Abstract
Development of the field of organ and tissue transplantation has accelerated remarkably since the human major histocompatibility complex (MHC) was discovered in 1967. Matching of donor and recipient for MHC antigens has been shown to have a significant positive effect on graft acceptance. The roles of the different components of the immune system involved in the tolerance or rejection of grafts and in graft-versus-host disease have been clarified. These components include: antibodies, antigen presenting cells, helper and cytotoxic T cell subsets, immune cell surface molecules, signaling mechanisms and cytokines that they release. The development of pharmacologic and biological agents that interfere with the alloimmune response and graft rejection has had a crucial role in the success of organ transplantation. Combinations of these agents work synergistically, leading to lower doses of immunosuppressive drugs and reduced toxicity. Reports of significant numbers of successful solid organ transplants include those of the kidneys, liver, heart and lung. The use of bone marrow transplantation for hematological diseases, particularly hematological malignancies and primary immunodeficiencies, has become the treatment of choice in many of these conditions. Other sources of hematopoietic stem cells are also being used, and diverse immunosuppressive drug regimens of reduced intensity are being proposed to circumvent the mortality associated with the toxicity of these drugs. Gene therapy to correct inherited diseases by infusion of gene-modified autologous hematopoietic stem cells has shown efficacy in two forms of severe combined immunodeficiency, providing an alternative to allogeneic tissue transplantation.
Keywords: bone marrow transplantation, solid organ transplantation, graft rejection, graft vs host disease
Efforts to transplant organs or tissues from one human to another had been unsuccessful for many decades until the discovery of the human major histocompatibility complex (MHC) in 1967.1 Identification of this genetic region launched the field of clinical organ and tissue transplantation. In 1968, the World Health Organization Nomenclature Committee designated that the leukocyte antigens controlled by the closely linked genes of the human MHC be named HLA (for human leukocyte antigen). This chapter reviews general immunologic concepts that have supported the success of human organ and tissue transplantation and summarizes current medical progress in the field of transplantation medicine.
TRANSPLANTATION ANTIGENS
The MHC
Histocompatibility antigens are tissue cell surface antigens capable of inducing an immune response in a genetically dissimilar (allogeneic) recipient, resulting in the rejection of the tissues or cells bearing those antigens. The genes that encode these antigens reside in the MHC region on the short arm of human chromosome 6 (Fig 1). The HLA complex contains more than 200 genes, more than 40 of which encode leukocyte antigens.2,3 These genes and their encoded cell surface and soluble protein products are divided into three classes (I, II, and III) on the basis of their tissue distribution, structure, and function.3–5 MHC class I and II genes encode codominantly expressed HLA cell surface antigens, and class III genes encode several components of the complement system; all of which share important roles in immune function. Class I MHC antigens are present on all nucleated cells and are each composed of a 45-kd α heavy chain encoded by genes of the HLA-A, HLA-B, or HLA-C loci on chromosome 6 and associated noncovalently with a 12-kd protein, β2 -microglobulin encoded by a gene on chromosome 15 (Fig 2)3 MHC class II antigens have a more limited tissue distribution and are expressed only on B lymphocytes, activated T lymphocytes, monocytes, macrophages, Langerhans cells, dendritic cells, endothelium, and epithelial cells.5 Each is a heterodimer composed of noncovalently associated α and β chains of approximately 230 amino acids encoded by genes of the HLA-D region (Fig 2). On cells expressing both class I and class II HLA antigens, there are three class I antigens and three or more (usually four) class II heterodimers. Class III genes are located between the HLA-B and HLA-D loci and determine the structure of three components of the complement system: C2, C4, and factor B.3,4 HLA antigens are inherited in a Mendelian dominant manner. Because of the closeness of the different loci of the MHC and the resultant low crossover frequency, however, HLA genes are almost always inherited together. To date, 3,756 different Class I and II HLA genes alleles have been identified.2 The fixed combination of these genetic determinants present in one chromosome of an individual is referred to as a haplotype. Chromosome 6 is an autosome, and therefore all individuals have two HLA haplotypes (one for each chromosome), and there are only four possible combinations of haplotypes among the offspring of any two parents. Thus, there is a 25% probability that biological siblings will have identical HLA alleles.
FIG 1.
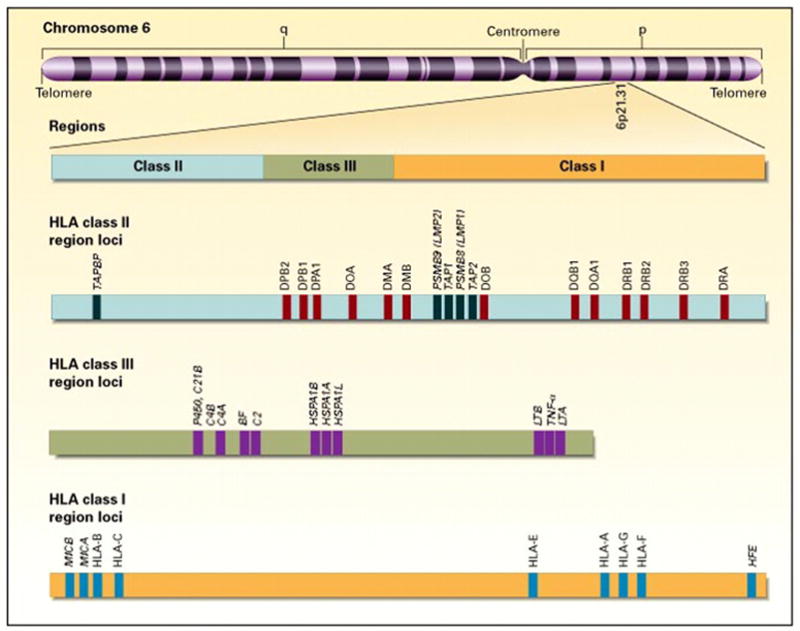
Location and organization of the HLA complex on chromosome 6. BF, Complement factor B; C2, complement component 2; C4A, complement component 4A; C4B, complement component 4B; TAP1, transporter of antigenic peptides 1; TAP2, transporter of antigenic peptides 2; LTA, lymphotoxin A; LTB, lymphotoxin B. From Klein J, Sato A. The HLA system: first of two parts. N Engl J Med 2000;343:703. Reprinted with permission.
FIG 2.
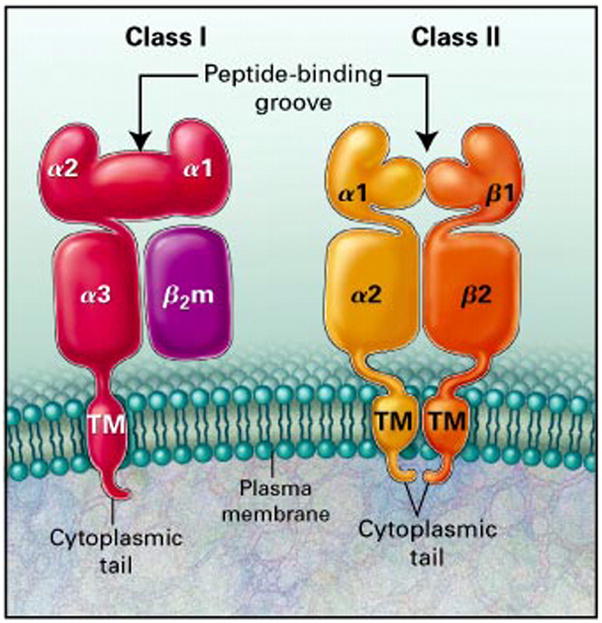
Structures of HLA class I and class II molecules. β2-Microglobulin (β2m) is the light chain of the class I molecule. TM, Transmembrane component. From Klein J, Sato A. The HLA system: first of two parts. N Engl J Med 2000;343:704. Reprinted with permission.
