Abstract
Myocardial infarction causes heterogeneity of noradrenergic transmission that contributes to the development of ventricular arrhythmias and sudden cardiac death. Ischemia-induced alterations in sympathetic transmission include regional variations in cardiac norepinephrine (NE) and in tyrosine hydroxylase, the rate-limiting enzyme in NE synthesis. Inflammatory cytokines that act through gp130 are elevated in the heart after myocardial infarction. These cytokines decrease expression of tyrosine hydroxylase in sympathetic neurons, and indirect evidence suggests they contribute to the local depletion of tyrosine hydroxylase in the damaged left ventricle. However, gp130 cytokines are also important for the survival of cardiac myocytes following damage to the heart. To examine the effect of cytokines on tyrosine hydroxylase and NE content in cardiac nerves we used gp130DBH-Cre/lox mice, which have a deletion of the gp130 receptor in neurons expressing dopamine beta hydroxylase. The absence of neuronal gp130 prevented the loss of tyrosine hydroxylase in cardiac sympathetic nerves innervating the left ventricle one week after ischemia-reperfusion. Surprisingly, restoring tyrosine hydroxylase in the damaged ventricle did not return neuronal NE content to normal levels. NE uptake into cardiac nerves was significantly lower in gp130 KO mice, contributing to the lack of neuronal NE stores. There were no significant differences in left ventricular peak systolic pressure, dP/dtMAX, or dP/dtMIN between the two genotypes after myocardial infarction, but ganglionic blockade revealed differences in autonomic tone between the genotypes. Stimulating the heart with dobutamine or releasing endogenous NE with tyramine generated similar responses in both genotypes. Thus, the removal of gp130 from sympathetic neurons prevents the post-infarct depletion of TH in the left ventricle, but does not alter NE content or cardiac function.
Keywords: cardiac, sympathetic, ischemia-reperfusion
Introduction
The sympathetic nervous system stimulates heart rate, cardiac conduction, and the force of contraction through the release of norepinephrine (NE) and activation of β1 adrenergic receptors (β1AR). Myocardial infarction commonly results in sympathetic dysfunction following the ischemic event (Barber et al., 1983;Zipes, 1990), and chronic alterations in cardiac NE content and noradrenergic transmission contribute to electrical remodeling and the development of ventricular arrhythmias and sudden cardiac death (Dae et al., 1997;Cao et al., 2000b;Cao et al., 2000a;Rubart & Zipes, 2005). Heterogeneity in noradrenergic transmission occurs due to the loss and gain of nerve fibers as well as regional variation in tyrosine hydroxylase, which is the rate-limiting enzyme in NE synthesis (Li et al., 2004).
Inflammatory cytokines that act through the gp130 receptor (Heinrich et al., 1998;Taga, 1996) are elevated in the heart after ischemia-reperfusion (Aoyama et al., 2000;Frangogiannis et al., 2002;Gwechenberger et al., 1999) and during heart failure (Kreusser et al., 2008;Frangogiannis et al., 2002). These cytokines suppress NE production and decrease the expression of tyrosine hydroxylase in sympathetic neurons (Cheng et al., 1997;Lewis et al., 1994;Li et al., 2003;Rao et al., 1993). Indirect evidence suggests these cytokines contribute to the local depletion of TH in the damaged left ventricle following cardiac ischemia-reperfusion (Li et al., 2003;Parrish et al., 2008;Dziennis & Habecker, 2003).
Cytokines that activate gp130 are critical survival factors for cardiac myocytes (Brar et al., 2001;Hirota et al., 1999;Yoshida et al., 1996;Zou et al., 2003), so in order to determine the effect of cytokines on TH content in cardiac neurons, we used a conditional knockout approach. gp130DBH-Cre/lox mice have a selective deletion of the gp130 cytokine receptor in all neurons expressing dopamine beta hydroxylase (DBH) (Stanke et al., 2006), including post-ganglionic sympathetic neurons. Sympathetic neurons in these mice do not respond to injury-induced inflammatory cytokines (Habecker et al., 2009). We asked if the absence of gp130 signaling in sympathetic neurons prevented the selective depletion of TH enzyme in the damaged left ventricle. We found that the absence of neuronal gp130 prevented the loss of TH content in cardiac sympathetic nerves innervating the left ventricle one week after ischemia-reperfusion. However, restoring TH content in the damaged left ventricle did not increase NE content or lead to significant differences in ventricular function between the two genotypes one week after myocardial infarction
Methods
Ethical Approval
All procedures were approved by the OHSU Institutional Animal Care and Use Committee and comply with the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH publication No. 85-23, revised 1996).
