1.0 Introduction
The Venezuelan equine encephalitis virus (VEEV) complex is composed of serologically related, mosquito-borne viruses belonging to the genus Alphavirus in the family Togaviridae. While generally associated with severe and fatal encephalitis in equines, humans are susceptible to VEEV with disease symptoms including fever, malaise, myalgia and severe headache [1]. Encephalitis occurs occasionally in adults, but more frequently in children [2]. Similar disease manifestations in laboratory workers accidentally exposed to VEEV confirm the highly infectious nature of the virus via the aerosol route [3]. In addition to natural or accidental exposure to the virus, the U.S. Department of Defense identified VEEV as a potential biological warfare agent since VEEV can be produced in unsophisticated culture systems, can be stored for extended periods of time and is highly infectious, requiring relatively few organisms to infect humans [4].
To address the aerosol threat of VEEV on public health, two vaccines were developed by the U.S. government during the 1960s and 1970s: TC-83, a cell-culture attenuated vaccine developed from the Trinidad donkey (VEEV TrD) strain of subtype IAB VEEV [5] and a formalin-inactivated vaccine derived from TC-83, designated C84 [6]. For several decades the TC-83 and C84 vaccines have been administered by the U.S. Army Special Immunizations Program to laboratory workers and animal health field workers at risk for exposure to VEEV. While TC-83 induces long-lasting immunity against closely related VEEV subtypes [7], major limitations of the vaccine exist including: only an approximately 80% response rate as assessed by plaque reduction neutralization test (PRNT) [8]; a 25% incidence of adverse reactions [9]; and reversion to virulence after mouse brain passages [5]. In addition, as a live virus vaccine, TC-83 cannot be used as a booster for subjects with waning antibody titers [10]. C-84 is currently used to boost antibody titers following vaccination with TC-83 and to immunize TC-83 non-responders. C-84 also has limitations in that protection is of short duration and thus requires multiple boosters.
The limitations of the TC-83 and C84 vaccines led to the development of an investigational live-attenuated VEEV vaccine, V3526, developed from a full-length cDNA clone of VEEV IAB Trinidad donkey strain (VEEV TrD) using site-directed mutagenesis. V3526 was attenuated by deleting a furin cleavage site from the PE2 glycoprotein and incorporating a single amino acid mutation in the E1 glycoprotein [11]. The V3526 vaccine is effective in protecting rodents, horses and nonhuman primates (NHP) against subcutaneous or aerosol challenge with fully virulent VEEV TrD (Subtype IAB), as well as other VEEV subtypes (IC, IE and IIIA) [12–15].
Based on the success of V3526 in nonclinical studies, a Phase 1 clinical trial was conducted to evaluate the safety and immunogenicity of V3526 in human subjects. The clinical findings from the Phase 1 trial showed robust immune responses in virtually all vaccine recipients, even those receiving very low dosages (~20 plaque forming units)[16]. However, a significant number of the vaccine recipients demonstrated mild to moderate adverse events (AE) including headache, fever, malaise and sore throat. Several of the vaccine recipients experienced fevers classified as grade 3, based on the current adverse event grading scale. Viral shedding that occurred in a subset of the recipients appeared to coincide with sore throat and/or fevers. Based on these findings, clinical testing of V3526 was discontinued.
Since a high frequency of adverse reactions has been associated with live-attenuated VEEV vaccines [9, 10, 16], licensure of a live-attenuated vaccine will likely be faced with significant regulatory obstacles relating to safety. Our strategy to develop a VEEV vaccine was revised to focus on a non-infectious virus vaccine. The use of C84 was not considered for further development because the Department of Defense, in 1996, deemed this vaccine in need of improvement. C84 was last manufactured between 1980 –1981 and the limited supply of C84 vaccine has been in storage for over 29 years and the recent potency and stability of this vaccine is unknown. Manufacture of new lots of C84 is unlikely to occur because this would require re-derivation of the TC-83 stock, followed by GMP production of the TC-83 in a certifiable cell line and further development of the entire TC-83/C84 manufacturing process. In addition, a technical review of the C84 manufacturing records failed to identify a credible source document describing the actual manufacturing process and testing scheme therefore this would also need to be devised.
Having a large inventory of GMP manufactured V3526 originally reserved for the clinical testing, the decision was made to inactivate V3526 for the production of VEEV vaccine candidates that would ultimately replace C84 and be used as a primary vaccine to protect personnel at risk to accidental or intentional VEEV exposure. Studies were initiated using formalin to inactivate V3526 with the intent of producing a vaccine with a significantly reduced adverse reaction profile compared to V3526, but one that retains potential as a protective immunogen against VEEV infection and performs similarly or better than C84.
Formalin inactivation of virus has been successfully used to develop safe and efficacious human and veterinary vaccines since 1955 [17] and most recently, an inactivated vaccine for Japanese encephalitis virus [18]. The use of formalin inactivation for virus vaccine development is attractive from a safety perspective in that the virus cannot revert to virulence, since there is no virus replication during immunization. The use of formalin to inactivate viruses is also attractive from a manufacturing perspective as the inactivation process is relatively simple to develop. In the development of a formalin inactivated VEEV vaccine candidate, we recently developed a method to inactivate V3526 using formalin and established a system of prioritized assays to evaluate residual infectivity and preservation of immunologically essential epitopes[19].
In that inactivated viral vaccines are non-replicating, formulation with adjuvants may be necessary to augment a protective immune response. To address this concern, we evaluated formalin-inactivated V3526 (fV3526) formulated with each of 4 adjuvants, Viprovex®, CpG oligodeoxynucleotides (ODN) 2395, Alhydrogel™ or CpG+Alhydrogel™. Viprovex® is a synthetically manufactured peptide analogue of Substance P that stimulates antigen presenting cells to utilize both the MHC Class I and II molecules and pathways, resulting in both T-helper (Th)-1 and Th2-mediated immune responses. CpG ODN 2395, is a type C CpG ODN that strongly activates B cells and induces high IFN-α production from plasmacytoid dendritic cells [20, 21]. CpG ODN2395 has demonstrated reactivity to human and murine TOLL-like receptor 9 (TLR9) ligand. Alhydrogel™ commonly known as aluminum hydroxide, binds antigen and incorporates into an insoluble, gel-like precipitate and is believed to continually stimulate the immune system by functioning as an antigen depot [22]. The use of CpG and Alhydrogel™ as a combination adjuvant is reported to enhance immune responses significantly greater than the use of either adjuvant alone [22–24] and was also evaluated.
The current study was designed to evaluate the immunogenicity and efficacy of fV3526 alone and in combination with adjuvants in BALB/c mice following subcutaneous (SC) or intramuscular (IM) administration. The protective efficacy of the immunological response was evaluated by challenge with VEEV TrD via the SC and aerosol routes. As the identification of a new VEEV vaccine candidate was dependent on it being as good as or better than the existing inactivated VEEV vaccine, C84 was included for comparison.
2.0 Materials and Methods
2.1 Test Material Information
Live V3526 bulk drug substance (BDS) was produced by Sigma Aldrich Fine Chemicals (SAFC Pharma), Carlsbad, CA. The titer of this material was 2.9 × 107 pfu/mL. The challenge virus, VEEV TrD, was produced by Commonwealth Biotechnologies Incorporated, Richmond, VA. For the negative control, process control material (PCM) was used, which consists of supernatant from mock infected cultures. C84 was used as a comparator and was manufactured at The Salk Institute, Government Service Division, Swiftwater, PA.
2.2 Formalin Inactivation and Testing
2.2.1 Inactivation of V3526
Virus inactivation studies were carried out at SAFC Pharma. V3526 virus was treated with 0.1% v/v formalin (USP grade, EMD Chemicals) in a calibrated shaking water bath set at 37°C for 24 hours. Residual formalin was reduced to less than 1 × 10−8 % using a tangential flow filtration system (GE Healthcare) with a 500 kDa molecular weight cutoff membrane.