The ABO system
ABO incompatibility does not cause stimulation in mixed leukocyte cultures, indicating that ABO compatibility is of much less importance than HLA compatibility in graft survival. However, ABO incompatibility can result in hyperacute rejection of primarily vascularized grafts, such as kidney and heart.6 This is thought to occur because (1) ABO blood group antigens are highly expressed on kidney and cardiac grafts, particularly those from patients who are blood group A or B antigen secretors, and (2) preformed naturally occurring antibodies to blood group substances are present in mismatched recipients. Advances in immunosuppressive therapies to prevent immune rejection of the graft have more recently allowed performing organ transplants across the ABO barrier.7
Donor-recipient HLA matching
Two laboratory methods are used to pair donors and recipients for transplantation. The first matching method involves the determination of HLA antigens on donor and recipient leukocytes by either serologic or DNA typing methods; the second method is functional and involves the measurement of the response of immunocompetent cells from the recipient to antigens present on donor cells (and vice versa for bone marrow transplantation). Results of both methods are generally consistent with each other. Disparities that are serologically detected are referred to as antigen mismatches, whereas differences that can be identified only by DNA-based typing are called allele mismatches. Since these methods take considerable time to perform, results are not known in time for some solid organ transplants, such as lung transplants, which are performed based on immediate organ availability. Since 2000, the National Donor Matching Program (NDMP) performs HLA typing of donor volunteers exclusively by a DNA-based method, the PCR-single-strand oligonucleotide probe (SSOP). Currently, approximately 60% of volunteer donors on the NMDP Registry had their HLA type determined by this method. Efforts continue to improve the efficiency of HLA typing as well as reduce costs of the assays.8
Donor recipient serologic cross-matching
Serologic cross-matching is of particular importance to the success of primarily vascularized grafts, such as kidney and heart. Serum from the prospective recipient is tested against cells from the potential donor for the presence of antibodies to red blood cell or HLA antigens. The presence of such antibodies correlates with hyperacute renal graft rejection.6 For this reason, a positive serologic cross-match has been considered a contraindication to renal transplantation, although therapeutic strategies, such as the use of plasmapheresis, are proposed when the mismatch cannot be avoided. 7
Usefulness of HLA typing in clinical organ and tissue transplantation
Although typing for intrafamilial transplants of all types is clearly of great value, the usefulness of HLA typing in cadaveric kidney grafting has been a point of controversy since cyclosporine became available.9 Although short term survival rates did not appear to be that different for closely or poorly matched cadaveric kidneys, the degree of HLA matching does correlate with long-term survival.10 Until 1980, only HLA-identical siblings could be used as bone marrow donors, because both graft rejection and lethal graft-versus-host disease (GVHD) were common complications if this was not the case.11 Fortunately, the development during the past 3 decades of techniques to rigorously deplete post-thymic T cells from donor marrow has permitted numerous successful half-HLA matched marrow transplants with no or minimal GVHD.12,13
MECHANISMS OF GRAFT REJECTION
Role of alloimmune antibodies
The strongest evidence for a role for antibodies in graft rejection is the hyperacute rejection of primarily vascularized organs, such as the kidney and heart. High titers of antidonor antibodies can be demonstrated in recipients presenting with these reactions.6 These antibodies combine with HLA antigens on endothelial cells, with subsequent complement fixation and accumulation of polymorphonuclear cells. Endothelial damage then occurs, probably as a result of enzymes released from polymorphonuclear leukocytes; platelets then accumulate, thrombi develop, and the result is renal cortical necrosis or myocardial infarction. 14
Leukocytes and cytokines in graft rejection
Allograft rejection results from the coordinated activation of alloreactive T cells and antigen-presenting cells (APCs). Although acute rejection is a T-cell–dependent process, the destruction of the allograft results from a broad array of effector immune mechanisms. Cell-cell interactions and the release by primed helper T cells of multiple types of cytokines (interleukin [IL]-2, IL-4, IL-5, IL-7, IL-10, IL-15, tumor necrosis factor-α, and interferon-γ) recruit not only immunocompetent donor-specific CD4+ T cells, CD8+ cytotoxic T cells, and antibody-forming B cells but also nonspecific inflammatory cells, which constitute the majority of cells infiltrating an allograft.15 Other cells specific to the transplanted organ may play a role in the balance of tolerance and rejections, such as the Kupffer cells and the sinusoidal epithelial cells in the liver.16 Stimulation of CD4+ T cells through their antigen receptors is not sufficient to initiate T-cell activation, unless costimulation is provided by interaction of other ligand-receptor pairs present on the surfaces of T cells and APCs during the encounter. Some of these interactive pairs include the T-cell surface molecule CD2 and its ligand CD58 on APCs; CD11a/CD18:CD54; CD5:CD72; CD40L:CD40; and CD28:CD80 or CD86. CD4+ T-cell anergy or tolerance induction occurs when the T-cell receptor interacts with the APC unless signals are provided through one or more of these receptor-ligand interactions (particularly through CD40L:CD40 and CD28:CD80 or CD86) or by cytokines (such as IL-1 and IL-6 from the APC). Thus, T-cell accessory proteins and their ligands on APCs are target molecules for antirejection therapy.17,18 If costimulation does occur, the CD4+ T cell becomes activated, which leads to stable transcription of genes important in T-cell activation. CD8+ T cells recognize antigenic peptides displayed on MHC class I molecules and represent a major cytotoxic effector lymphocyte population in graft rejection. Donor class I molecules on donor APCs in the graft directly activate cytotoxic effector lymphocytes. However, CD8 activation also requires a costimulatory second signal, as well as an IL-2 signal. Activated CD8+ T cells proliferate and mature into specific alloreactive clones capable of releasing granzyme (serine esterase), perforin, and toxic cytokines, such as tumor necrosis factor-α. More recently, the identification of TH17 effector cells (pro-inflammatory) and regulatory T cells (down-regulate immune activation) has improved our understanding of the development of graft tolerance or rejection. 19 Stimulation of the B cell by antigen occurs through its antigen receptor (surface immunoglobulin), but costimulation is also required for B-cell activation. This costimulation can be provided by cytokines released by T cells or through many of the same T-cell protein-ligand pairs important in T-cell–APC costimulation, because these ligands are also present on B cells. B cell contribution to the immune rejection of organ transplants is not limited to the production of alloimmune antibodies, but also involves antigen presentation and the secretion of proinflammatory cytokines.20
Once T-cell activation has occurred, autocrine T-cell proliferation continues as a consequence of the expression of the IL-2 receptor (IL-2R). Interaction of IL-2 with its receptor triggers the activation of protein tyrosine kinases and phosphatidylinositol-3-kinase, resulting in translocation into the cytosol of an IL-2R–bound serine-threonine kinase, Raf-1. This in turn leads to the expression of several DNA-binding proteins, such as c- Jun, c-Fos, and c-Myc, and to progression of the cell cycle. The consequence of all of these events is the development of graft-specific, infiltrating cytotoxic T cells. Cytokines from the T cells also activate macrophages and other inflammatory leukocytes and cause upregulation of HLA molecules on graft cells. The activated T cells also stimulate B cells to produce antigraft antibodies. Ultimately, if not recognized and managed, all these cellular and humoral factors constitute the rejection process that destroys the graft.
IMMUNOSUPPRESSION (Table I)
TABLE I.
Immunosuppresion regimens
| Immunosuppression regimen | Immunological Target | Specific use | Major adverse effects |
|---|---|---|---|
| Radiation, anti-metabolite agents | Hematopoietic Stem cells, Leukocytes | BMT | Cytopenias, opportunistic infections, diarrhea, alopecia, veno-occlusive disease, long-term organ damage: endocrine abnormalities, growth delay, hypodontia, cognitive delay, sterility |
| Calcineurin inhibitors, anti-lymphocyte antibodies, anti-cytokine antibodies, anti-metabolite agents and corticosteroids | Lymphocytes | In solid organ and BMT: prevention and treatment of graft rejection and of GVHD | Opportunistic infections, lymphopenia, renal dysfunction, seizures, hypertrichosis, hypertension, gastritis, osteoporosis, cataracts, growth delay |
Currently, there is no method that will suppress the host’s immune response to antigens of the graft and at the same time maintain other immune responses. Nonspecific immunosuppressive agents are needed to prevent rejection of the transplanted organ, which may occur even though HLA matched donors are used. The development of immunosuppressive strategies during the past 4 decades reflects enormous progress in understanding the cellular and molecular mechanisms that mediate allograft rejection.21 The success of transplantation between unrelated donors and recipients can be attributed to implementation of these strategies. Because these agents depress both specific and nonspecific immunity; however, they render the recipient more susceptible to both infection and malignancy. Indeed, infection is the most important cause of transplant recipient death. Thus, all patients must have the immunosuppressive regimen fine-tuned to prevent rejection yet minimize the risk of infection—too high a dose, and infection supervenes; too small a dose, and the graft is rejected.
The immunosuppressive agents initially used in most transplant centers for nearly 2 decades were corticosteroids, azathioprine and cyclosporine. Several new agents have been introduced during the past few years: mycophenolate mofetil, which has a similar but more effective mode of action to that of azathioprine; tacrolimus, which has a mode of action and side effects similar to those of cyclosporine, and sirolimus; which blocks IL-2–induced T-cell cycle progression. Immunosuppressive agents can be categorized by whether they (1) interrupt lymphocyte cell division, (2) deplete lymphocytes, (3) interfere with lymphocyte maturational events, (4) interfere with immune cell costimulation, (5) modulate ischemia-reperfusion injury, or (6) facilitate induction of tolerance.22 They can also be grouped into those used for induction therapy, for prophylaxis against rejection, for reversal of acute rejection episodes, and for maintenance of immunosuppression.
Monoclonal antibodies to lymphocytes and to cytokine receptors
Antibodies from animals immunized with human lymphoid cells are useful agents for induction therapy, as well as for reversal of acute rejection episodes.23 They consist of the IgG fraction of serum from horses or rabbits immunized with either human lymphocytes (antilymphocyte globulin [ALG]) or thymocytes (antithymocyte globulin [ATG, Thymoglobulin]) or of monoclonal antibodies (murine or humanized) to T-cell surface antigens (e.g., CD3, [OKT3]). In general, ALG, ATG, and OKT3 decrease the onset, severity, and number of rejection episodes. Prevention of graft rejection has also been approached by inhibiting cytokines from interacting with their receptors. Chimeric or humanized murine anti–IL-2R α chain antibodies (daclizumab and basiliximab) have been developed for clinical use. The advantage of these monoclonal antibodies to the IL-2R α chain is that such molecules are present only on activated T cells; therefore the main effect is on T cells possibly activated by graft antigens.
Calcineurin inhibitors
The main action of calcineurin inhibitors (cyclosporine and tacrolimus) is that they prevent the synthesis of IL-2 and other cytokines that might be produced by T cells activated by allografts.21 Through its hydrophobicity, cyclosporine enters cell membranes to gain access to and bind to the cytoplasmic isomerase protein cyclophilin. The complex then inhibits calcineurin, an intracellular phosphatase critical for the translocation of signals from the T-cell receptor to the nucleus. In this manner, it blocks transcription of the IL-2 gene. In addition, it also blocks the synthesis of other cytokines and thereby interferes with activated CD4+ helper T-cell function. As a consequence, T cell proliferation and differentiation of precursor cytotoxic lymphocytes are blocked. Tacrolimus binds to a cytoplasmic isomerase protein in the same way that cyclosporine does, but it binds to a different one, the FK-binding protein.24 The complex formed inhibits calcineurin to prevent T-cell receptor signal transduction to the cell nucleus, blocking cell activation. Tacrolimus thus inhibits synthesis of IL-2, IL-3, interferon-γ, and other cytokines; it was found to be 100 times more potent than cyclosporine as an immunosuppressive agent.24
Cytokine receptor signal transduction inhibitors
Sirolimus (Rapamune) has a structure similar to tacrolimus, and its activity is also dependent on its binding to the FK-binding protein. However, the complex formed does not inhibit calcineurin but instead prevents the phosphorylation of the p70S6 kinase. This action blocks signal transduction from many cell-surface cytokine receptors, including IL-2R, IL-4R, IL-15R, and IL-10R. Both in vitro and in vivo studies have shown a synergistic effect of sirolimus with cyclosporine, as would be expected because sirolimus prevents cytokine receptor signaling and cyclosporine inhibits cytokine production. In addition, sirolimus selectively preserves the development of T regulatory cells.25 No agent is the perfect nonspecific immunosuppressive drug. Anti-lymphocyte antibodies (including anti-CD3, anti-CD6 and anti CD52 antibodies), nucleoside synthesis inhibitors, steroids, cyclosporine (or tacrolimus), anti–IL-2Rα chain (anti-CD25), and sirolimus all affect allorecognition and antigen-driven T-cell proliferation at different points in the T-cell activation process. Thus the combined use of several of these types of agents provides a synergistic effect, rather than a merely additive effect.