Materials
Tyramine hydrochloride, and hexamethonium chloride were obtained from Sigma (St. Louis, MO). Dobutamine was obtained from Hospira Inc. (Lake Forest, IL). Antibodies were obtained as follows: rabbit anti-tyrosine hydroxylase (TH) was from Chemicon (Temecula, CA); rabbit anti-protein gene product 9.5 (PGP9.5) was from Accurate Chemicals (Westbury, NY); goat anti-rabbit HRP was from Pierce (Rockford, IL).
Animals
Wild type C57BL/6J were obtained from Jackson Laboratories West, Sacramento, CA. gp130DBH-Cre/lox mice were generated as previously described (Stanke et al., 2006). All mice were kept on a 12h: 12h- light dark cycle with ad libitum access to food and water. Male and female mice 12–18 weeks old were used for all experiments. Animals from the two genotypes were age and gender matched for each experiment
Myocardial ischemia-reperfusion
Ischemia-reperfusion was carried out as previously described (Gritman et al., 2006). Adult mice were placed in an induction chamber and anesthetized with 4% isoflurane. Mice were intubated, mechanically ventilated, and maintained with 1–2% isoflurane mixed with 100% oxygen. Core body temperature was maintained at ~37°C, and a 3-lead ECG was monitored throughout the surgery and experimental protocols using a PowerLab data acquisition system (ADInstruments, Inc., Colorado Springs, CO).
The mouse was turned to a right lateral decubitus position and a thoracotomy performed in the 4th intercostal space with the aid of a dissecting microscope. A ligature (8-0 nylon or equivalent on a taper needle) was placed around a proximal segment of the left anterior descending coronary artery and the ends of the suture were passed through a tube (PE10) with a blunted end to prevent tissue damage. The ligature was tightened to induce regional myocardial ischemia, which was confirmed by ECG changes, regional cyanosis, and wall motion abnormalities. After 45 minutes the coronary ligature was released, and reperfusion confirmed by visible epicardial hyperemia. The suture remained within the wound for identification of the ligature site, and the chest and skin were closed in layers. A small catheter was left in the thorax for 10–15 min to evacuate air and fluids. The mice were then removed from the ventilator, and repositioned every 30 min until able to maintain sternal recumbency. Mice were then returned singly to a cage and the cage was placed half on and half off a heating pad for ≥30 min before being returned to the animal room. Buprenex (0.1mg/kg) was administered as needed to ensure the animals were comfortable following surgery. All surgical procedures were performed under aseptic conditions.
Area at risk and infarct size
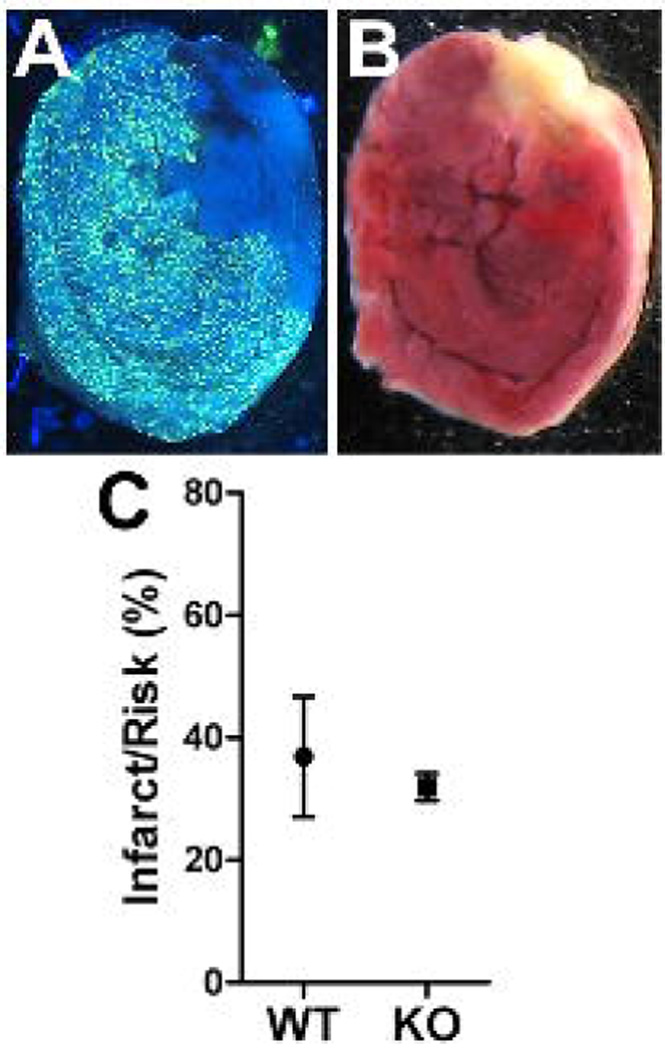
