Abstract
Aims
Myostatin inhibits myoblast differentiation/proliferation and may play a role in heart failure (HF) and reverse remodelling after left ventricular assist device (LVAD) support. This study sought to characterize myostatin expression and activation in advanced HF before and after LVAD support.
Methods and results
Left ventricular tissue pairs were collected at LVAD implantation (core) and at cardiac transplantation/LVAD explantation in patients with advanced ischaemic (ICM—ischaemic cardiomyopathy) and non-ischaemic (DCM—dilated cardiomyopathy) HF. Normal cardiac tissue (control) was obtained from hearts not placed for transplantation. Serum was collected independently from patients with stable DCM HF and from healthy controls. Full-length and cleaved propeptide myostatin levels were quantified by western blot analysis. Dilated cardiomyopathy propeptide levels at core were significantly higher than control and significantly increased after LVAD support. Ischaemic cardiomyopathy propeptide levels were higher than control, but did not change after LVAD support. No changes in full-length levels were seen. Serum myostatin levels were significantly higher in DCM HF patients than in healthy controls.
Conclusion
This is the first clinical evidence that myostatin activation is increased in HF. Myostatin may affect cardiac hypertrophy and may mediate regression of cellular hypertrophy after mechanical unloading.
Keywords: Protein, Myocyte regulation, Dilated cardiomyopathy, Mechanical circulation
See page 420 for the editorial comment on this article (doi:10.1093/eurjhf/hfq044)
Introduction
Myostatin is a member of the TGF-β superfamily that acts as a negative regulator of muscle growth. Highly conserved among species, the protein is synthesized as a full-length precursor (37 kDa) that dimerizes (75 kDa) and subsequently undergoes cleavage at a dibasic site to yield a biologically active, 25 kDa C-terminal dimer and two inactive N-terminal propeptides (25 kDa × 2) that associate with and inhibit the C-terminal dimer, forming a latent complex.1,2 This latent complex (75 kDa) is then secreted into the circulation and can be activated locally in target tissues, such as skeletal muscle and heart.
Proteolytic cleavage of the inhibitory N-terminal peptide occurs due to actions of specific metalloproteinases, such as bone morphogenic protein-1 (BMP-1),3 freeing the C-terminal dimer to stimulate the activin type II receptor (ActIIBR), SMAD2/3 pathways, and ultimately, TGF-β-responsive promoters.4,5 The actions of myostatin affect a variety of cellular processes, including decreased muscle cell growth and proliferation through the downregulation of transcriptional regulators, inhibition of myoblast cell cycle progression, increased deposition of extracellular matrix, and increased myofibroblast formation, leading to fibrosis.6–9
The potential role of myostatin in cardiac development was suggested after myostatin expression in foetal and adult sheep Purkinje fibres and cardiomyocytes was reported.10 Myostatin may also play an active role in cardiac remodelling after injury, given its expression after infarct and the wide diversity of pathways mediated by TGF-β members, such as inflammation, fibrosis, and hypertrophy.11–14 Myostatin may be abnormally regulated in heart failure (HF), but no prior clinical studies have measured myostatin expression in normal human cardiac tissue or in patients with HF. We hypothesized that cardiac myostatin would be abnormally regulated in the HF state and that it may play a role in ventricular remodelling and cachexia seen in patients with advanced HF.
Methods
Study design
Myocardial biopsies were prospectively collected from three patient cohorts: subjects with no known cardiopulmonary disease (control) (n = 5), and patients with advanced HF of ischaemic (n = 9) (ICM—ischaemic cardiomyopathy) and non-ischaemic aetiology (n = 9) (DCM—dilated cardiomyopathy) requiring left ventricular assist device (LVAD) support. Serum was prospectively collected independently from two additional patient cohorts: subjects with no known cardiopulmonary disease (n = 29) and patients with New York Heart Association (NYHA) Class III non-ischaemic HF (DCM HF) (n = 28). This study met all institutional guidelines and New York State organ donation guidelines regarding the use of clinical data, ethical treatment of patients adhering to the Declaration of Helsinki principles, and procurement of tissue for research.
Study subjects and myocardial tissue collection
Normal subjects
Subjects with no known cardiopulmonary disease whose organs were listed but were unable to be placed at the time of organ recovery for heart transplantation and who consented to the use of tissue for research purposes by the New York Organ Donor Network were included in this study from 2004 to 2006. Organs were unable to be placed primarily due to logistical and timing constraints and matching incompatibilities—no organ was rejected from transplantation due to depressed ventricular function. At the time of organ recovery, cardioplegia with Celsior solution was administered in the standard fashion. A section (40 mg) of the LV apex was immediately obtained at the conclusion of cardioplegia perfusion and flash-frozen in liquid nitrogen for analysis. Clinical data were obtained from chart review.
Left ventricular assist device patients
Patients with refractory HF underwent Heartmate I XVE (Thoratec Corp., Pleasanton, CA, USA) LVAD implantation as a bridge to transplant at the New York Presbyterian-Columbia Campus between 2001 and 2005 and met national criteria for device implantation. Heart failure medical regimens during LVAD support were similar among ICM and DCM groups and included diuretics and neurohormonal blockade with angiotensin-converting enzyme-inhibitors or angiotensin receptor blockers. Patient data were collected prospectively after informed consent and analysed retrospectively for the purposes of this study. Clinical data, including age, sex, weight, aetiology of HF, pre-LVAD EF, and duration of HF, were obtained prior to LVAD insertion. A core of LV apex (40 mg) was obtained at the time of LVAD implantation (pre-LVAD) and from the remaining apical area at the time of cardiac transplantation (post-LVAD).
Study subjects and serum collection
Normal subjects
Normal subjects with no pre-existing cardiopulmonary disease were enrolled at Albert Einstein College of Medicine between 2005 and 2007. Age, weight, and height were recorded. Following informed consent, 5 mL of serum was drawn via venepuncture after a 10 min period of rest. Samples were immediately spun down at 1200 r.p.m. for 10 min; plasma was flash-frozen for myostatin latent complex analysis and stored at −80°C.