SOLID ORGAN TRANSPLANTATION
The explosive growth of transplantation since the discovery of HLA in 1967 is attested by the fact that, according to the Global Database on Donation and Transplantation gathering data from 97 countries, in 2007 around 100,000 solid organ transplantations were performed per year worldwide: 68,250 are kidney transplantations (45% from living donors), 19,850 are liver transplantations (14% from living donors), 5,179 are heart transplantations, 3,245 are lung transplantations, and 2,797 are pancreas transplantations.26
Kidney transplantation
Despite major improvements in dialysis techniques, renal transplantation remains the treatment of choice for end-stage renal disease in patients of nearly all ages.27 Estimates of new cases of end-stage renal disease are at 300 cases/million people annually, with an increasing trend. 27 For adults and most children, the renal transplant operation has become standardized. The earlier practice of removing the patient’s diseased kidneys 2 to 3 weeks before transplantation has not been carried out routinely in recent years, except for patients with hypertension or infection, and nephrectomy is now performed at the time of transplantation.
Immunosuppressive regimens
Until cyclosporine became available in the early 1980s, most centers used a combination of azathioprine (Imuran) and prednisone to prevent graft rejection. Beginning in 1983, many centers began to use cyclosporine (in lieu of azathioprine) with lower doses of prednisone for immunosuppression.27,28 Cyclosporine has been given in varying doses at different centers but has generally been given intravenously during or just after transplantation and on the day after. It is then subsequently administered orally and gradually tapered, depending on signs of toxicity or rejection and blood levels. Trough blood levels are periodically monitored, and doses are adjusted to maintain levels above 200 ng/mL. Prednisone is given on the day of transplantation and gradually reduced during the course of 12 weeks. In many centers the induction agents consist of one of the anti–IL-2R α chain antibodies, daclizumab or basiliximab, along with steroids, mycophenolate mofetil (instead of azathioprine), and tacrolimus (instead of cyclosporine). Some transplant surgeons are combining plasmapheresis, IVIG and immunosuppressive drugs, for patients who are highly sensitized and have high titers of alloantibodies.29,30 Acute rejection episodes are treated with intravenous pulses of high-dose methylprednisolone. Among the most useful agents have been ALG for 5 days, ATG for 5 days, and OKT3 for 1 to 14 days. Another anti-lymphocyte monoclonal antibody, anti-CD52 or alemtuzumab, has also been used successfully, though with differences in the incidence of opportunistic infections.31,32
Rejection
Rejection is the most common problem during the 3 months immediately after kidney grafting.27 Except for hyperacute rejection, most such episodes can be partially or completely reversed by one of the previously described immunosuppressive agents. Rejection episodes are classified as follows (Table II):
TABLE II.
Solid organ rejection patterns: Renal rejection as an example
| Type | Time after transplantation | Signs & symptoms | Rapidity of onset | Immune component | Pathologic findings | Treatment | Success rate (%) |
|---|---|---|---|---|---|---|---|
| Hyperacute | <24 h | Fever, anuria | Hours | Antibody and complement | Polymorphonuclear neutrophil deposition and thrombosis | None | 0 |
| Accelerated | 3–5 d | Fever, graft swelling, oliguria, tenderness | 1 d | Non–complement-fixing antibody | Vascular disruption hemorrhage, | ALG, ATG, anti-CD3 | 60 |
| Acute | 6–90 d | Oliguria, salt retention, graft swelling, tenderness, sometimes fever | Days to weeks | T cells and antibody | Tubulitis, endovasculitis | Steroids, ALG, ATG, anti-CD3 | 60–90 |
| Chronic | >60 d | Edema, hyper-tention, proteinuria, occasional hematuria | Months to years | Antibody | Vascular onion skinning | None | 0 |
From Buckley R. Chapter 42: Transplantation. In: Stiehm ER, editor. Immunologic disorders in infants children and adults. 5th ed. Philadelphia: WB Saunders; 2006 Reprinted with permission.
Hyperacute rejection occurs within the first 48 hours after the anastomosis takes place in recipients with preformed antileukocyte antibodies. It is characterized by fever and anuria. The binding of cytotoxic antibodies to the vascular endothelium activates complement, with subsequent aggregation of neutrophils and platelets, resulting in thrombosis. This is an irreversible event, and the only treatment option is immediate graft removal.
Accelerated rejection occurs on the third to the fifth day after transplantation. It is accompanied by fever, graft swelling, oliguria, and tenderness. It is thought to be mediated by non–complement-fixing antibodies to antigens present in the donor kidney. Histopathologically, it is characterized by vascular disruption with hemorrhage. The most effective treatments are anti-lymphocyte reagents, with or without plasmapheresis; these have a success rate of about 60% in reversing this process.
Acute rejection, the most common form, is due to a primary allogeneic response occurring within the first 6 to 90 days after transplantation. It is mediated by both T cells and antibodies, which cause tubulitis and vasculitis, respectively. High-dose pulses of steroids and antilymphocyte reagents are effective in reversing the T-cell response about 80% to 90% of the time, but antilymphocyte antibodies only reverse the vasculitis about 60% of the time.
Chronic rejection occurs when the tenuous graft tolerance is disturbed 2 or more months after transplantation. It is characterized by marked proteinuria, occasional hematuria, hypertension, and the nephritic syndrome. The primary mediator of this type of rejection is antibody. A kidney biopsy is usually necessary to distinguish rejection from cyclosporine or tacrolimus nephrotoxicity. This process is usually treatment-resistant, although progression may be slowed by immunosuppressive regimens.
Efficacy
Renal grafts from HLA-identical sibling donors have a 10-year survival of about 74%. Those transplants from “6 HLA antigen–matched” cadavers have currently a 1 year survival of 95%. The estimated graft survival has slowly improved over time and the most recent data, from the 1998–1999 cohort, is estimated at 11.6 years, according to national statistics. Grafts from living donors have a higher estimated life, at 15 years.27,33
Liver and intestinal transplantation
Liver transplantation had its inception in 1963, when the diseased liver of a 3-year-old child with extrahepatic biliary atresia was replaced.34 Although that patient died, subsequent successes have established liver transplantation as standard therapy for advanced chronic liver disease.35 Since 1983, the 1-year survival rates have increased from 25% to 78%, depending on the age and health of the recipient, the underlying condition, and various clinical considerations. Liver transplantation is indicated for chronic end-stage liver disease, fulminant acute liver failure, and cancer limited to the liver.36 As with renal transplantation, combined therapy targeting several facets of the potential rejection process is used for liver transplantation. Anti–IL-2R α chain antibodies are given intravenously on the day of transplantation, followed by tacrolimus, which is given intravenously initially and orally thereafter, and then by mycophenolate mofetil and steroids tapered slowly over a year. Survival has increased by 20% in the last 2 decades with tacrolimus-based immunosuppression.37
Although this improvement may be the result of several factors, retransplantation as a result of\ acute or chronic rejection has not occurred in patients treated with tacrolimus. Similar to all solid organ transplantation, lack of suitable donors is a major problem for liver transplantation. Since 1988 this organ shortage has been approached by partial hepatectomies of living related donors. Donor safety is much greater with use of the left lateral segment.38
Intestinal transplantation is offered to patients who have intestinal failure (due to short bowel syndrome, mucosal disease, motility disorders, tumors) and who present with severe complications of parenteral nutrition, such as cholestatic liver disease and recurrent loss of central venous access.39 Advances in surgical techniques, control of immune rejection and treatment of infections have improved the outcomes over time. In 2008, 185 intestinal transplants were performed in the United States. The 1-year rate of patient survival has increased from 57% in 1997 to 80% in 2005, and to 90% if the data is limited to centers that perform the largest number of transplantations.
Heart, heart-lung, and lung transplantation
The various forms of cardiomyopathy are the most common indications for heart transplantation, followed by congenital heart disease. Approximately 25% of heart transplant recipients are infants.22 Immunosuppressive regimens for heart transplantation are similar in many respects to those already described for renal and hepatic grafts. Usually an anti–IL-2R α chain monoclonal antibody is given for induction therapy on the day of transplantation, along with high-dose intravenous methylprednisolone. Prednisone is given after the operation and maintained orally until it is discontinued after the first normal findings from an endomyocardial biopsy. Tacrolimus is then begun as the primary immunosuppressive agent with or without mycophenolate.22
Since the introduction of cyclosporine 26 years ago, the results of cardiac transplantation have improved greatly. The International Heart Transplantation Registry has shown a 4-year survival of 71% for patients receiving cyclosporine- or tacrolimus-based triple immunosuppression therapy. Survival is influenced by the age of the recipient; patients younger than 40 years have a better survival. 40 Lung transplantation has been performed for the following major diagnostic categories: cystic fibrosis, pulmonary vascular disease, bronchiolitis obliterans, pulmonary alveolar proteinosis, and pulmonary fibrosis, with 4-year survival at approximately 50%.22,41
BONE MARROW TRANSPLANTATION
Since 1955, more than 240,000 bone marrow transplantations have been performed worldwide at 450 centers in 47 countries, for the treatment of more than 50 different fatal diseases (Table III).42 Most of these transplants have been done by re-infusing stored autologous marrow cells collected prior to the patient receiving intensive chemotherapy or irradiation. Annually, 25,000 to 35,000 autologous transplants are performed, compared with approximately 15,000 allogeneic transplants. Certain unique problems distinguish bone marrow transplantation from transplantation of solid organs, such as the kidney, liver, and heart. The first problem is that immunocompetent cells, both in the recipient and in the donor marrow or blood have the potential to reject each other, resulting in graft rejection on one hand and GVHD on the other.43 The second concern is that successful unfractionated marrow grafting usually requires strict donor and recipient MHC class II antigen compatibility to minimize such reactions. Finally, except for patients with severe combined immunodeficiency (SCID), complete DiGeorge anomaly, or identical twin donors, even HLA-identical recipients must be pretreated with cytotoxic and myeloablative agents to prevent graft rejection.43 Diseases treated successfully by allogeneic bone marrow transplantation include radiation injury, primary immunodeficiencies, hemoglobinopathies, aplastic anemia, multiple myeloma, leukemia, neuroblastoma, non-Hodgkin lymphoma, inborn errors of metabolism, and certain autoimmune diseases.44 In addition, autologous marrow transplantation has been used following lethal irradiation or chemotherapy in the treatment of patients with some hematologic malignancies, solid tumors, or breast cancer, as well as for the treatment of several autoimmune diseases.45
TABLE III.