Twenty-four hours after the onset of reperfusion, mice were placed in an induction chamber and anesthetized with 4% isoflurane. Once unconscious, mice were re-intubated, maintained on 2% isoflurane, and the chest cavity was re-opened at the site of the initial incision. The coronary artery was then re-occluded and fluorescent particles (4 mg/ml in deionized water with 0.01% Tween 20; Duke Scientific #34-1, 2–8 µm size) were infused through a polyethylene catheter (PE10) with a 30-gauge needle tip in the left ventricle of the heart. Microspheres were infused at a rate of 400 µL/min for 5 minutes to delineate the area at risk (Fig. 1). The heart was then excised for infarct size analysis, and cut into transverse slices 1 mm thick using a cutting block. Both sides of all slices were photographed under UV light, and the captured images were saved in Photoshop for measurement of area at risk. The slices were then placed in 2,3,5-triphenyltetrazolium chloride solution (TTC, 1% w/v in sodium phosphate buffer at 37°C, pH 7.4) for 20 minutes. The staining procedure was carried out in the dark to prevent breakdown of the TTC by light. The slices were then placed in 10% neutral buffered formalin overnight to increase the contrast between stained and unstained tissue. Myocardium that did not stain red was presumed to be infarcted (Fig. 1). Both sides of each 1 mm section were photographed under white light, and images saved in Photoshop. Risk and infarct areas for each slice were traced and digitized. The volume of myocardium at risk and infarcted myocardium was calculated from the measured areas and slice thickness. Infarct size was normalized as fraction of the area-at-risk. All analyses were performed in a blinded fashion by two people. The data presented are the average of the two independent determinations of infarct/risk.
Figure 1.
Infarct size compared to area at risk. Area at risk (A) and Infarct (B) are shown from a representative section 24 hours after ischemia-reperfusion. The area at risk is devoid of fluorescent microspheres, while the infarct is yellow/white following TTC staining. C) Infarct and risk were quantified in 5 WT and 6 KO mice and the Mean±SEM is shown.
HPLC Analysis of Norepinephrine
NE levels were measured by HPLC with electrochemical detection as described previously (Li et al., 2004)(Parrish et al., 2009). Hearts were excised and cut in 1–2 mm transverse cross sections, excluding the area spanning the LAD ligation. The base was processed as a single sample that included the top 2 mm of both ventricles. Below the site of LAD occlusion the left and right ventricles (LV, RV) were separated and processed individually for NE analysis. Heart tissue was homogenized in perchloric acid (0.1 M) containing 1.0 µM of the internal standard dihydroxybenzylamine to correct for sample recovery. Catecholamines were purified by alumina extraction before analysis by HPLC. Detection limits were ~0.05 pmol with recoveries from the alumina extraction >60%.
