Abstract
BACKGROUND:
The phospholipase A2 (PLA2) family comprises multiple isoenzymes that vary in their physicochemical properties, cellular localizations, calcium sensitivities, and substrate specificities. Despite these differences, PLA2s share the ability to catalyze the synthesis of the precursors of the proinflammatory mediators. To investigate the potential of PLA2 as a biomarker in screening neuroinflammatory disorders in both clinical and research settings, we developed a PLA2 assay and determined the predominant types of PLA2 activity in cerebrospinal fluid (CSF).
METHODS:
We used liposomes composed of a fluorescent probe (bis-Bodipy® FL C11-PC [1,2-bis-(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-undecanoyl)-sn-glycero-3-phosphocholine]) and 1,2-dioleoyl-l-α-phosphatidylcholine as a substrate to measure CSF PLA2 activity in a 96-well microtiter plate format. We established the type of CSF PLA2 activity using type-specific inhibitors of PLA2.
RESULTS:
Using 5 μL CSF per assay, our PLA2 activity assay was reproducible with CVs <15% in 2 CSF samples and for recombinant secretory Ca2+-dependent PLA2 (sPLA2) in concentrations ranging from 0.25 to 1 μmol/L. This PLA2 assay allowed identification of sPLA2 activity in lumbar CSF from healthy individuals 20–77 years old that did not depend on either sex or age. Additionally, CSF sPLA2 activity was found to be increased (P = 0.0008) in patients with Alzheimer disease.
CONCLUSIONS:
Adult human CSF has sPLA2 activity that can be measured reliably with the assay described. This enzyme activity in the CSF is independent of both sex and age and might serve as a valuable biomarker of neuroinflammation, as we demonstrated in Alzheimer disease.
Nearly 22 different phospholipase A2 (PLA2,5 EC 3.1.1.4) enzymes have been identified and broadly classified into several families based on their structure, cellular localization, substrate specificity, and calcium requirement for catalytic activity: (1) the secretory (extracellular) Ca2+-dependent PLA2 (sPLA2) family of 10 isoenzymes with low molecular weight (14–19 kDa) that requires mmol/L calcium concentrations for catalytic activity; (2) the cytosolic Ca2+-dependent PLA2 (cPLA2) family of 3 isoenzymes with high molecular weight (61–114 kDa) that requires μmol/L calcium concentrations for binding to lipid substrates; (3) the intracellular Ca2+-independent PLA2 (iPLA2) family of 2 isoenzymes with high molecular weight (84–88 kDa); (4) the platelet-activating factor acetylhydrolase (PAF-AH) family of 4 isoenzymes with low molecular weight (26–45 kDa) that are calcium independent; and (5) the lysosomal PLA2 family of a 45-kDa isoform that does not require calcium for catalytic activity (1).
Despite their differences, PLA2s share the ability to catalyze the hydrolysis of the sn-2 ester bonds of glycerophospholipids, resulting in the production of free fatty acids (e.g., arachidonic acid and docosahexaenoic acid) and lysophospholipids. These metabolites have a variety of physiologic effects, e.g., regulation of gene expression and maintenance of neural membrane integrity, and serve as precursors for the synthesis of proinflammatory mediators such as eicosanoids (prostaglandins and leukotrienes) and platelet-activating factor (2). Although these PLA2-derived metabolites, at low concentrations, contribute to normal functions, their excessive production and accumulation can lead to pathological processes.
Second to adipose tissue, the brain has the highest lipid concentration. Dysregulation of lipid metabolism that results in an upregulation of PLA2 is of particular interest in the context of the central nervous system and has already been considered in a number of neurological, neurodegenerative, and psychiatric disorders including epilepsy, ischemia, Alzheimer disease (AD), Parkinson disease, schizophrenia, and mood disorder (2, 3). But, despite several studies, the functions of brain PLA2s are either unknown, diverse, or controversial.
The availability of a simple, reproducible, and sensitive assay is needed to facilitate studies regarding the role of PLA2 isoenzymes in regulating physiological and pathological functions in the central nervous system. We report here the development and validation of such an assay for measurement of PLA2 activity in cerebrospinal fluid (CSF).
Materials and Methods
CHEMICALS
Recombinant mouse group X secretory PLA2 (4, 5) was a kind gift from Tauseef R. Butt (LifeSensors Inc.). Bis-Bodipy® FL C11-PC [1,2-bis-(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-undecanoyl)-sn-glycero-3-phosphocholine] was purchased from Invitrogen; AACOCF3 (arachidonyl trifluoromethyl ketone), BEL (bromoenol lactone), and thioetheramide-PC (1-palmitylthio-2-palmitoylamido-1,2-dideoxy-sn-glycero-3-phosphorylcholine) were from Cayman Chemical.; PG (l-α-phosphatidylglycerol), DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine), and other chemical products were from Sigma Aldrich.
STUDY PARTICIPANTS AND CSF SAMPLING
We generated the study population from retrospectively collected lumbar CSFs available for research purposes. The study was approved by the ethics committees of Eastern Norway and the University of Gothenburg and by the Institutional Review Board of the New York State Institute for Basic Research in Developmental Disabilities. Informed consent was obtained from all participants in accordance with the provisions of the Helsinki Declaration. Forty-two healthy individuals, without any cognitive disturbance, were actively recruited and selected to participate in this study, plus an additional group of 33 AD patients diagnosed with probable AD according to criteria established by the work group of the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) (6). Mini-Mental State Examination (MMSE) scores were documented for most AD patients and for some controls (7). We determined the apolipoprotein E isoform by isoelectric focusing/immunoblotting using serum samples (8). The clinical features of the participants in this study are summarized in Tables 1 and 2. Venipuncture was performed to obtain serum, and CSF was obtained by lumbar puncture from the L3/L4 or L4/L5 intervertebral space; the first 12 mL CSF was collected, centrifuged at 2000g for 10 min at −4 °C, and aliquoted in 1-mL polypropylene tubes. All samples were sent in dry ice from Sahlgrenska University Hospital to New York State Institute for Basic Research and kept at −80 °C until used.