Conditions treated with hematopoietic stem cell transplants (HSCT)
| Leukemias | Acute lymphoblastic leukemia Acute myelogenous leukemia Chronic lymphocytic leukemia Chronic myelogenous leukemia |
| Lymphomas | Non-Hodgkin lymphoma Hodgkin disease |
| Plasma cell disorders | Multiple myeloma and related disorders |
| Solid organ neoplasias | Breast cancer, ovarian cancer, melanoma neuroblastoma, lung cancer, sarcoma |
| Myelodysplastic syndromes | |
| Severe aplastic anemia | |
| Autoimmune diseases | Multiple sclerosis, systemic sclerosis, systemic lupus erythematosus |
| Inherited erythrocyte abnormalities | Sickle cell disease, thalassemia |
| Inherited metabolic diseases | Mucopolysaccharidosis type I, adrenoleukodystrophy, osteopetrosis |
| Primary immunodeficiencies | Severe combined immunodeficiency (SCID) Wiskott-Aldrich syndrome Chronic granulomatous disease Leukocyte adhesion deficiency CD40 ligand deficiency X-linked lymphoproliferative disease Hemophagocytic lymphohistiocytosis |
Other sources of hematopoietic stem cells for transplantation
Bone marrow is not the only source of hematopoietic stem cells (HSC). These cells are capable of reconstituting all blood cell lineages and can also be obtained from peripheral blood or from cord blood. Peripheral blood-derived HSC are retrieved after the donor receives granulocyte-colony-stimulating factor (GCSF), usually at 5–10 ug/kg/day for 5 days, to allow mobilization of the HSC cells. These are then collected by leukapheresis, and the stem cells are positively selected using affinity columns containing antibodies to the cell surface markers CD34 or CD133, both of which are suggested to have the highest specificity for pluripotential hematopoiesis.46 Cord blood is increasingly being used, because of its availability, simplicity of procurement, and the potential of a lower severity of GVHD without full HLA-matching.47 The number of cells in cord blood units are a limiting factor that is currently being addressed by using more than one donor’s cord blood.
Clinical features of GVHD
Acute GVHD begins six or more days after transplantation (or after transfusion in the case of nonirradiated blood products).48 Signs of GVHD include fever, a morbilliform erythematous rash, and severe diarrhea.49 The rash becomes progressively confluent and may involve the entire body surface; it is both pruritic and painful and eventually leads to marked exfoliation. Eosinophilia and lymphocytosis develop, followed shortly by hepatosplenomegaly, exfoliative dermatitis, protein-losing enteropathy, bone marrow aplasia, generalized edema, increased susceptibility to infection, and death.50 Skin biopsy specimens reveal basal vacuolar degeneration or necrosis, spongiosis, single-cell dyskeratosis, eosinophilic necrosis of epidermal cells, and a dermal perivascular round cell infiltration. Similar necrotic changes may occur in the liver, intestinal tract, and eventually most other tissues.
Treatment of GVHD
Many regimens have been used to mitigate GVHD in both HLA-incompatible and HLA-compatible bone marrow transplants. In MHC-compatible bone marrow transplants into patients with SCID or complete DiGeorge anomaly, it is not usually necessary to give immunosuppressive agents to prevent or mitigate the mild GVHD that may occur, although occasionally steroids are used to treat more severe forms of this condition. For unfractionated HLA-identical marrow transplants into all patients for whom pretransplantation chemotherapy is given to prevent rejection, however, it is necessary to use prophylaxis against GVHD. Patients are usually given a combination of methotrexate, corticosteroids and a calcineurin inhibitor daily for 6 months.51–53 When GVHD becomes established, it is extremely difficult to treat. Anti-thymocyte serum, steroids, cyclosporine, tacrolimus, anti–IL-2R α chain antibodies, anti-TNF α inhibitors, mycophenolate mofetil, and murine monoclonal antibodies to human T-cell surface antigens have ameliorated some cases, but the course has been inexorably fatal in many patients similarly treated.54–56 The best approach to GVHD reactions is prevention, and by far the best preventive approach is the removal of all post-thymic T cells from the donor marrow or blood.
HLA-identical bone marrow transplantation for severe combined immunodeficiency (SCID)
The only adequate therapy for patients with severe forms of cellular immunodeficiency is immunologic reconstitution by transplantation of immunocompetent hematopoietic stem cells. Until 1980, only HLA-identical unfractionated bone marrow could be used for this purpose because of the lethal GVHD that ensued if mismatched donors were used.57 In most cases, both T-cell and B-cell immunity have been reconstituted by such fully matched transplants, with evidence of function detected very soon after unfractionated marrow transplantation.58 Analysis of the genetic origins of the immune cells in the engrafted patients has revealed that, although the T cells are all of donor origin, the B cells are often those of the recipient.12 Initially, it was considered that bone marrow was effective in conferring immunity in patients with SCID, because it provided normal stem cells, but it is apparent from later experience with T-cell–depleted marrow59 that the early restoration of immune function after unfractionated HLA-identical marrow transplantation is by adoptive transfer of mature T and B cells in the donor marrow. Unfortunately, because of the lack of HLA-identical related donors, unfractionated bone marrow transplantation has not been possible for more than 85% of the immunodeficient patients who could have benefited. As a consequence, before the year 1982, most such patients died with severe infections.
HLA-haploidentical bone marrow transplantation for severe combined immune deficiency
The fact that totally HLA-disparate fetal liver cells could correct the immune defect in a few such patients without causing GVHD gave hope that HLA-disparate marrow stem cells could do the same if all donor post-thymic T cells could be removed. Early success in T-cell depletion was achieved in experimental animals by treating donor marrow or spleen cells with anti–T-cell antiserum or agglutinating the unwanted cells with plant lectins.60 The remaining immature marrow or splenic non-T cells restored lymphohematopoietic function to lethally irradiated MHC-disparate recipients without lethal GVHD. This approach was applied to human beings in the early 1980s and has been highly successful in infants with SCID.12,59– 63
The time to development of immune function after haploidentical stem cell grafts is quite different from that after unfractionated HLA-identical marrow. Lymphocytes with mature T-cell phenotypes and functions fail to rise significantly until 3 to 4 months after transplantation; normal T-cell function is reached between 4 and 7 months.59 B-cell function develops much more slowly, averaging 2 to 2.5 years for normalization; many do not have B-cell function developed, despite normal T-cell function.12,13 Genetic analyses of the lymphocytes from such chimeric patients have revealed all T cells to be genetically from donor origin, whereas the B cells and APCs almost always remain those of the recipient.61,62 These observations indicate that the thymic microenvironment of most infants with SCID is capable of differentiating half-matched normal stem cells to mature and functioning T lymphocytes that can cooperate effectively with host B cells for antibody production. Thus, the genetic defect in SCID does not compromise the function of the thymus.
Efficacy of bone marrow transplantation in immunodeficiency diseases
Although precise figures are not available, during the past 40 years, more than 1,200 patients worldwide with different forms of genetically determined immunodeficiency have been given bone marrow transplants in attempts to correct their underlying immune defects. Possibly because of earlier diagnosis before untreatable opportunistic infections develop, the results have improved considerably during the last 2 decades.62–67 As would be expected, survival outcomes of HLA-matched related transplants have been superior to HLA-haploidentical or HLA-identical unrelated transplants in several series of patients treated in specialized centers worldwide.
SCID
Bone marrow transplantation (BMT) has been more widely applied and more successful in infants with SCID than any other primary immunodeficiency. The use of pretransplant myelosuppressive or myeloablative conditioning is advocated by some investigators to prevent graft rejection, but since infants with SCID lack T cells, there should be no need to give pretransplantation chemotherapy. The largest multi-center report of SCID cases who received BMT was an European collaborative study from 1968 to 1999, including 153 patients receiving an HLA matched related (from parent or sibling) transplant, with a survival rate of 77%, and 294 patients receiving haploidentical HLA-matched transplant, with a survival of 54%.63 Twenty- eight patients received an HLA-matched unrelated donor transplant, with a survival rate of 63%. These outcomes have improved in the last decade, likely by progress in early diagnosis and in medical care, specifically in the availability of newer antibacterial and antiviral agents, as well as immunosuppressive drugs for the control and prophylaxis of GVHD. In addition, difference in the use of myeloablative and rejection prophylaxis regimens with their inherent toxicity is a variable that affects survival rate. The largest series of SCID receiving BMT in the USA, reported 161 patients who did not receive pre-transplant conditioning.62,68 16 of them received a HLA-matched related donor transplant, with a 100% survival. The others received a haploidentical HLA-matched related donor transplant, with a long-term (up to 26 years) survival rate of 77%. Nevertheless, this is a major accomplishment, because SCID is 100% fatal without marrow transplantation or, in the case of adenosine deaminase-deficient SCIDs, enzyme-replacement therapy. Of note, those who were transplanted earlier than 3.5 months of age had a survival of 94%, possibly reflecting the influence of opportunistic infections as determinants of transplantation success. These studies and others have shown that such transplants can provide normal numbers of T cells and normalize T-cell function in all known molecular types of SCID. Thus, there appears to be no survival advantage in performing such transplants in utero69,70 as opposed to performing them soon after birth. In utero transplants carry the risks associated with the invasive procedure that involves accessing the fetus and the difficulty to monitor the possible development of GVHD during gestation.