Dilated cardiomyopathy heart failure patients
Heart failure patients were recruited from the HF outpatient clinic at Albert Einstein College of Medicine between 2005 and 2007. Patients were included if they were in stable NYHA Class III and on a maximal HF regimen; 80% were managed with angiotensin-converting enzyme-inhibitors or angiotensin receptor blockers, 80% were on β-blockade, and 27% were on diuretics. Only patients with non-ischaemic aetiologies of HF were included. Patients requiring inotropic support or in acute decompensated HF were excluded. Enrollment occurred on the day the patient presented for a routine cardiopulmonary exercise test. Following informed consent, 5 mL of serum was drawn via venepuncture after a 10 min period of rest prior to the exercise test. Samples were immediately spun down at 1200 r.p.m. for 10 min; plasma was flash-frozen for myostatin latent complex analysis and stored at −80° C.
Peak oxygen consumption (VO2) was measured during treadmill cardiopulmonary exercise testing according to standard protocols using a Medical Graphics 2001 (St Paul, MN, USA) cart, as described previously.15
Western blot analysis
Left ventricular specimens obtained for western blotting were snap-frozen in liquid nitrogen after removal from the patient without OCT fixative. Specimens were pulverized, homogenized in 10 volumes of triple-detergent lysis buffer [50 mM Tris, pH 8.0, 0.1% SDS, 1.0% Triton X-100, 0.5% DOC, 5 mM EDTA, 50 mM DTT, 0.4 tablet/10 mL Complete protease inhibitor (Roche, Indianapolis, IN, USA), pH 8.0], and centrifuged at 13 000 r.p.m. (15 700 relative centrifugal force) for 5 min at 4°C (Eppendorf centrifuge 5415R equipped with fixed angle rotor F45-24-11, Hauppauge, NY, USA). Protein concentration of the supernatant was determined using the BioRad Protein Assay (Hercules, CA, USA). Then, 50 μg of each tissue sample or 1.5 μL of serum were electrophoresed on a 10% SDS–polyacrylamide gel following the addition of 2X sample loading buffer (130 mM Tris, pH 8.0, 20% glycerol, 4.6% SDS, 2% DTT, 0.02% bromophenol blue; pH 8.0) and 5 min of denaturation at 100°C. Proteins were then transferred to Immobilon-P (Millipore, Bedford, MA, USA) using the iBlot transfer apparatus (Invitrogen, Carlsbad, CA, USA). The membrane was subsequently blocked with 5% non-fat dry milk in Tris-buffered saline containing 0.05% Tween 20 (pH 8.0). Immunoblotting was performed to detect myostatin (1:1000, polyclonal antibody provided by Dr S.J. Lee,16 phospho-Smad 2/3 (1:2000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), Smad 2/3 (1:1000, Cell Signaling Technology, Danvers, MA, USA), BMP1 (1:500, Abcam, Cambridge, MA, USA), ActIIBR (1:500, Sigma, St Louis, MO, USA), phospho-Erk1/2 and total Erk1/2 (1:1000, Cell Signaling Technology), and actin (1:2000, Sigma). The myostatin antibody was raised against an epitope in the N-terminus (propeptide region) of myostatin and therefore was able to detect full-length myostatin, cleaved propeptide, and latent complex. The BMP-1 antibody recognizes four isoforms of BMP-1 ranging from ∼70–100 kDa. Detection was performed using the Super Signal West Pico Chemiluminescent Substrate kit (Pierce, Rockford, IL, USA). Protein bands were quantified using densitometry software (Alpha Innotech, San Leandro, CA, USA), and bands were normalized to the actin band as a loading control. Phospho-protein bands were normalized to the relevant total protein (i.e. phospho-Smad to total Smad).
Histology
Left ventricular samples from normal subjects (n = 5) and DCM (n = 5) patients were placed in a 2% paraformaldehyde fixative and then in 75% ethanol. These samples were subsequently stained for trichrome and haematoxylin and eosin. Histological analysis was performed by two blinded, experienced investigators. Myocyte size (average of 10 cells measured at the level of the nucleus, repeated for four representative images) at ×10 was analysed on each subject using digital software (Image Pro Plus, v. 4.5.1, Silver Spring, MD, USA).
Statistics
Continuous variables are expressed as mean ± standard error and were compared using independent two-tailed t-testing or Wilcoxon–Mann–Whitney non-parametric testing, paired t-testing, and one-way ANOVA with post hoc Bonferroni analysis, when necessary. Categorical variables were compared by χ2. Pearson's product–moment correlation coefficient was used to determine the correlation between myostatin latent complex to body mass index (BMI) and peak VO2. A linear regression model was used to identify independent predictors of myostatin expression (pre-LVAD, HF) and activation (post-LVAD) in LVAD and HF cohorts; variables included age, sex, weight, number of myocardial infarctions, pre-LVAD ejection fraction, duration of CHF, duration of LVAD support, blood type, cytomegalovirus seropositivity, creatinine kinase level pre-LVAD, use of angiotensin-converting enzyme-inhibitor, intra-aortic balloon pump, steroids, digoxin, dobutamine, diuretic, or β-blocker, or peak VO2 on exercise testing. For all analyses, a P-value <0.05 was considered statistically significant. All data were analysed utilizing SPSS 11.5 (SPSS, Chicago, IL, USA).
Results
Demographics
Left ventricular assist device myocardial tissue study population
Clinical and demographic data for normal subjects and the LVAD patients from whom myocardial tissue samples were obtained are summarized in Table 1. Normal subjects (n = 5) had an average age of 39.8 ± 6.2 years, and three of the five subjects were female. The duration of HF was markedly longer in DCM compared with ICM patients (105.1 ± 23.1 vs. 14.0 ± 7.3 months) (P = 0.002), although pre-LVAD ejection fraction (EF) was similar among groups (15.8 ± 1.3 vs. 16.8 ± 1.7%, respectively, P = 0.715). Dilated cardiomyopathy patients were supported for a mean of 132.9 ± 44.4 days (range 15–437) vs. 117.0 ± 17.0 days (range 39–211) in ICM patients; no patient from either group exhibited myocardial recovery sufficient for LVAD explantation. All ICM and DCM patients were successfully bridged to transplantation (n = 18).
Table 1.
