Abstract
Aims
To assess the independent and combined effects of diabetes and hypertension on left ventricular (LV) diastolic function in a community-based cohort at high cardiovascular risk.
Methods and results
Two-dimensional echocardiography was performed in 708 subjects from the Cardiac Abnormalities and Brain Lesions (CABL) study. Peak diastolic early (E) and late (A) transmitral flow, and tissue Doppler-derived early mitral annulus velocity (E′) were recorded, and E/A and E/E′ ratios were calculated. The population was divided into four groups: those without hypertension or diabetes (HT−/DM−), those with only hypertension (HT), only diabetes (DM), and with hypertension plus diabetes (HT + DM). In multivariate analysis, hypertension and diabetes were independent predictors of worse diastolic function. The coexistence of hypertension and diabetes was associated with greater impairment of diastolic function (higher E/E′ ratio than HT−/DM−, HT, or DM, all P < 0.05), independent of covariates. The negative, synergistic effect of hypertension and diabetes on LV diastolic function was present both in lean participants and in overweight/obese ones. An E/E′ ratio >15, suggestive of increased LV filling pressure, was found in 2.2% of HT−/DM−, 8.9% of HT, 5.9% of DM, and 14.7% of HT + DM (P < 0.01).
Conclusion
Hypertension and diabetes are independently associated with impaired LV diastolic function, independent of the effect of overweight/obesity and other covariates. Their coexistence results in a negative synergistic effect on LV diastolic mechanics and is associated with higher LV filling pressures than either condition alone.
Keywords: Diabetes, Hypertension, Diastolic function, Echocardiography, Tissue Doppler
Introduction
Diastolic dysfunction is associated with future occurrence of heart failure, is a predictor of cardiovascular morbidity and mortality in the general population,1,2 and is associated with a reduced exercise performance in asymptomatic subjects.3 Diabetes mellitus is associated with high morbidity and mortality, mainly because of its association with arterial atherosclerosis and related complications, in particular coronary artery disease and congestive heart failure.4–7 Several reports have shown that impairment of left ventricular (LV) diastolic function can be present in subjects with diabetes even in the absence of alterations of LV systolic function.8–11 However, especially in the elderly, diabetes is often associated with arterial hypertension,6 which is in turn associated impaired diastolic dysfunction and unfavourable cardiovascular outcome.12–14 Also, hypertension and diabetes often share comorbidities and conditions, like obesity and LV hypertrophy, that can impact LV structure and mechanics.15 Therefore, it is difficult to quantify the individual and synergistic roles of diabetes and hypertension on LV diastolic function. So far, studies that have investigated this association have relied on echocardiographic Doppler flow methods that cannot distinguish between normal and pseudo-normal LV diastolic patterns, or did not take into account important variables that affect diastolic function, such as left ventricular mass (LVM) and geometry, and heart rate. A pseudo-normal diastolic function pattern has been demonstrated in up to 28% of asymptomatic patients with diabetes9,16 and in 27% of newly diagnosed hypertensive patients.17
Tissue Doppler imaging (TDI) is a newer echocardiographic tool that has been proved more sensitive in the early detection of LV diastolic function alterations than traditional Doppler flow methods.18 In particular, the combination of transmitral flow with TDI-derived diastolic phase parameters provides an estimation of LV diastolic performance that is less dependent on cardiac preload, and, unlike transmitral flow, is not affected by pseudo-normalization.19,20
The aim of this study was to assess, in a high-risk community sample without overt cardiac disease and with normal LV systolic function, the individual and combined effects of diabetes and hypertension on LV diastolic function, as evaluated by transmitral flow and TDI parameters.
Methods
Study population
The study cohort of the Cardiac Abnormalities and Brain Lesions (CABL) study was derived from the Northern Manhattan Study (NOMAS), an epidemiological study that evaluates the incidence, risk factors, and clinical outcome of stroke in the multiethnic population of Northern Manhattan. The study design and methodological details regarding NOMAS have been described previously.21 Briefly, community subjects from Northern Manhattan were eligible if they: (i) had never been diagnosed with a stroke, (ii) were aged 40 or older, and (iii) resided in Northern Manhattan for at least 3 months in a household with telephone. NOMAS subjects over age 50 that voluntarily agreed to undergo a brain MRI study and a more extensive echocardiographic evaluation including assessment of diastolic function were included in the CABL study. This subset of individuals constitutes the study population of the present report. Informed consent was obtained from all study participants. The study was approved by the Institutional Review Board of Columbia University Medical Center.
Risk factors assessment
Cardiovascular risk factors were ascertained through direct examination and interview by trained research assistants. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg at the time of the visit (mean of two readings), or patient's self-reported history of hypertension or of anti-hypertensive medications. Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dL or patient's self-reported history of diabetes or of diabetes medications. Hypercholesterolaemia was defined as total serum cholesterol >240 mg/dL, a patient's self-report of hypercholesterolaemia or of use of lipid-lowering treatment. Subjects with coronary artery disease, defined as a history of myocardial infarction, coronary artery bypass grafting, or percutaneous coronary intervention, were excluded from the study.
Echocardiographic assessment
Transthoracic echocardiography evaluation was performed using a commercially available system (iE 33, Philips, Andover, MA, USA) by a trained registered sonographer according to a standardized protocol. Left ventricular end-diastolic (LVDD) and end-systolic (LVSD) diameters, inter-ventricular septum thickness, and posterior wall thickness (PWT) were measured at end-diastole from a parasternal long-axis view according to the recommendations of the American Society of Echocardiography (ASE).22 Left ventricular mass, calculated with the Devereaux formula,23 and left atrial (LA) antero-posterior diameter were indexed by body surface area to account for the effect of body size. Left ventricular relative wall thickness (RWT), an index of LV geometry, was calculated with the formula: (2 × PWT)/LVDD.24 Left ventricular ejection fraction (LVEF) was calculated by the biplane modified Simpson's rule as recommended by the ASE,22 this was replaced by a semi-quantitative method or visual estimation in case of technically suboptimal images. Subjects with an EF ≤ 50% or with LV segmental wall motion abnormalities were excluded from analysis.