2.2.2 Testing for Residual Infectivity
The multi-system approach for evaluation of virus inactivation was developed to meet the expected regulatory requirements for documentation supporting the safety of new vaccines [25]. Inactivated virus preparations were tested for residual infectivity using a standard plaque assay previously described [12] and serial passage on baby hamster kidney (BHK)-21 cells [26]. The presence of residual infectivity was also assessed by intracranial inoculation of suckling mice with 10 μL of test virus (~2.9×107 pfu/ml prior to inactivation). As controls for the assay, additional suckling mice were intracranially inoculated with live V3526 or PCM. The brains from mice surviving 14 days post-inoculation were removed upon euthanasia, homogenized and frozen. A second set of suckling mice were inoculated intracranially with the brain homogenate from the corresponding group and observed for an additional 14 days.
2.2.3 Analysis of Epitope Integrity Following Formalin-Inactivation
A sandwich ELISA was developed utilizing monoclonal antibody (Mab) 1A4A-1 for the capture of antigen and horse anti-V3526 polyclonal serum for the detection of bound antigen [19]. Mab 1A4A-1 recognizes the E2c epitope on the VEEV IAB E2 glycoprotein, which has been identified as a critical virus neutralization site within the E2 envelope protein,[27] and allows for detection of VEEV IAB viruses including V3526, VEEV TrD and C84 as well as VEEV subtypes IC and ID. The Mab was coated on a 96-well plate overnight at 4°C at 0.5 μg/well. All subsequent incubations were performed at 37°C. Plates were then blocked with phosphate buffered saline (PBS) containing 0.5% Tween-20 and 5% skim milk (PBSTM) for 2 hours. Samples were diluted in PBSTM containing 1% inactivated fetal bovine serum (FBS), serially diluted 1:2 and incubated for 2 hours. Plates were washed six times with PBS containing Tween-20 (PBST) using the Bio-Rad 1550 Microplate washer. Bound virus was detected using horse anti-V3526 serum (1:1000) for 2 hours [12]. Following incubation, plates were washed six times with PBST. Bound equine antibody was quantitated by addition of peroxidase-labeled goat anti-horse antibody (KPL, Inc.) incubated for 1 hour, followed by six washes with PBST and the addition of ABTS substrate (KPL, Inc). After 30 minutes at room temperature, the optical density (OD) was determined at 410 nm using the SpectraMax 340PC (Molecular Devices). The per well background value was determined at 490 nm and subtracted from the 410 nm value to normalize differences in the non-optical quality of plastic of the round-bottom plates. All data were collected using SoftMaxPro 3.1 (Molecular Devices).
2.3 Adjuvants
Alhydrogel™ was purchased from Accurate Chemical and Scientific Corporation, Westbury, NY and diluted the day of use to achieve a final concentration of 0.2% v/v dose with sterile PBS. CpG ODN2395 was purchased from InvivoGen, San Diego, CA and reconstituted the day of use and diluted in sterile, endotoxin-free water to achieve a final concentration of 20 μg/dose. Viprovex® was purchased from ImmuneRegen, Scottsdale, AZ and reconstituted in sterile PBS the day of use to achieve a final concentration of 76 μg/dose. The concentration of CpG and Alhydrogel™ when used in combination were the same as when the adjuvants were prepared in the single adjuvant formulations.
2.4 Animal Information and Environmental Conditions
Six-week old female BALB/c mice were purchased from the National Cancer Institute, Fort Detrick, MD. Mice were group housed in polycarbonate cages with microisolator lids. The room temperature was 23°C ± 1°C and periods of light and dark were maintained on a 12 hour cycle. Animals were provided rodent diet and tap water ad libitum throughout the study. Research was conducted at the United States Army Medical Research Institute of Infectious Diseases (USAMRIID) and was in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals. USAMRIID is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
2.5 Vaccination and Challenge
Mice were vaccinated SC or IM with fV3526 alone or formulated with adjuvant on Day 0 and 28. Due to restrictions in the volume of inoculum that can be delivered to a mouse via the IM route, the SC vaccinated mice received five times more viral protein (0.2 μg) per dose than IM vaccinated mice (0.04 μg) (Table 1). For SC vaccination, 0.5 mL of inoculum was administered to the interscapular area. For IM vaccinations, 0.025 mL was administered into the muscle of each hind limb. C84 was administered according to the dosage (4 μg), route (SC), and schedule (0, 7, and 28) used in previously published animals studies [13, 28] and as administered to human vaccinees [8, 29] to allow comparisons between the data collected in this study to historical studies. Further, the dosage, route, schedule and use of adjuvants with C84 was not evaluated as the intent of the comparisons to be made with C84 were to show fV3526 formulations are as good as or better than C84 in its current formulation as the US government does not intend to fund further development of C84 as a VEEV vaccine. Sham-vaccinated mice received PCM either SC or IM and adjuvant control mice received Viprovex®, Alhydrogel™, CpG or CpG+Alhydrogel™ at the same concentrations and on the same schedule as administered in experimental groups with fV3526. On Day 21 and 49 post-primary vaccination, blood was collected from all mice for measurement of antibody responses. Mice were challenged on Day 56 with 1×104 pfu VEEV TrD by the aerosol or SC route. Aerosol exposures were conducted by putting mice in wire cages into a chamber where they were exposed to aerosolized virus for 10 minutes. Virus collected in an all-glass impinger was titrated to determine the concentration of virus (pfu/L) in air using a previously described plaque assay method [30] and the volume inhaled was estimated using Guyton’s formula [31]. Mice were monitored daily for signs of illness for 28 days post-challenge at which time surviving mice were euthanized. One iteration of each vaccination-challenge study was conducted, unless otherwise noted.
Table 1.
Protein concentration/dosea
| Antigen | Route of Vaccination | Day 0 | Day 7 | Day 28 | Total Protein |
|---|---|---|---|---|---|
| fV3526 | IM | 0.04 μg | -- | 0.04 μg | 0.08 μg |
| fV3526 | SC | 0.2 μg | -- | 0.2 μg | 0.4 μg |
| C84 | SC | 4 μg | 4 μg | 4 μg | 12 μg |
Protein concentration was determined as previously described [19].
2.6 Determination of Neutralizing Antibody Titers
Virus-neutralizing antibodies in the immunized and control mice were determined as previously described [32] using live VEEV TrD virus as target antigen. Sera were serially diluted two-fold with virus and incubated overnight at 4°C. The serum-virus mixtures were added to Vero cell monolayers for 1 hour at 37°C, after which the cells were overlaid with 0.6% agarose in Eagle’s basal medium with Earle’s salts supplemented with 10% FBS, 200 U/mL penicillin, 200 μg/mL streptomycin, 2 mM L-glutamine and 100 μM non-essential amino acids. Monolayers were stained with 5% Neutral Red stain one day later and plaques counted the following day. The endpoint titer was determined to be the highest dilution with an 80% or greater reduction of the number of plaques observed compared to control wells. Limit of quantitation for the plaque reduction neutralization test (PRNT) was at the initial 1:10 serum dilution (the most concentrated dilution tested) which was 1:20 following dilution of the serum with the virus. The endpoint titer was determined to be the reciprocal of the highest final dilution. Non-responders were assigned a value of one and geometric mean endpoint titers were calculated.