Immunoblot Analysis
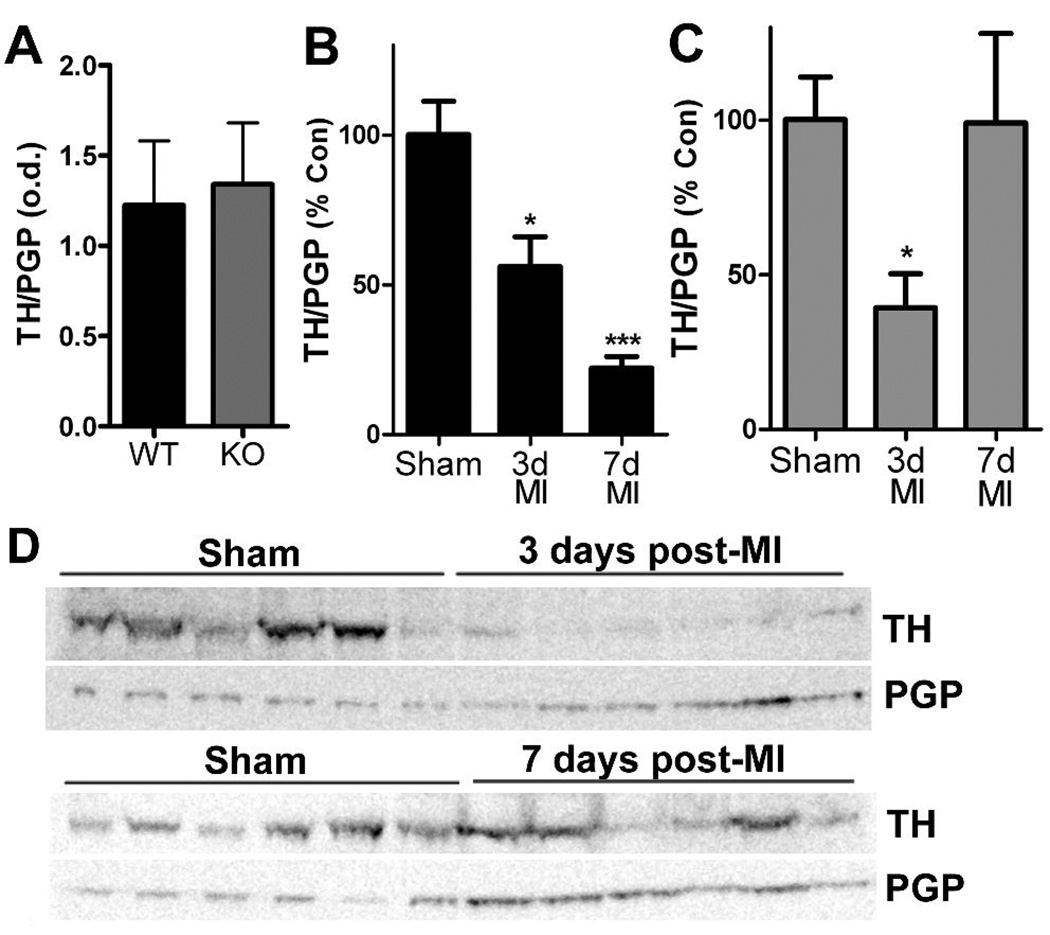
The left ventricle (LV) below the site of LAD occlusion was processed for western blot analysis. TH levels were normalized to the pan-neuronal marker PGP (protein gene product 9.5/ubiquitin carboxy-terminal hydrolase L1) to control for denervation/hyperinnervation in the ventricle (Li et al. 2004). LV samples included both infarct and peri-infarct tissue. Tissue stored in RNA later was homogenized in lysis buffer, sized fractionated on SDS-PAGE gels, and transferred to membranes for blotting. Controls from both genotypes were run on the same gel for a direct comparison of TH levels (Fig. 2A), and unoperated controls were compared to 3 day and 7 day shams for each genotype. All control groups had identical TH/PGP ratios, so the 3- and 7-day sham groups were merged. PGP content for all control groups was identical in both genotypes, and PGP content for all post-MI groups was increased compared to controls. Samples were assayed in duplicate, with six sham and six MI samples on a single gel. Each blot was incubated with rabbit polyclonal anti-TH (1:1000) followed by incubation with goat anti-rabbit horse radish peroxidase (1:5000). Immunoreactive bands were visualized by chemiluminescence using SuperSignal Dura (Pierce), and band intensity was recorded by a −40°C CCD camera. Blots were stripped and reblotted with polyclonal rabbit anti-PGP9.5 (1:1000) followed by incubation with goat anti-rabbit horse radish peroxidase (1:5000). Data were analyzed using LabWorks Software (UVP, Upland, CA). Sample blots are shown in Figure 2.
Figure 2.
Tyrosine hydroxylase content in the left ventricle. TH was quantified by western blot and normalized to the pan-neuronal marker PGP. A) Neuronal TH content is identical in the left ventricle of WT and gp130 KO mice. B) Neuronal TH content in WT mice declined 3 days after MI, and decreased further 7 days after ischemia-reperfusion surgery. Data shown are the Mean±SEM; n=6; * p<0.05, *** p<0.001. C) Neuronal TH content in gp130 KO mice decreased 3 days after MI but returned to control levels 7 days after ischemia-reperfusion. Data shown are the Mean±SEM; n=6; * p<0.05. D) Representative western blots showing TH and PGP in gp130 KO left ventricle from 6 sham and 6 post-MI mice.
