Abstract
Few studies have evaluated age and racial/ethnic differences in the prevalence of symptoms in HIV infection. Thus, the objective of this study was to compare the prevalence of gastrointestinal, metabolic, general malaise, neurologic, or other self-reported symptoms by age and race/ethnicity among 1,574 HIV-infected women enrolled in the Women’s Interagency HIV Study and 955 HIV-infected men who have sex with men (MSM) enrolled in the Multicenter AIDS Cohort Study. All patients had known dates of initiation of highly-active antiretroviral therapy. It was observed that women ≥50 years were less likely to experience gastrointestinal symptoms (24% vs. 27%, multivariable p=0.024), but more likely to experience general malaise (47% vs. 37%; multivariable p=0.004), neurologic (44% vs. 38%; multivariable p=0.048), or other symptoms (40% vs. 28%; multivariable P<0.001) compared to women <40 years of age. Only neurologic symptoms had a higher prevalence among older MSM (52% vs. 37%; multivariable P=0.002), largely driven by paresthesias (48% vs. 31%; multivariable p=0.004), the most common individual symptom reported by men. Caucasian women generally had the highest prevalence of symptoms and African-American women had the lowest prevalence. Few racial/ethnic differences were noted for MSM. Depression and a prior diagnosis of AIDS were the strongest and most consistent predictors of clinical symptoms in both cohorts. In summary, the prevalence of reported symptoms varies with patient race/ethnicity, age, and modifiable factors such as depression and HIV disease stage. Clinicians should consider these factors when counseling patients regarding potential adverse effects of antiretrovirals or symptoms associated with HIV disease.
Keywords: Highly active antiretroviral therapy, symptoms, gender, race/ethnicity, aging
Introduction
Reductions of 70% or more of new AIDS diagnoses and death have been reported in the highly-active antiretroviral therapy (HAART) era (1-5). As a result, older HIV-infected patients are one of the fastest growing demographics, with a quintupling of AIDS patients in the U.S. over 50 years of age between 1990-2000 (6). The HIV epidemic also has disproportionately affected minority populations, particularly African Americans. In the U.S., among the growing number of HIV/AIDS cases in women, 80% were from minority populations, consisting of African Americans (64%) and Hispanics (15%) (7).
Prior studies have justifiably focused on modifiers of the effectiveness of HAART (2-4), but few have focused on potential demographic differences in adverse effects of HAART (8). Serious, often life-threatening, adverse events have been attributed to antiretrovirals, including liver, kidney and cardiovascular dysfunction (8-13). Symptoms, however, are much more common and directly impact the quality of life of the majority of HIV-infected patients. The etiology of symptoms in HIV patients is complex and may be related to HIV infection, HAART use, or both, and further may vary according to demographic or clinical characteristics of HIV patients.
We recently evaluated the prevalence of symptoms among women enrolled in the Women’s Interagency HIV Study (WIHS), a multicenter cohort study primarily consisting of women from minority racial/ethnic groups. We reported that two-thirds of all HIV-infected women experienced at least one symptom, most commonly diarrhea, nausea/vomiting, body fat redistribution, myalgias and paresthesias (14). Few others have comprehensibly examined the prevalence of individual symptoms in the HAART era (9, 11, 12, 15, 16). A recent study confirmed the high prevalence of symptoms among HIV patients in palliative care, and further indicated that few symptoms were being adequately treated (17). Thus, we extend our previous work to HIV-infected and demographically similar HIV-uninfected subjects enrolled in the WIHS and the Multicenter AIDS Cohort Study (MACS), a multicenter cohort study of men who have sex with men (MSM). Our objective was to present a comprehensive analysis of factors associated with self-reported symptoms, with a specific focus on age and race/ethnicity.
Methods
Study Population, Design and Follow-Up
The WIHS (18) is an ongoing multicenter cohort study of HIV infection among women followed at six month intervals. A total of 2,791 HIV-infected and 975 HIV-uninfected women were enrolled in six U.S. sites: Bronx/Manhattan, NY; Brooklyn, NY; Chicago, IL; Los Angeles, CA; San Francisco, CA; and Washington, DC. The MACS (19) is an ongoing cohort study of HIV infection among MSM also followed at 6 month intervals. A total of 2,883 HIV-infected and 4,089 HIV-uninfected MSM were enrolled in the MACS in four US cities: Baltimore, MD; Pittsburgh, PA; Chicago, IL; and Los Angeles, CA. Semi-annual visits for each cohort involve physical exams and specimen collection, and the administration of detailed questionnaires to ascertain comprehensive sociodemographic, pharmacy, and clinical data including the prevalence of symptoms.
The study population for this analysis consisted of HIV-infected WIHS and MACS participants who provided self-report data on 13 symptoms and who reported HAART initiation between 1995-2004. HAART was defined according to DHHS/Kaiser Panel guidelines (20). Participants in racial/ethnic categories other than African American, Caucasian or Hispanic were excluded due to small sample size. Follow-up was also restricted to years when symptoms were comprehensibly assessed in WIHS (between October 1999 and April 2004) and MACS (between April 2001 and April 2004). Of 2,791 HIV-infected WIHS participants and 2,883 HIV-infected MACS participants, 1,867 and 1,312, respectively had initiated HAART by 2004. Study exclusions resulted in a final study population of 1,574 WIHS and 955 MACS participants. For comparison, and to help disentangle the effects of HIV and HAART use on the occurrence of symptoms, we also studied HIV-uninfected participants in WIHS and MACS with available symptom data and who were followed during same study years as HIV-infected participants. HIV-uninfected comparators included 711 WIHS and 1126 MACS participants.
Study Parameters
At each study visit, WIHS and MACS participants were asked if they experienced certain symptoms at any time since the last semi-annual study visit. Study endpoints consisted of the following 13 symptoms which were assessed in both cohorts: 1) gastrointestinal: nausea/vomiting, abdominal pains or cramps, diarrhea; 2) metabolic: fever, body fat redistribution (i.e., “shifting of your body fat” in WIHS and “fat maldistribution or abnormal changes in body fat” in MACS); 3) general malaise: fatigue (i.e., “drowsiness/tiredness”), myalgias (i.e., “muscle aches or pains”); 4) neurologic: paresthesias (i.e., “pain/tingling in feet or hands”), dizziness, headaches; and, 5) other: rash, weight loss, night sweats.