2.6.1 Determination of Serum Binding Antibody Titers
Antibody responses to VEEV TrD were evaluated by ELISA. Plates were coated with 0.5 μg purified VEEV TrD per well and incubated overnight at 4°C. All subsequent incubations were performed at 37°C. The following day, plates were blocked with PBS containing 0.05% Tween-20, 5% nonfat dry milk and 3% normal goat serum (Sigma) (PBSTMG) for 2 hours. The plates were washed three times with PBST. Mouse sera were serially diluted 1:3 in PBSTMG, and incubated for 2 hours. Plates were washed three times with PBST followed by addition of peroxidase-labeled goat anti-mouse IgG (KPL, Inc.). The plates were incubated with secondary antibody for 1 hour and subsequently washed three times with PBST. The ABTS Peroxidase substrate (KLP, Inc.) was applied to each well and color developed for approximately 20 minutes at which time the OD was determined at 410 nm using the SpectraMax 340PC. The per well background value was determined at 490 nm and subtracted from the 410 nm value to normalize differences in the non-optical quality of plastic of the round-bottom plates. All data were collected using SoftMaxPro 3.1. Endpoint titers were determined as the highest serum dilution that produced an optical density greater than the negative control OD (normal mouse serum, KPL, Inc.) plus 3 standard deviations of background values. The endpoint titer was determined to be the reciprocal of the highest final dilution. Non-responders were assigned a value of one and geometric mean endpoint titers (GMT) were calculated.
2.7 Statistical Analysis
All ELISA and PRNT values were log10-transformed for analysis. After transformation, the data met assumptions of normality and homogeneity of variance. ELISA and PRNT values were compared between groups using ANOVA with post-hoc Tukey’s tests for pairwise comparisons. Fisher’s Exact Test was employed to determine statistical significance of difference in survival rates between groups. Mean time to death comparisons were made using ANOVA with Fisher’s LSD post-hoc test. Correlations between antibody titers and survival were evaluated using logistic regression analysis. All data were analyzed using SAS Version 9.2.
3.0 Results
3.1 Inactivation testing of fV3526
The preparation of fV3526 used in this study was tested for inactivation by evaluating infectivity by Vero cell plaque assay, serial passage on BHK-21 cells and intracerebral (IC) inoculation of suckling mice. IC inoculation of suckling mice is recommended by the FDA for detecting adventitious agents [33], including alphaviruses and is used to evaluate attenuation of live alphavirus vaccines [34]. In this study, IC inoculation of suckling mice with live V3526 was uniformly lethal demonstrating the sensitivity of this model to the live vaccine strain. All suckling mice IC inoculated with fV3526 survived the 14 day observation period (Table 2). The brains from these mice were passaged into a second set of mice which also survived the post-inoculation observation period. In that live V3526 is known to replicate in mouse brain [35], this second passage was used to detect infectious virus that may have been present in undetectable levels in the first set of mice and subsequently undergone replication. Since all mice survived IC inoculations with fV3526 or brain homogenates from fV3526 mice, we conclude that no detectable levels of live virus were present in the preparaton. These data are supported by the in vitro testing for inactivation whereby serial passage of fV3526 on BHK-21 cells and plaque assay on Vero cells failed to detect infectious V3526 (Table 2). A critical component of inactivated vaccines is the retention of immunologically relevant epitopes. Excessive modification by formalin over-inactivation may destroy important epitopes thereby reducing vaccine immunogenicity. Using an ELISA to evaluate epitope preservation, the fV3526 vaccine showed greater binding activity than untreated V3526 suggesting formalin treatment may induce slight conformational changes to the V3526 envelope proteins making those determinants more available for antibody binding (Table 2).
Table 2.
Post-formalin inactivation test of fV3526
| Assay | Result |
|---|---|
| Plaque assay | Below detectable levelsa |
| Serial passage in BHK cells | Below detectable levels |
| Intracranical inoculation of suckling mice | All mice survivedb |
| Epitope retention | 117%c |
Detection limit of Vero cell assay is 3 pfu/mL
All V3526 inoculated suckling mice succumbed to infection
Percent binding of live V3526
3.2 Immunogenicity of Subcutaneous and Intramuscular Vaccination
Seroconversion
Mice were bled 3 weeks after each vaccination for assessment of antibody titers by PRNT and ELISA. Seroconversion rates ranged from 95–100% in groups of mice after receiving one dose of the fV3526 formulation regardless of route of administration and 100% of mice seroconverted by both assays by Day 49 (Table 3). SC vaccination with C84 resulted in 100% seroconversion by Day 21 for both ELISA and PRN. However, it is important to note, that these mice received 2 doses of C84 (8 μg total) prior to the Day 21 test, whereas mice that received fV3526 only received one dose prior to Day 21; 0.04 μg viral protein for mice vaccinated IM and 0.2 μg viral protein for mice vaccinated SC.
Table 3.
Seroconversion rates following vaccination with fV3526 formulations and C84
| PRNa,b | ELISAa,b | ||||||
|---|---|---|---|---|---|---|---|
| Group | Formulation | Dosagec (μg) | Route | Day 21 | Day 49 | Day 21 | Day 49 |
| 1 | fV3526 | 0.04 | IM | 100 | 100 | 100 | 100 |
| 2 | fV3526 | 0.2 | SC | 100 | 100 | 100 | 100 |
| 3 | fV3526 + Viprovex | 0.04 | IM | 95 | 100 | 100 | 100 |
| 4 | fV3526 + Viprovex | 0.2 | SC | 100 | 100 | 100 | 100 |
| 5 | fV3526 + CpG | 0.04 | IM | 100 | 100 | 100 | 100 |
| 6 | fV3526 + CpG | 0.2 | SC | 100 | 100 | 95 | 100 |
| 7 | fV3526 + Alhydrogel | 0.04 | IM | 85 | 95 | 100 | 100 |
| 8 | fV3526 + Alhydrogel | 0.2 | SC | 70 | 100 | 100 | 100 |
| 9 | fV3526 + CpG + Alhydrogel | 0.04 | IM | 90 | 100 | 100 | 100 |
| 10 | fV3526 + CpG + Alhydrogel | 0.2 | SC | 80 | 90 | 95 | 100 |
| 11 | C84 | 4.0 | SC | 100 | 100 | 100 | 100 |
| 12 | PCM | NA | IM | 0 | 0 | 0 | 0 |
| 13 | PCM | NA | SC | 0 | 0 | 0 | 0 |
Percent seroconversion is defined as follows: PRNT, the percentage of mice with PRN titers ≥20; ELISA, the percentage of mice with ELISA titers ≥100.
Percent seroconversion was calculated by combining groups of similarly vaccinated mice making a total of 20 mice per group or experimental groups and 10 mice per group for controls. Groups were later split in half with half of the mice challenged by the aerosol route and half of the mice challenged by the SC route.
All dosages were administered on a 2 dose schedule (day 0 and 28) except for C-84 which was administered on a 3 dose schedule (day 0, 7 and 28).
