28% weight decline over a 1-year period was most closely linked to plasma glucose. A threshold amount of weight loss is likely required before physiologic changes exert vascular effects, in line with National Heart, Lung, and Blood Institute guidelines that target at least a 10% weight decrease as an initial therapeutic goal (12). To support this, a small study that achieved <5% weight reduction failed to lower glucose or improve flow-mediated vasodilation (15).
The long-lasting positive effects of structured intervention are also germane to other aspects of endothelial function. In obese women, ≥10% weight loss reduced circulating proinflammatory cytokines such as tumor necrosis factor-α and interleukin-6, and lowered markers of endothelial activation including vascular cell adhesion molecule-1 and intracellular adhesion molecule-1. These changes occurred simultaneously with improved platelet aggregation, blood viscosity, and vasodilation to L-arginine (16). Constrictor and inflammatory mediators including endothelin-1 and E-selectin also decline with successful weight loss in parallel with blunted sympathetic activity (17). Taken together, the dysfunctional metabolic, inflammatory, thrombotic, and vasodilator properties of the endothelium may be reversed with long-term therapeutic intervention.
The association between glycemic status and vascular endothelial dysfunction has basis in both experimental and clinical studies. Acute exposure of blood vessels to hyperglycemia blunts arterial dilation and obesity reduces the glycemic threshold for impaired endothelial nitric oxide function in vivo (18,19). In healthy individuals, intra-arterial glucose acutely reduces forearm flow (20), and oral glucose loading rapidly suppresses endothelium-dependent brachial relaxation (21). There is strong evidence that the endothelium is also dysfunctional in chronic states of hyperglycemia. Diet-induced diabetic rodents exhibit abnormal vasomotion to endothelial agonists (22). Human diabetic vessels show increased vasoconstriction owing to deficient basal and stimulated nitric oxide (NO) production and endothelial nitric oxide expression (23). Similarly, forearm vascular reactivity is impaired in diabetic subjects (24).
Mechanisms that link glucose levels to vascular homeostasis are likely multifactorial. Hyperglycemia induces oxidative stress and superoxide production which inhibits endothelial nitric oxide activity, quenches NO, and promotes conversion of NO to peroxynitrite (25) whereas antioxidants augment NO bioavailability and NO-dependent vasorelaxation (26). Protein kinase C activation and NADPH oxidase play a role in tissue generation of reactive oxygen species in response to elevated glucose (27). Phosphoinositide 3-kinase/Akt insulin signaling pathways are also altered resulting in decreased endothelial nitric oxide action (28). Chronic hyperglycemia generates advanced end glycosylation products that facilitate inflammatory endothelial activation and cell surface expression of adhesion molecules that are key early atherosclerotic events in the vascular bed (29). Hyperglycemia has also been associated with the upregulation of potent vasoconstrictors including endothelin-1 (30) that have the potential to modulate ambient vascular tone.
Recent data show that accumulation of excess fat is associated with adipose tissue inflammation and systemic elaboration of adipocytokines that modulate insulin resistance. Low-grade tissue inflammation elicited by expanding toxic fat mass and infiltrating macrophages amplify a localized response that progresses to a state of heightened systemic inflammation (31). Although inflammatory activity in fat may be intertwined with mechanisms of glucose disposal, a proinflammatory fat phenotype and associated adipokine overexpression are also suspected to play a direct role in vascular injury (32,33). Thus, the overall inflammatory burden may not only govern insulin sensitivity but may also be integral to vascular phenotype. In the present study, plasma CRP as a marker of systemic inflammation was markedly elevated in our obese cohort and significantly decreased with weight loss, but we did not identify a direct correlation with endothelial function as previously reported in a larger population (34).
Although obesity is linked to insulin resistance and diabetes mellitus which are in turn strongly associated with cardiovascular risk (35), longitudinal studies examining cardiovascular outcomes in association of weight loss are largely lacking. A recent landmark study which prospectively followed obese patients over a mean of 10.9 years following bariatric surgery identified myocardial infarction as the most common cause of cardiovascular death, and blood glucose was a strong predictor of mortality (4). Thus, it seems plausible to consider that ambient glycemic status among other variables may govern vascular phenotype in obesity and measurement of endothelial function might identify at-risk individuals. Although degree of hyperglycemia is associated with cardiovascular disease (36), with the exception of the STOPNIDDM trial (37), studies focusing on tight glycemic control have failed to show reduced macrovascular outcomes or cardiovascular benefit (38,39). A seminal finding in these studies is the added weight gain, by up to >10 kg, that may offset metabolic gains and exacerbate insulin resistance (40). The interplay between excess fat mass and glycemic status in obese subjects is likely complex, and glucose control earlier in the atherosclerotic disease course and in parallel with weight loss may represent the optimal approach for cardiovascular benefit, although this issue remains an open question.