Clinical and demographic data for patients included in the left ventricular assist device myocardial tissue study
| Normal | LVAD non-ischaemic | LVAD ischaemic | |
|---|---|---|---|
| n | 5 | 9 | 9 |
| Age (years) | 39.8 ± 6.2 | 52.2 ± 3.7* | 55.4 ± 2.8* |
| Weight (kg) | 72.3 ± 9.4 | 86.4 ± 8.8 | 79.8 ± 3.9 |
| Male | 2 | 8 | 7 |
| Pre-LVAD duration of HF (months) | NA | 105.1 ± 23.1 | 14.0 ± 7.3** |
| Pre-LVAD EF (%) | 60 ± 4 | 16 ± 1* | 17 ± 2* |
| Status at LVAD implantation | |||
| Inotropes | 0 | 9 | 6 |
| IABP | 0 | 0 | 2 |
| Duration of LVAD support (days) | NA | 132 ± 44 | 117 ± 17 |
LVAD, left ventricular assist device; HF, heart failure; EF, ejection fraction; IABP, intra-aortic balloon pump.
*P < 0.05 vs. normal.
**P < 0.05 vs. non-ischaemic.
Dilated cardiomyopathy heart failure serum study population
As shown in Table 2, 28 patients with NYHA Class III DCM and 29 healthy, normal volunteers were prospectively enrolled for serum collection. The average age of the DCM HF patients (54.4 ± 2.0) was significantly higher than the normals (34.6 ± 1.9, P = 0.03), and the majority of patients in both cohorts were male. Dilated cardiomyopathy HF patients had a mean EF of 32 ± 2% by echocardiography.
Table 2.
Clinical and demographic data for subjects included in the dilated cardiomyopathy heart failure serum study
| Normal | DCM HF | |
|---|---|---|
| n | 29 | 28 |
| Age (years) | 34.6 ± 1.9 | 54.4 ± 2.0* |
| Weight (kg) | 75.1 ± 2.3 | 97.7 ± 5.0* |
| Male | 20 | 18 |
| Duration of HF (months) | — | 78.8 ± 12.0 |
| EF (%) | — | 32 ± 2 |
| LVEDD (cm) | 6.3 ± 0.2 | |
| Peak VO2 (mL/kg/min) | — | 15.4 ± 1.0 |
| Body mass index (kg/m2) | 26.4 ± 4.6 | 33.2 ± 7.8* |
| Medications | ||
| β-Blocker | 24 | |
| ACE-I/ARB | 24 | |
HF, heart failure; EF, ejection fraction; LVEDD, left ventricular end-diastolic dimension; VO2, oxygen consumption; ACE-I, angiotensin-converting enzyme-inhibitor; ARB, angiotensin receptor blocker.
Myostatin expression and activation in tissue
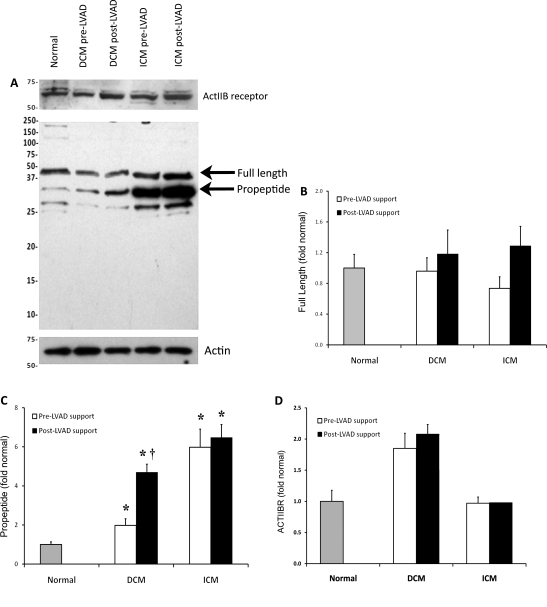
Figure 1 depicts full-length and propeptide myostatin levels in ICM and DCM patients before and after LVAD support compared with normal controls. Since propeptide is released upon myostatin cleavage and activation, propeptide levels were measured as a marker of myostatin activation. Full-length myostatin and propeptide myostatin, as well as the myostatin receptor (ActIIBR), were detected at measurable levels in all tissue samples (Figure 1A). Full-length myostatin ran at ∼50 kDa, whereas myostatin propeptide ran at ∼37 kDa. It is important to note that myostatin can be glycosylated at several N-linked sites, which may cause the various forms to run on a gel at a higher apparent molecular weight than predicted.2 No changes in full-length myostatin were detected in patients with ICM or DCM compared with normal subjects or after LVAD support (Figure 1B). Propeptide levels were significantly higher in both ICM and DCM patients before LVAD support compared with normal subjects (ICM: P = 0.005; DCM: P = 0.025) (Figure 1C). Although propeptide levels did not change in ICM patients with LVAD support, mean propeptide levels doubled in DCM patients (P = 0.043 vs. pre-LVAD) (Figure 1C). No correlation between full-length or propeptide myostatin level and clinical/demographic variables were found on regression analysis. Expression of the ActIIBR was not significantly upregulated in ICM patients before or after LVAD support; however, there was a trend towards increased expression of the receptor in DCM patients before and after LVAD support compared with normal (Figure 1D) (P = 0.069, P = 0.078, respectively).
Figure 1.
Expression of myostatin and its receptor, ActIIBR. (A) Representative western blot for full-length (∼50 kDa) and propeptide myostatin (∼37 kDa) and the ActIIB receptor from cardiac biopsies from normal subjects and patients with ischaemic cardiomyopathy (ICM) and dilated cardiomyopathy (DCM). No changes in the full-length myostatin protein were observed in patients with ischaemic cardiomyopathy or dilated cardiomyopathy when compared with normal subjects or after left ventricular assist device (LVAD) support (B). The myostatin propeptide was elevated in patients with both ischaemic cardiomyopathy and dilated cardiomyopathy before and after left ventricular assist device support compared with normal subjects (C). Left ventricular assist device support was associated with a significant increase in propeptide levels in dilated cardiomyopathy patients. Expression of the ActIIBR was not significantly upregulated in ischaemic cardiomyopathy patients before or after left ventricular assist device support, but there was a trend towards increased expression in dilated cardiomyopathy patients both before and after left ventricular assist device support compared with normal (D). (*P < 0.05 vs. normal, †P < 0.05 vs. pre-LVAD support).