Real-Time PCR
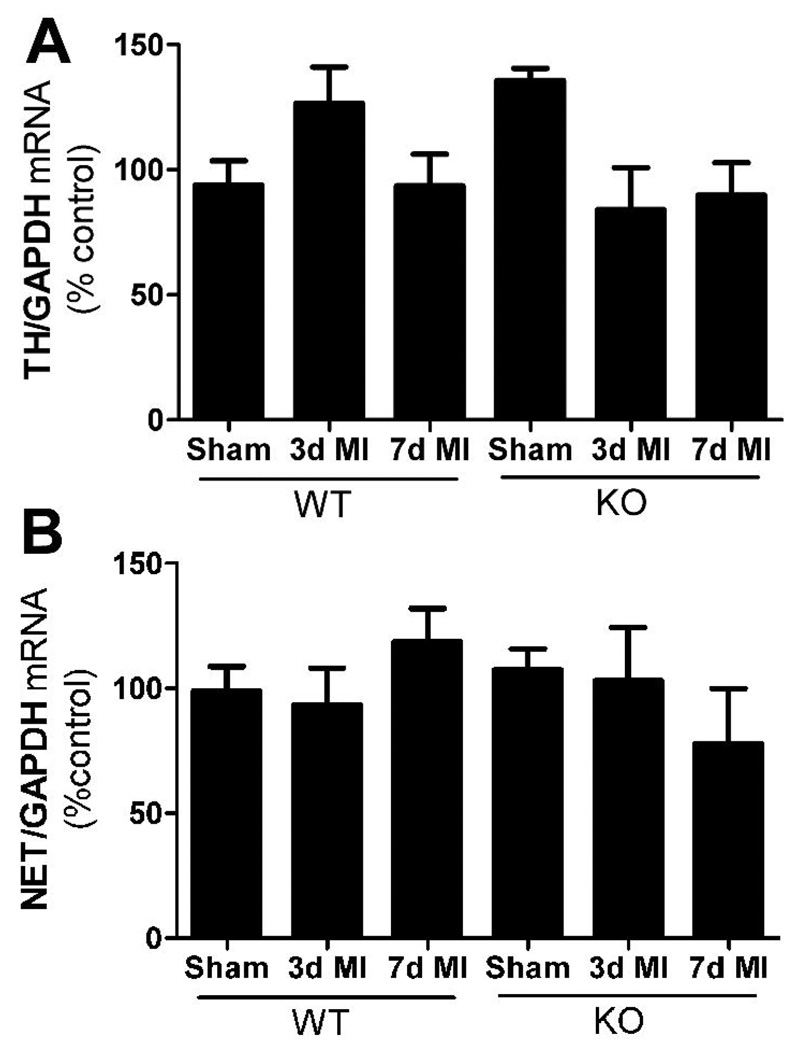
Stellate ganglia were harvested 3 or 7 days after sham or ischemia-reperfusion surgery and stored immediately in RNAlater. RNA was isolated from individual stellate ganglia using the Ambion RNAqueous micro kit. Total RNA quantified by OD260, and then 200 ng of total RNA was reverse transcribed and diluted for use. Real-time PCR was performed with ABI TaqMan Universal PCR master mix in the ABI 7500. Samples were assayed using ABI pre-validated TaqMan gene expression assays for mouse TH, NE Transporter (NET), GTP cyclohydrolase (GCH), and GAPDH. For the PCR amplification, 4 µl of RT reactions (representing 5 ng of RNA template) were used in a total volume of 20 µl, and each sample was assayed in duplicate. Standard curves for TH, NET, GCH, and GAPDH were generated with known amounts of RNA from control sympathetic ganglia, ranging from 0.8 ng – 100 ng. Values for TH, NET, and GCH were normalized to GAPDH from the same sample. 3 and 7 day sham controls were identical within each genotype, and were combined into a single sham group.