Transmitral diastolic flow was obtained by pulsed-wave Doppler from an apical four-chamber view. Colour Doppler was used to visualize the transmitral flow; the pulsed Doppler sample volume was placed at the level of mitral valve leaflet tips, with the ultrasonic beam perpendicular to the inflow jet. Doppler baseline and velocity scale were adjusted to obtain optimal visualization of the inflow spectrum. At least four cardiac cycles were recorded during patient apnoea, and the images were stored in digital format for off-line analysis. Peak velocities of the early (E-wave) and late (A-wave) phase of the mitral inflow pattern from Doppler recordings were measured and their ratio (E/A) was calculated. Left ventricular myocardial velocities were evaluated by pulsed TDI and sampled on the longitudinal axis from the apical four-chamber view. Two-dimensionally guided pulsed TDI sample volume was placed at the level of the lateral and septal mitral valve annulus, Doppler gain and wall filter were adjusted to reduce artefacts, and velocity scale was set to ±20 cm/s. Four consecutive beats were recorded at a sweep rate of 100 mm/s during patient apnoea and stored in digital format for off-line analysis. The peak systolic (S) and peak early diastolic (E′) velocities of the lateral and septal mitral annulus by pulsed-TDI were measured and the average value was calculated and used in all subsequent analyses.25 The ratio between the E and the E′ wave (E/E′) was calculated as a pre-load independent index of LV filling pressures. Diastolic dysfunction was defined, according to ASE guidelines25 and taking into consideration the mean age of our population, as: (i) E/A ratio ≤0.7 (impaired relaxation); (ii) E/A ratio >0.7 and ≤1.5 and E′ velocity <7 cm/s (pseudo-normalized pattern); or (iii) E/A ratio >1.5 and E′ velocity <7 cm/s (restrictive pattern).
Statistical analysis
Data are presented as mean ± standard deviation for continuous variables and as proportions for categorical variables. The χ2 test was used to test differences between proportions. Multiple linear regression were performed to assess the independent association of hypertension and diabetes with diastolic function parameters. Hypertension and diabetes were entered together in the models (see Results section). Differences between hypertension/diabetes groups were assessed by one-way analysis of variance. The Kruskal–Wallis non-parametric test was applied to confirm statistical findings when the normality assumptions were not met. Analysis of covariance was conducted separately for each diastolic function parameter to assess differences between groups after adjustment for covariates. Estimated marginal means adjusted for covariates and 95% confidence intervals were derived. Partial η2 was used as an effect size indicator to estimate the contribution of each factor to the overall model.
For all statistical analyses, a two-tailed P < 0.05 was considered significant. Statistical analyses were performed using SAS software version 9.1 (SAS Institute, Inc., Cary, NC, USA).
Results
Population characteristics
General characteristics of the study cohort are shown in Table 1. Our study cohort consisted of 708 subjects, mean age was 70.4 ± 9.6 years, and 450 subjects (63.6%) were women. Hypertension was present in 496 (70.1%) and diabetes in 204 (28.8%). According to the inclusion criteria, intended to avoid the possible interference of associated conditions on the assessment of diastolic parameters, no subjects had an LVEF <50% or coronary artery disease.
Table 1.
Demographic and clinical variables in the overall sample and according to the presence of hypertension and/or diabetes
| Overall sample (n = 708) | HT−/DM− (n = 178) | HT (n = 326) | DM (n = 34) | HT + DM (n = 170) | |
|---|---|---|---|---|---|
| Age (year) | 70.4 ± 9.6 | 67.3 ± 9.8 | 71.6 ± 10.0** | 70.6 ± 10.7 | 71.2 ± 7.5** |
| Women, n (%) | 450 (63.6) | 100 (56.2) | 214 (65.6)* | 22 (64.7) | 114 (67.1)* |
| Body mass index (kg/m2) | 28.2 ± 4.9 | 26.3 ± 4.7 | 28.3 ± 4.5** | 27.8 ± 4.8 | 30.2 ± 5.1**‡§§ |
| SBP (mmHg) | 135.2 ± 7.6 | 122.1 ± 11.8 | 140.7 ± 17.1** | 124.2 ± 10.4‡ | 140.6 ± 16.7**§§ |
| DBP (mmHg) | 78.1 ± 9.5 | 74.0 ± 7.8 | 80.6 ± 9.6** | 74.4 ± 8.4‡ | 78.3 ± 9.6**‡§ |
| Blood glucose (mg/dL) | 99.7 ± 32.5 | 87.0 ± 12.2 | 89.8 ± 12.0 | 132.1 ± 51.4**‡ | 124.5 ± 47.1**‡ |
| Creatinine (mg/dL) | 0.92 ± 0.26 | 0.88 ± 0.18 | 0.94 ± 0.26* | 0.88 ± 0.30 | 0.96 ± 0.33** |
| Hypercholesterolaemia, n (%) | 419 (59.3) | 98 (55.1) | 187 (57.5) | 15 (44.1) | 119 (70.0)**त |
| Heart rate (b.p.m.) | 70.3 ± 11.4 | 68.0 ± 10.7 | 70.6 ± 11.2* | 68.8 ± 10.4 | 72.3 ± 12.2** |
| LVEDD (cm/m2) | 2.55 ± 0.30 | 2.57 ± 0.31 | 2.55 ± 0.31 | 2.50 ± 0.23 | 2.53 ± 0.28 |
| LVMI (g/m2) | 102.7 ± 25.0 | 95.2 ± 22.2 | 105.0 ± 25.9** | 93.5 ± 20.2‡ | 108.1 ± 25.3**§§ |
| Relative wall thickness | 0.50 ± 0.09 | 0.47 ± 0.07 | 0.51 ± 0.09** | 0.49 ± 0.07 | 0.52 ± 0.08** |
| LVEF (%) | 64.3 ± 5.3 | 63.5 ± 4.7 | 64.5 ± 5.5 | 63.7 ± 5.2 | 65.0 ± 5.2** |
| S velocity (cm/s) | 8.7 ± 1.9 | 9.0 ± 1.7 | 8.6 ± 1.9** | 8.4 ± 1.9 | 8.6 ± 2.0 |
| Stroke index (mL/m2) | 35.0 ± 7.9 | 35.3 ± 8.2 | 34.9 ± 7.9 | 34.2 ± 8.2 | 34.9 ± 7.7 |
| LA size (mm/m2) | 22.2 ± 3.2 | 21.1 ± 2.8 | 22.6 ± 3.3** | 22.3 ± 3.0* | 22.6 ± 3.1** |
HT−/DM−, absence of hypertension and diabetes; HT, hypertension only; DM, diabetes only; HT + DM, hypertension and diabetes; SBP, systolic blood pressure; DBP, diastolic blood pressure; LVEDD, LV end-diastolic diameter; LVMI, LV mass index; LVEF, LV ejection fraction; LA, left atrial.