Table 1.
CSF vs serum sPLA2 activity.a
| Healthy individuals (n = 10) |
|
|---|---|
| Sex, F/M | 3/7 |
| Age, years | 53.0 (22.6) |
| CSF/serum sPLA2 activity ratiob | 0.4 (0.1) |
| CSF/serum protein ratioc | 0.013 (0.003) |
Categorical data (sex) and continuous variables (age, CSF/serum sPLA2 activity ratio, CSF/serum protein ratio) are expressed as number of individuals and as mean (SD), respectively.
Whereas CSFs were undiluted, sera were appropriately diluted to get similar protein concentration between CSF and serum-matched samples.
Protein concentrations were measured using bicinchoninic acid protein assay (Pierce).
Table 2.
Demographic and clinical features and CSF levels of sPLA2 activity of AD and age-matched controls.a
| Control | AD | Statistic value | P | |
|---|---|---|---|---|
| 19 | 33 | |||
| Male sex, n (%) | 12 (63) | 17 (52) | NA | 0.564 |
| Age, years | 62.6 (8.2) | 66.8 (3.6) | z = 212.5 | 0.055 |
| MMSE | 29.5 (0.5) | 22.7 (6.4) | z = 58.5 | 0.0008 |
| ApoE4 carrier, n (%) | 0 (0) | 19 (58) | NA | <0.0001 |
| sPLA2 activity, ΔFI/min | 3.8 (0.9) | 5.1 (1.3) | tdf=50 = 3.59 | 0.0008 |
Differences between groups were assessed using Fisher test. Continuous variables are expressed as mean (SD), and differences between groups were assessed using Mann–Whitney test (age, MMSE) or 2-tailed Student t-test (sPLA2 activity). NA, not applicable.
CONTINUOUS FLUORESCENT ASSAY FOR PLA2
We prepared fluorescence-labeled liposomes similarly to a described method (9). To optimize the conditions for the measurement of PLA2 activity in CSF, we adjusted parameters such as concentration and composition of phospholipids. Briefly, 388 μg DOPC and 97 nmol bis-Bodipy FL C11-PC were mixed in 970 μL chloroform. After drying under vacuum, we added 970 μL sucrose/Tris buffer (50 mmol/L Tris-HCl, pH 7.4, 250 mmol/L sucrose, 0.02% sodium azide), followed by thorough and repeated mixing with a Vortex-mix over 5 min at room temperature. The suspension was then ultrasonicated for 10 min on ice using an ultrasonic cell disrupter (Branson Digital Sonifier) at 50 W sonic energy. The liposomes were stored at −20 °C until used. Liposomes containing other proportions and/or types of phospholipids were prepared by comparable procedures.
We carried out PLA2 activity assays in triplicate using a continuous fluorescent measurement. In a 96-well microplate, we diluted 5 μL lumbar CSF (or 10 μmol/L recombinant sPLA2 or 5 μL of appropriately diluted serum) in 90 μL PLA2 assay buffer (10 mmol/L Tris-HCl, pH 7.4, 100 mmol/L KCl, 5 mmol/L CaCl2, 1 mmol/L dithiothreitol). We added 5 μL bis-Bodipy FL C11-PC-labeled 100% DOPC liposomes to each well and immediately placed the microplate in a temperature controlled (30 °C) cytofluor multiwell plate reader series 4000 (PerSeptive Biosystems). The fluorescence intensity was recorded over 90 min (91 cycles of 60 s each) at 485 nm excitation and 530 nm emission. Finally, we evaluated PLA2 activity using linear curve fitting with Graph Prism 3.0 (GraphPad).
METHOD VALIDATION
We assessed the linearity of the assay by measuring in triplicate the PLA2 activity at 5 different concentrations of recombinant sPLA2 (0–2 μmol/L) as well as 5 different CSF volumes (0–10 μL). We estimated the precision by measuring repeatability and intermediate precision. We assessed both measurements with 3 different concentrations of recombinant sPLA2 (0.25, 0.5, and 1 μmol/L) as well as with 3 different CSF volumes (2.5, 5, and 7.5 μL). We calculatedc repeatability by measuring PLA2 activity in sextuplicate. To evaluate intermediate precision, we analyzed each sample 3 times a day on 3 consecutive days.
PLA2 INHIBITION
We prepared stock solutions and serial dilutions of PLA2 inhibitors (AACOCF3, BEL, and thioetheramide-PC) in PLA2 assay buffer supplemented by 4.25 mmol/L Triton X-100. Each assay was carried out in the absence or presence of 5 μL of the appropriate inhibitor.
STATISTICAL ANALYSES
We performed statistical analyses with Statgraphics Centurion XV (StatPoint) and Graph Prism 3.0 (GraphPad). We considered variable distributions nonnormal when values of skewness and kurtosis were outside the range −2 to +2. Non–normally distributed variables were analyzed by nonparametric tests or were log-transformed before parametric tests. We compared frequency distributions using the Fisher test. We assessed differences between 2 means using unpaired, 2-tailed Student t-test or Mann–Whitney test. Pearson correlation coefficients were calculated for statistical analysis of correlation. The level of significance was defined as P < 0.05. Results are expressed as mean (SD).