To confirm a normal inflammatory response in the KO left ventricle, tissue was harvested from 3 mice of each genotype 24 hours after sham or ischemia-reperfusion surgery and stored immediately in RNAlater. RNA was isolated from ventricles using the Qiagen RNeasy Mini kit and samples assayed using ABI pre-validated TaqMan gene expression assays for mouse IL6, LIF, and GAPDH using standard curves as described above. Cytokine mRNAs were elevated to a similar extent in both genotypes compared to wild type shams (IL-6 % of control: WT 441±165, KO 550±99; LIF % of control: WT 410±165, KO 416±79).
Hemodyamics
Mice were anesthetized with 4% isoflurane and maintained with 2–3% isoflurane. Mice were intubated and placed on a rodent ventilator. Body temperature was monitored and maintained at 37±0.2 °C. A microtipped pressure transducer (1.0 French; Millar) was inserted into the right carotid artery and advanced into the left ventricle for measurement of left ventricular pressure using a PowerLab data acquisition system. A small polyvinyl catheter was placed in the left jugular vein for drug administration. When the animal was stable, it was given hexamethonium chloride (5 mg/kg) to abolish ganglionic transmission. After a new baseline was established, animals received a single bolus dose of the beta agonist dobutamine (32 µg/kg) to assess beta receptor sensitivity. After the dobutamine washed out another dose of hexamethonium was administered, followed by a bolus dose of tyramine hydrochloride (200 µg/kg) to assess the cardiac response to release of endogenous NE. After parameters returned to baseline, left ventricular peak systolic pressure (LVP), dP/dtMAX, and dP/dtMIN were analyzed using ChartPro software.
NE Uptake
Uptake of 3H-NE into sympathetic nerve terminals was assayed essentially as described by Sharma and Benerjee (Sharma & Banerjee, 1977) and Liang et. al. (Liang et al., 1989). Mouse ventricles were separated into sections 1 mm thick, with two sections from each heart used for total uptake and two for background uptake (defined by addition of 1 mM desipramine). The high concentration of desipramine is comparable to what is needed in binding assays to fully block non-specific NET binding in the heart (Backs et al., 2001;Li et al., 2004). Samples were preincubated for 10 minutes at 37°C in KRH buffer (KRH; 120 mM NaCl, 4.7 mM KCl, 2.2 mM CaCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 10 mM HEPES, 5 mM Tris Base pH 7.4) with or without desipramine, and then 50 nM 3H-NE was added. 10 or 20 minutes after addition of 3H-NE, reactions were stopped by placing the samples on ice and adding 1 mL of ice-cold KRH buffer. Tissue was rinsed 3 times in cold KRH, weighed, placed into ice-cold 0.4 M perchloric acid, minced, and extracted in the acid for 21 hours at 4°C (Sharma & Banerjee, 1977). 0.5 mL of the supernatant was quantified by liquid scintillation counting. Both time points generated similar results when calculated as the amount of uptake per minute.
Statistics
Student’s t-test was used for comparisons of just two samples. mRNA data that were normalized as percent of WT unoperated control were analyzed by one-way ANOVA using the Newman-Keuls post-hoc test. Western blots were analyzed within each genotype by one-way ANOVA using the Dunnett post-hoc test to compare each condition to sham control. NE data and hemodynamic data were analyzed across genotypes and surgical groups by two-way ANOVA using the Bonferroni post-hoc test. All statistical analyses were carried out using Prism 5.0.
Results
Infarct size compared to area-at-risk was identical in wild type and neuronal gp130 KO mice (Fig. 1). Left ventricular TH content normalized to the pan-neuronal marker PGP9.5 was similar in unoperated controls from both genotypes (Fig. 2A). Three days after ischemia-reperfusion, TH content in the left ventricle was significantly depleted in both genotypes, despite normalization to PGP to control for innervation density. However, a week after ischemia-reperfusion TH content had returned to control levels in the neuronal gp130 KO mice but was further decreased in wild type mice (Fig. 2B, C). The increased TH/PGP ratio in KO ventricle was due to increased TH content rather than a drop in PGP. Tyrosine hydroxylase mRNA levels in the sympathetic neurons of the stellate ganglia that project to the heart were similar in unoperated controls of each genotype and did not decrease after ischemia reperfusion (Fig. 3). This suggests that the loss of TH content in the left ventricle is due to a direct effect on the protein. Likewise, NE transporter mRNA levels in the stellate ganglia were identical in both genotypes and did not change after ischemia reperfusion (Fig. 3).