Antibody Titers
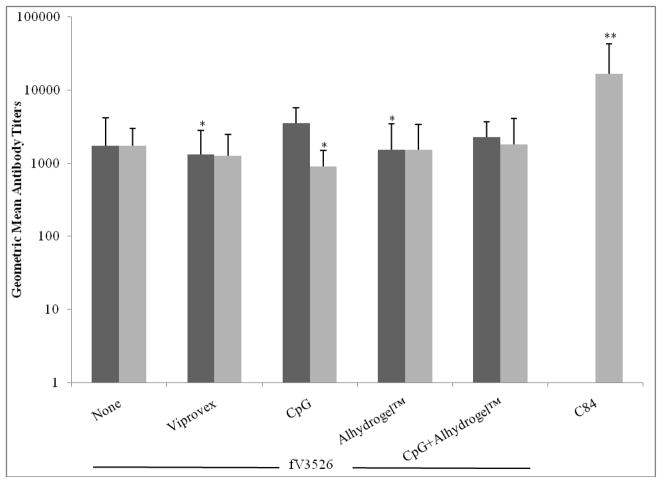
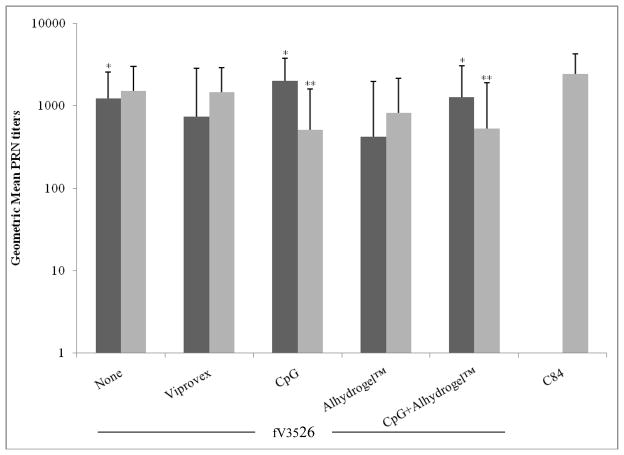
No differences were observed in ELISA or neutralizing antibody GMT induced by fV3526 formulations administered SC. However, following IM administration, fV3526+CpG induced significantly higher ELISA GMT compared to fV3526 formulated with Viprovex® or Alhydrogel™ (p<0.05). ELISA GMT on day 49 post-vaccination with C84 was significantly higher than all other ELISA GMT (p<0.01) (Figure 1). Neutralizing titers on day 49 showed few statistical differences between vaccine formulations (Figure 2). Interestingly, fV3526+Alhydrogel™ administered IM showed significantly lower neutralizing titers compared to IM administered fV3526, fV3526+CpG+Alhydrogel™ and fV3526+CpG (p<0.05). The neutralizing titers induced by C84 were only significantly higher than SC administered fV3526 formulations containing CpG (p<0.05) and IM administered fV3526+Alhydrogel™ on Day 49. No differences in ELISA or neutralizing antibody GMT were found between mice vaccinated with the same formulation administered IM versus SC except mice receiving fV3526+CpG. Mice vaccinated IM with fV3526+CpG had significantly higher ELISA and neutralizing antibody GMT on Day 49 compared to mice vaccinated SC with the same formulation (p<0.05) (Figures 1 and 2). Anti-VEEV antibodies were below detectaable levels in all sham-vaccinated mice.
Figure 1.
Virus-binding antibody responses induced to VEEV TrD virus by vaccination with fV3526 formulated with the indicated adjuvant and C84. Sera were obtained from mice on Day 49 post-primary vaccination. These data are reported as the geometric mean endpoint titers as determined by ELISA. Black bars: IM vaccinated, Gray bars: SC vaccinated. C84 administered IM was not evaluated. *statistically different from fV3526/CpG administered IM (p<0.05) **statistically different from all other groups (p<0.01). The titers presented are combined titers from similarly vaccinated mice (same route, same formulation) giving a total of 20 mice per group. Groups were later split into 10 mice challenged by the aerosol route and 10 challenged by the SC route.
Figure 2.
Neutralizing antibody responses induced to VEEV TrD virus by vaccinations with fV3526 formulated with the indicated adjuvant and C84. Sera were obtained from mice on Day 49 post-primary vaccination. These data are reported as the geometric mean endpoint titers that inhibited plaque formation by 80%. Black bars: IM vaccinated, Gray bars: SC vaccinated. C84 administered IM was not evaluated. * statistically different from fV3526/Alhydrogel™ administered IM (p<0.05). ** statistically different from C84 (p<0.05). The titers presented are combined titers from similarly vaccinated mice (same route, same formulation) giving a total of 20 mice per group. Groups were later split into 10 mice challenged by the aerosol route and 10 challenged by the SC route.
3.3 Efficacy of Subcutaneous Vaccination
The immunogenicity and protective efficacy of SC vaccination with fV3526 formulations against challenge on day 56 with VEEV TrD administered by the SC or aerosol route was evaluated. All mice receiving fV3526 formulations survived SC VEEV TrD challenge (Table 4). Further, no clinical signs of disease, including changes in body weight, were observed following SC challenge, demonstrating vaccination with the fV3526 formulations protected mice not only against death but also from development of overt signs of illness. In this study, vaccination with C84 protected 80% of mice from SC challenge with VEEV TrD. The only C84 vaccinated mice that showed clinical signs of disease were those that ultimately succumbed to challenge. In sham-vaccinated mice, decreased body weight and mild signs of illness were first observed on Day 2 and 3 post-SC challenge, respectively. All sham-vaccinated mice succumbed to disease between Day 5 and 7 post-challenge.
Table 4.
Suvival Data from SC Vaccinated Mice following SC or Aerosol Challenge.
| Formulation | Dosagea | Survived | Died | Total | MTTDb |
|---|---|---|---|---|---|
| Subcutaneous Challenge | |||||
| fV3526 | 0.2 | 10 | 0 | 10 | --- |
| fV3526 + Viprovex | 0.2 | 10 | 0 | 10 | --- |
| fV3526 + CpG | 0.2 | 9 | 0 | 9c | --- |
| fV3526 + Alhydrogel™ | 0.2 | 10 | 0 | 10 | --- |
| fV3526 + CpG + Alhydrogel™ | 0.2 | 10 | 0 | 10 | --- |
| C84 | 4.0 | 8 | 2 | 10 | 8.3 |
| PCM | NA | 0 | 5 | 5 | 6.6 |
| Aerosol Challenge | |||||
| fV3526 | 0.2 | 3 | 7 | 10 | 8.0 |
| fV3526 + Viprovex | 0.2 | 4 | 6 | 10 | 7.8 |
| fV3526 + CpG | 0.2 | 3 | 6 | 9c | 7.0 |
| fV3526 + Alhydrogel™ | 0.2 | 8 | 1 | 9c | 7.0 |
| fV3526 + CpG + Alhydrogel™ | 0.2 | 7 | 3 | 10 | 9.0d |
| C84 | 4.0 | 7 | 3 | 10 | 7.5 |
| PCM | NA | 0 | 5 | 5 | 7.0 |
All dosages were administered on a 0 and 28 day schedule expect for C84 which was administered on a 0, 7 and 28 day schedule.
MTTD: mean time to death of mice that succumbed to infection
The total number of mice in this group was 9 at the time of challenge. One mouse succumbed to a non-study related reason between study day 21 and 49.
MTTD significantly longer than sham-vaccinated mice (p<0.05)
Although SC vaccination induced a high level of protection against SC challenge, SC vaccination did not protect all mice against an aerosol challenge (Table 4). High percentages of surviving mice were observed in groups of mice vaccinated with fV3526+Alhydrogel™ and fV3526+CpG+ Alhydrogel™ where 8 of 9 and 7 of 10 mice, respectively, survived following aerosol challenge. In contrast, ≤ 40% of mice administered fV3526, fV3526/Viprovex® and fV3526+CpG survived aerosol challenge when vaccinated SC at the tested dosages. SC vaccination with C84 at 4 μg/dose protected 70% of mice from death. The mean time to death was only significantly different from sham-vaccinated mice when the fV3526 was formulated with CpG+ Alhydrogel™ (p<0.05). Regardless of vaccine formulation, mice in all groups displayed mild clinical signs of disease (decreased grooming) and decreased body weight within 2 days post-challenge that resolved in surviving mice between Day 8 and 15 post-challenge, with mice vaccinated with fV3526+CpG+ Alhydrogel™ showing resolution of symptoms first (Day 8) followed by mice vaccinated with fV3526 on Day 10. Similar to SC challenge, sham-vaccinated mice showed early signs of disease by Day 2 post-aerosol challenge and the disease appeared to progress more rapidly in these animals compared to vaccinated mice. Logistic regression analysis of Day 49 antibody titers as determined by ELISA and PRNT failed to find a correlation between circulating antibody titers and survival for any of the fV3526 formulations indicating, in this study, that antibody titers were not predictive of survival.