The primary predictors of interest were age (<40, 40-49, ≥50 years) and race/ethnicity (African American, Caucasian, Hispanic). Additional parameters examined were CD4+ T-cell counts (per 100 cell increase), HIV RNA levels (per 1 log increase), prior report of clinical AIDS, years since initiation of HAART (≤1, 1-4, ≥4 years), HAART class (no antiretrovirals, protease inhibitor [PI]-containing, PI-sparing), alcohol intake since the last visit (0, 1, ≥2 drinks per week), body mass index (BMI) (<25, 25-30, ≥30 kg/m2 ), education level (<high school, high school, some college or graduate training), and presence of depression defined as Center for Epidemiological Studies Depression (CES-D) Scale ≥16. Additionally, HAART use was categorized as: (1) stable (i.e., reporting the same HAART regimen between study visits); (2) switched (i.e., reporting a different HAART regimen between study visits); (3) discontinued all antiretrovirals, or; (4) discontinued some but not all antiretrovirals (i.e., reporting a transition from HAART use to either monotherapy or two-drug regimens). Since CES-D is measured only at every other visit in WIHS, we examined depression only at HAART initiation. All other parameters were time-updated over follow-up.
Statistical Methods
The unit of observation for analyses was a pair of study visits one year or less apart after the initiation of HAART. WIHS participants contributed up to eight pairs to analyses between WIHS visits 11 (October 1999 - March 2000) and 19 (October 2003 - March 2004). MACS participants contributed up to five pairs between MACS visits 35 (April 2001 - September 2001) and 40 (October 2003 - March 2004). Time-updated exposures were assessed at the first visit in a pair of visits and symptoms at the second visit in the pair, thus ensuring the assessment of exposure prior to the outcome. The exception to this convention was the measurement of the pattern of HAART use (e.g., stable, switched or discontinued HAART), which was assessed during the interval defined by the pair of visits.
Since there were substantial differences in demographics between cohorts, analyses were stratified, and, therefore, symptoms were not directly compared by gender. Analyses compared the prevalence of each of 13 symptoms by age and race/ethnicity within each cohort. We also determined the prevalence of symptoms by category (i.e., gastrointestinal, metabolic, general malaise, neurologic, and other) and overall as presence of one or more symptoms. For each outcome measure, fully adjusted logistic regression models were constructed to evaluate the association of age and race/ethnicity. Models for HIV-uninfected participants adjusted for all factors except HIV or antiretroviral specific parameters. Generalized estimating equation methods (21) using PROC GENMOD in SAS (Version 9.0, Cary, NC) accounted for within-individual correlation between repeated observations, using robust standard errors and an exchangeable working correlation structure.
Results
Study participants included 1,574 HIV-infected women enrolled in WIHS who contributed 8,253 paired visits (median five pairs per person), and 955 HIV-infected MSM enrolled in MACS who contributed 2,919 paired visits (median 3 pairs per person). As demonstrated in Table 1, there were substantial differences in clinical and demographic characteristics between groups, which reflect the very different target populations for the WIHS and MACS. Compared to WIHS participants, MACS participants were older, more likely Caucasian, had higher education levels, had more alcohol consumption, had lower levels of depression, and had lower levels of obesity. MACS participants also had more stable HAART use, higher use of PIs, and improved HIV parameters.
Table 1. Baseline Comparison of HIV-Infected Participants a.
| WIHS | MACS | |
|---|---|---|
| Number of participants | 1574 | 955 |
| Number of paired visits | 8253 | 2919 |
| Age category, % | ||
| <40 years | 56.6 | 29.2 |
| 40 - 50 years | 34.5 | 46.3 |
| ≥50 years | 8.9 | 24.5 |
| Race/ethnicity, % | ||
| African American | 55.8 | 26.4 |
| Hispanic | 30.0 | 12.6 |
| Caucasian | 14.2 | 61.1 |
| Baseline education | ||
| < High school education | 38.4 | 6.8 |
| Completed high school | 29.4 | 17.2 |
| > High school education | 32.2 | 76.0 |
| Alcohol consumption | ||
| none | 59.9 | 23.4 |
| 1 drink/week | 18.4 | 28.7 |
| ≥2 drink/week | 21.7 | 47.9 |
| Baseline CES-D score ≥16 | 51.1 | 27.6 |
| Body Mass Index | ||
| <24.9 | 37.3 | 56.1 |
| 25.0-29.9 | 32.7 | 35.2 |
| ≥30.0 | 30.1 | 8.7 |
| Prior diagnosis of AIDS, % | 36.5 | 15.6 |
| Mean CD4+ cell count, cells/μl | 438.2 | 526.0 |
| Mean log HIV RNA, copies/ml | 7.1 | 5.7 |
| Mean years since HAART initiation | 2.4 | 3.8 |
| Pattern of HAART use | ||
| Stable use | 54.7 | 62.9 |
| Switched regimens | 14.7 | 18.3 |
| Discontinue some ARVs | 24.8 | 14.1 |
| Discontinue all ARVs | 5.8 | 4.6 |
| HAART class | ||
| No ARVs | 16.9 | 9.2 |
| PI-containing | 43.1 | 50.1 |
| PI-sparing | 39.9 | 40.7 |
ARV = antiretrovirals; CES-D = Center for Epidemiological Studies Depression; HAART = highly-active antiretroviral therapy; PI = protease inhibitor.
All comparisons by cohortwere P<0.001 using Pearson’s Chi-square test for categorical variables and Wilcoxon rank-sum test for continuous variables.