Myostatin latent complex in serum
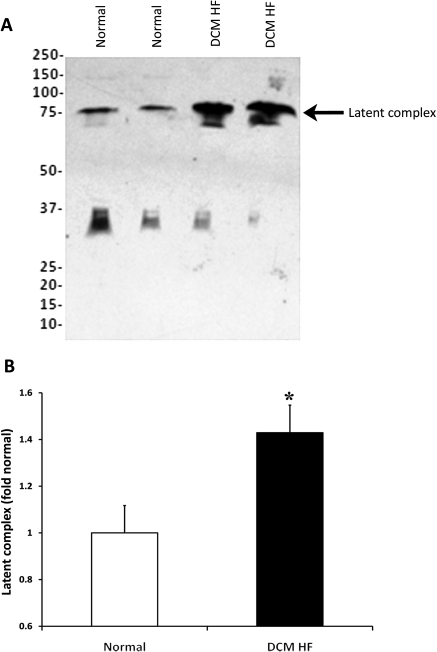
Figure 2A depicts myostatin levels in the serum of DCM HF patients compared with normal controls. Serum levels of myostatin latent complex were significantly higher (43%) in DCM HF patients compared with normals (P = 0.014) (Figure 2B). The range of peak oxygen consumption (VO2) in DCM HF patients was 5.7–25.9 (mean 15.4 ± 1.0) mL/kg/min (Table 2). Both age and BMI were significantly greater in the DCM HF group than normals. Although we had speculated that myostatin levels would be higher in patients with more advanced HF who manifest skeletal muscle dysfunction and decreased exercise tolerance (lower peak VO2), no correlation was seen between myostatin latent complex levels and peak VO2 (r = 0.02, P = 0.9) in this cohort of DCM HF patients. Furthermore, there was no correlation between myostatin latent complex levels and BMI in this cohort (r = 0.05, P = 0.7). Myostatin propeptide was detected in serum in both normal and HF patients at low levels, though no significant difference between the groups was seen. No correlation between myostatin latent complex and clinical/demographic variables were found on regression analysis.
Figure 2.
Expression of myostatin latent complex. (A) Representative western blot for myostatin latent complex (75 kDa) in the serum of patients with non-ischaemic heart failure (DCM HF—dilated cardiomyopathy heart failure) vs. normal controls. Myostatin latent complex is increased significantly in dilated cardiomyopathy heart failure (B). (*P < 0.05 vs. normal).
Smad2/3 signalling
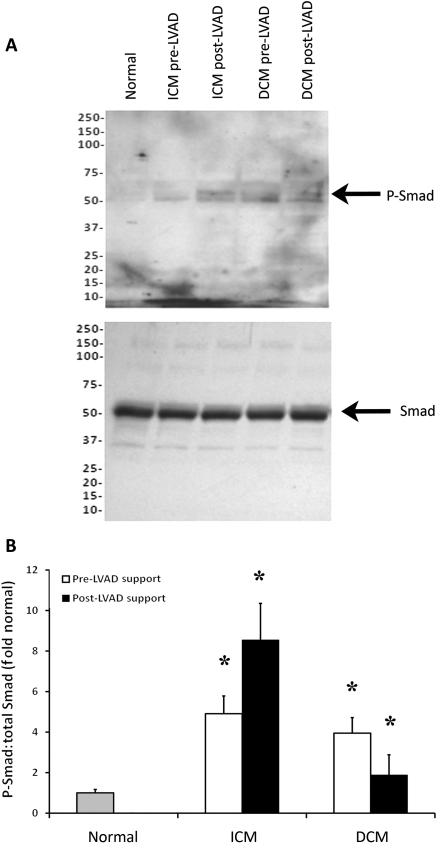
Since myostatin is a member of the TGF-β family, increases in myostatin activation should be accompanied by increases in Smad phosphorylation. Smad2/3 phosphorylation was quantified in myocardial tissue samples from normal subjects and from ICM and DCM patients before and after LVAD support (Figure 3A). Smad phosphorylation was significantly increased in both ICM (P = 0.013) and DCM (P = 0.023) patients compared with normal controls (Figure 3B). Smad phosphorylation was not significantly changed after LVAD support in either ICM or DCM patients but continued to be significantly elevated compared with normal controls (ICM: P = 0.015; DCM: P = 0.022).
Figure 3.
Smad 2/3 phosphorylation. As shown in western blots (A), Smad2/3 phosphorylation was significantly increased in patients with ischaemic cardiomyopathy (ICM) and dilated cardiomyopathy (DCM) vs. normal subjects before left ventricular assist device (LVAD) support. Although levels did not change significantly with left ventricular assist device support, they remained higher than normal subjects (B). (*P < 0.005 vs. normal).
Bone morphogenic protein-1 expression
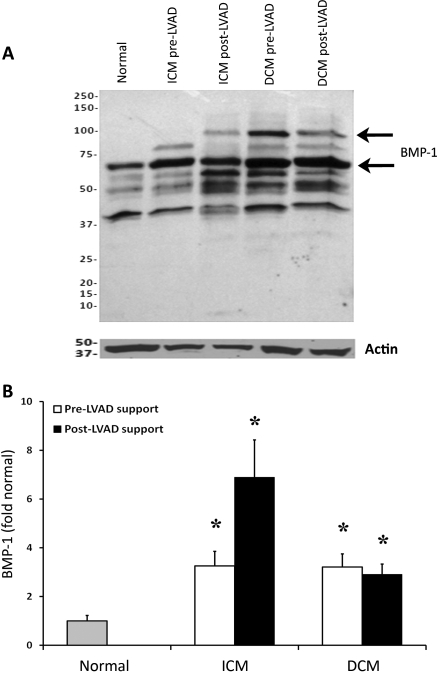
Bone morphogenic protein-1 is a matrix metalloprotease that is capable of cleaving and activating myostatin in skeletal muscle.3 Bone morphogenic protein-1 expression was quantified in myocardial tissue samples from normal subjects and from ICM and DCM patients before and after LVAD support (Figure 4A). Expression of BMP-1 isoforms was significantly increased in both ICM (P = 0.027) and DCM (P = 0.019) patients compared with normal controls (Figure 4B). Bone morphogenic protein-1 expression was not affected by LVAD support in either ICM or DCM patients but continued to be significantly elevated compared with normal controls (ICM: P = 0.022; DCM: P = 0.015). The BMP-1 antibody recognizes the two major isoforms of BMP-1, running at ∼75 and 100 kDa. These bands are displayed in Figure 4A. For simplicity, we show the quantitative data from one of the isoforms, although results were similar for the two isoforms.
Figure 4.
