Introduction
Cardiac cachexia occurs in severe heart failure (HF).1,2 Heart transplantation (HTx) is associated with weight gain and the frequent development of obesity. Although this weight gain has been attributed to glucocorticoid therapy, it is more dramatic than after other solid organ transplants, despite similar steroid regimens, and also unrelated to steroid dose,3 suggesting that other mechanisms are responsible.
Leptin is an adipo(cyto)kine produced primarily by adipocytes that acts on the hypothalamus to decrease appetite and increases energy expenditure.4 Plasma leptin is thought to reflect adiposity, and obesity is associated with elevated leptin. Resistance to the appetite-suppressing effects of leptin is thought to co-exist with obesity and insulin resistance in the metabolic syndrome, but the mechanisms are poorly understood.
Aims
To determine the relations of leptin and insulin resistance to adipose tissue mass, body composition, and caloric intake. We hypothesized that the dramatic weight gain post-HTx is associated with leptin resistance, insulin resistance, and central obesity.
Methods
We performed a cross-sectional study in 12 HF patients awaiting HTx, 12 patients 12.7 ± 8.6 months of post-HTx, and 7 control subjects (Table 1). We then performed a longitudinal study of 6 of the 12 HF patients 5.8 ± 3.3 months after HTx. Subjects were age and gender matched. Heart failure and HTx patients were matched by aetiology, and controls were matched by body mass index (BMI) to the HTx patients. The protocol complied with the Declaration of Helsinki and was approved by the institutional review board of Columbia University Medical Center, and written informed consent was obtained from all patients.
Table 1.
Baseline characteristics of the cross-sectional cohort
| Group | Control (n = 7) | HF (n = 12) | HTx (n = 12) | P-value (overall) |
|---|---|---|---|---|
| Age (years) | 42 ± 14 | 52 ± 16 | 47 ± 18 | >0.2* |
| Gender, n (%) | >0.2 | |||
| Male | 5 (71) | 10 (83) | 11 (92) | |
| Female | 2 (29) | 2 (17) | 1 (8) | |
| BMI (kg/m2) | 27.8 ± 4.2 | 23.1 ± 3.1 | 31.5 ± 5.5 | <0.001 |
| Cachexia presenta | NA | 7 (58%) | NA | NA |
| Diabetes | 0 | 3 (25%) | 2 (17%) | >0.2b |
| Weight gain since HTx (kg) | NA | NA | 18.0 ± 7.7 | NA |
| Time since HTx (months) | NA | NA | 12.7 ± 8.6 | NA |
| Aetiology | NA | >0.2 | ||
| Ischaemic | 4 (33%) | 6 (50%) | ||
| Dilated | 6 (50%) | 5 (42%) | ||
| Valvular | 2 (17%) | 1 (8%) | ||
| HF drug therapy | NA | NA | NA | |
| Loop diuretic | 12 (100%) | |||
| ACE inhibitor | 12 (100%) | |||
| β-Blocker | 5 (42%) | |||
| Inotropes | 7 (58%) | |||
| HTx drug therapy | NA | NA | NA | |
| Prednisone (mg) | 6 ± 4 | |||
| Cyclosporine A | 9 (75%) | |||
| Tacrolimus | 3 (25%) | |||
| Mycophenolate mofetil | 12 (100%) |
Bold denotes statistical significance (P < 0.05). BMI, body mass index; HTx, heart transplantation; ACE, angiotensin-converting enzyme; NA, not applicable.
aCachexia was defined as non-oedematous weight loss ≥7.5% over the preceding 6 months.10
bComparison HF vs. HTx only.
*P < 0.05 considered significant. P < 0.20 cited since may reflect trends. P-values >0.2 listed as >0.2.
Dual energy X-ray absorptiometry (DEXA) scanning was performed with a QDR 4500 A Delphi W densitometer (Hologic Inc., Bedford, MA, USA) to analyse body composition. Waist circumference was measured in duplicate at the level of the umbilicus. Measurement of resting energy expenditure (REE) and cardiopulmonary exercise testing with measurement of peak oxygen consumption (peak VO2) was performed using a metabolic cart (Medical Graphics, Minneapolis, MN, USA).