3.4 Efficacy of Intramuscular Vaccination
In this study, we evaluated the immunogenicity and efficacy of fV3526 formulations administered IM as an alternative to SC vaccination. Despite receiving less fV3526 per dose, all IM vaccinated mice survived SC challenge with 1×104 pfu VEEV TrD regardless of fV3526 formulation (Table 5). Similar to SC vaccination, mice in this arm of the study did not display signs of illness or loss of body weight following SC challenge. All sham-vaccinated mice succumbed to infection on Day 7 post-challenge.
Table 5.
Suvival Data from IM Vaccinated Mice following SC or Aerosol Challenge.
| Formulation | Dosagea | Survived | Died | Total | MTTDb |
|---|---|---|---|---|---|
| Subcutaneous Challenge | |||||
| fV3526 | 0.04 | 10 | 0 | 10 | --- |
| fV3526 + Viprovex | 0.04 | 10 | 0 | 10 | --- |
| fV3526 + CpG | 0.04 | 9 | 0 | 9c | --- |
| fV3526 + Alhydrogel™ | 0.04 | 10 | 0 | 10 | --- |
| fV3526 + CpG + Alhydrogel™ | 0.04 | 10 | 0 | 10 | --- |
| PCM | NA | 0 | 10 | 10 | 6.8 |
| Aerosol Challenge | |||||
| fV3526 | 0.04 | 6 | 4 | 10 | 7 |
| fV3526 + Viprovex | 0.04 | 6 | 4 | 10 | 8.3 |
| fV3526 + CpG | 0.04 | 9 | 1 | 10 | 8.0 |
| fV3526 + Alhydrogel™ | 0.04 | 7 | 3 | 10 | 10.0d |
| fV3526 + CpG + Alhydrogel™ | 0.04 | 7 | 3 | 10 | 8.3 |
| PCM | NA | 0 | 5 | 5 | 6.4 |
All dosages were administered on a 0 and 28 day schedule expect for C84 which was administered on a 0, 7 and 28 day schedule.
MTTD: mean time to death of mice that succumbed to infection
The total number of mice in this group was 9 at the time of challenge. One mouse succumbed to a non-study related reason prior to challenge.
MTTD significantly longer than sham-vaccinated mice (p<0.05)
Similar to SC vaccination, induction of protective immunity to infectious aerosols following IM vaccination was more difficult to achieve compared to SC challenge. No statistically significant differences were observed in survival among the vaccinated groups, however, the mean time to death in mice vaccinated with fV3526 + Alhydrogel™ was longer compared to other formulations (p<0.01). The onset of clinical signs of disease was closely associated with decreases in body weight and was similar for 3 of the 4 vaccine formulations with the onset of symptoms being Day 2 post-challenge and continuing through Day 13. In the group of mice vaccinated with fV3526+CpG+Alhydrogel™, signs of disease were not observed until Day 3 and were resolved by Day 9. All sham vaccinated mice were clinically ill by Day 2 post-challenge and all succumbed to disease between Day 4 and 7.
In general, IM vaccinated mice showed a trend toward higher survival rates following aerosol challenge compared to mice vaccinated SC with the same formulations (compare Tables 4 and 5). In fact, survival was statistically higher in mice vaccinated IM with fV3526+CpG (9 of 10 survived) compared to mice vaccinated with the same formulation SC (3 of 9 survived) (p<0.05, Logistic regression analysis).
The reproducibility of the efficacy data following aerosol challenge was evaluated for fV3526 formulated with adjuvants containing CpG. In 1 or 2 independent iterations, mice were IM vaccinated with fV3526+CpG+Alhydrogel™ or fV3526+CpG and challenged by the aerosol route using the same dosages and schedules as in earlier studies. In each group, survival percentages ranged from 70–90% with an average 80% survival for fV3526+CpG and 85% survival for fV3526+CpG+ Alhydrogel™ following aerosol challenge (Figure 3). As observed in the preliminary studies, comparison of PRN and ELISA titers with survival following aerosol challenge using logistic regression failed to find an association between antibody titer and survival in this study.
Figure 3.
Average survival among fV3526+CpG and fV3526+CpG+Alhydrogel™ vaccinated mice following aerosol challenge. Data represent 2 vaccination/challenge iterations for fV3526+CpG and 3 vaccination/challenge iterations for fV3526+CpG+Alhydrogel™. Each iteration consisted of 10 mice/group and a group of 10 sham-vaccinated. All mice were challenged by the aerosol route.
4.0 Discussion
Currently, an FDA licensed vaccine for prevention of Venezuelan equine encephalitis virus does not exist. V3526 was recently evaluated in a Phase I clinical trial and was found to be highly immunogenic in vaccine recipients but due to the development of adverse events, further development of V3526 as a live vaccine was stopped. In this study, formalin was used to inactivate V3526 and the inactivated virus was formulated with adjuvants to evaluate the immunogenicity and efficacy of these vaccine formulations in mice as compared to the existing inactivated VEEV vaccine, C84.
One of our goals in inactivating V3526 was to reduce the potential for adverse events as seen with the live V3526 and with TC-83. As demonstrated in this study and others, following intracranial inoculation of live V3526 in suckling mice, the virus replicates to high titers and is uniformly lethal [34]. In this study, we inoculated suckling mice with fV3526 and observed 100% survival, suggesting the V3526 was inactivated. These in vivo data are supported by the lack of cytopatholgy following serial passage of fV3526 on BHK cells and examination of infectivity on Vero cells. The absence of detectable infectivity and lack of lethality in suckling suggest the fV3526 will be a safer vaccine as compared to V3526. Recently, an inbred mouse model with telemetry implants was developed and shown to be a sensitive model for detecting adverse responses to vaccination, specifically V3526 [16]. To ensure the safety of fV3526, the inactivated virus should be evaluated in this model prior to evaluating the formulations in large animal models and humans.
An assessment of the immunogenicity of the fV3526 with different adjuvants was conducted by determining the level of circulating antibodies after one and two doses of the vaccine. Neutralizing antibodies were induced after one dose with nearly 100% seroconversion following vaccination for all vaccine formulations. However, the level of antibody, particularly neutralizing antibody, present one week prior to challenge did not correlate with a protective status post-challenge. Studies previously conducted in hamsters [36] and mice [37] also report that the level of circulating neutralizing antibodies are not predictive of protection following aerosol challenge. Rather, the protection may be dependent on development of antibody in the nasal mucosa [36–38]. The lack of a correlation between neutralizing antibody titers and SC challenge was more surprising, as this finding contradicts the widely reported association between neutralizing antibody titers in serum and protection against systemic VEEV challenge [36, 39, 40]. The protective immune response induced by vaccination with the fV3526 formualtions may be attributable to induction of an alternative immune mechanism such as protective T cells. Recently, Paessler et al used γδ TCR-deficient mice, IFN-γ deficient mice and μMT deficient mice to evaluate the association between T cells in vaccinated mice and a protective status post-intranasal challenge with a lethal dose of VEEV [41]. In that study, it was demonstrated that neutralizing antibodies are not required for survival following lethal VEEV challenge. In this same report, Paessler et al evaluated the contribution of T cells subsets in the brain in protecting mice against lethal VEEV challenge and found αβ T cells are required for protection against a lethal VEEV challenge but that γδ T cells are not. This finding was supported by adoptive transfer studies where CD3+ T cells derived from vaccinated wild-type mice were able to restore protective immunity in αβ TCR deficient mice following a lethal VEEV challenge [41]. The findings from these studies are supported by other reports demonstrating T cell immunity as a key component to protection against VEEV infection [42, 43]. Based on these reports, it is conceivable that T cell responses may be the predominant protective response following vaccination with the fV3526 formulations and that neutralizing antibodies play a secondary role in protection of the host. Dissecting the specific immune responses induced by the fV3526 formulations which are required for protection were beyond of scope of this study but should be investigated upon down-selection of a fV3526 formulation.