Bone morphogenic protein-1 (BMP-1) expression. Bone morphogenic protein-1 isoforms are shown in the western blot (A). Bone morphogenic protein-1 demonstrated no changes after left ventricular assist device (LVAD) support, although levels in patients with dilated cardiomyopathy (DCM) and ischaemic cardiomyopathy (ICM) were higher than in normal subjects pre- and post-LVAD (B). (*P < 0.05 vs. normal).
Erk phosphorylation
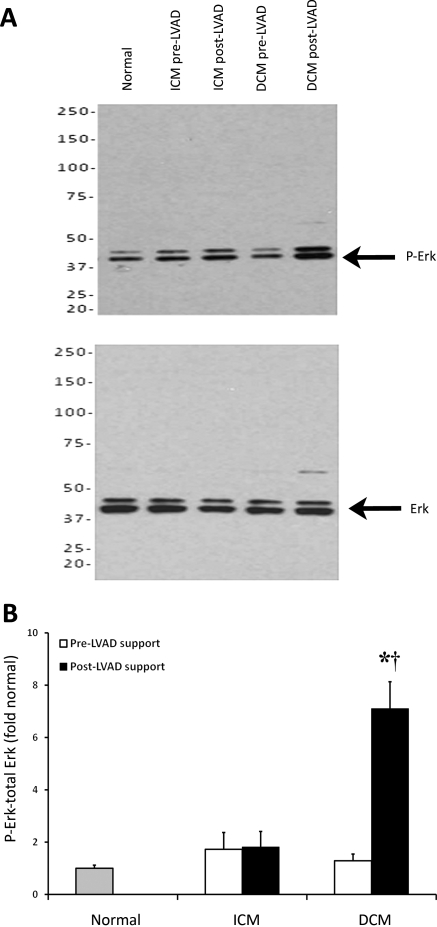
Erk is a mitogen-activated protein kinase that is activated in the heart in response to pathological stress. Erk1/2 phosphorylation was quantified in myocardial tissue samples from normal subjects and from ICM and DCM patients before and after LVAD support (Figure 5A). Erk phosphorylation was significantly increased in DCM patients following LVAD support (P = 0.003), and this activation was also significantly elevated compared with normal controls (P = 0.002) (Figure 5B). There was no change in Erk phosphorylation in the ICM group compared with control or after LVAD support.
Figure 5.
Erk phosphorylation. As shown in western blots (A), Erk 1/2 phosphorylation was not significantly altered in patients with ischaemic cardiomyopathy (ICM) compared with normal subjects or after left ventricular assist device (LVAD) support. In patients with dilated cardiomyopathy (DCM), Erk phosphorylation was not significantly altered before left ventricular assist device support compared with normal but was significantly elevated after left ventricular assist device support compared with both normal subjects and before left ventricular assist device support (B). (*P < 0.05 vs. normal, †P < 0.05 vs. pre-LVAD support).
Histology
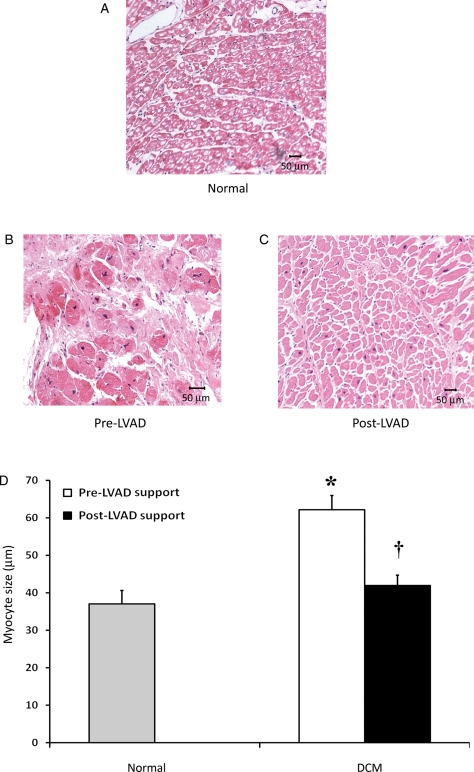
Histological analysis was performed on normal subjects (Figure 6A) and on five patients with DCM supported with an LVAD (duration of HF prior to LVAD 144.3 ± 22.7 months), as shown in Figure 6B and C (pre-LVAD support and post-LVAD support, respectively). Dilated cardiomyopathy samples demonstrated significant cellular hypertrophy prior to LVAD support compared with normals (myocyte size: 62.2 ± 4.2 vs. 37.0 ± 3.6 μm, respectively, P < 0.001) and a significant reduction in myocyte size after 125.1 ± 40.7 days of LVAD support (42.0 ± 3.0 μm, P < 0.001 vs. pre-LVAD) (Figure 6D).
Figure 6.
Myocyte size. Haematoxylin and eosin staining was performed on myocardial samples from normal subjects (A) and heart failure patients at left ventricular assist device (LVAD) implantation (pre-LVAD) (B) and explantation (post-LVAD) (C). Myocyte size in patients with dilated cardiomyopathy (DCM) was significantly larger (pre-LVAD) than normal controls and was reduced after left ventricular assist device support (D). *P < 0.001 vs. normal, †P < 0.0001 vs. pre-LVAD support.
Discussion
Myostatin plays a pivotal role in skeletal muscle growth, as demonstrated by impressive muscle hypertrophy with inhibition1 or cachexia with overexpression.17 Its potential role in cardiac muscle regulation is only partially understood, and it is unclear whether myostatin is associated with changes in ventricular function, cardiomyocyte size, or clinical status in cardiac disease.
The primary findings of our study in this small cohort of patients were the following: (i) levels of the cardiac myostatin propeptide (marker of activation) were increased in HF and increased after LVAD support in patients with DCM, (ii) levels of myostatin latent complex were increased in the serum of DCM HF patients compared with healthy controls but did not correlate with BMI or exercise tolerance, (iii) Smad phosphorylation and Erk phosphorylation were increased in HF patients, suggestive of increased downstream activity of myostatin, (iv) BMP-1 expression was increased in patients with advanced HF, correlating with higher levels of the cleaved propeptide, (v) myostatin activity was associated both with compensatory myocyte hypertrophy in HF and with myocyte size regression after LVAD support in DCM patients. These findings represent the first report of human myostatin regulation in cardiac disease.