All subjects reported to the lab at 8 a.m. in the fasting state and underwent blood sampling, completed a visual analogue scale (0–10) assessing hunger, and then consumed a yoghurt-based breakfast test meal where they were encouraged to eat until absolute satiety. Eating until absolute satiety was intended to ensure that postprandial assessment of hormones, appetite, and caloric intake would not be affected by differences in postprandial satiety. Postprandial serial blood testing and hunger assessment were performed hourly for 5 h. Subjects were then given an unlimited buffet lunch meal of regular food of their choosing and were neither encouraged nor dissuaded to eat. Caloric intake at each meal was estimated using computerized software NDS-R 4.05-33 (University of Minnesota, Minneapolis, MN, USA).
Blood was collected into chilled EDTA tubes and centrifuged at 2500 g for 15 min at 4°C. Plasma was stored at −70°C. Radioimmunoassays were performed for insulin (Linco Research Inc., St Charles, MO, USA) and leptin (Phoenix Pharmaceuticals, Belmont, CA, USA). Serum glucose was measured by the hexokinase method. The homeostasis model assessment (HOMA, mMmU/L2) for insulin resistance was calculated as fasting insulin (µU/mL) × fasting glucose (mg/dL)/405.3.5
Continuous variables were compared by one-way analysis of variance (ANOVA). Pair-wise comparisons between cross-sectional groups were done by Fischer's least significance test. Paired comparisons between longitudinal groups were done by Student's t-test. Serial data over time were used to calculate the area under the curve (AUC) for leptin. Pearson's correlations were calculated. A value of P < 0.05 was considered statistically significant. All results are reported as mean ± SD.
Results
Clinical characteristics and medical regimens of the cross-sectional population are summarized in Table 1. Heart transplantation patients had gained 18.0 ± 7.7 kg over the 12.7 ± 8.6 months since HTx. The 6 HF patients followed longitudinally had similar characteristics compared with the 12 cross-sectional HTx patients.
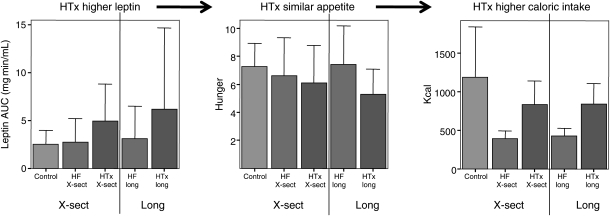
In the cross-sectional study (Table 2), HTx patients had higher BMI, fat mass, body fat %, abdominal fat mass and %, waist circumference, and leptin AUC. Despite higher fasting leptin, HTx patients had higher breakfast caloric intake than HF patients, and despite higher leptin AUC between breakfast and lunch, HTx patients had a trend towards a higher caloric intake at lunch than the HF patients (Figure 1). The REE was similar between the groups crudely and after adjusting for body surface area. Longitudinal results were similar to cross-sectional.
Table 2.
Body composition, resting energy expenditure, exercise tolerance, hormones, appetite, and caloric intake in the cross-sectional cohort
| Group | Control (n = 7) | HF cross-sectional (n = 12) | HTx cross-sectional (n = 12) |
P-value |
|||
|---|---|---|---|---|---|---|---|
| All | Control vs. HF | Control vs. HTx | HF vs. HTx | ||||
| BMI (kg/m2) | 27.8 ± 4.2 | 23.1 ± 3.1 | 31.5 ± 5.5 | <0.001 | 0.03 | 0.08 | <0.001 |
| Total mass (kg) | 87 ± 15 | 69 ± 6 | 92 ± 15 | <0.001 | <0.004 | >0.2 | <0.001 |
| Fat mass (kg)a | 24 ± 9 | 17 ± 2 | 31 ± 9 | <0.006 | <0.13 | 0.10 | <0.002 |
| Lean mass (kg)a | 66 ± 8 | 55 ± 4 | 61 ± 11 | 0.12 | 0.04 | >0.2 | 0.17 |