Other primary immunodeficiencies
The second largest group of patients with immunodeficiency given bone marrow transplants since 1968 are those with the Wiskott-Aldrich syndrome.71,72 In a report from the International Bone Marrow Transplant Registry, 170 patients with Wiskott-Aldrich syndrome had undergone transplantation, and the 5-year probability of survival for all subjects was 70% (95% confidence interval 63%– 77%). Probabilities differed according to donor type: 87% (95% confidence interval 74%–93%) with HLA identical sibling donors, 52% (95% confidence interval 37%–65%) with other related donors, and 71% (95% confidence interval 58%– 80%) with matched unrelated donors (MUDs, P = .0006). Boys who had received a MUD transplant before 5 years of age had survivals similar to those receiving HLA-identical sibling transplants. Of note, the incidence of autoimmunity in these patients after bone marrow transplant is up to 20%.72
Patients with combined immunodeficiencies characterized by less severe T-cell defects than in SCID, such as ZAP70 deficiency, constitute the third largest group of patients given bone marrow transplants. Forty-five patients with Omenn syndrome were reported as having received marrow transplants, and 23 or 51% were alive at the time of the report.61 Fourteen of 26 patients with the bare lymphocyte syndrome (54%) were alive after having been given marrow transplants.73,74 Other disorders treated successfully by bone marrow transplantation include X-linked hyper-IgM,75 reticular dysgenesis,76 purine nucleoside phosphorylase deficiency,77 cartilage hair hypoplasia, and X-linked lymphoproliferative syndrome.
Patients with the complete DiGeorge syndrome have undergone both marrow and thymic transplantations. Six of 9 such patients were reported to have survived 2 to 24 years after having received unfractionated HLA-identical sibling marrow;78 however, possible publication bias was suggested, proposing that a number of patients who may not have survived had not been taken into account.79 Since the underlying defect in this condition is absence of the thymus, a more direct approach is to perform thymus transplantation. To this end, 54 infants with complete DiGeorge syndrome have undergone thymic transplantation using cultured HLA-unmatched unrelated thymic tissue, with a survival rate of 69%.80 An important immunological difference is that the transplanted thymus allows the development of naïve T cells even with a disparate HLA haplotype between donor and recipient. In contrast, complete DiGeorge syndrome patients who receive bone marrow transplants survive with a reduced T cell number, and absent naïve T cell population.
Patients with primarily phagocyte disorders also have shown to benefit from bone marrow transplant. Recently, a report from Europe included data from 24 patients with chronic granulomatous disease (CGD) who had received bone marrow transplants, with 19 patients surviving.81 At Texas Children’s Hospital (Houston, Texas), eleven patients with CGD (9 X-linked CGD and 2 autosomal-recessive CGD) have been transplanted, with 10 patients surviving and immunoreconstituted, and a median follow-up of 25 months (unpublished data). Four of these received HLA-matched related transplants, and 6 received HLA-matched unrelated grafts. One patient who received a mismatched related (HLA 5/6 matched) transplant did not survive. Other leukocyte disorders that have been successfully treated with bone marrow transplantation include: pigmentary dilution (Griscelli) syndrome, Chediak-Higahi syndrome, familial hemophagocytic hystiocytosis, severe congenital neutropenia and leukocyte adhesion deficiency.61,82
Efficacy of bone marrow transplantation in malignancy
Bone marrow transplantation is the therapy of choice for leukemia, lymphoma and myelodysplatic proliferative disorders.83 The success of marrow transplantation in curing malignancy depends on a number of factors, the most important of which are the type of malignant disease, the stage of that disease, and the age of the recipient. Most patients with acute myelogenous leukemia achieve remission after chemotherapy, however approximately 65% of patients will relapse within 2 years. 84 During the first complete remission, consolidation chemotherapy or bone marrow transplantation are possible alternatives. In patients with intermediate risk disease, the projected disease-free survivals at 5 years are 52% for allogeneic transplantation and 45% for autologous transplantation.85 For patients with chronic myelogenous leukemia, allogenic bone marrow transplantation is considered primarily for pediatric patients, with a success rate over 80% and for those adults who have failed medical treatment with tyrosine kinase inhibitors.83,86 Three-year overall survival is variable among different series, reaching up to 80%. The best survival rates with the lowest probability of relapse occurs in patients younger than 20 years who had acute nonlymphocytic leukemia and underwent transplantation in first remission and in patients with chronic myelogenous leukemia who underwent transplantation in the chronic phase.87
The rationale for allogeneic bone marrow transplantation in leukemia is the hope that the leukemic cells can be reduced or eliminated by irradiation or chemotherapy and that the grafted allogeneic normal T cells can then reject any remaining leukemic cells.88 Supporting a need for T cells in the graft is the fact that T-cell–depleted bone marrow transplants have been associated with a higher degree of leukemia recurrence. 89
Efficacy of bone marrow transplantation in hemoglobinopathies, osteopetrosis, metabolic storage diseases and severe autoimmunity
Bone marrow transplantation has been highly effective for the treatment of homozygous β-thalassemia, with survivals reaching 70% to 80% for marrow transplants from HLA-identical siblings.90 Likewise, HLA-identical bone marrow transplantation has also been successful for patients with sickle cell disease, with 59 patients known to have been treated, 55 of whom were surviving with 50 free of sickle cell disease.91 The European Bone Marrow Transplantation Group reported on 69 patients with autosomal recessive osteopetrosis who were given HLA identical or haploidentical bone marrow transplants between 1976 and 1994.92,93 Recipients of genotypically HLA-identical marrow had an actuarial probability for 5- year survival up to 60% with osteoclast function of 79% of the survivors. Mucopolysaccharidosis type I (Hurler’s disease) and adrenoleukodystrophy, but not other lysosomal storage diseases, have been successfully treated with bone marrow transplantation when performed before significant organ damage occurs, as an alternative to enzyme replacement.94 Autologous and allogeneic bone marrow transplantation protocols have been used with relative success in severe autoimmunity. In a large collaborative study of over 500 patients with autoimmune conditions, survival was 80%, with sustained improvement in 70% of the survivors.95
Nonmyeloablative bone marrow transplantation
For patients with preexisting organ damage, there is significant morbidity and mortality from traditional conditioning regimens with busulfan and cyclophosphamide or irradiation. Because of this, there has been increasing interest in developing conditioning regimens that are less toxic.96 This has been accomplished by either total lymphoid irradiation or a combination of nucleoside analogs and anti–lymphocyte antibody preparations. Although these regimens are significantly less cytotoxic than high-dose alkylating agents and total-body irradiation, they are profoundly immunosuppressive. Opportunistic infections, such as the reactivation of cytomegalovirus, remain clinical obstacles when nonmyeloablative stem cell transplants are performed with these agents, especially in elderly and previously immunosuppressed patients. GVHD prophylaxis with cyclosporine and methotrexate, with added mycophenolate mofetil in some cases, has been necessary, because GVHD is common after nonmyeloablative transplantation.
Gene therapy for primary immunodeficiencies
Gene therapy trials in the last decade have shown —proof of concept, that genetic disorders can be modified and even cured. Significant progress was made in X-linked severe combined immunodeficiency (SCID), adenosine deaminase (ADA)-deficiency SCID and in X-linked CGD. The reports by Cavazzana-Calvo et al.97 and Hacein- Bey-Abina et al.98,99 of successful gene therapy in infants with X-linked SCID represented a major step forward, because repeated efforts to achieve gene correction of adenosine deaminase-deficient SCID had failed during the decade before 2000. Subsequently, Gaspar et al. reported a similar gene therapy protocol for X-linked SCID conducted in London, confirming the efficacy of this novel approach.100 The group at the Hôpital Necker in Paris treated 11 patients with X-linked SCID with gene-corrected autologous bone marrow cells. Nine infants had normal T and B-cell functions develop after the treatments. Two did not improve and were given allogeneic bone marrow transplants. The nine patients who did acquire normal immune function did not require intravenous immunoglobulin infusions and were at home without any medication. Four of the 10 patients treated in London have poor B cell reconstitution and are dependent on immunoglobulin supplementation. Natural killer cell reconstitution in this molecular type of SCID is also poor, similar to patients who receive bone marrow transplantation.
However, serious adverse events with this therapy occurred in four patients treated at the Hôpital Necker and one patient treated in London.99 Shortly before varicella developed, the first patient was discovered to have a high white blood cell count as a result of an expanded clonal population of circulating γδ-positive T cells. The white blood cell count became much higher and became a leukemic-like process that was treated with chemotherapy. The T-cell clone was shown to carry the inserted retroviral gene vector, within an intron in a gene on chromosome 11 called LMO2. LMO2 is an oncogene that is aberrantly expressed in acute lymphoblastic leukemia of childhood.101 Similarly, the other three patients in that protocol and one of the ten patients treated in London developed T-cell proliferation with upregulation of the expression of not only LMO2, but also of other oncogenes. Fortunately, four of these patients responded to conventional chemotherapy regimens and are presently in remission, leading a relatively normal quality of life. Insertional oncogenesis has long been known to be a potential complication of retroviral vector gene transfer, because retrovirus integration might occur within oncogenes in the genome. This complication has been thought to be unlikely with such vectors, because the vectors cannot reproduce themselves and cannot repeatedly insert into the cell’s chromosomes to increase the likelihood of malignant change. Before these cases, malignant changes had not been seen in any human beings given retroviral vectors for gene transfer. Considering the success of bone marrow transplantation for recipients of HLA-matched related donor grafts and for those who are treated in early infancy, new gene therapy trials for X-linked SCID are now being developed with the objective of reducing their oncogenesis potential, such as with the use of lentivirus-based gene vectors.102
Gene therapy trials for ADA deficiency were initiated in the early 1990’s, with targeting of peripheral lymphocytes and later CD34-enriched bone marrow cells. The success of these trials was modest, resulting in detection of a small proportion of gene-modified cells in peripheral blood, but no evidence of immunological benefits.103 The required concomitant use of polyethylene glycol modified bovine adenosine deaminase (PEG-ADA) is considered to have been a contributing cause to the failures in the US trials. Recently, two European research groups reported gene therapy trials for ADA deficiency using low dose busulfan pre-therapy without PEG-ADA or (in those patients who were on it) withdrawing the enzyme for a few weeks before infusion of the gene-modified cells.104,105 Eleven of the 15 patients treated with this approach (10 in Italy and 5 in London) showed good immunoreconstitution. Of note, there have not been cases of leukemia or lymphoma in the ADA-deficient SCIDs who have been corrected by gene therapy, although insertions of gene vectors near oncogenes similar to the X-linked SCID trials have been observed.