This study has several limitations. Although adequately powered for the entire group, the sample size was relatively small which limits our ability to perform subgroup analyses in diabetic vs. nondiabetic subjects, and individuals treated surgically or with dietary intervention alone. We did not measure insulin levels in this cohort and thus cannot speculate on the relative effect of insulin resistance on vascular responses. Although exercise regimens were not specifically prescribed, we did not quantify physical activity in follow-up although individuals with such severe obesity tend to have limited activity. Waist circumference was not available for all subjects, and thus we cannot specifically comment on the effects of regional fat distribution, although central adiposity tends to be present in nearly all subjects with this level of severe obesity (5). The limitations are counterbalanced by the opportunity to longitudinally study endothelial function in a group of severely obese individuals who lost significant weight providing novel data where limited information exists.
In summary, long-term sustained weight loss in severely obese individuals improved vascular endothelial function at 1 year. The findings suggest that the effect of obesity on vascular function is reversible and restoration of arterial homeostasis may partly explain reduced cardiovascular risk associated with weight change. The results also suggest that parallel metabolic changes are stronger determinants of vascular phenotype than magnitude of weight reduction.
ACKNOWLEDGMENT
The study was funded by NIH grants HL074097 and HL084213 (to N.G.).
Footnotes
DISCLOSURE
C.M.A. serves as a consultant for Novo Nordisk, Arena Pharmaceuticals, Merck Pharmaceuticals, Amylin Pharmaceuticals, GI Dynamics, Johnson & Johnson Inc., Sanofi Aventis Groupe, Orexigen Pharmaceuticals, and Pfizer. She reports research funding from Amylin Pharmaceuticals, Sanofi Aventis Groupe, Pfizer, Orexigen Therapeutics, MetaProteomics LLC, Atkins Foundation, and Arena Pharmaceuticals. Other authors declared no conflict of interest.
REFERENCES
- 1.Madala MC, Franklin BA, Chen AY, et al. CRUSADE Investigators Obesity and age of first non-ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2008;52:979–985. doi: 10.1016/j.jacc.2008.04.067. [DOI] [PubMed] [Google Scholar]
- 2.McGill HC, Jr, McMahan CA, Herderick EE, et al. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation. 2002;105:2712–2718. doi: 10.1161/01.cir.0000018121.67607.ce. [DOI] [PubMed] [Google Scholar]
- 3.Prospective Studies Collaboration Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. [Google Scholar]
- 4.Sjöström L, Narbro K, Sjöström CD, et al. Swedish Obese Subjects Study Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 5.Arkin JM, Alsdorf R, Bigornia S, et al. Relation of cumulative weight burden to vascular endothelial dysfunction in obesity. Am J Cardiol. 2008;101:98–101. doi: 10.1016/j.amjcard.2007.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinberg HO, Chaker H, Leaming R, et al. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40:505–510. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- 8.Bergholm R, Tiikkainen M, Vehkavaara S, et al. Lowering of LDL cholesterol rather than moderate weight loss improves endothelium-dependent vasodilatation in obese women with previous gestational diabetes. Diabetes Care. 2003;26:1667–1672. doi: 10.2337/diacare.26.6.1667. [DOI] [PubMed] [Google Scholar]
- 9.Gokce N, Vita JA, McDonnell M, et al. Effect of medical and surgical weight loss on endothelial vasomotor function in obese patients. Am J Cardiol. 2005;95:266–268. doi: 10.1016/j.amjcard.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Raitakari M, Ilvonen T, Ahotupa M, et al. Weight reduction with very-low-caloric diet and endothelial function in overweight adults: role of plasma glucose. Arterioscler Thromb Vasc Biol. 2004;24:124–128. doi: 10.1161/01.ATV.0000109749.11042.7c. [DOI] [PubMed] [Google Scholar]
- 11.Sciacqua A, Candigliota M, Ceravolo R, et al. Weight loss in combination with physical activity improves endothelial dysfunction in human obesity. Diabetes Care. 2003;26:1673–1678. doi: 10.2337/diacare.26.6.1673. [DOI] [PubMed] [Google Scholar]
- 12.NHLBI Obesity Education Initiative . Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. National Institutes of Health; Washington: 1998. [PubMed] [Google Scholar]
- 13.Vita JA. Nitric oxide-dependent vasodilation in human subjects. Meth Enzymol. 2002;359:186–200. doi: 10.1016/s0076-6879(02)59183-7. [DOI] [PubMed] [Google Scholar]