Results
DEVELOPMENT AND VALIDATION OF THE PLA2 ACTIVITY ASSAY
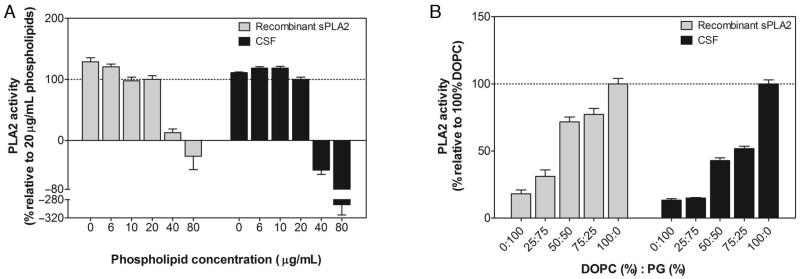
To measure PLA2 activity in human CSF, we developed a continuous fluorescence assay using an already well-known PLA2 specific substrate, self-quenched fluorescent bis-Bodipy FL C11-PC (9). This substrate (because of its 2 Bodipy fluorophores added to the sn-1 and sn-2 acyl chains of phosphatidylcholine), once incorporated into artificial cell membranes such as liposomes, exhibits attenuated fluorescence due to energy transfer between the 2 Bodipy fluorophores. On cleavage by PLA2, the Bodipy from the sn-2 acyl chain is released and an increase in fluorescence results from the separation of the 2 Bodipy moieties. Because PLA2 activity has been reported to depend on phospholipid concentration and composition from liposomes (9), we assessed the effect of these 2 parameters on CSF as well as on recombinant sPLA2 activities to optimize the conditions for measurement. We observed a maximum activity of the enzymatic reaction, for CSF as well as recombinant sPLA2, when using liposomes made from 20 μg/mL 100% DOPC and labeled with 5 μmol/L bis-Bodipy FL C11-PC (Fig. 1). These conditions allowed us to record a time-dependent increase in fluorescence intensity (FI) from lumbar CSF (Fig. 2A), whereas bis-Bodipy FL C11-PC–labeled 100% DOPC liposomes alone did not show such increases. Because no artifactual change in FI was associated with the substrate, the observed changes in FI during the reaction can be associated with artifactual hydrolysis of bis-Bodipy FL C11-PC by CSF PLA2, which produces fluorescence-labeled free fatty acid and lysophosphatidylcholine. Similar results were obtained with recombinant sPLA2 (data not shown).
Fig. 1. Effect of the liposome phospholipid concentration and composition on recombinant and lumbar CSF PLA2 activities.
(A), Effect of increasing the liposome phospholipid concentration on the rate of the initial reaction of 0.5 μmol/L recombinant sPLA2 or 5 μL CSF. The activity was determined using Bodipy-labeled liposomes composed of 50% DOPC and 50% PG in 10 mmol/L Tris-HCl (pH 7.4) supplemented by 5 mmol/L CaCl2. (B), Effect of varying proportions of DOPC and PG on the rate of the initial reaction of 0.5 μmol/L recombinant sPLA2 and 5 μL CSF. The activity was determined using Bodipy-labeled liposomes made up of 20 μg/mL phospholipids in 10 mmol/L Tris-HCl (pH 7.4) supplemented by 5 mmol/L CaCl2. Data are expressed as mean (SD) (n = 3).
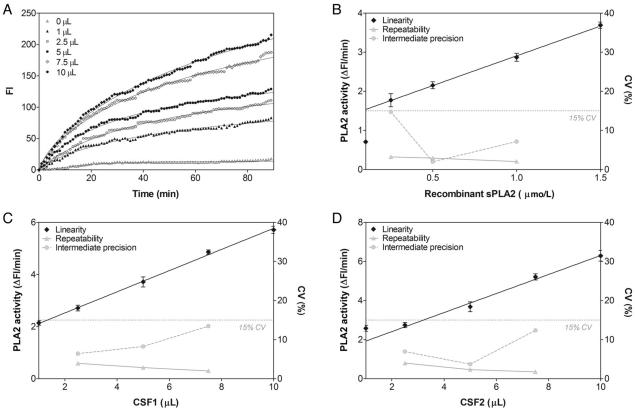
Fig. 2. Linearity and precision profiles for recombinant and lumbar CSF PLA2 activities.
(A), Time dependence of the PLA2 activity in a representative experiment using different volumes of lumbar CSF (0–10 μL). Data are expressed as mean of 3 independent experiments. (B–D), Signal-to-concentration linearity performance and CV for repeatability and intermediate precision assessment for recombinant sPLA2 (B) and CSFs from 2 different cases (C and D). Linearity data are shown as mean (SD) (n = 3), and precision are shown as CV resulting from 6 consecutive replicates for repeatability and from 3 measurements on 3 consecutive days (n = 9) for intermediate precision.
In line with the recommendations of the International Conference on Harmonisation guideline on validation of analytical procedures (10), we validated this new continuous fluorescence assay using both recombinant sPLA2 (Fig. 2B) and lumbar CSF (Fig. 2C and D). The assay imprecision was <15% of CV, which meets the recommended guidelines (11). The CV range for imprecision was 2.0%–14.8% for recombinant sPLA2 in concentrations 0.25–1 μmol/L and 1.8%–13.4% for 2 different CSF samples with sizes ranging from 2.5 to 7.5 μL per assay. Moreover, the initial rate of reaction depended on recombinant sPLA2 concentration (Fig. 2B) and CSF volume (Fig. 2C and D), giving a recombinant sPLA2 signal-to-concentration linearity over the range 0.25–1.5 μmol/L (r2 = 0.999, P < 0.001) and a linear signal-to-CSF volume relationship over the range 2.5–10 μL for the CSF1 (r2 = 0.997, P = 0.001) as well as for the CSF2 (r2 = 0.992, P = 0.004) tested.