*P < 0.05 and **P < 0.01 vs. HT−/DM−.
§P < 0.05 and §§P < 0.01 vs. DM.
To test the individual and combined effects of diabetes and hypertension on diastolic function, we divided the population into four groups: those who had neither hypertension nor diabetes (HT−/DM−), those with hypertension but not diabetes (HT), those with diabetes but not hypertension (DM), and those with both hypertension and diabetes (HT + DM). Clinical and echocardiographic characteristics of the four groups are shown in Table 1. Age, BMI, creatinine, heart rate, and LV RWT were higher in HT and HT + DM than in HT−/DM−. Left ventricular mass index was higher in HT and HT + DM than in DM and HT−/DM−. S velocity was lower in HT than in HT−/DM−, and LVEF was higher in HT + DM than in HT−/DM−. Prevalence of hypercholesterolaemia was higher in HT + DM than in the other groups. Left atrial size was significantly higher in HT, DM, and HT + DM than in HT−/DM−.
Correlations of hypertension and diabetes with diastolic function parameters
The independent association between diastolic function parameters and diabetes and hypertension were tested in three models (Table 2): (i) a model only including hypertension and diabetes; (ii) a model adjusted for age, sex, and body mass index (BMI), and (iii) a model adjusted for clinical and echocardiographic covariates. In the final multivariate model (Table 2, Model 3), hypertension was an independent predictor of lower E′-wave [B = −0.60, standard error (SE) 0.13] and higher E/E′ ratio (B = 0.70, SE 0.24; both P < 0.01). In the same model, diabetes was an independent predictor of higher E-wave (B = 4.00, SE 1.40, P < 0.05), higher A-wave (B = 5.64, SE 1.57, P < 0.01), and higher E/E′ ratio (B = 0.79, SE 0.24, P < 0.01).
Table 2.
Association of diabetes and hypertension with diastolic function parameters
| E-wave | A-wave | E/A ratio | E′-wave | E/E′ ratio | |
|---|---|---|---|---|---|
| Model 1 | R2 = 0.01 | R2 = 0.06 | R2 = 0.02 | R2 = 0.10 | R2 = 0.08 |
| Hypertension | −1.36 (1.38) | 6.43 (1.62)** | −0.07 (0.02)** | −1.15 (0.14)** | 1.44 (0.25)** |
| Diabetes | 4.10 (1.40)** | 7.62 (1.63)** | −0.02 (0.02) | −0.20 (0.14) | 0.97 (0.26)** |
| Model 2 | R2 = 0.03 | R2 = 0.17 | R2 = 0.06 | R2 = 0.27 | R2 = 0.18 |
| Hypertension | −1.85 (1.43) | 2.59 (1.58) | −0.04 (0.02) | −0.79 (0.13)** | 0.89 (0.25)** |
| Diabetes | 3.66 (1.41)* | 6.17 (1.56)** | −0.01 (0.02) | −0.10 (0.13) | 0.81 (0.25)** |
| Model 3 | R2 = 0.13 | R2 = 0.23 | R2 = 0.14 | R2 = 0.36 | R2 = 0.29 |
| Hypertension | −1.05 (1.45) | 1.13 (1.63) | −0.02 (0.03) | −0.60 (0.13)** | 0.70 (0.24)** |
| Diabetes | 4.00 (1.40)* | 5.64 (1.57)** | −0.005 (0.02) | −0.07 (0.13) | 0.79 (0.24)** |
Values are unstandardized estimate coefficients (B) and relative standard errors. Model 1: univariate. Model 2: adjusted for age, sex, and BMI. Model 3: adjusted for age, BMI, sex, heart rate, LVEF, S velocity, LVMI, relative wall thickness, creatinine, hypercholesterolaemia.
*P < 0.05; **P < 0.01.
Blood pressure, blood glucose, and left ventricular diastolic function
Partial correlations, accounting for age, were assessed between systolic blood pressure, diastolic blood pressure, blood glucose, and diastolic function parameters (Table 3). Systolic blood pressure was significantly correlated to A-wave, E/A ratio, E,′ and E/E′ ratio (all P < 0.01); diastolic blood pressure showed significant correlations with E-wave, A-wave, E′ velocity (all P < 0.01), and E/A ratio (P < 0.05). Blood glucose was significantly correlated to A-wave, E′ velocity (both P < 0.01), and E/E′ ratio (P < 0.05).
Table 3.
Partial correlations of systolic blood pressure, diastolic blood pressure, and blood glucose with diastolic function parameters
| E-wave | A-wave | E/A ratio | E′-wave | E/E′ ratio | |
|---|---|---|---|---|---|
| Systolic BP | 0.03 | 0.17** | −0.10** | −0.19** | 0.16** |
| Diastolic BP | −0.15** | 0.12** | −0.22* | −0.24** | 0.05 |
| Blood glucose | 0.04 | 0.16** | −0.06 | −0.10** | 0.12* |
Values are partial correlation coefficients adjusted for age.