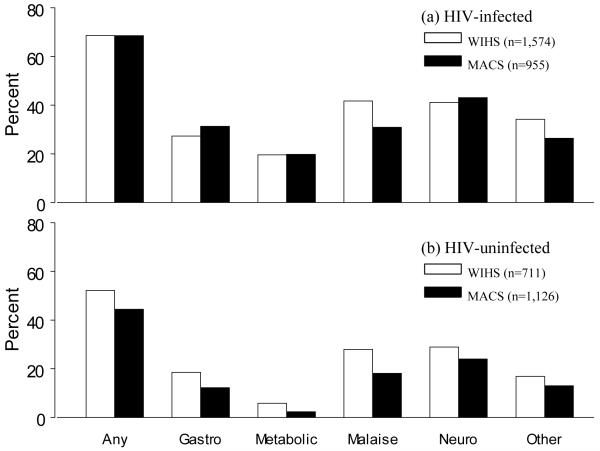
The frequency and type of reported symptoms among HIV-infected WIHS and MACS study participants were similar (Figure 1; top panel). The most commonly reported categories of symptoms overall were neurologic (41.4% in WIHS, 43.1% in MACS) and general malaise (41.7% in WIHS and 30.9% in MACS). The average number of symptoms reported by participants at each visit were 2.2 (standard deviation [SD] 2.5) and 1.9 (SD 2.1) in WIHS and MACS, respectively. Overall, more than two-thirds of participants in each cohort reported at least one symptom at a given study visit, with 24.5% and 19.6% in WIHS and MACS, respectively, reporting four or more symptoms. The frequency and type of reported symptoms among HIV-uninfected participants were also similar by cohort, but at lower levels compared to HIV-infected participants (Figure 1; bottom panel).
Figure 1.
Symptoms reported by HIV-infected and HIV-uninfected study participants. Top panel presents percent of HIV-infected participants with any, gastrointestinal (gastro), metabolic, malaise, neurologic (neuro), or other symptoms in Women’s Interagency HIV Study (WIHS) (white bars) and Multicenter AIDS Cohort Study (MACS) (black bars). Bottom panel presents the same for HIV-uninfected participants.
Association of Age and Symptoms in HIV Patients
In WIHS, the average number of symptoms reported by age were 1.9 (SD 2.3), 2.5 (SD 2.6), and 2.4 (SD 2.5) among those <40, 40-50 and ≥50 years, respectively. The corresponding ORs by age for the prevalence of one or more symptoms in WIHS were 1.2 (95% CI= 1.0, 1.4) and 1.2 (95% CI= 0.9, 1.6) in the two older age groups, respectively, compared to those younger than 40 years (Table 2). In MACS, the average number of symptoms reported by age were 1.7 (SD 2.1), 1.9 (SD 2.2), and 2.1 (SD 2.1) among those <40, 40-50 and ≥50 years, respectively. The corresponding ORs by age for the prevalence of one or more symptoms in MACS were 1.2 (95% CI= 0.8, 1.7) and 1.8 (95% CI= 1.2, 2.7) in the two older age categories, respectively (Table 2).
Table 2. Prevalence of Symptoms by Age.
| Age in WIHS |
Age in MACS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <40 |
40-50 |
≥50 |
<40 |
40-50 |
≥50 |
|||||
| % | % | OR a | % | OR a | % | % | OR a | % | OR a | |
| Gastrointestinal | 26.7 | 28.8 | 0.91 | 24.3 | 0.77 b | 29.9 | 30.9 | 1.35 | 32.8 | 1.45 |
| Nausea/vomiting | 12.7 | 12.9 | 0.86 | 10.6 | 0.78 | 15.4 | 13.8 | 1.11 | 13.0 | 1.01 |
| Abdominal pain or cramps | 15.2 | 15.9 | 0.83 b | 12.6 | 0.73 b | 13.5 | 12.8 | 1.12 | 15.5 | 1.54 |
| Diarrhea | 9.3 | 11.7 | 1.02 | 10.6 | 0.91 | 15.2 | 19.9 | 1.35 | 19.3 | 1.27 |
| Metabolic | 15.9 | 23.0 | 1.20 b | 21.8 | 1.27 | 14.7 | 19.1 | 1.02 | 24.2 | 1.24 |
| Fever | 6.0 | 8.6 | 1.13 | 7.4 | 1.11 | 4.7 | 5.4 | 0.65 | 3.7 | 0.49 |
| Body fat redistribution | 11.1 | 17.0 | 1.27 b | 16.4 | 1.34 | 10.5 | 15.0 | 1.18 | 21.3 | 1.60 |
| General Malaise | 36.5 | 45.8 | 1.26 b | 46.6 | 1.40 b | 28.1 | 30.1 | 1.33 | 34.1 | 1.56 |
| Fatigue | 29.2 | 34.4 | 1.08 | 31.3 | 1.14 | 21.9 | 23.3 | 1.14 | 25.0 | 1.33 |
| Myalgias | 20.3 | 29.9 | 1.46 c | 34.2 | 1.70 c | 14.2 | 16.2 | 1.30 | 20.6 | 1.63 |
| Neurologic | 37.9 | 43.7 | 1.04 | 44.0 | 1.25 b | 37.2 | 39.7 | 1.07 | 51.9 | 1.92 b |
| Paresthesias | 15.5 | 23.7 | 1.36 b | 29.0 | 1.86 c | 30.6 | 35.8 | 0.97 | 47.9 | 1.84 b |
| Dizziness | 9.7 | 13.5 | 1.13 | 13.4 | 1.27 | 3.8 | 4.3 | 1.60 | 6.0 | 2.10 |
| Headaches | 28.3 | 27.6 | 0.82 b | 22.9 | 0.88 | 10.5 | 6.9 | 0.87 | 6.0 | 0.87 |
| Other | 28.0 | 38.9 | 1.40 c | 40.3 | 1.49 c | 26.9 | 27.8 | 1.03 | 23.9 | 0.92 |
| Rash | 8.7 | 10.8 | 1.08 | 8.8 | 0.94 | 12.5 | 16.6 | 1.27 | 11.9 | 0.94 |
| Weight loss | 13.1 | 18.8 | 1.35 c | 20.7 | 1.50 b | 9.0 | 6.7 | 0.66 | 7.5 | 0.80 |
| Night sweats | 13.9 | 23.5 | 1.40 c | 23.9 | 1.51 b | 12.2 | 10.0 | 0.80 | 10.3 | 0.98 |
| One or more symptoms | 64.0 | 72.6 | 1.19 b | 72.0 | 1.22 | 64.6 | 65.8 | 1.15 | 74.8 | 1.77 b |
OR Odds ratio; WIHS, Women’s Interagency HIV Study; MACS, Multicenter AIDS Cohort Study.