CHARACTERIZATION OF THE CSF PLA2 ACTIVITY
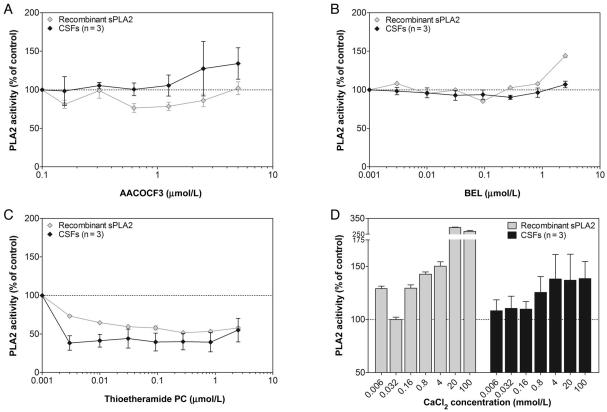
Although 22 different PLA2 enzymes classified in 5 families (sPLA2, cPLA2, iPLA2, PAF-AH, and lysosomal PLA2) have been identified (1), no specific tool allowing the specific characterization of each of these isoenzyme is available to date. Thus, with regard to a new PLA2 activity identification, the current approach is to determine the family instead of the concerned specific isoenzyme. For this purpose, a battery of inhibitors more or less specific to each PLA2 family is generally used to discriminate 1 family from another. Thus, to identify the type of PLA2 activity in the CSF measured using our assay, we performed incubation of 5 μL lumbar CSF in the presence of 3 different PLA2 inhibitors (AACOCF3, BEL, and thioetheramide-PC) that are known to exhibit different relative potencies for the 3 major families of the enzyme (sPLA2, cPLA2, and iPLA2). Indeed, whereas AACOCF3 is a selective inhibitor of both cPLA2 and iPLA2 (12, 13), BEL inhibits specifically isoenzymes from the iPLA2 family (12), and thioetheramide-PC functions as a competitive inhibitor toward sPLA2 (14). The pharmacological profile of the PLA2 activity in lumbar CSF was found to be most consistent with the sPLA2 subtype (Fig. 3). Indeed, wheres the sPLA2-specific inhibitor thioetheramide-PC dose-dependently inhibited the PLA2 activity from lumbar CSF as well as from the recombinant sPLA2 (Fig. 3C), the 2 other inhibitors failed to do so (Fig. 3A and B).
Fig. 3. Effect of PLA2 family-specific inhibitors on recombinant and lumbar CSF PLA2 activities.
(A–C), Effect of different concentrations of 3 PLA2 inhibitors: 0.16–5 μmol/L AACOCF3 (A); 0.003–2.5 μmol/L BEL (B); 0.003–2.5 μmol/L thioetheramide-PC (C). (D), Effect of increasing concentration of calcium (6.4 μmol/L to 100 mmol/L). PLA2 activity is expressed as percentage of the activity relative to the same sample without any inhibitor or any calcium supplementation. Recombinant sPLA2 and lumbar CSF data are expressed as mean (SD) from 3 experiments and from 3 different CSFs each analyzed in triplicate, respectively.
Although calcium is known to be an absolute requirement for the activity of both sPLA2 and cPLA2, its role in the 2 activations is different. Calcium resides at the catalytic center of sPLA2 and is directly involved in substrate–enzyme interaction (15), whereas it is not required for cPLA2 catalytic activity but rather involved in the translocation of cPLA2 from cytosol to the membrane where the substrates are located (16). Thus, to further characterize the CSF PLA2 and to confirm its type, we assessed PLA2 activity in the presence of various concentrations of calcium. With increase in the concentration of calcium, we observed an increase of PLA2 activity in lumbar CSF as well as recombinant sPLA2 (Fig. 3D). Thus, the PLA2 activity measured in human CSF appears to be mainly sPLA2 activity. The PLA2 activity observed in CSF was unlikely to be due to either PAF-AH or lysosomal PLA2, since both of them are calcium-independent (17, 18).
MEASUREMENT OF sPLA2 ACTIVITY IN NORMAL CSF
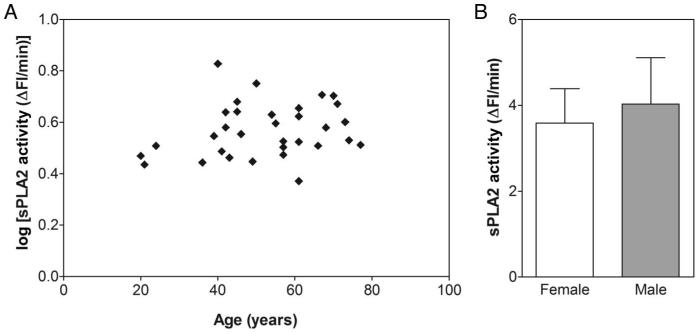
To determine whether CSF sPLA2 activity depends on age or sex, we assessed its normal physiologic levels from 32 healthy volunteers (16 women and 16 men; age 52.3 (15.2) years; MMSE score 29.5 (0.5); sPLA2 activity 3.8 (1.0) ΔFI/min). As shown in Fig. 4A, sPLA2 activity in CSF does not seem to correlate with age. Moreover, no significant difference in sPLA2 activity was noted between men and women (Fig. 4B).