*P < 0.05; **P < 0.01.
Separate and combined effect of hypertension and diabetes on left ventricular diastolic function
Diastolic parameters were compared between the four groups after adjusting for covariates (Table 4). The HT group had significantly lower E′ velocity (P < 0.01) and higher E/E′ ratio (P < 0.05) compared with HT−/DM−. DM subjects had a significantly higher A-wave (P < 0.05) and a non-significant trend towards a lower E/A ratio, lower E′ and higher E/E′ than HT−/DM−. No significant differences were observed between HT and DM groups in any of the diastolic function parameters. The HT + DM group showed a significantly higher E/E′ ratio compared with the HT−/DM−, HT (both P < 0.01), and DM (P < 0.05) groups. HT + DM also had significantly higher A-wave and lower E′ than HT−/DM− (both P < 0.01), and higher E- and A-waves than HT (both P < 0.01). Partial η2 was 0.072 for age, 0.021 for sex, 0.018 for heart rate, 0.010 for BMI, 0.011 for LVM, 0.004 for RWT, and 0.036 for the combination of hypertension and diabetes.
Table 4.
Adjusted means and 95% confidence intervals of diastolic function parameters by the presence of hypertension and/or diabetes
| HT−/DM− | HT | DM | HT + DM | |
|---|---|---|---|---|
| E-wave (cm/s) | 70.9 (68.2–73.5) | 68.7 (66.9–70.6) | 71.3 (65.5–77.0) | 73.7 (71.1–76.4)‡ |
| A-wave (cm/s) | 85.6 (82.7–88.6) | 86.8 (84.7–88.9) | 92.7 (86.3–99.1)* | 92.6 (89.6–95.6)**‡ |
| E/A ratio | 0.86 (0.82–0.91) | 0.83 (0.79–0.86) | 0.79 (0.69–0.89) | 0.84 (0.79–0.89) |
| E′-wave (cm/s) | 7.9 (7.6–8.1) | 7.2 (7.0–7.4)** | 7.6 (7.0–8.1) | 7.2 (6.9–7.4)** |
| E/E′ ratio | 9.6 (9.1–10.0) | 10.2 (9.8–10.5)* | 10.0 (9.1–11.0) | 11.1 (10.7–11.6) **‡§ |
HT−/DM−, absence of hypertension and diabetes; HT, hypertension only; DM, diabetes only; HT + DM, hypertension and diabetes. Standard set of covariates: age, BMI, sex, LV mass index, LV relative wall thickness, LV ejection fraction, and heart rate.
*P < 0.05 and **P < 0.01 vs. HT−/DM−.
‡P < 0.01 vs. HT.
§P < 0.05.
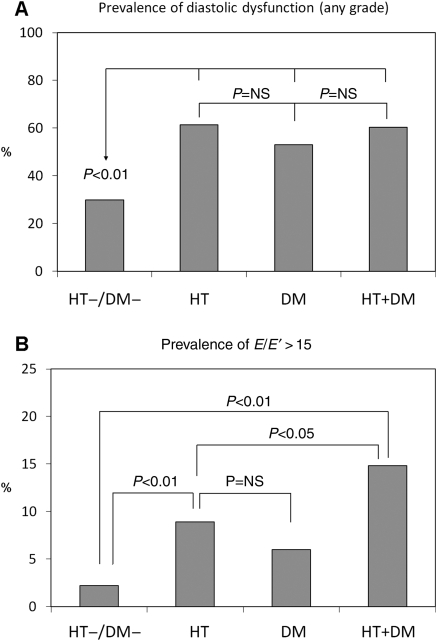
Prevalence of diastolic dysfunction of any grade, in the entire study population was 52.8%. HT, DM, and HT + DM had significantly higher prevalence of diastolic dysfunction than HT−/DM− (61.4% in HT, 53.0% in DM, 60.2% in HT + DM vs. 30.1% in HT−/DM−; all P < 0.01 vs. HT−/DM−). The differences between HT, DM, and HT + DM were not statistically significant (Figure 1A). Of note, the prevalence of a pseudo-normal filling pattern, which would not have been unmasked without the use of TDI, was 20.7% in HT, 14.7% in DM, and 20.5% in HT + DM, but only 8.5% in HT−/DM− (P < 0.01). An E/E′ ratio >15, suggestive of increased LV filling pressure, was found in 2.2% of HT−/DM−, 8.9% of HT, 5.9% of DM, and 14.7% of HT + DM (P < 0.01 for HT + DM and HT vs. HT−/DM−; P < 0.05 for HT + DM vs. HT, Figure 1B).
Figure 1.
Prevalence of LV diastolic dysfunction of any grade (A) and of an E/E′ ratio >15 (B) according to the presence of hypertension, diabetes, or both. HT−/DM−: absence of hypertension and diabetes; HT: hypertension only; DM: diabetes only; HT + DM: hypertension and diabetes.