A small number of patients with X-linked CGD have been treated with gene therapy approaches.106 In the USA, initial efforts in 1997 by Malech and collaborators resulted in the detection of genetically corrected cells, but in minimal proportion (<1% of granulocytes). A more recent European trial adding a myeloablative regimen prior to infusion of the gene-corrected cells showed a larger proportion of gene-modified cells; however with only transient expression of the gene. The treatment provided initial clinical benefit, including resolution of severe and chronic fungal and bacterial infections. Patients in one of the trials demonstrated cell expansion as a result of insertional mutagenesis, and needed bone marrow transplantation, which was curative in one of two patients.107 Efforts aimed to improve the expression of the gene and to reduce oncogenesis are underway.
CONCLUSIONS
Advances in transplantation immunology have allowed the exponential growth of organ and tissue transplantation in medicine over the last three decades. Newer immunosuppressive agents have allowed the control of solid organ and tissue rejection and of graft-versus-host disease even when HLA incompatibility is present. For the treatment of hematological disorders, including primary immunodeficiencies, hematopoietic stem cell transplantation is not only feasible but it is the treatment of choice in many cases. Future developments in the field of transplantation immunology will hopefully include novel immunosuppressors with less toxicity and more specificity to control graft rejection while sparing overall immunity and thereby enabling better infection control. Gene therapy has shown promise in curing severe primary immunodeficiencies; however, problems with this approach urgently need to be addressed, the most important of which is insertional mutagenesis seen with the gene vectors used to date.
Abbreviations
- ALG
Antilymphocyte globulin
- APC
Antigen-presenting cell
- ATG
Antithymocyte globulin
- GVHD
Graft-versus-host disease
- IL
Interleukin
- MHC
major histocompatibility complex
- HLA
Human leukocyte antigen
- MUD
Matched unrelated donor
- SCID
Severe combined immunodeficiency
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Javier Chinen, Department of Pediatrics, Allergy/Immunology, Baylor College of Medicine, Houston, TX.
Rebecca H. Buckley, Department of Pediatrics, Allergy/Immunology, Duke University Medical Center, Durham, NC.
References
- 1.Bach FH, Amos DB. Hu-1: major histocompatibility locus in man. Science. 1967;156:1506–8. doi: 10.1126/science.156.3781.1506. [DOI] [PubMed] [Google Scholar]
- 2.Holdsworth R, Hurley CK, Marsh SG, Lau M, Noreen HJ, Kempenich JH, et al. The HLA dictionary 2008: a summary of HLA-A, -B, -C, -DRB1/3/4/5, and -DQB1 alleles and their association with serologically defined HLA-A, -B, -C, -DR, and -DQ antigens. Tissue Antigens. 2009;73:95–170. doi: 10.1111/j.1399-0039.2008.01183.x. [DOI] [PubMed] [Google Scholar]
- 3.Klein J, Sato A. The HLA system: first of two parts. N Engl J Med. 2000;343:702–9. doi: 10.1056/NEJM200009073431006. [DOI] [PubMed] [Google Scholar]
- 4.Marsh SG. WHO Nomenclature Committee for Factors of the HLA System. Nomenclature for factors of the HLA system, update June 2009. Tissue Antigens. 2009 Oct;74:364–6. doi: 10.1111/j.1399-0039.2009.01330.x. [DOI] [PubMed] [Google Scholar]
- 5.Klein J, Sato A. The HLA system: second of two parts. N Engl J Med. 2000;343:782–6. doi: 10.1056/NEJM200009143431106. [DOI] [PubMed] [Google Scholar]
- 6.Kissmeyer-Nielsen F, Olsen S, Petersen VP, Fjeldborg O. Hyperacute rejection of kidney allografts associated with pre-existing humoral antibodies against donor cells. Lancet. 1966;2:662–5. doi: 10.1016/s0140-6736(66)92829-7. [DOI] [PubMed] [Google Scholar]
- 7.Thielke J, Kaplan B, Benedetti E. The role of ABO-incompatible living donors in kidney transplantation: state of the art. Semin Nephrol. 2007;27:408–13. doi: 10.1016/j.semnephrol.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Spellman S, Setterholm M, Maiers M, Noreen H, Oudshoorn M, Fernandez-Viña M, et al. Advances in the selection of HLA-compatible donors: refinements in HLA typing and matching over the first 20 years of the National Marrow Donor Program Registry. Biol Blood Marrow Transplant. 2008 Sep;14(9 Suppl):37–44. doi: 10.1016/j.bbmt.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Kahan BD. Cyclosporine: a revolution in transplantation. Transplant Proc. 1999;31:14S–5S. doi: 10.1016/s0041-1345(98)02074-0. [DOI] [PubMed] [Google Scholar]
- 10.Zou Y, Stastny P. The role of major histocompatibility complex class I chain-related gene A antibodies in organ transplantation. Curr Opin Organ Transplant. 2009;14:414–8. doi: 10.1097/mot.0b013e32832d835e. [DOI] [PubMed] [Google Scholar]
- 11.Mickelson EM, Fefer A, Storb R, Thomas ED. Correlation of the relative response index with marrow graft rejection in patients with aplastic anemia. Transplantation. 1976;22:294–330. doi: 10.1097/00007890-197609000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Buckley RH, Schiff SE, Schiff RI, Markert L, Williams LW, Roberts JL, et al. Hematopoietic stem cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340:508–16. doi: 10.1056/NEJM199902183400703. [DOI] [PubMed] [Google Scholar]
- 13.Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol. 2004;22:625–55. doi: 10.1146/annurev.immunol.22.012703.104614. [DOI] [PubMed] [Google Scholar]
- 14.Al-Lamki RS, Bradley JR, Pober JS. Endothelial cells in allograft rejection. Transplantation. 2008;86:1340–8. doi: 10.1097/TP.0b013e3181891d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karczewski J, Karczewski M, Glyda M, Wiktorowicz K. Role of TH1/TH2 cytokines in kidney allograft rejection. Transplant Proc. 2008;40:3390–2. doi: 10.1016/j.transproceed.2008.07.125. [DOI] [PubMed] [Google Scholar]
- 16.Knechtle SJ, Kwun J. Unique aspects of rejection and tolerance in liver transplantation. Semin Liver Dis. 2009;29:91–101. doi: 10.1055/s-0029-1192058. [DOI] [PubMed] [Google Scholar]
- 17.Snanoudj R, de Préneuf H, Créput C, Arzouk N, Deroure B, Beaudreuil S, et al. Costimulation blockade and its possible future use in clinical transplantation. Transpl Int. 2006;19:693–704. doi: 10.1111/j.1432-2277.2006.00332.x. [DOI] [PubMed] [Google Scholar]
- 18.Li XC, Rothstein DM, Sayegh MH. Costimulatory pathways in transplantation: challenges and new developments. Immunol Rev. 2009 May;229(1):271–93. doi: 10.1111/j.1600-065X.2009.00781.x. [DOI] [PubMed] [Google Scholar]
- 19.Atalar K, Afzali B, Lord G, Lombardi G. Relative roles of Th1 and Th17 effector cells in allograft rejection. Curr Opin Organ Transplant. 2009;14:23–9. doi: 10.1097/MOT.0b013e32831b70c2. [DOI] [PubMed] [Google Scholar]
- 20.Balin SJ, Platt JL, Cascalho M. Noncognate function of B cells in transplantation. Transpl Int. 2009;22:593–8. doi: 10.1111/j.1432-2277.2008.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahan BD. Forty years of publication of transplantation proceedings--the second decade: the cyclosporine revolution. Transplant Proc. 2009;41:1423–37. doi: 10.1016/j.transproceed.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Webber SA, McCurry K, Zeevi A. Heart and lung transplantation in children. Lancet. 2006;368:53–69. doi: 10.1016/S0140-6736(06)68969-1. [DOI] [PubMed] [Google Scholar]
- 23.Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D Thymoglobulin Induction Study Group. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355:1967–77. doi: 10.1056/NEJMoa060068. [DOI] [PubMed] [Google Scholar]
- 24.Webster AC, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. BMJ. 2005;331:810. doi: 10.1136/bmj.38569.471007.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coenen JJ, Koenen HJ, van Rijssen E, Hilbrands LB, Joosten I. Rapamycin, and not cyclosporin A, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells. Blood. 2006;107:1018–23.22. doi: 10.1182/blood-2005-07-3032. [DOI] [PubMed] [Google Scholar]
- 26.Matesanz R, Mahillo B, Alvarez M, Carmona M. Global observatory and database on donation and transplantation: world overview on transplantation activities. Transplant Proc. 2009;41:2297–301. doi: 10.1016/j.transproceed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Magee CC, Pascual M. Update in renal transplantation. Arch Intern Med. 2004;164:1373–88. doi: 10.1001/archinte.164.13.1373. [DOI] [PubMed] [Google Scholar]
- 28.Knoll G. Trends in kidney transplantation over the past decade. Drugs. 2008;68(Suppl 1):3–10. doi: 10.2165/00003495-200868001-00002. [DOI] [PubMed] [Google Scholar]
- 29.Lefaucheur C, Nochy D, Andrade J, Verine J, Gautreau C, Charron D, et al. Comparison of combination Plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. Am J Transplant. 2009;9:1099–107. doi: 10.1111/j.1600-6143.2009.02591.x. [DOI] [PubMed] [Google Scholar]
- 30.Uchida J, Iwai T, Kato M, Machida Y, Naganuma T, Kumada N, et al. A novel approach to successful ABO-incompatible high-titer renal transplantation. Transplant Proc. 2008;40:2285–8. doi: 10.1016/j.transproceed.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 31.Kauffman HP, Stormstad AS, Sampson D, Stawicki AT. Randomized steroid therapy of human kidney transplant rejection. Transplant Proc. 1979;11:36–8. [PubMed] [Google Scholar]