Fig. 4. CSF sPLA2 activity as function of age and sex.
(A), Pearson correlation between log(sPLA2 activity) and age (r = 0.214; P = 0.239; 95% CI −0.145 to 0.524). (B), No significant difference between healthy women and healthy men for sPLA2 activity (tdf=30 = 1.312; P = 0.200). Data are shown as mean (SD).
CSF sPLA2 ACTIVITY VS SERUM sPLA2 ACTIVITY
To better understand the origin of the sPLA2 activity measured in lumbar CSF, we simultaneously assessed sPLA2 activity in 10 undiluted lumbar CSFs as well as diluted sera from the same cases (Table 1). We found that the sPLA2 activity relative to protein concentration was 2.5-fold higher in serum than in lumbar CSF which, based on the basic feature of both brain- and blood-derived proteins (19), seems to be of brain origin with a blood-derived fraction in it.
CSF sPLA2 ACTIVITY IN ALZHEIMER DISEASE
Evidence from epidemiological, clinical, and experimental studies supports the hypothesis that inflammatory events are part of the neuropathology of AD (20, 21). Several markers of neuroinflammation have been measured in biological fluids, but their specificity for AD remains controversial (22). To assess the relevance of CSF sPLA2 activity measurement as a neuroinflammatory biomarker for AD, we performed its measurement in CSF from 33 AD patients and 19 age-matched controls. As shown in Table 2, a significant increase in CSF sPLA2 activity was noted for AD compared with the control group.
Discussion
We have developed a simple continuous fluorescence assay for measuring PLA2 activity in CSF using a 96-well microplate format that allows for high-throughput analysis of samples. In addition to fluoro-metric assays, titrimetric, colorimetric, and radiometric methods can be used to determine PLA2 activity in biological fluids (23). With the exception of the radio-metric, however, these methods are not sufficiently sensitive. Although radiometric methods are more sensitive, they are hazardous, laborious, and impractical for assaying large numbers of samples. Thus, albeit with a sensitivity that is second to radiometric methods (23), fluorescence-based assays offer the advantage of being suitable for the analysis of a large number of biological fluid samples in a clinical setting.
The performance of a fluorescence-based assay is highly dependent on the conditions and in particular on the type of fluorescent PLA2 substrate used. Several substrates, differentiated by type, number, and localization of the fluorophores, are commercially available. The 2 more frequently used substrates are NBD C6-HPC® [2-(6-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)hexanoyl-1-hexadecanoyl-sn-glycero-3-phosphocholine] and bis-Bodipy FL C11-PC. The former exhibits 1 fluorophore on the sn-2 phospholipid acyl chain, whereas the latter exhibits an additional fluorophore on the sn-1 phospholipid acyl chain, which contributes to its self-quenching. When using NBD C6-HPC, an additional laborious step of separation of the remainder substrate from the product is required (24). In the case of bis-Bodipy FL C11-PC, this step is not required (9), allowing the continuous monitoring of PLA2 activity. When combined, these characteristics make our approach the most attractive methodology currently available.
We also show that the use of liposomes made up of 20 μg/mL phospholipids containing 100% DOPC and labeled with 5 μmol/L bis-Bodipy FL C11-PC as substrate in 10 mmol/L Tris-HCl (pH 7.4) supplemented by 5 mmol/L CaCl2 is optimal to measure PLA2 activity in 5 μL lumbar CSF per assay. The linear signal-to-CSF volume relationship over the range 2.5–10 μL CSF demonstrates the sensitivity of this approach. Another strength of our assay is its reliability as shown by CV <15% for repeatability and intermediate precisions (11).
In the present study, for the first time, we identified the secretory Ca2+-dependent PLA2 activity in lumbar CSF. The observations that 3 mmol/L thioetheramide-PC inhibited >60% of substrate cleavage and that the FI increase was calcium dependent suggest that the CSF activity is predominantly due to sPLA2 type. Additionally, negative results from the pharmacologic experiments using AACOCF3 and BEL, 2 inhibitors of cPLA2/iPLA2 and iPLA2, respectively, confirm this conclusion. Based on our data, however, we cannot identify which specific isoenzymes give rise to this sPLA2 activity. The identification of sPLA2 isoenzymes from CSF using selective antibodies should be considered. At present, however, the lack of availability of specific antibodies against each sPLA2 isoenzyme makes this identification difficult. In addition, we cannot rule out the presence of other PLA2 types in human CSF. To our knowledge, only 1 other study has reported on this subject to date (25); but the authors of that study claimed that the PLA2 activity identified in their study was most likely from cPLA2 and iPLA2 types. Thus, because the PLA2 type targeted in a specific assay in part depends on the methodology used, we can expect the future identification of other CSF PLA2 types as has been seen in studies of human serum using independent methodologies (9, 24, 26).
Phospholipase A2 enzymes are well known to play a role in neuroinflammation (2). Concerning the specific secretory Ca2+-dependent PLA2 family, a large variety of additional biological functions have been proposed. These include, among others, reactive oxygen species generation (27), apoptosis (28), and cell proliferation (29). To date, however, the in vivo biological functions, in physiological as well as pathological conditions, of each brain sPLA2 remain unknown. We believe that the development of this simple and sensitive assay as well as the demonstration of the secretory (extracellular) Ca2+-dependent PLA2 activity in CSF will stimulate studies that will enhance the understanding of the role of these isoenzymes in neurological disorders.