Logistic regression models accounted for correlated data and adjusted for age, race,AIDS, CD4+ cell count, viral load, years on highly-active antiretroviral therapy (HAART), pattern of HAART use, HAART class, alcohol, BMI, CES-D, education. OR compared to <40 years (reference group) within gender.
P-value < 0.05.
P-value < 0.001.
In WIHS, individual symptoms elevated in older age categories included body fat redistribution, myalgias, paresthesias, weight loss and night sweats. Each of these symptoms, except body fat redistribution, was significantly elevated in both older age groups with evidence of an increasing trend with older age. Other symptoms including abdominal pain or cramps, and headaches were significantly lower for older age categories among women. In MACS, the only individual symptom associated with older age was paresthesias: 47.9% of MSM ≥50 years of age reported paresthesias compared to 30.6% of MSM <40 years of age (OR=1.8; 95% CI=1.2, 2.8) (Table 2).
Association of Race/Ethnicity and Symptoms in HIV Patients
The average number of symptoms reported at each WIHS visit by race/ethnicity were 2.1 (SD 2.4), 2.2 (SD 2.5), and 2.6 (SD 2.6) for African Americans, Hispanics, and Caucasians, respectively. The corresponding ORs by race/ethnicity for one or more symptoms were 1.4 (95% CI= 1.1, 1.8) comparing Caucasians to African Americans, and 1.4 (95% CI= 1.1, 1.8) comparing Caucasians to Hispanics (Table 3). The average number of symptoms reported at each MACS visit by race/ethnicity were 1.7 (SD 2.0), 2.1 (SD 2.2), and 2.0 (SD 2.1) for African Americans, Hispanics, and Caucasians, respectively. The ORs for one or more symptoms by race/ethnicity in MACS did not reach statistical significance (p>0.05).
Table 3. Prevalence of Symptoms by Race / Ethnicity.
| Race in WIHS |
Race in MACS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AA |
H |
C |
AA |
H |
C |
|||||
| % | % | OR a | % | OR a | % | % | OR a | % | OR a | |
| Gastrointestinal | 25.5 | 27.0 | 1.18 | 34.2 | 1.61 b | 27.2 | 32.0 | 1.22 | 32.4 | 1.13 |
| Nausea/vomiting | 10.8 | 13.9 | 1.56 b | 16.1 | 1.75 b | 11.0 | 21.5 | 2.33* | 13.7 | 1.58 |
| Abdominal pain or cramps | 14.9 | 14.5 | 0.98 | 17.3 | 1.25 | 12.2 | 15.5 | 1.08 | 14.1 | 0.92 |
| Diarrhea | 8.9 | 10.0 | 1.28 | 16.9 | 2.02 b | 15.0 | 14.8 | 0.92 | 20.4 | 1.03 |
| Metabolic | 18.3 | 20.2 | 1.24 c | 23.5 | 1.39 c | 10.7 | 23.6 | 2.27 c | 22.0 | 1.38 |
| Fever | 7.2 | 7.0 | 1.03 | 8.0 | 1.24 | 3.8 | 4.9 | 0.96 | 4.9 | 1.19 |
| Body fat redistribution | 12.8 | 15.0 | 1.35 c | 18.0 | 1.39 c | 7.2 | 19.0 | 2.78 c | 18.3 | 1.64 |
| General Malaise | 40.3 | 41.3 | 1.17 | 47.5 | 1.40 c | 28.4 | 31.7 | 1.32 | 31.6 | 1.12 |
| Fatigue | 29.2 | 32.8 | 1.34 c | 38.0 | 1.57 b | 20.7 | 26.1 | 1.27 | 24.1 | 1.20 |
| Myalgias | 26.0 | 25.4 | 1.05 | 28.0 | 1.15 | 16.0 | 16.9 | 1.39 | 17.6 | 0.93 |
| Neurologic | 39.6 | 41.0 | 1.16 | 46.9 | 1.46 c | 37.7 | 41.9 | 1.18 | 44.9 | 1.32 |
| Paresthesias | 20.6 | 18.9 | 1.02 | 24.4 | 1.29 | 33.1 | 33.5 | 1.05 | 41.0 | 1.26 |
| Dizziness | 10.0 | 13.9 | 1.64 b | 14.3 | 1.68 c | 3.2 | 6.3 | 2.61 | 5.0 | 1.44 |
| Headaches | 25.8 | 27.9 | 1.17 | 31.2 | 1.51 c | 8.4 | 12.3 | 1.48 | 6.3 | 1.06 |
| Other | 35.9 | 31.9 | 0.84 c | 32.7 | 0.96 | 28.7 | 28.9 | 0.94 | 25.3 | 0.77 |
| Rash | 9.4 | 9.6 | 1.14 | 10.5 | 1.25 | 14.4 | 13.0 | 1.05 | 14.4 | 0.85 |
| Weight loss | 17.8 | 15.9 | 0.85 | 12.7 | 0.64 c | 9.5 | 8.5 | 0.91 | 6.7 | 0.68 |
| Night sweats | 20.4 | 15.8 | 0.73 c | 21.6 | 1.32 | 11.9 | 13.7 | 0.93 | 9.7 | 0.76 |
| 1 or more symptoms | 68.1 | 67.2 | 1.03 | 73.1 | 1.43 c | 62.3 | 70.1 | 1.64 | 70.0 | 1.19 |
AA = African American; H = Hispanic; C = Caucasian; OR = Odds ratio; WIHS = Women’s Interagency HIV Study; MACS = Multicenter AIDS Cohort Study.
Logistic regression models accounted for correlated data and adjusted for age, race,AIDS, CD4+ cell count, viral load, years on highly-active antiretroviral therapy (HAART,) pattern of HAART use, HAART class , alcohol, BMI, CES-D, education. OR compared to AA (reference group) within gender.
P-value < 0.001.
P-value < 0.05.