Myostatin may exert a direct effect on cardiomyocyte size and hypertrophy in animal models. As shown by Morissette et al.,12 when myostatin was overexpressed in neonatal cardiomyocytes in the presence of the α-agonist phenylephrine, protein synthesis of the cell and cell size were reduced via the reduction of Akt.18 Lower heart weight is associated with myostatin overexpression in mice,19 consistent with its effects in skeletal muscle; this finding was also confirmed by Artaza et al.,14 who observed lower heart weight in a transgenic model of myostatin overexpression in 7-week-old mice.
The increase in myostatin activation in HF (pre-LVAD), represented by elevated levels of cleaved propeptide and supported by increased Smad phosphorylation, is most likely stimulated by myocyte stretch and increased myocardial stress. A state of pathological stress, induced by pressure–volume overload, produces a maladaptive response to overcome organ hypoperfusion: myocytes hypertrophy, generate greater force per unit stretch, and ultimately the ventricle dilates. Specifically, the process of ventricular hypertrophy in response to pressure–volume overload induces expression of insulin-like growth factor-1 (IGF-1).11,20 Insulin-like growth factor-1 appears to counteract many of the actions of myostatin in vitro.21
Myostatin activation, we postulate, may be highly dependent on a tightly controlled positive feedback pathway between IGF-1 and myostatin: as pressure–volume-induced stress occurs, IGF-1 increases, and myostatin activation increases as a compensatory function. Insulin-like growth factor-1 may serve to increase cardiomyocyte growth and hypertrophy in an effort to maintain haemodynamics or as a pathological response to myocardial stress, whereas myostatin may act in an opposite fashion to limit unrestrained cellular growth, possibly to prevent the untoward effects of overcompensated myocardial growth as a homeostatic function, as suggested by other investigators.22 This hypothesis is supported by several in vitro and in vivo studies. Shyu et al.11 have demonstrated that cyclic stretch increases IGF-1, but also increases myostatin expression. Further evidence for a counter-regulatory mechanism is found in the control of Akt activation, a serine threonine kinase critical to enhanced myocyte growth and size.18 Myostatin decreases Akt activity,12,18 and IGF-1, via binding to IGF1-R, a tyrosine kinase receptor, ultimately leads to Akt activation.22
With LVAD support and resultant loss in pressure–volume overload stimulus, IGF-1-mediated signalling that limits the actions of myostatin may be lost, allowing the inhibition of myocyte growth and regression of hypertrophy. Indeed, profound changes in myocyte size correlate with the levels of the propeptide in DCM patients—myocyte size was reduced by >30% after LVAD support, a finding that may potentially be attributed to the increased activation of myostatin that was observed. Our hypothesis also predicts reduced myostatin activation after longer durations of mechanical support—as systemic haemodynamics and ventricular stress normalize with longer duration of support, myostatin activation and, conversely, IGF-1 activation are expected to decrease.22 We did, in fact, observe lower myostatin propeptide levels in ICM and DCM patients with LVAD support for >150 days, as predicted by our hypothesis, but larger sample sizes are required to confirm this preliminary finding.
An increase in myostatin activation was not found, however, in the ICM patients. This may be due to the extensive scarring typically found in the apical and ventricular free walls after large myocardial infarction which prevents substantial ventricular reverse remodelling during LVAD support; geometric and size changes are less pronounced in these patients and contribute to the low rate of myocardial recovery after LVAD support.23,24 Finally, it must be noted that age, duration of CHF or LVAD support, pre-LVAD ejection fraction, and other clinical demographics did not correlate with myostatin activation, despite differences in the LVAD, CHF, and normal populations. Prior reports have described no variance in myostatin levels based upon age.25
We observed that levels of full-length myostatin were comparable with normal in both the ICM and DCM groups in the HF state (i.e. pre-LVAD). Cleaved propeptide levels, in contrast, were significantly elevated in both ICM and DCM in HF, suggesting that full-length myostatin is maintained at a constant level with a self-renewal mechanism, regardless of the rate of cleavage (activation); both the myostatin propeptide or the active C-terminal dimer may be involved in feedback stimulus for the full-length protein.26,27
Although Smad2/3 continued to be activated compared with normal controls after mechanical unloading, activation did not increase compared with pre-LVAD levels. However, we did observe an increase in Erk1/2 activation following mechanical unloading, and it has been demonstrated that Erk1/2 can mediate cellular effects downstream of myostatin stimulation.28 These effects may be independent of Smad signalling,28,29 which may explain why a further increase in Smad activation was not observed with mechanical unloading; neither Smad nor Erk signalling are direct markers of myostatin activation. It is also possible that myostatin activates different downstream pathways (Smad vs. Erk) depending on the local environment (HF vs. mechanical unloading).
The increased levels of circulating myostatin latent complex may serve as a reservoir for local activation in the heart by BMP-1, which has been previously implicated as an activator of the myostatin latent complex.3 Indeed, we observed that elevated expression of BMP-1 correlates with increased activation of myostatin in HF. Bone morphogenic protein-1 expression did not further increase with myostatin activation after LVAD support in the DCM patients, but this may indicate that the upregulated BMP-1 enzyme was not yet saturated or that another matrix metalloprotease may be involved in myostatin activation with mechanical unloading vs. HF.
Although the actions of myostatin on the remodelling heart may be beneficial, circulating myostatin may have deleterious effects on skeletal muscle of HF patients, which may contribute to cardiac cachexia.30 Increased serum myostatin has also been observed in other disease states, such as HIV-associated cachexia31 and a subset of patients with Duchenne muscular dystrophy.32 In this study, we could not show a relationship between circulating myostatin and either exercise capacity or BMI. Our study cohort of predominantly obese HF patients do not reflect the cachectic state of more advanced disease. However, these findings remain important, as this is the first report of increased circulating myostatin associated with HF. The role of circulating myostatin in regulating the periphery will require further study in more advanced disease patient populations.