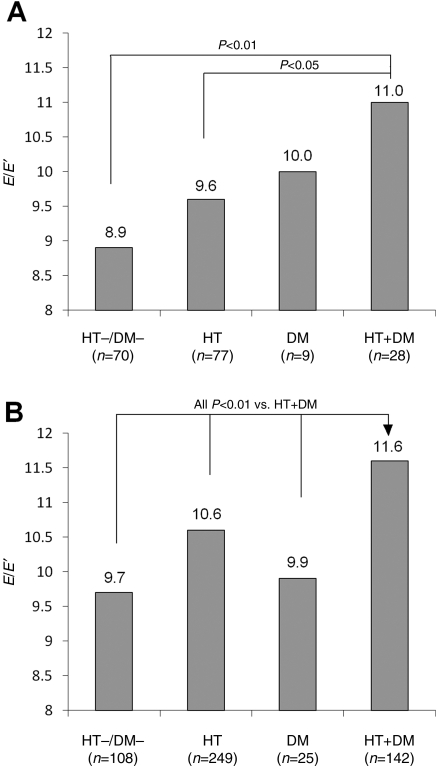
In a sub-analysis aimed to evaluate the impact of hypertension and diabetes on the E/E′ ratio separately in lean patients and in overweight/obese patients, hypertensives and diabetics were stratified according to a BMI< or ≥25 kg/m2. The analysis was adjusted for age, gender, and heart rate. As can be seen in Figure 2, within the lean patients (Figure 2A), E/E′ ratio was higher in the HT + DM group (11.0, SE 0.52) than in HT (9.6, SE 0.32, P = 0.03), DM (10.0, SE 0.93, P = 0.38), and HT−/DM− (8.9, SE 0.34, P = 0.001). Similarly, in the overweight/obese patients (Figure 2B), E/E′ was significantly higher in HT + DM (11.6, SE 0.24) than in HT (10.6, SE 0.18, P = 0.002), DM (9.9, SE 0.55, P = 0.006), and HT−/DM− (9.7, SE 0.28, P < 0.001).
Figure 2.
E/E′ ratio (adjusted for age, gender, and heart rate) according to the presence of hypertension, diabetes, or both in patients with body mass index <25 kg/m2 (A) or ≥25 kg/m2 (B). HT−/DM−: absence of hypertension and diabetes; HT: hypertension only; DM: diabetes only; HT + DM: hypertension and diabetes.
Discussion
Diastolic dysfunction is a risk factor for the development of congestive heart failure, and has prognostic value in population settings.1,26 In our study, we demonstrated that in a community-based unselected cohort without overt cardiac disease, diabetes, and hypertension have an independent negative impact on LV diastolic function. We showed that diabetes and hypertension were independently associated with a higher E/E′ ratio, an index of LV end-diastolic pressure, even after adjustment for covariates that significantly affect diastolic function such as age, LVM, geometry, heart rate, and LVEF. The finding of a higher LV end-diastolic pressure when diabetes and hypertension coexist, compared with either condition alone, could explain in part the additional risk of developing heart failure in patients with combined diabetes and hypertension compared with patients with hypertension alone.5,7 In this respect, the exclusion, from our study, of subjects with evidence of coronary artery disease or with abnormal LV systolic function allowed us to derive important information on the effect of the two most prevalent cardiovascular risk factors on diastolic function at early stages of disease (Stages A and B of the American Heart Association classification of heart failure development). To better separate the effect of diabetes and hypertension, as well as to assess their combined effect, we evaluated the diastolic function in groups with only diabetes, only hypertension, or both, compared with subjects without either condition. The prevalence of diastolic dysfunction was higher in HT, DM, and HT + DM than in HT−/DM−. When we considered the proportion of subjects with elevated LV end-diastolic pressure (identified by an E/E′ ratio >15), we found it to be significantly greater in the HT + DM group (14.7%) than in HT and DM groups (8.9 and 5.9%, respectively). As expected, the HT + DM group was older and had higher BMI, whereas the HT and DM groups were in an intermediate position between HT + DM and HT−/DM−. Interestingly, LVM index was higher in the groups with hypertension, but was not significantly different between HT−/DM− and DM groups. This finding is consistent with previous studies that reported no increase in LVM in diabetic compared with non-diabetic subjects when the effect of hypertension is removed.18,27–29 After adjustment for all the covariates, we found that the group with both hypertension and diabetes had significantly worse diastolic function than the others. E′ velocity was lower in HT + DM than in HT−/DM−, but not significantly different from HT and DM, whereas the E/E′ ratio was significantly higher in HT + DM than in HT and DM, suggesting an additive effect of hypertension and diabetes on the LV end-diastolic pressure independent of other covariates. However, although the E/A ratio showed a trend toward lower values in patients with hypertension and/or diabetes, no significant differences were found among the four groups. This observation may appear surprising, but it is consistent with the pathophysiology of the LV diastolic dynamics. The E/A ratio is strongly dependent on cardiac load, and follows a U-shaped curve in the natural history of LV diastolic dysfunction, with a reduction in the earlier stages of dysfunction. In this stage, the relaxation of the LV is delayed and the early diastolic flow (E-wave) becomes slower; therefore, as a compensatory mechanism, the atrial contribution to the LV filling increases, and the E/A ratio decreases (diastolic dysfunction, Stage 1). With the progression of diastolic dysfunction, the increasing pressure gradient between the left atrium and the LV acts as a propelling force, causing an increase in the E-wave velocity. As a consequence, the E/A ratio increases as well, becoming indistinguishable from a normal flow pattern. In this regard, the analysis of other Doppler flow-derived parameters (mitral isovolumic relaxation time and E-wave deceleration time) that are load-dependent is not useful for identifying the apparent reversal of the mitral inflow to a normal pattern30,31 (also known as ‘pseudo-normalization’ of the mitral inflow) as actual progression of the disease (diastolic dysfunction, Stage 2). The mitral annulus early diastolic velocity assessed by pulsed TDI (E′) has been shown to be less pre-load dependent, and its decrease with the progressive impairment of the LV diastolic mechanics allows the identification of LV abnormal relaxation and reduced LV compliance, and can identify diastolic dysfunction even in the presence of a pseudo-normal mitral flow.32 In fact, the combination of flow and tissue Doppler analysis allowed us to detect subjects with impaired LV relaxation and increased LV stiffness who would have been considered normal by simple transmitral flow analysis in a relevant proportion of hypertensive and diabetic patients (20.7, 14.7%) and in the control group (8.5%). These proportions are comparable to those reported in other studies that used different LV diastolic parameters in similar populations.9,17,18