In WIHS, Caucasian women had the highest prevalence for virtually all individual symptoms. In multivariable models, Caucasian women, compared to African Americans, had significantly higher odds of abdominal cramps, diarrhea, body fat redistribution, fatigue, dizziness, and headaches, and a lower odds of weight loss. Hispanic women had a higher odds of nausea/vomiting, body fat redistribution, fatigue, and dizziness, and a lower odds of night sweats compared to African Americans. Finally, Caucasian women had significantly increased odds of only diarrhea and night sweats compared to Hispanics. Fewer differences were noted for individual symptoms by race among MACS participants. Hispanic MSM, compared to African-American MSM, had a higher odds of reporting nausea/vomiting (OR=2.3; 95% CI= 1.1, 5.1), and body fat redistribution (OR=2.8; 95% CI= 1.2, 6.2). No statistically significant differences were noted for symptoms comparing Caucasians to Hispanic or African-American MSM.
Other Factors Associated with Symptoms in HIV Patients
The strongest predictors of symptoms were depression (i.e., CES-D score ≥16) and a prior diagnosis of clinical AIDS (Table 4 for WIHS and Table 5 for MACS). Depression was associated with ORs for one or more symptoms of 2.5 (95% CI= 2.1, 2.9) and 1.8 (95% CI= 1.3, 2.5) in WIHS and MACS, respectively. Clinical AIDS was associated with ORs of 1.7 (95% CI= 1.4, 2.0) and 2.1 (95% CI= 1.4, 3.0) in WIHS and MACS, respectively. These factors were also associated with all symptom categories (except depression for metabolic symptoms in MACS). Other consistent predictors of symptoms in each cohort included PI-containing HAART regimens, discontinuation or switching of HAART, and higher HIV RNA levels.
Table 4. Adjusteda Odds Ratios for Symptoms Reported by HIV-Infected Women.
| Gastro | Metabolic | Malaise | Neuro | Other | ≥ One symptom(s) | |
|---|---|---|---|---|---|---|
| Age, years | ||||||
| ≥50 | 0.77 b | 1.27 | 1.40 b | 1.25 b | 1.49 c | 1.22 |
| 40 to 50 | 0.91 | 1.20 b | 1.26 b | 1.04 | 1.40 c | 1.19 b |
| <40 | 1 | 1 | 1 | 1 | 1 | 1 |
| Race/ethnicity | ||||||
| Caucasian | 1.61 c | 1.39 b | 1.40 b | 1.46 b | 0.96 | 1.43 b |
| Hispanic | 1.18 | 1.24 b | 1.17 | 1.16 | 0.84 b | 1.03 |
| African American | 1 | 1 | 1 | 1 | 1 | 1 |
| Baseline education | ||||||
| ≥Some college | 1.38 b | 1.38 b | 1.30 b | 1.22 | 0.84 | 1.17 |
| Completed high school | 1.02 | 1.08 | 0.94 | 0.94 | 0.89 | 0.94 |
| <Less than high school | 1 | 1 | 1 | 1 | 1 | 1 |
| Alcoholic drinks/week | ||||||
| ≥2 | 0.98 | 0.89 | 1.08 | 0.96 | 1.00 | 0.98 |
| 1 | 0.97 | 0.95 | 1.01 | 1.00 | 0.97 | 0.92 |
| 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| CES-D score ≥16 | 1.98 c | 1.51 c | 2.42 c | 2.33 c | 1.96 c | 2.49 c |
| BMI | ||||||
| ≥30 | 1.02 | 1.12 | 1.13 | 1.25 b | 0.76 b | 1.03 |
| 25.0-29.9 | 0.91 | 0.99 | 0.96 | 1.05 | 0.75 c | 0.88 |
| <25.0 | 1 | 1 | 1 | 1 | 1 | 1 |
| AIDS | 1.48 c | 1.79 c | 1.46 c | 1.45 c | 1.80 c | 1.69 c |
| CD4 T-cells/μl (100) | 0.99 | 1.01 | 1.00 | 0.98 | 0.96 b | 1.00 |
| HIV RNA (log) | 1.04 b | 1.01 | 1.04 b | 1.02 | 1.05 c | 1.03 b |
| Years HAART use | ||||||
| ≥4 | 0.79 b | 0.97 | 0.80 b | 0.74 b | 0.68 c | 0.71 b |
| 1 to 4 | 0.92 | 1.04 | 1.00 | 0.92 | 0.79 b | 0.91 |
| ≤1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Pattern of HAART use | ||||||
| Switched regimens | 1.29 b | 1.38 c | 1.17 b | 1.09 | 0.97 | 1.21 b |
| Discontinue some ARVs | 1.01 | 1.05 | 1.11 | 1.19 | 1.03 | 1.27 |
| Discontinue all ARVs | 1.33 b | 1.32 b | 1.22 b | 1.42 c | 1.52 c | 1.59 c |
| Stable HAART | 1 | 1 | 1 | 1 | 1 | 1 |
| HAART class | ||||||
| No ARVs | 0.79 | 0.78 | 0.95 | 0.81 | 0.80 b | 0.79 |
| PI-containing | 1.35 c | 1.12 | 1.02 | 1.15 b | 0.99 | 1.15 b |
| PI-sparing | 1 | 1 | 1 | 1 | 1 | 1 |
ARV = antiretrovirals; HAART = highly-active antiretroviral therapy; BMI = body mass index.
Multivariable logistic regression models adjusted for all factors simultaneously.
P-value < 0.05.
P-value < 0.001.
Table 5. Adjusteda Odds Ratios for Symptoms Reported by HIV-Infected Men Who Have Sex with Men.