The clinical application of a myostatin inhibitor may not be appropriate in the advanced HF state, but its application after LVAD support to promote myocardial recovery is an intriguing and important concept, as only 5% of patients with chronic HF demonstrate sufficient recovery to allow LVAD explantation without transplantation.24 There is evidence to suggest that prolonged support may not be the optimal strategy for patients to recover function. When examining data from the LVAD Working Group Study,23 ejection fraction initially improves after LVAD implantation, but after 120 days, falls significantly. Although it is currently unknown whether myostatin levels return to pre-HF levels after prolonged support, inhibition of myostatin before further functional deterioration takes place may represent a method to improve heart function. Adjunctive pharmacological therapy during LVAD support formed the basis for the use of clenbuterol (a β-adrenergic agonist with potent anabolic properties) to produce physiological myocyte hypertrophy during LVAD unloading.33 Myostatin inhibition may therefore offer an alternative approach to clenbuterol to promote recovery by altering growth signalling pathways and attenuating cellular changes during LVAD support. Close and cautious study will be required, especially in ICM patients or patients with prolonged LVAD support, as inhibition of compensatory mechanisms could worsen the HF state.
In summary, we have shown that cardiac myostatin propeptide is elevated in HF patients of both ICM and DCM aetiology, and that LVAD support increases propeptide levels in DCM patients. Increased myostatin activation by propeptide cleavage was suggested by the presence of increased Smad phosphorylation and associated with an increase in BMP1 expression. These findings correlate with a reduction in cellular size that may be attributable to downstream effects of myostatin.
Funding
This work was supported in part by a grant from the National Institutes of Health T32-HL07854 (I.G.) and T32-HL007748 (L.T.B), by a Wellstone Muscular Dystrophy Cooperative Center Grant (U54-AR052646) to H.L.S, and by a grant from Parent Project Muscular Dystrophy (S.M. and H.L.S.).
Conflict of interest: none declared.
References
- 1.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-B superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. doi:10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 2.Jiang MS, Liang LF, Wang S, Ratovitski T, Holmstrom J, Barker C, Stotish R. Characterization and identification of the inhibitory domain of GDF-8 propeptide. Biochem Biophys Res Commun. 2004;315:525–531. doi: 10.1016/j.bbrc.2004.01.085. doi:10.1016/j.bbrc.2004.01.085. [DOI] [PubMed] [Google Scholar]
- 3.Wolfman NM, McPherron AC, Pappano WM, Davies MV, Song K, Tomkinson KN, Wright JF, Zhao L, Sebald SM, Greenspan DS, Lee SJ. Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases. PNAS. 2003;100:15842–15846. doi: 10.1073/pnas.2534946100. doi:10.1073/pnas.2534946100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L. Myostatin signals through a transforming growth factor β-like signaling pathway to block adipogenesis. Mol Cell Biol. 2003;23:7230–7242. doi: 10.1128/MCB.23.20.7230-7242.2003. doi:10.1128/MCB.23.20.7230-7242.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu X, Topouzis S, Liang L, Stotish R. Myostatin signaling through Smad2 Smad3, and Smad4 is regulated by the inhibitory Smad7 by a negative feedback mechanism. Cytokine. 2004;26:262–272. doi: 10.1016/j.cyto.2004.03.007. doi:10.1016/j.cyto.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Joulia D, Bernardi H, Garandel V, Rabenoelina F, Vernus B, Cabello G. Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin. Exp Cell Res. 2003;286:263–275. doi: 10.1016/s0014-4827(03)00074-0. doi:10.1016/S0014-4827(03)00074-0. [DOI] [PubMed] [Google Scholar]
- 7.Yang W, Zhang Y, Li Y, Wu Z, Zhu D. Myostatin induces cyclin D1 degradation to cause cell cycle arrest through a phosphatidylinositol 3-kinase/AKT/GSK-3 beta pathway and is antagonized by insulin-like growth factor 1. J Biol Chem. 2007;282:3799–3808. doi: 10.1074/jbc.M610185200. doi:10.1074/jbc.M610185200. [DOI] [PubMed] [Google Scholar]
- 8.Zhu J, Li Y, Shen W, Qiao C, Ambrosio F, Lavasani M, Nozaki M, Branca MF, Huard J. Relationships between transforming growth factor-beta1, myostatin, and decorin: implications for skeletal muscle fibrosis. J Biol Chem. 2007;282:25852–25863. doi: 10.1074/jbc.M704146200. doi:10.1074/jbc.M704146200. [DOI] [PubMed] [Google Scholar]
- 9.Li ZB, Kollias HD, Wagner KR. Myostatin directly regulates skeletal muscle fibrosis. J Biol Chem. 2008;283:19371–19378. doi: 10.1074/jbc.M802585200. doi:10.1074/jbc.M802585200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma M, Kambadur R, Matthews KG, Somers WG, Devlin GP, Conaglen JV, Fowke PJ, Bass JJ. Myostatin, a transforming growth factor-β superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J Cell Phys. 1999;180:1–9. doi: 10.1002/(SICI)1097-4652(199907)180:1<1::AID-JCP1>3.0.CO;2-V. doi:10.1002/(SICI)1097-4652(199907)180:1<1::AID-JCP1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 11.Shyu KG, Ko WH, Yang WS, Wang BW, Kuan P. Insulin-like growth factor-1 mediates stretch induced upregulation of myostatin expression in neonatal rat cardiomyocytes. Cardiovasc Res. 2005;68:405–414. doi: 10.1016/j.cardiores.2005.06.028. doi:10.1016/j.cardiores.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 12.Morissette MR, Cook SA, Foo SY, McKoy G, Ashida N, Novikov M, Scherrer-Crosbie M, Li L, Matsui T, Brooks G, Rosenweig A. Myostatin regulates cardiomyocyte growth through modulation of Akt signaling. Circ Res. 2006;99:15–24. doi: 10.1161/01.RES.0000231290.45676.d4. doi:10.1161/01.RES.0000231290.45676.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shyu KG, Lu MJ, Wang BW, Sun HY, Chang H. Myostatin expression in ventricular myocardium in a rat model of volume-overload heart failure. Eur J Clin Invest. 2006;36:713–719. doi: 10.1111/j.1365-2362.2006.01718.x. doi:10.1111/j.1365-2362.2006.01718.x. [DOI] [PubMed] [Google Scholar]