- 32.Clatworthy MR, Friend PJ, Calne RY, Rebello PR, Hale G, Waldmann H, Watson CJ. Alemtuzumab (CAMPATH-1H) for the treatment of acute rejection in kidney transplant recipients: long-term follow-up. Transplantation. 2009;87:1092–5. doi: 10.1097/TP.0b013e31819d3353. [DOI] [PubMed] [Google Scholar]
- 33.Paramesh A, Zhang R, Yau CL, Balamuthusamy S, Shenava R, Killackey M, et al. Long-term outcome of single pediatric donor kidney transplants between African-American and non-African-American adults. Clin Nephrol. 2009;72:55–61. doi: 10.5414/cnp72055. [DOI] [PubMed] [Google Scholar]
- 34.Starzl TE, Esquivel C, Gordon R, Todo S. Pediatric liver transplantation. Transplant Proc. 1987;19:3230–5. [PMC free article] [PubMed] [Google Scholar]
- 35.Jain A, Mazariegos G, Kashyap R, Kosmach-Park B, Starzl TE, Fung J, et al. Pediatric liver transplantation. A single center experience spanning 20 years. Transplantation. 2002;73:941–7. doi: 10.1097/00007890-200203270-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mowat AP. Liver disorders in children: the indications for liver replacement in parenchymal and metabolic diseases. Transplant Proc. 1987;19:3236–41. [PubMed] [Google Scholar]
- 37.Pillai AA, Levitsky J. Overview of immunosuppression in liver transplantation. World J Gastroenterol. 2009;15:4225–33. doi: 10.3748/wjg.15.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong JC, Yersiz H, Farmer DG, Duffy JP, Ghobrial RM, Nonthasoot B, et al. Long term outcomes for whole and segmental liver grafts in adult and pediatric liver transplant recipients: a 10- year comparative analysis of 2,988 cases. J Am Coll Surg. 2009;208:682–9. doi: 10.1016/j.jamcollsurg.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 39.Fishbein TM. Intestinal transplantation. New Engl J Med. 2009;361:998–1008. doi: 10.1056/NEJMra0804605. [DOI] [PubMed] [Google Scholar]
- 40.Aurora P, Edwards LB, Christie J, Dobbels F, Kirk R, Kucheryavaya AY, et al. Registry of the International Society for Heart and Lung Transplantation: eleventh official pediatric lung and heart/lung transplantation report--2008. J Heart Lung Transplant. 2008;27:978–83. doi: 10.1016/j.healun.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 41.Hertz MI, Aurora P, Christie JD, Dobbels F, Edwards LB, Kirk R, et al. Registry of the International Society for Heart and Lung Transplantation: a quarter century of thoracic transplantation. J Heart Lung Transplant. 2008;27:937–42. doi: 10.1016/j.healun.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 42.Horowitz M. The role of registries in facilitating clinical research in BMT: examples from the Center for International Blood and Marrow Transplant Research. Bone Marrow Transplant. 2008;42 (Suppl 1):S1–S2. doi: 10.1038/bmt.2008.101. [DOI] [PubMed] [Google Scholar]
- 43.Martin PJ, Hansen JA, Storb R, Thomas ED. Human marrow transplantation: an immunological perspective. Adv Immunol. 1987;40:379–438. doi: 10.1016/s0065-2776(08)60243-6. [DOI] [PubMed] [Google Scholar]
- 44.Appelbaum FR. Hematopoietic-cell transplantation at 50. N Engl J Med. 2007;357:1472–5. doi: 10.1056/NEJMp078166. [DOI] [PubMed] [Google Scholar]
- 45.Ljungman P, Urbano-Ispizua A, Cavazzana-Calvo M, Demirer T, Dini G, Einsele H, et al. European Group for Blood and Marrow. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: definitions and current practice in Europe. Bone Marrow Transplant. 2006;37:439–49. doi: 10.1038/sj.bmt.1705265. [DOI] [PubMed] [Google Scholar]
- 46.Padmanabhan A, Reich-Slotky R, Jhang JS, Dael S, Crowder T, Colovai AI, Schwartz J. Use of the haematopoietic progenitor cell parameter in optimizing timing of peripheral blood stem cell harvest. Vox Sang. 2009;97:153–9. doi: 10.1111/j.1423-0410.2009.01183.x. [DOI] [PubMed] [Google Scholar]
- 47.Kurtzberg J. Update on umbilical cord blood transplantation. Curr Opin Pediatr. 2009;21:22–9. doi: 10.1097/mop.0b013e32832130bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dwyre DM, Holland PV. Transfusion-associated graft-versus-host disease. Vox Sang. 2008;95:85–93. doi: 10.1111/j.1423-0410.2008.01073.x. [DOI] [PubMed] [Google Scholar]
- 49.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HLA-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 50.Deeg HJ, Antin JH. The clinical spectrum of acute graft-versus-host disease. Semin Hematol. 2006;43:24–31. doi: 10.1053/j.seminhematol.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Carnevale-Schianca F, Leisenring W, Martin PJ, Furlong T, Schoch G, Anasetti C, Appelbaum FR, et al. Longitudinal assessment of morbidity and acute graft-versus-host disease after allogeneic hematopoietic cell transplantation: retrospective analysis of a multicenter phase III study. Biol Blood Marrow Transplant. 2009;15:749–56. doi: 10.1016/j.bbmt.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. ATG-Fresenius Trial Group. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–64. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- 53.Ogawa H, Soma T, Hosen N, Tatekawa T, Tsuboi A, Oji Y, et al. Combination of tacrolimus, methotrexate, and methylprednisolone prevents acute but not chronic graft-versus-host disease in unrelated bone marrow transplantation. Transplantation. 2002;74:236–43. doi: 10.1097/00007890-200207270-00015. [DOI] [PubMed] [Google Scholar]
- 54.Adkins D, Ratanatharathorn V, Yang H, White B. Safety profile and clinical outcomes in a phase I, placebo-controlled study of siplizumab in acute graft-versus-host disease. Transplantation. 2009;88:198–202. doi: 10.1097/TP.0b013e3181abfbf7. [DOI] [PubMed] [Google Scholar]
- 55.Pidala J, Kim J, Anasetti C. Sirolimus as primary treatment of acute graft-versus-host disease following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15:881–5. doi: 10.1016/j.bbmt.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-vs-host disease. Lancet. 2009;373:1550–61. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bortin MM, Rimm AA. Severe combined immunodeficiency disease. Characterization of the disease and results of transplantation. JAMA. 1977;238:591–600. [PubMed] [Google Scholar]
- 58.Sindel LJ, Buckley RH, Schiff SE, Ward FE, Mickey GH, Huang AT, et al. Severe combined immunodeficiency with natural killer cell predominance: abrogation of graft-versus-host disease and immunologic reconstitution with HLA-identical bone marrow cells. J Allergy Clin Immunol. 1984;73:829–36. doi: 10.1016/0091-6749(84)90455-x. [DOI] [PubMed] [Google Scholar]
- 59.Buckley RH, Schiff SE, Sampson HA, Schiff RI, Markert ML, Knutsen AP, et al. Development of immunity in human severe primary T cell deficiency following haploidentical bone marrow stem cell transplantation. J Immunol. 1986;136:2398–407. [PubMed] [Google Scholar]
- 60.Reisner Y, Itzicovitch L, Meshorer A, Sharon N. Hematopoietic stem cell transplantation using mouse bone marrow and spleen cells fractionated by lectins. Proc Nat Acad Sci USA. 1978;75:2933–6. doi: 10.1073/pnas.75.6.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buckley RH, Fischer A. Bone marrow transplantation for primary immunodeficiency diseases. In: Ochs HD, Smith CIE, Puck JM, editors. Primary immunodeficiency diseases: a molecular and genetic approach. 2. New York: Oxford University Press; 2006. pp. 669–683. [Google Scholar]
- 62.Sarzotti-Kelsoe M, Win CM, Parrott RE, Cooney M, Moser BK, Roberts JL, et al. Thymic output, T-cell diversity, and T-cell function in long-term human SCID chimeras. Blood. 2009;114:1445–53. doi: 10.1182/blood-2009-01-199323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Antoine C, Muller S, Cant A, Cavazzana-Calvo M, Veys P, Vossen J, et al. Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968–99. Lancet. 2003;361:553–60. doi: 10.1016/s0140-6736(03)12513-5. [DOI] [PubMed] [Google Scholar]
- 64.Grunebaum E, Mazzolari E, Porta F, Dallera D, Atkinson A, Reid B, et al. Bone marrow transplantation for severe combined immune deficiency. JAMA. 2006;295:508–18. doi: 10.1001/jama.295.5.508. [DOI] [PubMed] [Google Scholar]
- 65.Mazzolari E, Forino C, Guerci S, Imberti L, Lanfranchi A, Porta F, et al. Long-term immune reconstitution and clinical outcome after stem cell transplantation for severe T-cell immunodeficiency. J Allergy Clin Immunol. 2007;120:892–9. doi: 10.1016/j.jaci.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 66.Neven B, Leroy S, Decaluwe H, Le Deist F, Picard C, Moshous D, et al. Long-term outcome after haematopoietic stem cell transplantation of a single-centre cohort of 90 patients with severe combined immunodeficiency: Long-term outcome of HSCT in SCID. Blood. 2009;113(17):4114–24. doi: 10.1182/blood-2008-09-177923. [DOI] [PubMed] [Google Scholar]
- 67.Patel NC, Chinen J, Rosenblatt HM, Hanson IC, Brown BS, Paul ME, et al. Long-term outcomes of 48 patients with severe combined immunodeficiency transplanted in one center. J Allergy Clin Immunol. 2009 doi: 10.1016/j.jaci.2008.10.030. (In Press) [DOI] [PubMed] [Google Scholar]
- 68.Railey MD, Lokhnygina Y, Buckley RH. Long term clinical outcome of patients with severe combined immunodeficiency who received related donor bone marrow transplants without pre-transplant chemotherapy or post-transplant GVHD prophylaxis. Journal of Pediatrics. doi: 10.1016/j.jpeds.2009.07.049. Published online 10/9/2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wengler GS, Lanfranchi A, Frusca T, Verardi R, Neva A, Brugnoni D, et al. In-utero transplantation of parental CD34 haematopoietic progenitor cells in a patient with X-linked severe combined immunodeficiency (SCIDX1) Lancet. 1996;348:1484–7. doi: 10.1016/s0140-6736(96)09392-0. [DOI] [PubMed] [Google Scholar]