In the present study, all fV3526 formulations induced an immune response that solidly protected mice against a SC challenge with VEEV TrD. While not statistically different from vaccination with fV3526 formulations, vaccination with C84 did not induce a protective immune response in all mice as has been previously reported [37]. While this result was unexpected, so were the findings in similar studies where C84 also failed to solidly protect mice from SC challenge [19, 44]. One possible explanation for this discrepancy may be a loss of C84 potency. C84 was manufactured nearly 29 years ago and the loss of potency may be due to the prolonged storage. Stability and potency studies were conducted on C84 for several years following manufacture but this testing ended in the late 1990’s, and no current potency data on the inactivated vaccine are available. Differences in the protective immune responses induced by the fV3526 formulations were more apparent when mice were challenged by the aerosol route but those differences failed to reach statistical significance. Survival rates in mice vaccinated with the fV3526 formulations following aerosol challenge were also similar to those for C84, however similar to SC challenge, C84 again failed to induce a protective response in all mice providing additional support to a loss of C84 vaccine potency.
In contrast to mice vaccinated with live V3526, mice vaccinated with fV3526 formulations displayed mild clinical signs of disease following aerosol challenge. This difference may be attributable to the presence of IgA antibodies in the nasal mucosa and high levels of circulating neutralizing antibodies induced by vaccination with live V3526 [45]. In that fV3526 vaccinations did not induce high levels of circulating neutralizing antibodies, it is tempting to speculate that fV3526 did not induce sufficient levels of nasal mucosal IgA antibodies resulting in VEEV infection in the brain. This supposition is supported by the transient illness observed in vaccinated mice following aerosol challenge. Further, as a high percentage of mice ultimately recovered, the involvement of a protective immune mechanisms in the brain [41], that can control and eliminate the VEEV, is supported.
In the present study, we found IM vaccination with fV3526+CpG induced a stronger antibody response and afforded a higher level of protection against an aerosol challenge compared to mice vaccinated SC with the same formulation. This finding is particularly interesting as IM vaccinated mice received 5 times less viral protein than did SC vaccinated mice. It is not clear why fV3526+CpG administered by the IM route induced a more protective immune response than SC vaccination. Previously, it has been suggested that IM vaccination can overcome immune compartmentalization and generate robust mucosal T cell responses [46]. In that study, IM vaccination with a recombinant adenovirus resulted in potent, durable and functional CD8+ T lymphocyte responses at multiple mucosal effector sites, including the pulmonary compartment, in both mice and rhesus macaques. Similarly, IM vaccination with an inactivated, whole-virus vaccine for influenza also showed remarkable protection against respiratory challenge [47] further suggesting IM vaccination may play a role in the induction of mucosal immunity. Since the induction of mucosal immunity is believed to be critically important for protection against an aerosolized VEEV infection [38, 45, 48] it is possible that vaccinating mice IM with the fV3526+CpG induced a robust mucosal immune response involving T cells that failed to be induced by SC vaccination. To gain a better understanding of the contribution of IM and SC vaccination in inducing protective immunity, additional studies administering equivalent concentrations by the SC and IM route are needed.
The success of fV3526 will likely be dependent on co-administration with adjuvant. In this study, adjuvants did not significantly increase the immune responses measured following vaccination or increase survival following aerosol challenge as compared to unadjuvanted fV3526. Although the adjuvants did not appear to play a critical role in this study, it is likely that the benefit of these adjuvants will not be realized until more rigorous efficacy studies evaluating onset and duration of protection and dose titration studies to evaluate potency are conducted or immune responses more relevant to protection are more clearly defined.
A limited number of studies are reported that use CpG to augment VEEV-specific immune responses. In 2008, CpG was used as an adjuvant with an adenoviral vector expressing VEEV proteins (RAd/VEE#3)[49]. The results from this study are similar to the data present here in that the addition of CpG did not have a remarkable effect on measured VEEV-specific immune responses or significantly increased survival following challenge. The lack of an enhanced VEEV-specific response following vaccination with RAd/VEE#3 may be attributable to the generation of an immune response to the vector [50] which is supported by the lack of a significant increase in survival. However, in our study, the lack of a significant increase in VEEV-specific immune responses may be due to the induction of an immune response that was not measured and should be further investigated. The lack of a significant increase in survival in the CpG containing fV3526 formulations is due to a high survival rate induced by fV3526 in the absence of adjuvant and the true adjuvant effect of CpG can only be answered by increasing the number of animals per group, evaluating additional immune responses and conducting more rigorous efficacy studies as described above.
The present study identified four fV3526 formulations that could potentially serve as a next generation inactivated VEEV vaccine to replace C84. All formulations, including fV3526 without adjuvant, induced protective immune responses statistically similar to C84. This finding is particularly noteworthy in that the concentration of viral protein administered with each dose of the fV3526 formulations was 20 (SC administration) and 100 (IM administration) times less than the C84 concentration. Further, C84 was administered on a 3 dose schedule as compared to 2 doses administered for the fV3526 formulations resulting in a total dosage per mouse of 12 μg C84 and 0.4 μg (SC) and 0.08 μg (IM) for fV3526. The ability to induce similar protective responses with the fV3526 formulations with less viral protein and fewer doses as compared to C84 is a feature of the fV326 formulation that demonstrates superiority of fV3526 over C84. Furthermore, a comparison of additional vaccine characteristics related to the development and manufacturing demonstrate that fV3526 formulations are more amenable to licensure in the US (Table 6) and warrant their further evaluation for advanced development.
Table 6.
Comparison of fV3526 and C84
| Points of Consideration | fV3526 | C84 |
|---|---|---|
| Characterization of attenuating mutation | V3526: Well-defined; Result of site directed mutagenesis [11] | TC-83: Not well-defined; Result of serial passage in guinea pig cultures[51] |
| Purity of attenuated virus stock | Homogeneous [11] | Heterogeneous[52] |
| Certified cell lines for virus propagation | MRC-5 cells, FDA certified | Chick embryo cultures[6]; not FDA certifiable |
| Propagation process of live attenuated virus | Uses current technology | Uses 1970’s technology and would require updating |
| GMP manufacturing process of live attenuated virus | Established | GMP manufacturing process does not exist |
| Formalin inactivation | 0.1% v/v formalin, 24 hrs, 37°C | 0.1% v/v formalin, 24 hrs, 37°C |
| Number of dose/schedule | 2 (0, 28 days) | 3 (0, 7, 28 days) |
| Protein conc/dose | 0.04 μg (IM), 0.2μg (SC) | 4 μg (SC) |
| Seroconversion rate in mice | 100% | 100% |
| Protects mice from aerosol challenge | Yes | Yes |
In summary, the data presented in this report demonstrate that vaccination with fV3526 formulations induce immune responses in mice that afford high levels of protection against aerosol and subcutaneous challenge. Survival outcomes in fV3526 vaccinated mice were similar to survival outcomes in mice vaccinated with C84. Given the similarities in protection afforded by the fV3526 formulations and C84 and the multitude of hurdles that would need to be overcome to manufacture new lots of C84 for further development and optimization, we believe that fV3526 shows potential as a replacement vaccine for C84. At this time, one fV3526 formulation cannot be identified as superior to the others and will need to be a goal of future studies.