| Gastro | Metabolic | Malaise | Neuro | Other | ≥1 symptoms | |
|---|---|---|---|---|---|---|
| Age, years | ||||||
| ≥50 | 1.45 | 1.24 | 1.56 | 1.92 b | 0.92 | 1.77 b |
| 40 to 50 | 1.35 | 1.02 | 1.33 | 1.07 | 1.03 | 1.15 |
| <40 | 1 | 1 | 1 | 1 | 1 | 1 |
| Race/ethnicity | ||||||
| Caucasian | 1.13 | 1.38 | 1.12 | 1.32 | 0.77 | 1.19 |
| Hispanic | 1.22 | 2.27 b | 1.32 | 1.18 | 0.94 | 1.64 |
| African-American | 1 | 1 | 1 | 1 | 1 | 1 |
| Baseline education | ||||||
| ≥Some college | 2.30 | 5.81 b | 2.01 | 0.54 | 1.80 | 1.33 |
| Completed high school | 2.38 | 3.83 | 2.49 | 0.78 | 1.60 | 1.49 |
| <Less than high school | 1 | 1 | 1 | 1 | 1 | 1 |
| Alcoholic drinks/week | ||||||
| ≥2 | 0.85 | 0.84 | 0.82 | 0.78 | 0.78 | 0.72 b |
| 1 | 0.97 | 0.88 | 0.98 | 0.89 | 0.97 | 0.83 |
| 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| CES-D score ≥16 | 1.89 c | 1.26 | 2.20 c | 1.59 b | 2.01 c | 1.78 c |
| BMI | ||||||
| ≥30 | 1.12 | 1.28 | 1.03 | 0.93 | 0.81 | 0.79 |
| 25.0-29.9 | 0.95 | 0.93 | 0.93 | 0.97 | 0.68 b | 0.77 b |
| <25.0 | 1 | 1 | 1 | 1 | 1 | 1 |
| AIDS | 1.82 c | 1.39 | 2.02 c | 2.13 c | 1.45 b | 2.07 c |
| CD4 T-cells/μl (100) | 0.95 b | 1.04 | 0.97 | 0.99 | 0.96 | 1.00 |
| HIV RNA (log) | 1.07 b | 1.06 | 1.08 b | 1.06 b | 1.07 b | 1.09 b |
| Years HAART use | ||||||
| ≥4 | 0.71 | 1.66 | 1.48 | 0.94 | 0.87 | 1.03 |
| 1 to 4 | 0.71 | 0.87 | 1.65 | 0.97 | 0.77 | 0.96 |
| ≤1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Pattern of HAART use | ||||||
| Switched regimens | 1.33 b | 1.36 b | 1.26 | 1.05 | 1.06 | 0.99 |
| Discontinue some ARVs | 1.26 | 1.00 | 0.77 | 1.25 | 1.21 | 1.03 |
| Discontinue all ARVs | 1.74 b | 1.34 | 2.84 c | 1.42 | 2.08 b | 1.73 |
| Stable HAART | 1 | 1 | 1 | 1 | 1 | 1 |
| HAART class | ||||||
| No ARVs | 0.51 | 0.65 | 0.26 c | 0.64 | 0.53 | 0.48 b |
| PI-containing | 1.62 c | 1.26 | 0.99 | 1.29 b | 0.94 | 1.32 b |
| PI-sparing | 1 | 1 | 1 | 1 | 1 | 1 |
ARV= antiretrovirals; HAART = highly-active antiretroviral therapy; BMI = body mass index.
Multivariable logistic regression models adjusted for all factors simultaneously.
P-value < 0.05.
P-value < 0.001.
Symptoms Among HIV-Uninfected Participants
In WIHS, HIV-uninfected women ≥50 years of age were more likely to report one or more symptoms compared to younger HIV-uninfected women (OR= 1.6; 95% CI= 1.1, 2.3). Caucasian women were also more likely to report one or more symptoms compared to African Americans (OR= 1.8; 95% CI= 1.3, 2.7) and Hispanics (OR= 1.6; 95% CI= 1.1, 2.3). Depression was the only other significant predictor of symptoms for HIV-uninfected women. In MACS, age and race/ethnicity were not significant predictors of one or more symptoms. However, similar to HIV-infected MSM, paresthesias was more common in MSM ≥50 years of age compared to younger MSM (OR= 2.3; 95% CI= 1.4, 3.8). Other significant predictors of one or more symptoms for HIV-uninfected MSM included depression and BMI ≥30.
Discussion
We report on the high prevalence of symptoms among HAART users in two distinct but key segments of the current HIV/AIDS epidemic, that is, MSM and women from minority racial/ethnic groups. Two-thirds of participants receiving HAART in each cohort experienced at least one symptom with about 20-25% in each cohort reporting four or more symptoms. Older age and Caucasian race were associated with a higher prevalence of certain symptoms, particularly among women. However, other factors were stronger and more consistent predictors of symptoms, including depression, clinical AIDS diagnoses, higher HIV RNA levels, changing HAART regimens and PI-containing HAART.
Older age was associated with a higher likelihood of reporting several symptoms among HIV-infected women, including body fat redistribution, myalgias, paresthesias, weight loss and night sweats but only paresthesias was increased among older MSM. Older women were also less likely to experience abdominal pain/cramps and headaches, but these were not observed for MSM. Other studies have also indicated an association of older age and a higher probability of paresthesias (11, 15), weight loss (15), and body fat changes or lipodystrophy (11, 22-24), and a lower probability of headaches (15) and diarrhea (15). Others have found no association of age and symptoms for body fat redistribution (15), dizziness (15), headaches, myalgias (15), diarrhea (11), and other symptoms (11, 15, 25-27). Of these studies, only one large cross-sectional study of 3,745 HIV-infected patients was specifically designed to assess age differences in the prevalence of symptoms, although most study participants had never received HAART (15).
Racial/ethnic differences in the prevalence of symptoms were more prominent in WIHS, as Caucasian women had a higher prevalence of most symptoms compared to Hispanics or African Americans. In MACS, Hispanic MSM had the highest probability of nausea/vomiting, and African-American MSM reported a substantially lower prevalence of body fat redistribution compared to other racial/ethnic groups. Other symptoms were generally similar by race/ethnicity in the MACS. Few others have examined racial/ethnic differences in symptoms. Zingmond et al. (15) reported a lower prevalence of most symptoms for non-Caucasians besides weight loss which was higher in non-Caucasians. Others have reported a lower risk of lipodystrophy in Hispanics compared to Caucasians, and no association with race/ethnicity for nausea/vomiting (27) and fatigue (25, 26).
These results highlight important differences in the prevalence of certain symptoms by age and race/ethnicity with long-term use of HAART, independent of major confounding factors. However, further study is warranted to determine why symptoms vary by age and race/ethnicity in HIV patients. It is conceivable, for example, that there are host genetic differences by race that influence risk of symptoms in HIV-infected patients receiving HAART, but this has not been reported in the literature. It is also possible that a higher comorbidity burden in older patients, and/or reduced tolerability to HAART contributes to the increased prevalence of symptoms. On the other hand, our study results may reflect general differences in the occurrence of symptoms not specific to HIV infection. For example, the relationship of age and certain symptoms among women are also consistent with age-related hormonal changes. For example, the higher prevalence of abdominal pain or cramps and headaches in younger women may reflect menstrual symptoms and the higher prevalence of body fat redistribution and night sweats in older women may reflect menopausal symptoms. In support of this possibility, results were similar for these symptoms with respect to age among women in our parallel analysis among HIV-uninfected participants, albeit at much lower levels.