In elderly populations with high cardiovascular risk profile, several cofactors are often associated to both conditions. In particular, obesity is strictly linked to both high blood pressure and insulin-resistance and diabetes. We were able to demonstrate that the synergistic effect of hypertension and diabetes on LV diastolic function is present in both lean and overweight/obese patients. We also demonstrated that a BMI ≥25 kg/m2 was associated with worse diastolic function, even in the HT−/DM− group. Therefore, our data confirm the negative effect of increased BMI on LV diastolic mechanics,33,34 but also show that the impact of hypertension and diabetes on LV diastole recognizes disease-specific mechanisms that go beyond the simple effect of obesity. These findings suggest several considerations. First, it is known that diabetes is associated with changes in myocardial metabolism and structure that contribute to diastolic abnormalities. Multiple metabolic alterations have been identified that contribute to diabetic cardiomyopathy. In diabetic hearts, glucose metabolism is reduced in favour of energy production from beta-oxidation of free fatty acids.35,36 The altered glucose metabolism results in an accumulation of toxic intermediates that affect calcium handling, promote apoptosis, and affect myocardial mechanics.37,38 Hyperglycaemia has also been linked to the activation of the renin–angiotensin system, which in turn is responsible for increased oxidative damage and cell apoptosis and necrosis, resulting in increased interstitial fibrosis.39 In addition, an increased resting tension of the cardiomyocytes has been documented in diabetic patients with diastolic heart failure as a prominent cause of increased LV stiffness.40 Therefore, it is reasonable, to hypothesize that the myocardial damage secondary to diabetes may add to the hypertensive cardiac disease, resulting in a more severe impairment of LV diastolic function. In autopsy studies, interstitial and perivascular fibrosis was more represented in diabetic than in hypertensive hearts, and it was greatest in hypertensive-diabetic hearts.41 Second, the observation that the negative effect of diabetes and hypertension on LV diastole is independent of LVM has therapeutic implications. Recent data from the VALIDD (Valsartan in Diastolic Dysfunction) study showed that, in patients with hypertension, a better control of blood pressure resulted in an improvement of diastolic function regardless of the type of anti-hypertensive drug used, even in the absence of LV hypertrophy.42 On the other hand, despite the good correlation found in our and other studies between glucose and LV diastolic parameters, it is not clear whether tight glycaemic control would exert beneficial effects on LV diastolic function in diabetic patients. After some encouraging results from preliminary experiences,43 negative data were recently reported from the DADD (Diabetes mellitus And Diastolic Dysfunction) study, in which the authors failed to demonstrate an improvement in diastolic function in diabetic patients under strict insulin-based glycaemic control.44 Given these combined observations, the therapeutic goals of LVM reduction and glycaemic control may not be enough to obtain improvement in diastolic function when diabetes and hypertension coexist.
Surprisingly, our finding of a decreased diastolic function when hypertension and diabetes coexisted was not associated with a parallel increase in LA size in the HT + DM group. Left atrial size is related to LV diastolic function and is considered to be an indicator of a chronic exposure to elevated LV filling pressures.45 However, as the main determinant of LA size is the body size, and given the elevated mean BMI in the HT, DM, and HT + DM groups, a significant overlap in the distribution of the LA size exists in those groups that may have prevented the detection of disease-specific effects on LA diameter. Moreover, it is known that LA antero-posterior diameter, although prognostically relevant in the population, is not an accurate measure of LA size, because the expansion of the LA in the antero-posterior axis is limited by the anatomical constraints of the spine and sternum.46 Therefore, atrial enlargements over the latero-lateral and cranio-caudal axes are not identified by the LA antero-posterior diameter. In this regard, the evaluation of LA volumes might be more sensitive in detecting smaller differences in LA size than the antero-posterior dimension.
Previous studies have investigated the relations of LV diastolic function with diabetes and hypertension. These studies, however, used older criteria for the definition of diabetes,47,48 or did not use TDI for the assessment of diastolic function,47 or did not adjust the comparisons for important covariates such as LVM.18,28 A report from the Strong Heart Study showed that the combination of hypertension and diabetes was associated with greater impairment of diastolic relaxation after adjusting for covariates such as age and LVM.47 That study, however, used transmitral flow parameters only, which could not distinguish a pseudo-normal pattern from truly normal LV diastolic function, resulting in an underestimation of the prevalence of diastolic dysfunction. In fact, a pseudo-normal diastolic filling pattern has been observed in a significant proportion of asymptomatic hypertensive and diabetic subjects (up to 27 and 28%, respectively).9,16,17 Another study showed that in hypertensive patients the presence of diabetes was associated with worse diastolic function, but the lack of a control group without hypertension and the high prevalence of coronary artery disease made the results difficult to interpret.29
Our study has several limitations. The population of the study is at high cardiovascular risk because of the high prevalence of diabetes and hypertension. Therefore, the results of our study should not be extrapolated to different populations with lower risk profiles. Because of the high prevalence of hypertension in the cohort, the group with diabetes alone was small, and this could have prevented the detection of potentially significant differences in the pair-wise comparisons between DM and HT−/DM−. Finally, we did not perform measurement of mitral valve deceleration time, pulmonary venous flow velocities, and Valsalva manoeuvre for the diastolic function evaluation. However, the combination of traditional flow Doppler with tissue Doppler data is considered adequate in the assessment of diastolic function in large cohort studies, and in our study it was able to detect a pseudonormalized transmitral flow in a significant number of patients.
In conclusion, our study demonstrates that hypertension and diabetes have an independent negative impact on LV diastolic function that goes beyond the effect of an increased body size and is independent of other covariates possibly affecting LV diastolic function. The combination of hypertension and diabetes exerts a synergistic effect on the parameters of LV diastolic function, and results in higher LV filling pressure than either condition alone.
Funding
This work was supported by the National Institute of Neurological Disorders and Stroke (NINDS), part of the National Institutes of Health [grant number R01 NS36286 to M.R.D.T. and NS29993 to R.L.S./M.S.V.E.].