Acknowledgments
This study was funded by the National Institute of Allergy and Infectious Diseases, (1UC1AI062538-01) and Joint Science and Technology Office-Chemical, Biological Defense (Plan1.1C0041_09_RD_B). We thank the aerobiology staff at USAMRIID for their contributions to the aerosol challenge components of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ehrenkranz NJ, Ventura AK. Venezuelan equine encephalitis virus infection in man. Annu Rev Med. 1974;25:9–14. doi: 10.1146/annurev.me.25.020174.000301. [DOI] [PubMed] [Google Scholar]
- 2.Hinman AR, McGowan JE, Jr, Henderson BE. Venezuelan equine encephalomyelitis: surveys of human illness during an epizootic in Guatemala and El Salvador. Am J Epidemiol. 1971;93(2):130–6. doi: 10.1093/oxfordjournals.aje.a121233. [DOI] [PubMed] [Google Scholar]
- 3.Smith DG, Mamay HK, Marshall RG, Wagner JC. Venezulean equine encephalomyelitis; laboratory aspects of fourteen human cases following vaccination and attempts to isolate the virus from the vaccine. Am J Hyg. 1956;63(2):150–64. [PubMed] [Google Scholar]
- 4.Kortepeter M, Christopher G, Cieslak T, Culpepper R, Darling R. USAMRIID’s Medical Management of Biological Casualities Handbook. 4. Fort Detrick, MD: 2001. [Google Scholar]
- 5.McKinney RW, Berge TO, Sawyer WD, Tigertt WD, Crozier D. Use of an attenuated strain of venezuelan equine encephalomyelitis virus for immunization in man. Am J Trop Med Hyg. 1963;12(4):597–603. doi: 10.4269/ajtmh.1963.12.597. [DOI] [PubMed] [Google Scholar]
- 6.Cole FE, Jr, May SW, Eddy GA. Inactivated Venezuelan equine encephalomyelitis vaccine prepared from attenuated (TC-83 strain) virus. Appl Microbiol. 1974;27(1):150–3. doi: 10.1128/am.27.1.150-153.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke DS, Ramsburg HH, Edelman R. Persistence in humans of antibody to subtypes of Venezuelan equine encephalomyelitis (VEE) virus after immunization with attenuated (TC- 83) VEE virus vaccine. J Infect Dis. 1977;136(3):354–9. doi: 10.1093/infdis/136.3.354. [DOI] [PubMed] [Google Scholar]
- 8.Pittman PR, Makuch RS, Mangiafico JA, Cannon TL, Gibbs PH, Peters CJ. Long-term duration of detectable neutralizing antibodies after administration of live-attenuated VEE vaccine and following booster vaccination with inactivated VEE vaccine. Vaccine. 1996;14(4):337–43. doi: 10.1016/0264-410x(95)00168-z. [DOI] [PubMed] [Google Scholar]
- 9.McKinney RW. Inactivated and live VEE vaccines - A review. PAHO Scientific Publication: Proc of the workshop symposium on Venezuelan Encephalitis; 1972. pp. 369–389. [Google Scholar]
- 10.Edelman R, Ascher MS, Oster CN, Ramsburg HH, Cole FE, Eddy GA. Evaluation in humans of a new, inactivated vaccine for Venezuelan equine encephalitis virus (C-84) J Infect Dis. 1979;140(5):708–15. doi: 10.1093/infdis/140.5.708. [DOI] [PubMed] [Google Scholar]
- 11.Davis NL, Brown KW, Greenwald GF, et al. Attenuated mutants of Venezuelan equine encephalitis virus containing lethal mutations in the PE2 cleavage signal combined with a second-site suppressor mutation in E1. Virology. 1995;212(1):102–10. doi: 10.1006/viro.1995.1458. [DOI] [PubMed] [Google Scholar]
- 12.Fine DL, Roberts BA, Teehee ML, et al. Venezuelan equine encephalitis virus vaccine candidate (V3526) safety, immunogenicity and efficacy in horses. Vaccine. 2007;25(10):1868–76. doi: 10.1016/j.vaccine.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Hart MK, Lind C, Bakken R, Robertson M, Tammariello R, Ludwig GV. Onset and duration of protective immunity to IA/IB and IE strains of Venezuelan equine encephalitis virus in vaccinated mice. Vaccine. 2002;20(3–4):616–22. doi: 10.1016/s0264-410x(01)00337-1. [DOI] [PubMed] [Google Scholar]
- 14.Pratt WD, Davis NL, Johnston RE, Smith JF. Genetically engineered, live attenuated vaccines for Venezuelan equine encephalitis: testing in animal models. Vaccine. 2003;21(25–26):3854–62. doi: 10.1016/s0264-410x(03)00328-1. [DOI] [PubMed] [Google Scholar]
- 15.Reed DS, Larsen T, Sullivan LJ, et al. Aerosol exposure to western equine encephalitis virus causes fever and encephalitis in cynomolgus macaques. J Infect Dis. 2005;192(7):1173–82. doi: 10.1086/444397. [DOI] [PubMed] [Google Scholar]
- 16.Martin SS, Bakken RR, Lind CM, et al. Telemetric analysis to detect febrile responses in mice following vaccination with a live-attenuated virus vaccine. Vaccine. 2009;27(49):6814–23. doi: 10.1016/j.vaccine.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furesz J. Developments in the production and quality control of poliovirus vaccines -- historical perspectives. Biologicals. 2006;34(2):87–90. doi: 10.1016/j.biologicals.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Japanese Encephalitis Virus Vaccine, Inactivated, Adsorbed Approval Letter. 2009 http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm142577.htm.
- 19.Fine DL, Jenkins E, Martin SS, Glass P, Parker MD, Grimm B. A multisystem approach for development and evaluation of inactivated vaccines for Venezuelan equine encephalitis virus (VEEV) J Virol Methods. 2009;163:424–32. doi: 10.1016/j.jviromet.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krug A, Rothenfusser S, Hornung V, et al. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 2001;31(7):2154–63. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 21.Marshall JD, Fearon KL, Higgins D, et al. Superior activity of the type C class of ISS in vitro and in vivo across multiple species. DNA Cell Biol. 2005;24(2):63–72. doi: 10.1089/dna.2005.24.63. [DOI] [PubMed] [Google Scholar]
- 22.Gupta RK, Siber GR. Adjuvants for human vaccines--current status, problems and future prospects. Vaccine. 1995;13(14):1263–76. doi: 10.1016/0264-410x(95)00011-o. [DOI] [PubMed] [Google Scholar]
- 23.Gu M, Hine PM, James JW, Giri L, Nabors GS. Increased potency of BioThrax anthrax vaccine with the addition of the C-class CpG oligonucleotide adjuvant CPG 10109. Vaccine. 2007;25(3):526–34. doi: 10.1016/j.vaccine.2006.07.056. [DOI] [PubMed] [Google Scholar]
- 24.Linghua Z, Xingshan T, Fengzhen Z. The efficacy of CpG oligodinucleotides, in combination with conventional adjuvants, as immunological adjuvants to swine streptococcic septicemia vaccine in piglets in vivo. Int Immunopharmacol. 2006;6(8):1267–76. doi: 10.1016/j.intimp.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Content and Format of Chemistry, Manufacturing and Controls Information and Establishment Description Information for a Vaccine or Related Product. U.S. Dept. Health and Human Services, Food and Drug Administration, Center for Biological Evaluation and Research; 1999. FDA Guidance. [Google Scholar]
- 26.Beaty BJ, Calisher CH, Shope RE, Schmidt NJ, Emmons RW. Arboviruses. 6. Washington, DC: American Public Health Association; 1989. Diagnostic procedures for viral, rickettsial and chlamydial infections; pp. 797–855. [Google Scholar]
- 27.Roehrig JT, Mathews JH. The neutralization site on the E2 glycoprotein of Venezuelan equine encephalomyelitis (TC-83) virus is composed of multiple conformationally stable epitopes. Virology. 1985;142(2):347–56. doi: 10.1016/0042-6822(85)90343-5. [DOI] [PubMed] [Google Scholar]