| Fat % | 26 ± 7 | 23 ± 2 | 33 ± 7 | <0.009 | >0.2 | <0.03 | <0.004 |
| Abdominal fat mass (kg) | 11.7 ± 5 | 8.4 ± 2 | 16.8 ± 6 | <0.01 | >0.2 | <0.06 | <0.004 |
| Abdominal fat % | 27 ± 8 | 23 ± 4 | 33 ± 7 | <0.009 | >0.2 | 0.05 | <0.003 |
| Waist circumference (cm) | 99 ± 10 | 86 ± 4 | 111 ± 13 | <0.001 | <0.10 | <0.05 | <0.001 |
| REE (kcal/day) | 1710 ± 384 | 1812 ± 352 | 2036 ± 528 | >0.2 | >0.2 | <0.13 | >0.2 |
| REE (kcal/m2/h) | 35 ± 5 | 41 ± 9 | 41 ± 10 | >0.2 | <0.14 | <0.15 | >0.2 |
| Peak VO2 (mL/kg/min)a | 27 ± 9 | 11 ± 2 | 15 ± 3 | <0.001 | <0.001 | <0.001 | 0.18 |
| Glucose (mg/dL) | 86.4 ± 5.8 | 105.4 ± 16.8 | 96.4 ± 16.1 | <0.05 | <0.02 | 0.17 | <0.19 |
| Insulin (µU/mL) | 11.6 ± 7.1 | 21.8 ± 10.9 | 18.1 ± 6.9 | 0.08 | <0.03 | 0.14 | >0.2 |
| HOMA (mMmU/L2)b | 2.5 ± 1.6 | 5.7 ± 3.0 | 4.3 ± 1.9 | <0.04 | 0.01 | 0.12 | 0.20 |
| Leptin (ng/mL)c | 8.56 ± 5.2 | 8.14 ± 7.6 | 16.65 ± 11.6 | <0.06 | >0.2 | 0.07 | <0.03 |
| Leptin per fat % (ng/mL/%)a | 0.27 ± 0.1 | 0.29 ± 0.1 | 0.48 ± 0.3 | <0.10 | >0.2 | 0.06 | <0.10 |
| Hunger fasting (0–10) | 5.8 ± 2.5 | 5.0 ± 2.4 | 3.4 ± 1.3 | <0.05 | >0.2 | 0.02 | 0.07 |
| Breakfast (kcal) | 907 ± 260 | 832 ± 364 | 1238 ± 421 | 0.03 | >0.2 | <0.07 | 0.01 |
| Hunger after breakfast (0–10) | 1.1 ± 1.5 | 2.0 ± 2.0 | 1.9 ± 2.8 | >0.2 | >0.2 | >0.2 | >0.2 |
| Leptin AUC (mg min/mL) | 2.54 ± 1.4 | 2.73 ± 2.5 | 5.41 ± 3.7 | 0.05 | >0.2 | <0.05 | 0.03 |
| Hunger before lunch (0–10) | 7.3 ± 1.6 | 6.6 ± 2.8 | 6.1 ± 2.7 | >0.2 | >0.2 | >0.2 | >0.2 |
| Lunch (kcal) | 1185 ± 650 | 391 ± 103 | 831 ± 309 | <0.008 | <0.002 | 0.13 | 0.06 |
Bold denotes statistical significance (P < 0.05). BMI, body mass index; HTx, heart transplantation.
aPeak VO2 and DEXA scans were not performed in the seven HF patients on inotropic therapy and thus leptin per fat % was not available.
bHOMA was not measured in patients with diabetes (n = 3 HF and n = 2 HTx); upper limit of normal for HOMA is 2.5 mMmU/L2.5
cNormal range leptin for this assay: men, 3.8 ± 1.8; women, 7.4 ± 3.7 ng/mL (Phoenix Pharmaceuticals).
Figure 1.
Leptin, appetite, and caloric intake. Subjects consumed a breakfast until absolute satiety to ensure that postprandial assessments were not affected by differences in postprandial satiety. For 5 h thereafter, the leptin area under the curve (AUC, mg min/mL) was significantly higher in heart transplantation patients than in control or heart failure patients. Despite higher leptin, hunger was no different in heart transplantation patients compared with control or heart failure patients. At the ad lib lunch meal, when subjects were neither encouraged nor dissuaded to eat, caloric intake was significantly and dramatically higher in heart transplantation patients than in heart failure patients, and similar to controls. Hunger was measured on a 0–10 visual analogue scale. X-sect, cross-sectional; long, longitudinal.
Correlations for cross-sectional and longitudinal patients together are listed in Table 3. Leptin correlated with overall and abdominal fat % and with waist circumference in controls and HTx patients [P < 0.02 (for all except waist circumference in controls, P < 0.18)] but not in HF patients. Body mass index, overall and abdominal fat, and waist circumference tended to correlate positively with insulin resistance in control and HTx patients but negatively in HF patients.
Table 3.