Conflict of interest: none declared.
Acknowledgements
The authors wish to thank Janet De Rosa, MPH, for the coordination of the study activities; Rui Liu, MD and Michele Alegre, RDCS, for the performance and preliminary interpretation of the echocardiographic studies; Rafael Cabral, MD and Palma Gervasi-Franklin for their help in the collection and management of the data.
References
- 1.Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2001;37:1042–1048. doi: 10.1016/s0735-1097(01)01110-x. [DOI] [PubMed] [Google Scholar]
- 2.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. J Am Med Assoc. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 3.Palmieri V, Russo C, Palmieri EA, Arezzi E, Pezzullo S, Minichiello S, Martino S, Migliaresi P, Celentano A. Isolated left ventricular diastolic dysfunction: implications for exercise left ventricular performance in patients without congestive heart failure. J Am Soc Echocardiogr. 2006;19:491–498. doi: 10.1016/j.echo.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Garcia MJ, McNamara PM, Gordon T, Kannel WB. Morbidity and mortality in diabetics in the Framingham population. Sixteen year follow-up study. Diabetes. 1974;23:105–111. doi: 10.2337/diab.23.2.105. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 6.Grossman E, Messerli FH. Diabetic and hypertensive heart disease. Ann Intern Med. 1996;125:304–310. doi: 10.7326/0003-4819-125-4-199608150-00009. [DOI] [PubMed] [Google Scholar]
- 7.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 8.Celentano A, Vaccaro O, Tammaro P, Galderisi M, Crivaro M, Oliviero M, Imperatore G, Palmieri V, Iovino V, Riccardi G. Early abnormalities of cardiac function in non-insulin-dependent diabetes mellitus and impaired glucose tolerance. Am J Cardiol. 1995;76:1173–1176. doi: 10.1016/s0002-9149(99)80330-0. [DOI] [PubMed] [Google Scholar]
- 9.Zabalgoitia M, Ismaeil MF, Anderson L, Maklady FA. Prevalence of diastolic dysfunction in normotensive, asymptomatic patients with well-controlled type 2 diabetes mellitus. Am J Cardiol. 2001;87:320–323. doi: 10.1016/s0002-9149(00)01366-7. [DOI] [PubMed] [Google Scholar]
- 10.Boyer JK, Thanigaraj S, Schechtman KB, Perez JE. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol. 2004;93:870–875. doi: 10.1016/j.amjcard.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 11.Fang ZY, Schull-Meade R, Leano R, Mottram PM, Prins JB, Marwick TH. Screening for heart disease in diabetic subjects. Am Heart J. 2005;149:349–354. doi: 10.1016/j.ahj.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. J Am Med Assoc. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 13.Fouad FM, Slominski JM, Tarazi RC. Left ventricular diastolic function in hypertension: relation to left ventricular mass and systolic function. J Am Coll Cardiol. 1984;3:1500–1506. doi: 10.1016/s0735-1097(84)80289-2. [DOI] [PubMed] [Google Scholar]
- 14.Inouye I, Massie B, Loge D, Topic N, Silverstein D, Simpson P, Tubau J. Abnormal left ventricular filling: an early finding in mild to moderate systemic hypertension. Am J Cardiol. 1984;53:120–126. doi: 10.1016/0002-9149(84)90695-7. [DOI] [PubMed] [Google Scholar]
- 15.Palmieri V, Bella JN, Arnett DK, Liu JE, Oberman A, Schuck MY, Kitzman DW, Hopkins PN, Morgan D, Rao DC, Devereux RB. Effect of type 2 diabetes mellitus on left ventricular geometry and systolic function in hypertensive subjects: Hypertension Genetic Epidemiology Network (HyperGEN) Study. Circulation. 2001;103:102–107. doi: 10.1161/01.cir.103.1.102. [DOI] [PubMed] [Google Scholar]
- 16.Poirier P, Bogaty P, Garneau C, Marois L, Dumesnil JG. Diastolic dysfunction in normotensive men with well-controlled type 2 diabetes: importance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. Diabetes Care. 2001;24:5–10. doi: 10.2337/diacare.24.1.5. [DOI] [PubMed] [Google Scholar]
- 17.Almuntaser I, Brown A, Murphy R, Crean P, King G, Mahmud A, Feely J. Comparison of echocardiographic measures of left ventricular diastolic function in early hypertension. Am J Cardiol. 2007;100:1771–1775. doi: 10.1016/j.amjcard.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 18.Di Bonito P, Moio N, Cavuto L, Covino G, Murena E, Scilla C, Turco S, Capaldo B, Sibilio G. Early detection of diabetic cardiomyopathy: usefulness of tissue Doppler imaging. Diabet Med. 2005;22:1720–1725. doi: 10.1111/j.1464-5491.2005.01685.x. [DOI] [PubMed] [Google Scholar]
- 19.Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–480. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 20.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 21.Sacco RL, Roberts JK, Boden-Albala B, Gu Q, Lin IF, Kargman DE, Berglund L, Hauser WA, Shea S, Paik MC. Race-ethnicity and determinants of carotid atherosclerosis in a multiethnic population. The Northern Manhattan Stroke Study. Stroke. 1997;28:929–935. doi: 10.1161/01.str.28.5.929. [DOI] [PubMed] [Google Scholar]
- 22.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 24.Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, Saba PS, Vargiu P, Simongini I, Laragh JH. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–1558. doi: 10.1016/0735-1097(92)90617-v. [DOI] [PubMed] [Google Scholar]