- 14.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 15.Skilton MR, Sieveking DP, Harmer JA, et al. The effects of obesity and non-pharmacological weight loss on vascular and ventricular function and structure. Diabetes Obes Metab. 2008;10:874–884. doi: 10.1111/j.1463-1326.2007.00817.x. [DOI] [PubMed] [Google Scholar]
- 16.Ziccardi P, Nappo F, Giugliano G, et al. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105:804–809. doi: 10.1161/hc0702.104279. [DOI] [PubMed] [Google Scholar]
- 17.Pontiroli AE, Pizzocri P, Paroni R, Folli F. Sympathetic overactivity, endothelial dysfunction, inflammation, and metabolic abnormalities cluster in grade III (World Health Organization) obesity: reversal through sustained weight loss obtained with laparoscopic adjustable gastric banding. Diabetes Care. 2006;29:2735–2738. doi: 10.2337/dc06-1417. [DOI] [PubMed] [Google Scholar]
- 18.Bohlen HG, Lash JM. Topical hyperglycemia rapidly suppresses EDRF-mediated vasodilation of normal rat arterioles. Am J Physiol. 1993;265:H219–H225. doi: 10.1152/ajpheart.1993.265.1.H219. [DOI] [PubMed] [Google Scholar]
- 19.Bohlen HG, Nase GP. Obesity lowers hyperglycemic threshold for impaired in vivo endothelial nitric oxide function. Am J Physiol Heart Circ Physiol. 2002;283:H391–H397. doi: 10.1152/ajpheart.00019.2002. [DOI] [PubMed] [Google Scholar]
- 20.Williams SB, Goldfine AB, Timimi FK, et al. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998;97:1695–1701. doi: 10.1161/01.cir.97.17.1695. [DOI] [PubMed] [Google Scholar]
- 21.Kawano H, Motoyama T, Hirashima O, et al. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J Am Coll Cardiol. 1999;34:146–154. doi: 10.1016/s0735-1097(99)00168-0. [DOI] [PubMed] [Google Scholar]
- 22.Molnar J, Yu S, Mzhavia N, et al. Diabetes induces endothelial dysfunction but does not increase neointimal formation in high-fat diet fed C57BL/6J mice. Circ Res. 2005;96:1178–1184. doi: 10.1161/01.RES.0000168634.74330.ed. [DOI] [PubMed] [Google Scholar]
- 23.Okon EB, Chung AW, Rauniyar P, et al. Compromised arterial function in human type 2 diabetic patients. Diabetes. 2005;54:2415–2423. doi: 10.2337/diabetes.54.8.2415. [DOI] [PubMed] [Google Scholar]
- 24.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27:567–574. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- 25.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 26.Title LM, Cummings PM, Giddens K, Nassar BA. Oral glucose loading acutely attenuates endothelium-dependent vasodilation in healthy adults without diabetes: an effect prevented by vitamins C and E. J Am Coll Cardiol. 2000;36:2185–2191. doi: 10.1016/s0735-1097(00)00980-3. [DOI] [PubMed] [Google Scholar]
- 27.Gutterman DD. Vascular dysfunction in hyperglycemia: is protein kinase C the culprit? Circ Res. 2002;90:5–7. [PubMed] [Google Scholar]
- 28.Montagnani M, Golovchenko I, Kim I, et al. Inhibition of phosphatidylinositol 3-kinase enhances mitogenic actions of insulin in endothelial cells. J Biol Chem. 2002;277:1794–1799. doi: 10.1074/jbc.M103728200. [DOI] [PubMed] [Google Scholar]
- 29.Yan SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond. Circ Res. 2003;93:1159–1169. doi: 10.1161/01.RES.0000103862.26506.3D. [DOI] [PubMed] [Google Scholar]
- 30.Deng D, Evans T, Mukherjee K, Downey D, Chakrabarti S. Diabetes-induced vascular dysfunction in the retina: role of endothelins. Diabetologia. 1999;42:1228–1234. doi: 10.1007/s001250051296. [DOI] [PubMed] [Google Scholar]
- 31.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Apovian CM, Bigornia S, Mott M, et al. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28:1654–1659. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishimura S, Manabe I, Nagasaki M, et al. In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J Clin Invest. 2008;118:710–721. doi: 10.1172/JCI33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shechter M, Beigel R, Freimark D, Matetzky S, Feinberg MS. Short-term sibutramine therapy is associated with weight loss and improved endothelial function in obese patients with coronary artery disease. Am J Cardiol. 2006;97:1650–1653. doi: 10.1016/j.amjcard.2005.12.059. [DOI] [PubMed] [Google Scholar]
- 35.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 36.Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141:421–431. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 37.Chiasson JL, Josse RG, Gomis R, et al. STOP-NIDDM Trial Research Group Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486–494. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 38.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 40.Cefalu WT. Glycemic targets and cardiovascular disease. N Engl J Med. 2008;358:2633–2635. doi: 10.1056/NEJMe0803831. [DOI] [PubMed] [Google Scholar]