- 28.Pratt WD, Gibbs P, Pitt ML, Schmaljohn AL. Use of telemetry to assess vaccine-induced protection against parenteral and aerosol infections of Venezuelan equine encephalitis virus in non-human primates. Vaccine. 1998;16(9–10):1056–64. doi: 10.1016/s0264-410x(97)00192-8. [DOI] [PubMed] [Google Scholar]
- 29.Engler RJ, Mangiafico JA, Jahrling P, Ksiazek TG, Pedrotti-Krueger M, Peters CJ. Venezuelan equine encephalitis-specific immunoglobulin responses: live attenuated TC-83 versus inactivated C-84 vaccine. J Med Virol. 1992;38(4):305–10. doi: 10.1002/jmv.1890380414. [DOI] [PubMed] [Google Scholar]
- 30.Pushko P, Parker M, Ludwig GV, Davis NL, Johnston RE, Smith JF. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology. 1997;239(2):389–401. doi: 10.1006/viro.1997.8878. [DOI] [PubMed] [Google Scholar]
- 31.Guyton AC. Measurement of the respiratory volumes oflaboratory animals. American Journal of Physiology. 1947;150:70–77. doi: 10.1152/ajplegacy.1947.150.1.70. [DOI] [PubMed] [Google Scholar]
- 32.Earley E, Peralta PH, Johnson KM. A plaque neutralization method for arboviruses. Proc Soc Exp Biol Med. 1967;125(3):741–7. doi: 10.3181/00379727-125-32194. [DOI] [PubMed] [Google Scholar]
- 33.Food and Drug Administration. Points to Consider in the Characterization of Cell Lines Used to Produce Biologicals. Food and Drug Administration, Center for Biologics Evaluation and Research; Rockville, MD: 1993. [Google Scholar]
- 34.Wang E, Petrakova O, Adams AP, et al. Chimeric Sindbis/eastern equine encephalitis vaccine candidates are highly attenuated and immunogenic in mice. Vaccine. 2007;25(43):7573–81. doi: 10.1016/j.vaccine.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ludwig GV, Turell MJ, Vogel P, et al. Comparative neurovirulence of attenuated and non-attenuated strains of Venezuelan equine encephalitis virus in mice. Am J Trop Med Hyg. 2001;64(1–2):49–55. doi: 10.4269/ajtmh.2001.64.49. [DOI] [PubMed] [Google Scholar]
- 36.Jahrling PB, Stephenson EH. Protective efficacies of live attenuated and formaldehyde-inactivated Venezuelan equine encephalitis virus vaccines against aerosol challenge in hamsters. J Clin Microbiol. 1984;19(3):429–31. doi: 10.1128/jcm.19.3.429-431.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hart MK, Pratt W, Panelo F, Tammariello R, Dertzbaugh M. Venezuelan equine encephalitis virus vaccines induce mucosal IgA responses and protection from airborne infection in BALB/c, but not C3H/HeN mice. Vaccine. 1997;15(4):363–9. doi: 10.1016/s0264-410x(96)00204-6. [DOI] [PubMed] [Google Scholar]
- 38.Phillpotts RJ, Wright AJ. TC-83 vaccine protects against airborne or subcutaneous challenge with heterologous mouse-virulent strains of Venezuelan equine encephalitis virus. Vaccine. 1999;17(7–8):982–8. doi: 10.1016/s0264-410x(98)00315-6. [DOI] [PubMed] [Google Scholar]
- 39.Griffin D, Levine B, Tyor W, Ubol S, Despres P. The role of antibody in recovery from alphavirus encephalitis. Immunol Rev. 1997;159:155–61. doi: 10.1111/j.1600-065x.1997.tb01013.x. [DOI] [PubMed] [Google Scholar]
- 40.Mathews JH, Roehrig JT, Trent DW. Role of complement and the Fc portion of immunoglobulin G in immunity to Venezuelan equine encephalomyelitis virus infection with glycoprotein-specific monoclonal antibodies. J Virol. 1985;55(3):594–600. doi: 10.1128/jvi.55.3.594-600.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paessler S, Yun NE, Judy BM, et al. Alpha-beta T cells provide protection against lethal encephalitis in the murine model of VEEV infection. Virology. 2007;367(2):307–23. doi: 10.1016/j.virol.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosas CT, Paessler S, Ni H, Osterrieder N. Protection of mice by equine herpesvirus type 1 based experimental vaccine against lethal Venezuelan equine encephalitis virus infection in the absence of neutralizing antibodies. Am J Trop Med Hyg. 2008;78(1):83–92. [PubMed] [Google Scholar]
- 43.Bennett AM, Elvin SJ, Wright AJ, Jones SM, Phillpotts RJ. An immunological profile of Balb/c mice protected from airborne challenge following vaccination with a live attenuated Venezuelan equine encephalitis virus vaccine. Vaccine. 2000;19(2–3):337–47. doi: 10.1016/s0264-410x(00)00123-7. [DOI] [PubMed] [Google Scholar]
- 44.Martin SS, Bakken RR, Lind CM, et al. Comparison of the immunological responses and efficacy of gamma-irradiated V3526 vaccine formulations against subcutaneous and aerosol challenge with Venezuelan equine encephalitis virus subtype IAB. Vaccine. 2010;28:1031–1040. doi: 10.1016/j.vaccine.2009.10.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hart MK, Caswell-Stephan K, Bakken R, et al. Improved mucosal protection against Venezuelan equine encephalitis virus is induced by the molecularly defined, live-attenuated V3526 vaccine candidate. Vaccine. 2000;18(26):3067–75. doi: 10.1016/s0264-410x(00)00042-6. [DOI] [PubMed] [Google Scholar]
- 46.Kaufman DR, Liu J, Carville A, et al. Trafficking of antigen-specific CD8+ T lymphocytes to mucosal surfaces following intramuscular vaccination. J Immunol. 2008;181(6):4188–98. doi: 10.4049/jimmunol.181.6.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hagenaars N, Mastrobattista E, Glansbeek H, et al. Head-to-head comparison of four nonadjuvanted inactivated cell culture-derived influenza vaccines: effect of composition, spatial organization and immunization route on the immunogenicity in a murine challenge model. Vaccine. 2008;26(51):6555–63. doi: 10.1016/j.vaccine.2008.09.057. [DOI] [PubMed] [Google Scholar]
- 48.Greenway TE, Eldridge JH, Ludwig G, et al. Induction of protective immune responses against Venezuelan equine encephalitis (VEE) virus aerosol challenge with microencapsulated VEE virus vaccine. Vaccine. 1998;16(13):1314–23. doi: 10.1016/s0264-410x(98)00008-5. [DOI] [PubMed] [Google Scholar]
- 49.Perkins SD, Williams AJ, O’Brien LM, Laws TR, Phillpotts RJ. CpG used as an adjuvant for an adenovirus-based Venezuelan equine encephalitis virus vaccine increases the immune response to the vector, but not to the transgene product. Viral Immunol. 2008;21(4):451–7. doi: 10.1089/vim.2008.0052. [DOI] [PubMed] [Google Scholar]
- 50.Kass-Eisler A, Falck-Pedersen E, Elfenbein DH, Alvira M, Buttrick PM, Leinwand LA. The impact of developmental stage, route of administration and the immune system on adenovirus-mediated gene transfer. Gene Ther. 1994;1(6):395–402. [PubMed] [Google Scholar]
- 51.Berge TO, Banks IS, Tigertt WD. Attenuation of Venezuelan Equine Encephalomyelitis virus by In Vitro cultivation in guinea-pig heart cells. Am J Hyg. 1961;73:209–18. [Google Scholar]
- 52.Jahrling PB, Scherer WF. Homegeneity of Venezuelan encephalitis virion populations of hamster-virulent and benign strains, including the attenuated TC83 vaccine. Infect Immun. 1973;7(6):905–10. doi: 10.1128/iai.7.6.905-910.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]