The discovery of the sPLA2 activity in lumbar CSF allows us to envisage the use of our assay in the diagnosis of neurological disorders associated with inflammation. Such a consideration raises the question of whether an increase of sPLA2 activity in CSF reflects the cause or the outcome of a neuroinflammatory process. In other words, is the sPLA2 activity measured in lumbar CSF of central nervous system origin or of blood origin or both? Because sPLA2 activity is present in both serum (9) and brain (2), both origins can be considered equally. The simultaneous measurement of sPLA2 activity in both CSF and serum from the same cases, at equal protein concentrations, revealed a CSF: serum sPLA2 activity ratio of 1:2.5. In considering the basic features of both brain- and blood-derived proteins as described by Reiber (19), such a ratio is consistent with a brain origin associated with a blood-derived fraction of the enzyme activity. Furthermore, the fact that the CSF sPLA2 activity did not increase from age 20 to 77 whereas the CSF/serum albumin ratio does increase with age (30), also supports the main brain origin of the CSF sPLA2 activity we have observed. However, the discrimination between brain and blood origin is difficult to assess once the multiplicity of serum and brain PLA2 isoenzymes characterized by a sPLA2 activity is taken into consideration.
The presence of activated glial cells and the increase in inflammation-associated proteins in AD brain had led to the consideration that chronic inflammation plays a role in the pathophysiology of AD (20, 21). For these reasons, several mediators of inflammation such as interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α) have been extensively studied in CSF of AD patients, but conflicting results have been observed (22). Indeed, although the majority of studies report no difference (31, 32), others showed an increase (33, 34) or even a decrease (35) in levels of these inflammatory markers in CSF of AD patients compared with controls. Due to this low specificity, the use of cytokines as biomarkers of AD is questionable. Other key molecules involved in neuroinflammation, such as constituents of the arachidonic acid cascade, have been investigated in the CSF. The downstream events of this pathway result in enzymatic oxidation by cyclooxygenase and lipoxygenase and in the nonenzymatic peroxidation of arachidonic acid (AA) in proinflammatory eicosanoids (i.e., prostaglandins, leukotrienes, and thromboxanes) and isoprostanes, respectively (36, 37). The concentrations of eicosanoids and isoprostanes in CSF are of particular interest in the biomarker field, and increases in CSF prostaglandin E2 (PGE2) and F2-isoprostane have been reported in AD patients (38, 39). However, such analyses require highly specialized equipment and expertise, e.g., GC/MS, and, the measurements of CSF PGE2 and F2-isoprostane have not, to date, been widely used in practice. The upstream events of the arachidonic acid cascade corresponding to AA synthesis by PLA2 are of major interest in neuroinflammation. Whereas the quantification of 11C-AA incorporation into human brain by positron emission tomography has provided promising results (40), little is known about phospholipase A2 activity in CSF. In this study, we have shown for the first time a significant increase of sPLA2 activity in AD compared with age-matched controls, which may allow consideration of such measurement as a neuroinflammatory biomarker.
In conclusion, we have developed a new continuous fluorescence method using commercially available reagents to measure the CSF activity of a well-known inflammatory key enzyme, i.e., the secretory (extracellular) Ca2+-dependent PLA2. We have also demonstrated that this microplate assay meets several of the ideal biomarker requirements such as reliability, reproducibility, and simplicity to perform (41). The discovery of the sPLA2 activity in human lumbar CSF should facilitate investigations of this enzyme activity in CSF from a variety of neurological diseases, especially neurodegenerative and neuroinflammatory disorders. The increase in the CSF level of sPLA2 activity in Alzheimer disease compared with age-matched healthy controls discovered in the present study is consistent with the involvement of neuroinflammation in this disease.
Acknowledgments
We are grateful to Ved Chauhan (NYS Institute for Basic Research, Staten Island, NY) for his valuable advice in the preparation of liposomes and to Janet Murphy for secretarial assistance. This work was supported by the New York State Office of Mental Retardation and Developmental Disabilities, the NIH National Institute of Aging (AG028538), and the Alzheimer's Association (IIRG-06-25836).
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
Footnotes
Nonstandard abbreviations: PLA2, phospholipase A2; sPLA2, secretory Ca2+-dependent PLA2; cPLA2, cytosolic Ca2+-dependent PLA2; iPLA2, intracellular Ca2+-independent PLA2; PAF-AH, platelet-activating factor acetylhydrolase; AD, Alzheimer disease; CSF, cerebrospinal fluid; bis-Bodipy FL C11-PC, 1,2-bis-(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-undecanoyl)-sn-glycero-3-phosphocholine; AACOCF3, arachidonyl trifluoromethyl ketone; BEL, bromoenol lactone; thioetheramide-PC, 1-palmitylthio-2-palmitoylamido-1,2-dideoxy-sn-glycero-3-phosphorylcholine; PG, l-α-phosphatidylglycerol; DOPC, 1,2-dioleoyl-sn-glycero-3-phosphocholine; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association; MMSE, Mini-Mental State Examination; FI, fluorescence intensity; NBD C6-HPC, 2-(6-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino)hexanoyl-1-hexadecanoyl-sn-glycero-3-phosphocholine; IL, interleukin; TNF-α, tumor necrosis factor-α; AA, arachidonic acid; PGE2, prostaglandin E2.
Authors' Disclosures of Potential Conflicts of Interest: No authors declared any potential conflicts of interest.