- 25.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 26.Fox ER, Han H, Taylor HA, Walls UC, Samdarshi T, Skelton TN, Pan J, Arnett D. The prognostic value of the mitral diastolic filling velocity ratio for all-cause mortality and cardiovascular morbidity in African Americans: the Atherosclerotic Risks in Communities (ARIC) study. Am Heart J. 2006;152:749–755. doi: 10.1016/j.ahj.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Fang ZY, Yuda S, Anderson V, Short L, Case C, Marwick TH. Echocardiographic detection of early diabetic myocardial disease. J Am Coll Cardiol. 2003;41:611–617. doi: 10.1016/s0735-1097(02)02869-3. [DOI] [PubMed] [Google Scholar]
- 28.Kosmala W, Kucharski W, Przewlocka-Kosmala M, Mazurek W. Comparison of left ventricular function by tissue Doppler imaging in patients with diabetes mellitus without systemic hypertension versus diabetes mellitus with systemic hypertension. Am J Cardiol. 2004;94:395–399. doi: 10.1016/j.amjcard.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 29.Wachter R, Luers C, Kleta S, Griebel K, Herrmann-Lingen C, Binder L, Janicke N, Wetzel D, Kochen MM, Pieske B. Impact of diabetes on left ventricular diastolic function in patients with arterial hypertension. Eur J Heart Fail. 2007;9:469–476. doi: 10.1016/j.ejheart.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician's Rosetta Stone. J Am Coll Cardiol. 1997;30:8–18. doi: 10.1016/s0735-1097(97)00144-7. [DOI] [PubMed] [Google Scholar]
- 31.Oh JK, Appleton CP, Hatle LK, Nishimura RA, Seward JB, Tajik AJ. The noninvasive assessment of left ventricular diastolic function with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 1997;10:246–270. doi: 10.1016/s0894-7317(97)70062-2. [DOI] [PubMed] [Google Scholar]
- 32.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–1533. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 33.Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, Barzilai B, vila-Roman VG. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol. 2004;43:1399–1404. doi: 10.1016/j.jacc.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 34.Wong CY, O'Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110:3081–3087. doi: 10.1161/01.CIR.0000147184.13872.0F. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigues B, Cam MC, McNeill JH. Metabolic disturbances in diabetic cardiomyopathy. Mol Cell Biochem. 1998;180:53–57. [PubMed] [Google Scholar]
- 36.Diamant M, Lamb HJ, Groeneveld Y, Endert EL, Smit JW, Bax JJ, Romijn JA, de RA, Radder JK. Diastolic dysfunction is associated with altered myocardial metabolism in asymptomatic normotensive patients with well-controlled type 2 diabetes mellitus. J Am Coll Cardiol. 2003;42:328–335. doi: 10.1016/s0735-1097(03)00625-9. [DOI] [PubMed] [Google Scholar]
- 37.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young ME, Guthrie PH, Razeghi P, Leighton B, Abbasi S, Patil S, Youker KA, Taegtmeyer H. Impaired long-chain fatty acid oxidation and contractile dysfunction in the obese Zucker rat heart. Diabetes. 2002;51:2587–2595. doi: 10.2337/diabetes.51.8.2587. [DOI] [PubMed] [Google Scholar]
- 39.Fiordaliso F, Li B, Latini R, Sonnenblick EH, Anversa P, Leri A, Kajstura J. Myocyte death in streptozotocin-induced diabetes in rats in angiotensin II- dependent. Lab Invest. 2000;80:513–527. doi: 10.1038/labinvest.3780057. [DOI] [PubMed] [Google Scholar]
- 40.van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJ, Schalkwijk CG, Bronzwaer JG, Diamant M, Borbely A, van der Velden J, Stienen GJ, Laarman GJ, Niessen HW, Paulus WJ. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 41.van Hoeven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation. 1990;82:848–855. doi: 10.1161/01.cir.82.3.848. [DOI] [PubMed] [Google Scholar]
- 42.Solomon SD, Janardhanan R, Verma A, Bourgoun M, Daley WL, Purkayastha D, Lacourciere Y, Hippler SE, Fields H, Naqvi TZ, Mulvagh SL, Arnold JM, Thomas JD, Zile MR, Aurigemma GP. Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet. 2007;369:2079–2087. doi: 10.1016/S0140-6736(07)60980-5. [DOI] [PubMed] [Google Scholar]
- 43.von Bibra H, Hansen A, Dounis V, Bystedt T, Malmberg K, Ryden L. Augmented metabolic control improves myocardial diastolic function and perfusion in patients with non-insulin dependent diabetes. Heart. 2004;90:1483–1484. doi: 10.1136/hrt.2003.020842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jarnert C, Landstedt-Hallin L, Malmberg K, Melcher A, Ohrvik J, Persson H, Ryden L. A randomized trial of the impact of strict glycaemic control on myocardial diastolic function and perfusion reserve: a report from the DADD (Diabetes mellitus And Diastolic Dysfunction) study. Eur J Heart Fail. 2009;11:39–47. doi: 10.1093/eurjhf/hfn018. [DOI] [PubMed] [Google Scholar]
- 45.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90:1284–1289. doi: 10.1016/s0002-9149(02)02864-3. [DOI] [PubMed] [Google Scholar]
- 46.Lester SJ, Ryan EW, Schiller NB, Foster E. Best method in clinical practice and in research studies to determine left atrial size. Am J Cardiol. 1999;84:829–832. doi: 10.1016/s0002-9149(99)00446-4. [DOI] [PubMed] [Google Scholar]
- 47.Liu JE, Palmieri V, Roman MJ, Bella JN, Fabsitz R, Howard BV, Welty TK, Lee ET, Devereux RB. The impact of diabetes on left ventricular filling pattern in normotensive and hypertensive adults: the Strong Heart Study. J Am Coll Cardiol. 2001;37:1943–1949. doi: 10.1016/s0735-1097(01)01230-x. [DOI] [PubMed] [Google Scholar]
- 48.Mogelvang R, Sogaard P, Pedersen SA, Olsen NT, Schnohr P, Jensen JS. Tissue Doppler echocardiography in persons with hypertension, diabetes, or ischaemic heart disease: the Copenhagen City Heart Study. Eur Heart J. 2009;30:731–739. doi: 10.1093/eurheartj/ehn596. [DOI] [PubMed] [Google Scholar]