Correlation of leptin and insulin resistance with selected parameters in the combined cross-sectional and longitudinal cohorts
| Control (n = 7) |
HF (n = 12) |
HTx (n = 18) |
||||
|---|---|---|---|---|---|---|
| R | P-value | R | P-value | R | P-value | |
| Leptin | ||||||
| Male gender | −0.82 | 0.02 | −0.75 | <0.005 | −0.79 | <0.001 |
| BMI | 0.36 | 0.42 | 0.80 | <0.002 | 0.49 | <0.04 |
| Fat mass | 0.94 | <0.006 | 0.18 | 0.73 | 0.67 | <0.005 |
| Fat % | 0.93 | <0.007 | −0.002 | 1.0 | 0.64 | <0.008 |
| Abdominal fat mass | 0.92 | <0.009 | 0.53 | <0.28 | 0.46 | <0.11 |
| Abdominal fat % | 0.91 | 0.01 | 0.58 | 0.23 | 0.75 | <0.003 |
| Waist circumference | 0.71 | <0.18 | 0.32 | 0.61 | 0.60 | 0.02 |
| Insulin resistance | ||||||
| BMI | 0.49 | 0.26 | −0.35 | 0.36 | 0.65 | 0.01 |
| Weight gain since HTx (kg) | NA | NA | 0.56 | <0.04 | ||
| Weight gain since HTx (%) | NA | NA | 0.58 | <0.03 | ||
| Fat mass | 0.62 | 0.19 | −0.46 | 0.54 | 0.45 | <0.12 |
| Fat % | 0.65 | 0.16 | −0.87 | 0.13 | 0.45 | 0.12 |
| Abdominal fat mass | 0.57 | 0.24 | −0.73 | 0.27 | 0.67 | 0.02 |
| Abdominal fat % | 0.59 | 0.21 | −0.81 | 0.19 | 0.44 | 0.17 |
| Waist circumference | 0.28 | 0.64 | −0.33 | 0.67 | 0.72 | 0.01 |
All R and P values are listed whether significant or not. Bold denotes statistical significance (P < 0.05). Leptin or insulin resistance did not correlate with each other, hunger, caloric intake, or steroid dose in any group. NA, not applicable; BMI, body mass index; HTx, heart transplantation.
Conclusion
In HF patients, leptin was elevated despite normal BMI, confirming some6,7 but not other8 previous studies. Heart failure patients had lower caloric intake than the other groups. This may reflect inappropriately high leptin and resulting appetite suppression. The degree of insulin resistance in HF patients was high, confirming previous reports.9 Neither leptin nor insulin resistance correlated with traditional markers such as total or abdominal fat or waist circumference (components of the metabolic syndrome). These data suggest that leptin and insulin regulation in HF patients may be perturbed secondary to the hormonal and inflammatory aspects of HF patients rather than to obesity. Leptin and insulin dysregulation may themselves contribute to cachexia, although neurohormonal and cytokine activation10 and/or resistance to growth hormone11 and the appetite-stimulating hormone ghrelin12 may also be important.
Post-HTx, this is the first study to show that (i) leptin is elevated and (B) despite higher leptin, caloric intake was increased, consistent with leptin resistance. Leptin may be elevated post-HTx due to the increased % body fat, but even leptin adjusted for fat % trended towards being higher post-HTx. Insulin resistance was reduced but did not resolve post-HTx. In controls and HTx patients but not in HF patients, leptin correlated with markers of adiposity. These data taken together suggest that HTx is associated with abdominal obesity and leptin and insulin resistance, components of the metabolic syndrome. Leptin resistance may contribute to weight gain, but the cause and effect relationship cannot be established. Weight gain post-HTx may also be due to resolution of HF-related catabolic states such as neurohormonal and cytokine activation10 and/or ghrelin12 and growth hormone resistance.11 High-dose dexamethasone has been shown to increase leptin;13 however, in our study, steroid doses were low, and we have previously shown that steroids are unrelated to weight gain post-HTx.
Limitations
The small patient population is the major limitation of the study. It did not allow detection of differences between different HF aetiologies and it rendered the longitudinal findings statistically non-significant. However, the consistency of findings and magnitude of differences in the cross-sectional and longitudinal analysis support the conclusions. The complexity of the measurements made it difficult to involve other HTx centres in the study. Our findings must be considered preliminary and need to be confirmed in a larger population in a multicentre study.
Summary
These preliminary data suggest that HTx, independent of steroid dosing, is associated with the development of leptin resistance and abdominal obesity and persistence of insulin resistance, states consistent with the metabolic syndrome. This may be secondary to and/or contribute to the dramatic weight gain observed post-HTx.