References
- 1.Burke JE, Dennis EA. Phospholipase A(2) Biochemistry. Cardiovasc Drugs Ther. 2009;23:49–59. doi: 10.1007/s10557-008-6132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farooqui AA, Horrocks LA. Phospholipase A2-generated lipid mediators in the brain: the good, the bad, and the ugly. Neuroscientist. 2006;12:245–60. doi: 10.1177/1073858405285923. [DOI] [PubMed] [Google Scholar]
- 3.Farooqui AA, Ong WY, Horrocks LA. Inhibitors of brain phospholipase A2 activity: their neuropharmacological effects and therapeutic importance for the treatment of neurologic disorders. Pharmacol Rev. 2006;58:591–620. doi: 10.1124/pr.58.3.7. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson B, Leach CA, Goldenberg SJ, Francis DM, Kodrasov MP, Tian X, et al. Characterization of ubiquitin and ubiquitin-like-protein isopeptidase activities. Protein Sci. 2008;17:1035–43. doi: 10.1110/ps.083450408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morioka Y, Saiga A, Yokota Y, Suzuki N, Ikeda M, Ono T, et al. Mouse group X secretory phospholipase A2 induces a potent release of arachidonic acid from spleen cells and acts as a ligand for the phospholipase A2 receptor. Arch Biochem Biophys. 2000;381:31–42. doi: 10.1006/abbi.2000.1977. [DOI] [PubMed] [Google Scholar]
- 6.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 7.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 8.Kataoka S, Paidi M, Howard BV. Simplified isoelectric focusing/immunoblotting determination of apoprotein E phenotype. Clin Chem. 1994;40:11–3. [PubMed] [Google Scholar]
- 9.Tsao FH, Shanmuganayagam D, Zachman DK, Khosravi M, Folts JD, Meyer KC. A continuous fluorescence assay for the determination of calcium-dependent secretory phospholipase A2 activity in serum. Clin Chim Acta. 2007;379:119–26. doi: 10.1016/j.cca.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 10.ICH Harmonised Tripartite Guideline—Validation of Analytical Procedures: Text and Methodology Q2(R1); International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use.2005. [Google Scholar]
- 11.Food and Drug Administration Guidance for Industry: Bioanalytical Method Validation. 2001 http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf (Accessed October 14, 2009)
- 12.Ackermann EJ, Conde-Frieboes K, Dennis EA. Inhibition of macrophage Ca(2+)-independent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones. J Biol Chem. 1995;270:445–50. doi: 10.1074/jbc.270.1.445. [DOI] [PubMed] [Google Scholar]
- 13.Street IP, Lin HK, Laliberte F, Ghomashchi F, Wang Z, Perrier H, et al. Slow- and tight-binding inhibitors of the 85-kDa human phospholipase A2. Biochemistry. 1993;32:5935–40. doi: 10.1021/bi00074a003. [DOI] [PubMed] [Google Scholar]
- 14.Plesniak LA, Boegeman SC, Segelke BW, Dennis EA. Interaction of phospholipase A2 with thioether amide containing phospholipid analogues. Biochemistry. 1993;32:5009–16. doi: 10.1021/bi00070a006. [DOI] [PubMed] [Google Scholar]
- 15.Scott DL, White SP, Browning JL, Rosa JJ, Gelb MH, Sigler PB. Structures of free and inhibited human secretory phospholipase A2 from inflammatory exudate. Science (Wash DC) 1991;254:1007–10. doi: 10.1126/science.1948070. [DOI] [PubMed] [Google Scholar]
- 16.Nalefski EA, Sultzman LA, Martin DM, Kriz RW, Towler PS, Knopf JL, Clark JD. Delineation of two functionally distinct domains of cytosolic phospholipase A2, a regulatory Ca(2+)-dependent lipid-binding domain and a Ca(2+)-independent catalytic domain. J Biol Chem. 1994;269:18239–49. [PubMed] [Google Scholar]
- 17.Abe A, Shayman JA. Purification and characterization of 1-O-acylceramide synthase, a novel phospholipase A2 with transacylase activity. J Biol Chem. 1998;273:8467–74. doi: 10.1074/jbc.273.14.8467. [DOI] [PubMed] [Google Scholar]
- 18.Stafforini DM. Biology of platelet-activating factor acetylhydrolase (PAF-AH, lipoprotein associated phospholipase A(2)) Cardiovasc Drugs Ther. 2009;23:73–83. doi: 10.1007/s10557-008-6133-8. [DOI] [PubMed] [Google Scholar]
- 19.Reiber H. Dynamics of brain-derived proteins in cerebrospinal fluid. Clin Chim Acta. 2001;310:173–86. doi: 10.1016/s0009-8981(01)00573-3. [DOI] [PubMed] [Google Scholar]
- 20.McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev. 1995;21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 21.McGeer PL, Rogers J. Anti-inflammatory agents as a therapeutic approach to Alzheimer's disease. Neurology. 1992;42:447–9. doi: 10.1212/wnl.42.2.447. [DOI] [PubMed] [Google Scholar]
- 22.Teunissen CE, de Vente J, Steinbusch HW, De Bruijn C. Biochemical markers related to Alzheimer's dementia in serum and cerebrospinal fluid. Neurobiol Aging. 2002;23:485–508. doi: 10.1016/s0197-4580(01)00328-1. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds LJ, Washburn WN, Deems RA, Dennis EA. Assay strategies and methods for phospholipases. Methods Enzymol. 1991;197:3–23. doi: 10.1016/0076-6879(91)97129-m. [DOI] [PubMed] [Google Scholar]