- 70.Flake AW, Roncarolo MG, Puck JM, Almeida-Porada G, Evans MI, Johnson MP, et al. Treatment of X-linked severe combined immunodeficiency by in utero transplantation of paternal bone marrow. N Engl J Med. 1996;335:1806–10. doi: 10.1056/NEJM199612123352404. [DOI] [PubMed] [Google Scholar]
- 71.Ozsahin H, Cavazzana-Calvo M, Notarangelo LD, Schulz A, Thrasher AJ, Mazzolari E, et al. Long-term outcome following hematopoietic stem-cell transplantation in Wiskott-Aldrich syndrome: collaborative study of the European Society for Immunodeficiencies and European Group for Blood and Marrow Transplantation. Blood. 2008;111(1):439–45. doi: 10.1182/blood-2007-03-076679. [DOI] [PubMed] [Google Scholar]
- 72.Filipovich AH, Stone JV, Tomany SC, Ireland M, Kollman C, Pelz CJ, et al. Impact of donor type on outcome of bone marrow transplantation for Wiskott-Aldrich syndrome: collaborative study of the International Bone Marrow Transplant Registry and the National Marrow Donor Program. Blood. 2001;97:1598–603. doi: 10.1182/blood.v97.6.1598. [DOI] [PubMed] [Google Scholar]
- 73.Klein C, Lisowska-Grospierre B, LeDeist F, Fischer A, Griscelli C. Major histocompatibility complex class II deficiency: clinical manifestations, immunologic features, and outcome. J Pediatr. 1993;123:921–8. doi: 10.1016/s0022-3476(05)80388-9. [DOI] [PubMed] [Google Scholar]
- 74.Klein C, Cavazzana-Calvo M, Le Deist F, Jabado N, Benkerrou M, Blanche S, et al. Bone marrow transplantation in major histocompatibility complex class II deficiency: a single center study of 19 patients. Blood. 1995;85:580–7. [PubMed] [Google Scholar]
- 75.Duplantier JE, Seyama K, Day NK, Hitchcock R, Nelson RP, Jr, Ochs HD, et al. Immunologic reconstitution following bone marrow transplantation for X-linked hyper IgM syndrome. Clin Immunol. 2001;98:313–865. doi: 10.1006/clim.2000.4994. [DOI] [PubMed] [Google Scholar]
- 76.Bertrand Y, Müller SM, Casanova JL, Morgan G, Fischer A, Friedrich W. Reticular dysgenesis: HLA non-identical bone marrow transplants in a series of 10 patients. Bone Marrow Transplant. 2002;29:759–62. doi: 10.1038/sj.bmt.1703531. [DOI] [PubMed] [Google Scholar]
- 77.Broome CB, Graham ML, Saulsbury FT, Hershfield MS, Buckley RH. Correction of purine nucleoside phosphorylase deficiency by transplantation of allogeneic bone marrow from a sibling. J Pediatr. 1996;128:373–6. doi: 10.1016/s0022-3476(96)70285-8. [DOI] [PubMed] [Google Scholar]
- 78.McGhee SA, Lloret MG, Stiehm ER. Immunologic reconstitution in 22q deletion (DiGeorge) syndrome. Immunol Res. 2009;45:37–45. doi: 10.1007/s12026-009-8108-7. [DOI] [PubMed] [Google Scholar]
- 79.Markert ML. Treatment of infants with complete DiGeorge anomaly. J Allergy Clin Immunol. 2008;121:1063. doi: 10.1016/j.jaci.2007.12.1181. [DOI] [PubMed] [Google Scholar]
- 80.Markert ML, Devlin BH, Alexieff MJ, Li J, McCarthy EA, Gupton SE, et al. Review of 54 patients with complete DiGeorge anomaly enrolled in protocols for thymus transplantation: outcome of 44 consecutive transplants. Blood. 2007;109:4539–47. doi: 10.1182/blood-2006-10-048652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van den Berg JM, van Koppen E, Ahlin A, Belohradsky BH, Bernatowska E, Corbeel L, et al. Chronic granulomatous disease: the European experience. PLoS One. 2009;4:e5234. doi: 10.1371/journal.pone.0005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qasim W, Cavazzana-Calvo M, Davies EG, Davis J, Duval M, Eames G, et al. Allogeneic hematopoietic stem-cell transplantation for leukocyte adhesion deficiency. Pediatrics. 2009;123:836–40. doi: 10.1542/peds.2008-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rowley S, Friedman TM, Korngold R. Hematopoietic stem cells transplantation for malignant diseases. In: Rich RR, editor. Clinical immunology. Principles and Practice. 3. Chapter 82. Elsevier; Philadephia: 2008. pp. 1223–1236. [Google Scholar]
- 84.Shipley JL, Butera JN. Acute myelogenous leukemia. Exp Hematol. 2009;37:649–58. doi: 10.1016/j.exphem.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 85.Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–61. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fernandez HF, Kharfan-Dabaja MA. Tyrosine kinase inhibitors and allogeneic hematopoietic cell transplantation for chronic myeloid leukemia: targeting both therapeutic modalities. Cancer Control. 2009;16:153–7. doi: 10.1177/107327480901600207. [DOI] [PubMed] [Google Scholar]
- 87.Sharathkumar A, Thornley I, Saunders EF, Calderwood S, Freedman MH, Doyle J. Allogeneic bone marrow transplantation in children with chronic myelogenous leukemia. J Pediatr Hematol Oncol. 2002;24:215–9. doi: 10.1097/00043426-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 88.Barrett AJ. Understanding and harnessing the graft-versus-leukaemia effect. Br J Haematol. 2008;142:877–88. doi: 10.1111/j.1365-2141.2008.07260.x. [DOI] [PubMed] [Google Scholar]
- 89.Goerner M, Weber-Nordt R, Hoepfner S, Benner A, Luft T, Ho AD. Addition of a low fixed number of CD3+ cells to CD34-enriched allografts: effects on engraftment, graft-versus-host disease, and survival after related and unrelated peripheral stem cell transplantation. J Hematother Stem Cell Res. 2003;12:309–20. doi: 10.1089/152581603322023043. [DOI] [PubMed] [Google Scholar]
- 90.Lucarelli G, Andreani M, Angelucci E. The cure of thalassemia by bone marrow transplantation. Blood Rev. 2002;16:81–5. doi: 10.1054/blre.2002.0192. [DOI] [PubMed] [Google Scholar]
- 91.Walters MC, Patience M, Leisenring W, Rogers ZR, Aquino VM, Buchanan GR, et al. Stable mixed hematopoietic chimerism after bone marrow transplantation for sickle cell anemia. Biol Blood Marrow Transplant. 2001;7:665–73. doi: 10.1053/bbmt.2001.v7.pm11787529. [DOI] [PubMed] [Google Scholar]
- 92.Gerritsen EJ, Vossen JM, Fasth A, Friedrich W, Morgan G, Padmos A, et al. Bone marrow transplantation for autosomal recessive osteopetrosis. J Pediatr. 1994;125:896–902. doi: 10.1016/s0022-3476(05)82004-9. [DOI] [PubMed] [Google Scholar]
- 93.Mazzolari E, Forino C, Razza A, Porta F, Villa A, Notarangelo LD. A single-center experience in 20 patients with infantile malignant osteopetrosis. Am J Hematol. 2009;84:473–9. doi: 10.1002/ajh.21447. [DOI] [PubMed] [Google Scholar]
- 94.Orchard PJ, Blazar BR, Wagner J, Charnas L, Krivit W, Tolar J. Hematopoietic cell therapy for metabolic disease. J Pediatr. 2007;151:340–6. doi: 10.1016/j.jpeds.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 95.Gratwohl A, Passweg J, Bocelli-Tyndall C, Fassas A, van Laar JM, Farge D, et al. Autologous hematopoietic stem cell transplantation for autoimmune diseases. Bone Marrow Transplant. 2005;35:869–79. doi: 10.1038/sj.bmt.1704892. [DOI] [PubMed] [Google Scholar]
- 96.Sandmaier BM, Mackinnon S, Childs RW. Reduced intensity conditioning for allogeneic hematopoietic cell transplantation: current perspectives. Biol Blood Marrow Transplant. 2007;13(1 Suppl 1):87–97. doi: 10.1016/j.bbmt.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cavazzana-Calvo M, Hacein-Bey S, deSaint Basile G, Gross F, Yvon E, Nusbaum P, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–72. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 98.Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346:1185–93. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- 99.Hacein-Bey-Albina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy for SCID-X1. J Clin Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gaspar HB, Parsley KL, Howe S, King D, Gilmour KC, Sinclair J, et al. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet. 2004;364:2181–7. doi: 10.1016/S0140-6736(04)17590-9. [DOI] [PubMed] [Google Scholar]
- 101.Rabbitts TH. LMO T-cell translocation oncogenes typify genes activated by chromosomal translocations that alter transcription and developmental processes. Genes Dev. 1998;12:2651–7. doi: 10.1101/gad.12.17.2651. [DOI] [PubMed] [Google Scholar]
- 102.Throm RE, Ouma AA, Zhou S, Chandrasekaran A, Lockey T, Greene M, et al. Efficient construction of producer cell lines for a SIN lentiviral vector for SCID-X1 gene therapy by concatemeric array transfection. Blood. 2009;113:5104–10. doi: 10.1182/blood-2008-11-191049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Onodera M, Nelson DM, Sakiyama Y, Candotti F, Blaese RM. Gene therapy for severe combined immunodeficiency caused by adenosine deaminase deficiency: improved retroviral vectors for clinical trials. Acta Haematol. 1999;101:89–96. doi: 10.1159/000040930. [DOI] [PubMed] [Google Scholar]
- 104.Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360:447–58. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- 105.Santilli G, Thornhill SI, Kinnon C, Thrasher AJ. Gene therapy of inherited immunodeficiencies. Expert Opin Biol Ther. 2008;8:397–407. doi: 10.1517/14712598.8.4.397. [DOI] [PubMed] [Google Scholar]
- 106.Kang EM, Malech HL. Advances in treatment for chronic granulomatous disease. Immunol Res. 2009;43(1–3):77–84. doi: 10.1007/s12026-008-8051-z. [DOI] [PubMed] [Google Scholar]
- 107.Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12(4):401–9. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]