Funding
This work was supported by Stockholms Läns Landsting and the Swedish Heart-Lung Foundation in Stockholm, Sweden, in the form of grants to L.H.L., and by the Division of Research Resources, General Clinical Research Centers Program, National Institutes of Health [5 MO1 RR00645], Bethesda, MD, USA, the Foundation for Cardiac Therapies (FACT Fund), New, York, NY, USA, and the Altman Fund, New York, NY, USA, in the form of grants to D.M.M.
Conflict of interest: none declared.
References
- 1.Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 2.Pocock SJ, McMurray JJ, Dobson J, Yusuf S, Granger CB, Michelson EL, Ostergren J, Pfeffer MA, Solomon SD, Anker SD, Swedberg KB. Weight loss and mortality risk in patients with chronic heart failure in the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:2641–2650. doi: 10.1093/eurheartj/ehn420. [DOI] [PubMed] [Google Scholar]
- 3.Williams JJ, Lund LH, LaManca J, Kunavarapu C, Cohen DJ, Heshka S, Heymsfield SB, Mancini DM. Excessive weight gain in cardiac transplant recipients. J Heart Lung Transplant. 2006;25:36–41. doi: 10.1016/j.healun.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 5.Nakai Y, Nakaishi S, Kishimoto H, Seino Y, Nagasaka S, Sakai M, Taniguchi A. The threshold value for insulin resistance on homeostasis model assessment of insulin sensitivity. Diabet Med. 2002;19:346–347. doi: 10.1046/j.1464-5491.2002.00712_3.x. [DOI] [PubMed] [Google Scholar]
- 6.Schulze PC, Kratzsch J, Linke A, Schoene N, Adams V, Gielen S, Erbs S, Moebius-Winkler S, Schuler G. Elevated serum levels of leptin and soluble leptin receptor in patients with advanced chronic heart failure. Eur J Heart Fail. 2003;5:33–40. doi: 10.1016/s1388-9842(02)00177-0. [DOI] [PubMed] [Google Scholar]
- 7.Doehner W, Pflaum CD, Rauchhaus M, Godsland IF, Egerer K, Cicoira M, Florea VG, Sharma R, Bolger AP, Coats AJ, Anker SD, Strasburger CJ. Leptin, insulin sensitivity and growth hormone binding protein in chronic heart failure with and without cardiac cachexia. Eur J Endocrinol. 2001;145:727–735. doi: 10.1530/eje.0.1450727. [DOI] [PubMed] [Google Scholar]
- 8.Murdoch DR, Rooney E, Dargie HJ, Shapiro D, Morton JJ, McMurray JJ. Inappropriately low plasma leptin concentration in the cachexia associated with chronic heart failure. Heart. 1999;82:352–356. doi: 10.1136/hrt.82.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doehner W, Rauchhaus M, Ponikowski P, Godsland IF, von Haehling S, Okonko DO, Leyva F, Proudler AJ, Coats AJ, Anker SD. Impaired insulin sensitivity as an independent risk factor for mortality in patients with stable chronic heart failure. J Am Coll Cardiol. 2005;46:1019–1026. doi: 10.1016/j.jacc.2005.02.093. [DOI] [PubMed] [Google Scholar]
- 10.Anker SD, Chua TP, Ponikowski P, Harrington D, Swan JW, Kox WJ, Poole-Wilson PA, Coats AJ. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96:526–534. doi: 10.1161/01.cir.96.2.526. [DOI] [PubMed] [Google Scholar]
- 11.Lund LH, Freda P, Williams JJ, Lamanca JJ, Lejemtel TH, Mancini DM. Growth hormone resistance in severe heart failure resolves after cardiac transplantation. Eur J Heart Fail. 2009;11:525–528. doi: 10.1093/eurjhf/hfp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lund LH, Williams JJ, Freda P, Lamanca JJ, Lejemtel TH, Mancini DM. Ghrelin resistance occurs in severe heart failure and resolves after heart transplantation. Eur J Heart Fail. 2009;11:789–794. doi: 10.1093/eurjhf/hfp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsson H, Ahren B. Short-term dexamethasone treatment increases plasma leptin independently of changes in insulin sensitivity in healthy women. J Clin Endocrinol Metab. 1996;81:4428–4432. doi: 10.1210/jcem.81.12.8954054. [DOI] [PubMed] [Google Scholar]