Our results also highlight the complex etiology of symptoms in HIV-infected patients. We identified several factors suggesting symptoms were related to both HIV disease (i.e., AIDS diagnosis and HIV RNA levels) and HAART use (i.e., discontinuation of antiretrovirals and PI-containing HAART). The observation that symptoms cannot be attributed solely to HIV infection or HAART use alone is consistent with our prior study on this topic which demonstrated a high prevalence of symptoms among HIV-uninfected women and HIV-infected women not receiving HAART (14).
The main strengths of the current study compared to other studies on this topic are the longitudinal study design, the inclusion of HIV-infected MSM and women with known dates of initiation of HAART, and the inclusion of demographically similar HIV-uninfected controls. Furthermore, the analyses were carried out in two well-established cohorts using similar methods to assess a wide range of potential confounding factors. Analyses were adjusted not only for factors related to HAART use including duration and changes in regimens over time, but also for important individual characteristics including clinical depression, HIV disease stage, BMI and alcohol use.
There were several study limitations. First, the survey questions for clinical symptoms and years administered were not identical in WIHS and MACS. These cohorts were also very different with respect to many characteristics, particularly age, race/ethnicity, education, and income levels. Despite these differences, the prevalence of reported symptoms was remarkably similar between cohorts. Nevertheless, differences in study results between cohorts should not be attributed to differences by gender, and may instead reflect sociodemographic differences, or perhaps different interpretation of survey questions between men and women (e.g., shifting of body fat). Further study of gender differences in symptoms is warranted. A second limitation was that our analysis evaluated only 13 symptoms assessed in both cohorts. It is possible that other symptoms common among HIV-infected patients will have a different association with age, race/ethnicity and other factors. A third potential limitation is recall bias since the symptoms were assessed by self-report. However, we do not feel this was a major source of bias since symptoms were assessed in the recent past (prior six months), and it is not likely that in this time frame there would be substantial differential recall of symptoms by age or race/ethnicity. Furthermore, substantial efforts were made in WIHS and MACS to ensure the collection of standardized data for all participants and across visits. Finally, given statistical power issues, we were unable to look at the association of particular drugs or regimens, which may contribute to our findings.
In summary, we identified a higher prevalence of reported body fat redistribution, myalgias, paresthesias, weight loss and night sweats among older women receiving HAART, and a higher prevalence of paresthesias among older MSM receiving HAART. Caucasian women also generally reported more symptoms compared to other race/ethnicities, with few racial/ethnic differences for MSM. The most consistent predictors of symptoms across cohorts and symptom categories were depression and advanced HIV disease stage. Thus, treatment of depression and control of HIV disease remain the key factors for reducing the very high burden of symptoms in HIV patients. Despite adjustment for these and other factors, the prevalence of many symptoms remained substantially higher among older and Caucasian women, raising concerns of adverse effects on quality of life in these groups with continued and potentially lifelong use of HAART.
Acknowledgments
Data in this manuscript were collected by the WIHS and MACS Collaborative Study Groups with centers (Principal Investigators) at:
WIHS: New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases with supplemental funding from the National Cancer Institute, and the National Institute on Drug Abuse (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590). Funding is also provided by the National Institute of Child Health and Human Development (grant UO1-HD-23632) and the National Center for Research Resources (grants MO1-RR-00071, MO1-RR-00079, MO1-RR-00083).
MACS: The Johns Hopkins Bloomberg School of Public Health (Joseph Margolick); Howard Brown Health Center and Northwestern University Medical School (John Phair); University of California, Los Angeles (Roger Detels); University of Pittsburgh (Charles Rinaldo); and Data Analysis Center (Lisa Jacobson). The MACS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute; and the National Heart, Lung, and Blood Institute: UO1-AI-35042, 5-M01-RR-00052 (GCRC), UO1-AI-35043, UO1-AI-37984, UO1-AI-35039, UO1-AI-35040, UO1-AI-37613, and UO1-AI-35041.
The authors also acknowledge the assistance of Zheng Hu and Gayle Springer in data management and data analysis.
Sources of support: The Women’s Interagency HIV Study is funded by the National Institute of Allergy and Infectious Diseases with supplemental funding from the National Cancer Institute and the National Institute on Drug Abuse (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590). Funding is also provided by the National Institute of Child Health and Human Development (UO1-HD-32632) and the National Center for Research Resources (MO1-RR-00071, MO1-RR-00079, MO1-RR-00083). The Multicenter AIDS Cohort Study is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute and the National Heart, Lung and Blood Institute. UO1-AI-35042, 5-MO1-RR-00722 (GCRC), UO1-AI-35043, UO1-AI-37984, UO1-AI-35039, UO1-AI-35040, UO1-AI-37613, UO1-AI-35041.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors do not have any associations that might pose a conflict of interest.