- 24.Smesny S, Kinder D, Willhardt I, Rosburg T, Lasch J, Berger G, Sauer H. Increased calcium-independent phospholipase A2 activity in first but not in multiepisode chronic schizophrenia. Biol Psychiatry. 2005;57:399–405. doi: 10.1016/j.biopsych.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 25.Smesny S, Stein S, Willhardt I, Lasch J, Sauer H. Decreased phospholipase A2 activity in cerebro-spinal fluid of patients with dementia. J Neural Transm. 2008;115:1173–9. doi: 10.1007/s00702-008-0081-0. [DOI] [PubMed] [Google Scholar]
- 26.Kosaka T, Yamaguchi M, Miyanaga K, Mizuno K. Serum platelet-activating factor acetylhydrolase (PAF-AH) activity in more than 3000 healthy Japanese. Clin Chim Acta. 2001;312:179–83. doi: 10.1016/s0009-8981(01)00612-x. [DOI] [PubMed] [Google Scholar]
- 27.Muralikrishna AR, Hatcher JF. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med. 2006;40:376–87. doi: 10.1016/j.freeradbiomed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 28.Taketo MM, Sonoshita M. Phospolipase A2 and apoptosis. Biochim Biophys Acta. 2002;1585:72–6. doi: 10.1016/s1388-1981(02)00326-8. [DOI] [PubMed] [Google Scholar]
- 29.Masuda S, Yamamoto K, Hirabayashi T, Ishikawa Y, Ishii T, Kudo I, Murakami M. Human group III secreted phospholipase A2 promotes neuronal outgrowth and survival. Biochem J. 2008;409:429–38. doi: 10.1042/BJ20070844. [DOI] [PubMed] [Google Scholar]
- 30.Reiber H. Proteins in cerebrospinal fluid and blood: barriers, CSF flow rate and source-related dynamics. Restor Neurol Neurosci. 2003;21:79–96. [PubMed] [Google Scholar]
- 31.Galimberti D, Venturelli E, Fenoglio C, Guidi I, Villa C, Bergamaschini L, et al. Intrathecal levels of IL-6, IL-11 and LIF in Alzheimer's disease and frontotemporal lobar degeneration. J Neurol. 2008;255:539–44. doi: 10.1007/s00415-008-0737-6. [DOI] [PubMed] [Google Scholar]
- 32.Lanzrein AS, Johnston CM, Perry VH, Jobst KA, King EM, Smith AD. Longitudinal study of inflammatory factors in serum, cerebrospinal fluid, and brain tissue in Alzheimer disease: interleukin-1beta, interleukin-6, interleukin-1 receptor antagonist, tumor necrosis factor-alpha, the soluble tumor necrosis factor receptors I and II, and alpha1-antichymotrypsin. Alzheimer Dis Assoc Disord. 1998;12:215–27. doi: 10.1097/00002093-199809000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Blum-Degen D, Muller T, Kuhn W, Gerlach M, Przuntek H, Riederer P. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer's and de novo Parkinson's disease patients. Neurosci Lett. 1995;202:17–20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- 34.Tarkowski E, Blennow K, Wallin A, Tarkowski A. Intracerebral production of tumor necrosis factor-alpha, a local neuroprotective agent, in Alzheimer disease and vascular dementia. J Clin Immunol. 1999;19:223–30. doi: 10.1023/a:1020568013953. [DOI] [PubMed] [Google Scholar]
- 35.Yamada K, Kono K, Umegaki H, Yamada K, Iguchi A, Fukatsu T, et al. Decreased interleukin-6 level in the cerebrospinal fluid of patients with Alzheimer-type dementia. Neurosci Lett. 1995;186:219–21. doi: 10.1016/0304-3940(95)11318-q. [DOI] [PubMed] [Google Scholar]
- 36.Farooqui AA, Horrocks LA, Farooqui T. Modulation of inflammation in brain: a matter of fat. J Neurochem. 2007;101:577–99. doi: 10.1111/j.1471-4159.2006.04371.x. [DOI] [PubMed] [Google Scholar]
- 37.Nourooz-Zadeh J. Key issues in F2-isoprostane analysis. Biochem Soc Trans. 2008;36:1060–5. doi: 10.1042/BST0361060. [DOI] [PubMed] [Google Scholar]
- 38.Montine TJ, Sidell KR, Crews BC, Markesbery WR, Marnett LJ, Roberts LJ, Morrow JD. Elevated CSF prostaglandin E2 levels in patients with probable AD. Neurology. 1999;53:1495–8. doi: 10.1212/wnl.53.7.1495. [DOI] [PubMed] [Google Scholar]
- 39.Montine TJ, Beal MF, Robertson D, Cudkowicz ME, Biaggioni I, O'Donnell H, et al. Cerebrospinal fluid F2-isoprostanes are elevated in Huntington's disease. Neurology. 1999;52:1104–5. doi: 10.1212/wnl.52.5.1104. [DOI] [PubMed] [Google Scholar]
- 40.Esposito G, Giovacchini G, Liow JS, Bhattacharjee AK, Greenstein D, Schapiro M, et al. Imaging neuroinflammation in Alzheimer's disease with radiolabeled arachidonic acid and PET. J Nucl Med. 2008;49:1414–21. doi: 10.2967/jnumed.107.049619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The Ronald and Nancy Reagan Research Institute of the Alzheimer's Association and National Institute on Aging Working Group Consensus Report of the Working Group on Molecular and Biochemical Markers of Alzheimer's Disease. Neurobiology of Aging. 1998;19:109–16. [PubMed] [Google Scholar]