- 14.Artaza JN, Reisz-Porsasz S, Dow JS, Kloner RA, Tsao J, Bhasin S, Gonzalez-Cadavid NF. Alterations in myostatin expression are associated with changes in cardiac left ventricular mass but not ejection fraction in the mouse. J Endocrin. 2007;194:63–74. doi: 10.1677/JOE-07-0072. doi:10.1677/JOE-07-0072. [DOI] [PubMed] [Google Scholar]
- 15.George I, Xydas S, Mancini DM, Lamanca J, DiTullio M, Marboe CC, Shane E, Schulman AR, Colley PM, Petrilli CM, Naka Y, Oz MC, Maybaum S. Effect of Clenbuterol on cardiac and skeletal muscle function during left ventricular assist device support. J Heart Lung Transplant. 2006;9:1084–1090. doi: 10.1016/j.healun.2006.06.017. doi:10.1016/j.healun.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA. 2001;98:9306–9311. doi: 10.1073/pnas.151270098. doi:10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296:1486–1488. doi: 10.1126/science.1069525. doi:10.1126/science.1069525. [DOI] [PubMed] [Google Scholar]
- 18.Cook SA, Matsui T, Ling L, Rosenweig A. Transcriptional effects of chronic Akt activation in the heart. J Biol Chem. 2002;277:22528–22533. doi: 10.1074/jbc.M201462200. doi:10.1074/jbc.M201462200. [DOI] [PubMed] [Google Scholar]
- 19.Reisz-Porszasz S, Bhasin S, Artaza JN, Shen R, Sinha-Hikim I, Hogue A, Fielder TJ, Gonzalez-Cadavid NF. Lower skeletal muscle mass in male transgenic mice with muscle-specific overexpression of myostatin. Am J Physiol Endocrinol Metab. 2003;285:E876–E888. doi: 10.1152/ajpendo.00107.2003. [DOI] [PubMed] [Google Scholar]
- 20.Zafeiridis A, Jeevanandam V, Houser SR, Margulies KB. Regression of cellular hypertrophy after left ventricular assist device support. Circulation. 1998;98:656–662. doi: 10.1161/01.cir.98.7.656. [DOI] [PubMed] [Google Scholar]
- 21.Nagoshi T, Matsui T, Aoyama T, Leri A, Anversa P, Li L, Ogawa W, del Monte F, Gwathmey JK, Grazette L, Hemming BA, Kass DA, Champion HC, Rosenzweig A. PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J Clin Invest. 2005;115:2128–2138. doi: 10.1172/JCI23073. doi:10.1172/JCI23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaussin V, Depre C. Myostatin, the cardiac chalone of insulin-like growth factor-1. Cardiovasc Res. 2005;68:347–349. doi: 10.1016/j.cardiores.2005.09.007. doi:10.1016/j.cardiores.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Maybaum S, Mancini D, Xydas S, Starling RC, Aaronson K, Pagani FD, Miller LW, Margulies K, McRee S, Frazier OH, Torre-Amione G LVAD Working Group. Cardiac improvement during mechanical circulatory support: a prospective multicenter study of the LVAD Working Group. Circulation. 2007;115:2497–2505. doi: 10.1161/CIRCULATIONAHA.106.633180. doi:10.1161/CIRCULATIONAHA.106.633180. [DOI] [PubMed] [Google Scholar]
- 24.Mancini DM, Beniaminovitz A, Levin H, Catanese K, Flannery M, DiTullio M, Savin S, Cordisco ME, Rose E, Oz MC. Low incidence of myocardial recovery after left ventricular assist device implantation in patients with chronic heart failure. Circulation. 1998;98:2383–2389. doi: 10.1161/01.cir.98.22.2383. [DOI] [PubMed] [Google Scholar]
- 25.Welle S, Bhatt K, Shah B, Thornton CA. Insulin-like growth factor-1 and myostatin mRNA expression in muscle: comparison between 62-77 and 21-1 yr old men. Exp Gerontol. 2002;37:833–839. doi: 10.1016/s0531-5565(02)00025-6. doi:10.1016/S0531-5565(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 26.Forbes D, Jackman M, Bishop A, Thomas M, Kambadur R, Sharma M. Myostatin auto-regulates its expression by feedback loop through Smad7 dependent mechanism. J Cell Physiol. 2006;206:264–272. doi: 10.1002/jcp.20477. doi:10.1002/jcp.20477. [DOI] [PubMed] [Google Scholar]
- 27.Zhu X, Topouzis S, Liang LF, Stotish RL. Myostatin signaling through Smad2, Smad3 and Smad4 is regulated by the inhibitory Smad7 by a negative feedback mechanism. Cytokine. 2004;26:262–272. doi: 10.1016/j.cyto.2004.03.007. doi:10.1016/j.cyto.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Yang W, Chen Y, Zhang Y, Wang X, Yang N, Zhu D. Extracellular signal-regulated kinase ½ mitogen-activated protein kinase pathway is involved in myostatin-regulated differentiation repression. Cancer Res. 2006;66:1320–1326. doi: 10.1158/0008-5472.CAN-05-3060. doi:10.1158/0008-5472.CAN-05-3060. [DOI] [PubMed] [Google Scholar]
- 29.Philip B, Lu Z, Gao Y. Regulation of GDF-8 signaling by the p39 MAPK. Cell Signal. 2005;17:365–375. doi: 10.1016/j.cellsig.2004.08.003. doi:10.1016/j.cellsig.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Hoenig MR. Hypothesis: myostatin is a mediator of cardiac cachexia. Int J Cardiol. 2007;124:131–133. doi: 10.1016/j.ijcard.2007.07.058. doi:10.1016/j.ijcard.2007.07.058. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Cadavid NF, Taylor WE, Yarasheski K, Sinha-Hikim I, Ma K, Ezzat S, Shen R, Lalani R, Asa S, Mamita M, Nair G, Arver S, Bhasin S. Organization of the human myostatin gene and expression in healthy men and HIV-infected men with muscle wasting. Proc Natl Acad Sci USA. 2008;95:14938–14943. doi: 10.1073/pnas.95.25.14938. doi:10.1073/pnas.95.25.14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Awano H, Takeshima Y, Okizuka Y, Saiki K, Yagi M, Matsuo M. Wide ranges of serum myostatin concentrations in Duchenne muscular dystrophy patients. Clinica Chimica Acta. 2008;391:115–117. doi: 10.1016/j.cca.2008.01.024. doi:10.1016/j.cca.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 33.Birks EJ, Tansley PD, Hardy J, George RS, Bowles CT, Burke M, Banner NR, Khaghani A, Yacoub MH. Left ventricular assist device and drug therapy for the reversal of heart failure. New Eng J Med. 2006;355:1873–1884. doi: 10.1056/NEJMoa053063. doi:10.1056/NEJMoa053063. [DOI] [PubMed] [Google Scholar]