References
- 1.Detels R, Munoz A, McFarlane G, et al. Multicenter AIDS Cohort Study Investigators Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. JAMA. 1998;280:1497–1503. doi: 10.1001/jama.280.17.1497. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Hoyos S, del Amo J, Muga R, et al. Effectiveness of highly active antiretroviral therapy in Spanish cohorts of HIV seroconverters: differences by transmission category. AIDS. 2003;17:353–359. doi: 10.1097/00002030-200302140-00009. [DOI] [PubMed] [Google Scholar]
- 3.Porter K, Babiker A, Bhaskaran K, et al. Determinants of survival following HIV-1 seroconversion after the introduction of HAART. Lancet. 2003;362:1267–1274. doi: 10.1016/s0140-6736(03)14570-9. [DOI] [PubMed] [Google Scholar]
- 4.Silverberg MJ, Wegner SA, Milazzo MJ, et al. Effectiveness of highly-active antiretroviral therapy by race/ethnicity. AIDS. 2006;20:1531–1538. doi: 10.1097/01.aids.0000237369.41617.0f. [DOI] [PubMed] [Google Scholar]
- 5.Tarwater PM, Mellors J, Gore ME, et al. Methods to assess population effectiveness of therapies in human immunodeficiency virus incident and prevalent cohorts. Am J Epidemiol. 2001;154:675–681. doi: 10.1093/aje/154.7.675. [DOI] [PubMed] [Google Scholar]
- 6.Mack KA, Ory MG. AIDS and older Americans at the end of the Twentieth Century. J Acquir Immune Defic Syndr. 2003;33(Suppl 2):S68–75. doi: 10.1097/00126334-200306012-00003. [DOI] [PubMed] [Google Scholar]
- 7.Espinoza L, Hall HI, Campsmith ML, Lee LM. Trends in HIV/AIDS diagnoses--33 states, 2001-2004. MMWR Morb Mortal Wkly Rep. 2005;54:1149–1153. [PubMed] [Google Scholar]
- 8.Tedaldi EM, Absalon J, Thomas AJ, Shlay JC, van den Berg-Wolf M. Ethnicity, race, and gender. Differences in serious adverse events among participants in an antiretroviral initiation trial: results of CPCRA 058 (FIRST Study) J Acquir Immune Defic Syndr. 2008;47:441–448. doi: 10.1097/QAI.0b013e3181609da8. [DOI] [PubMed] [Google Scholar]
- 9.Bonfanti P, Valsecchi L, Parazzini F, et al. Coordinamento Italiano Studio Allergia e Infezione da HIV (CISAI) Group Incidence of adverse reactions in HIV patients treated with protease inhibitors: a cohort study. J Acquir Immune Defic Syndr. 2000;23:236–245. doi: 10.1097/00126334-200003010-00004. [DOI] [PubMed] [Google Scholar]
- 10.Currier JS, Spino C, Grimes J, et al. The Aids Clinical Trials Group 175 Team Differences between women and men in adverse events and CD4+ responses to nucleoside analogue therapy for HIV infection. J Acquir Immune Defic Syndr. 2000;24:316–324. doi: 10.1097/00126334-200008010-00003. [DOI] [PubMed] [Google Scholar]
- 11.Fellay J, Boubaker K, Ledergerber B, et al. Prevalence of adverse events associated with potent antiretroviral treatment: Swiss HIV Cohort Study. Lancet. 2001;358:1322–1327. doi: 10.1016/s0140-6736(01)06413-3. [DOI] [PubMed] [Google Scholar]
- 12.Lucas GM, Chaisson RE, Moore RD. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions. Ann Intern Med. 1999;131:81–87. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- 13.Moore RD, Fortgang I, Keruly J, Chaisson RE. Adverse events from drug therapy for human immunodeficiency virus disease. Am J Med. 1996;101:34–40. doi: 10.1016/s0002-9343(96)00077-0. [DOI] [PubMed] [Google Scholar]
- 14.Silverberg MJ, Gore ME, French AL, et al. Prevalence of clinical symptoms associated with highly active antiretroviral therapy in the Women’s Interagency HIV Study. Clin Infect Dis. 2004;39:717–724. doi: 10.1086/423181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zingmond DS, Kilbourne AM, Justice AC, et al. Differences in symptom expression in older HIV-positive patients: The Veterans Aging Cohort 3 Site Study and HIV Cost and Service Utilization Study experience. J Acquir Immune Defic Syndr. 2003;33(Suppl 2):S84–92. [PubMed] [Google Scholar]
- 16.Skolasky RL, Phair J, Detels R, et al. Thrush and fever as markers of immune competence in the era of highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 2001;17:1311–1316. doi: 10.1089/08892220152596551. [DOI] [PubMed] [Google Scholar]
- 17.Karus D, Raveis VH, Alexander C, et al. Patient reports of symptoms and their treatment at three palliative care projects servicing individuals with HIV/AIDS. J Pain Symptom Manage. 2005;30:408–417. doi: 10.1016/j.jpainsymman.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Barkan SE, Melnick SL, Preston-Martin S, et al. WIHS Collaborative Study Group The Women’s Interagency HIV Study. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 19.Kaslow RA, Ostrow DG, Detels R, et al. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 20.US Department of Health and Human Services Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 2006 Available at: http://aidsinfo.nih.gov/Guidelines/
- 21.Diggle PJ, Liang KY, Zeger SL. Analysis of longitudinal data. Oxford University Press; New York, NY: 1995. [Google Scholar]
- 22.Lichtenstein KA, Ward DJ, Moorman AC, et al. Clinical assessment of HIV-associated lipodystrophy in an ambulatory population. AIDS. 2001;15:1389–1398. doi: 10.1097/00002030-200107270-00008. [DOI] [PubMed] [Google Scholar]
- 23.Martinez E, Mocroft A, Garcia-Viejo MA, et al. Risk of lipodystrophy in HIV-1-infected patients treated with protease inhibitors: a prospective cohort study. Lancet. 2001;357:592–598. doi: 10.1016/S0140-6736(00)04056-3. [DOI] [PubMed] [Google Scholar]
- 24.Thiebaut R, Daucourt V, Mercie P, et al. Groupe d’Epidemiologie Clinique du Syndrome d’Immunodeficience Acquise en Aquitaine Lipodystrophy, metabolic disorders, and human immunodeficiency virus infection: Aquitaine Cohort, France, 1999. Clin Infect Dis. 2000;31:1482–1487. doi: 10.1086/317477. [DOI] [PubMed] [Google Scholar]
- 25.Henderson M, Safa F, Easterbrook P, Hotopf M. Fatigue among HIV-infected patients in the era of highly active antiretroviral therapy. HIV Med. 2005;6:347–352. doi: 10.1111/j.1468-1293.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan PS, Dworkin MS. Prevalence and correlates of fatigue among persons with HIV infection. J Pain Symptom Manage. 2003;25:329–333. doi: 10.1016/s0885-3924(02)00676-0. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds NR, Neidig JL. Characteristics of nausea reported by HIV-infected patients initiating combination antiretroviral regimens. Clin Nurs Res. 2002;11:71–88. doi: 10.1177/105477380201100106. [DOI] [PubMed] [Google Scholar]