Figure 3.
TH and NET mRNA in cardiac sympathetic neurons. All values are normalized to WT unoperated control. A) TH mRNA normalized to GAPDH in WT stellate ganglia. Mean±SEM, n=4–8. B) NET mRNA normalized to GAPDH in WT stellate ganglia. Mean±SEM, n=4–8.
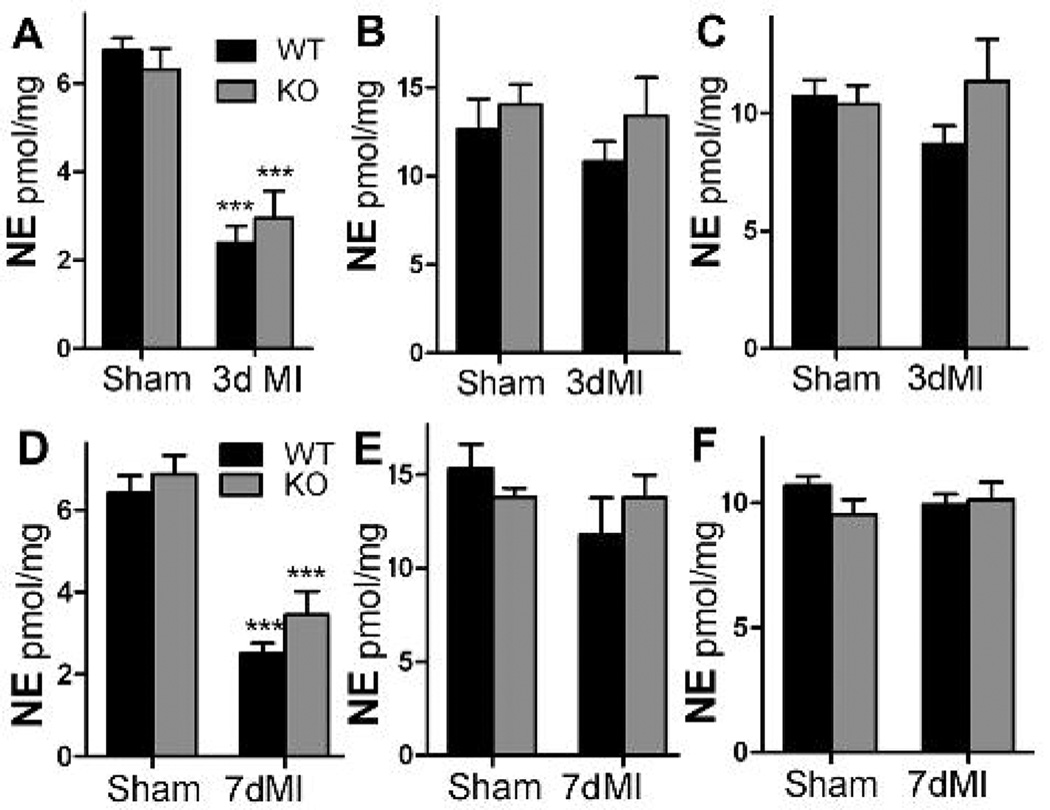
Tyrosine hydroxylase is the rate-limiting enzyme in NE synthesis, so we expected that NE content would be restored in the left ventricle of the neuronal gp130 KO hearts which had normal TH content per nerve fiber. In contrast, we found that NE content was identical in the left ventricles of both genotypes both 3 and 7 days after myocardial infarction, and was decreased significantly compared to sham animals (Fig. 4A,D). NE content in the base of the ventricles above the coronary artery ligation was unchanged compared to sham operated mice (Fig.4B,E), consistent with retention of TH enzyme in the base of the ventricle (Li et al., 2004;Parrish et al., 2008). NE content in the right ventricle was also unchanged compared to sham animals (Fig. 4C,F). NE content throughout the heart was indistinguishable between the two genotypes. Thus NE content in the damaged left ventricle did not change with the level of tyrosine hydroxylase enzyme.
Figure 4.
NE content in WT and KO hearts. Black bars are WT, grey bars are KO mice. NE content in the left ventricle below the occlusion (A,D), the base of the ventricles (B,E), or the right ventricle (C,F) 3 or 7 days after sham or ischemia-reperfusion surgery. Data are Mean±SEM, n=6, *** p< 0.001.
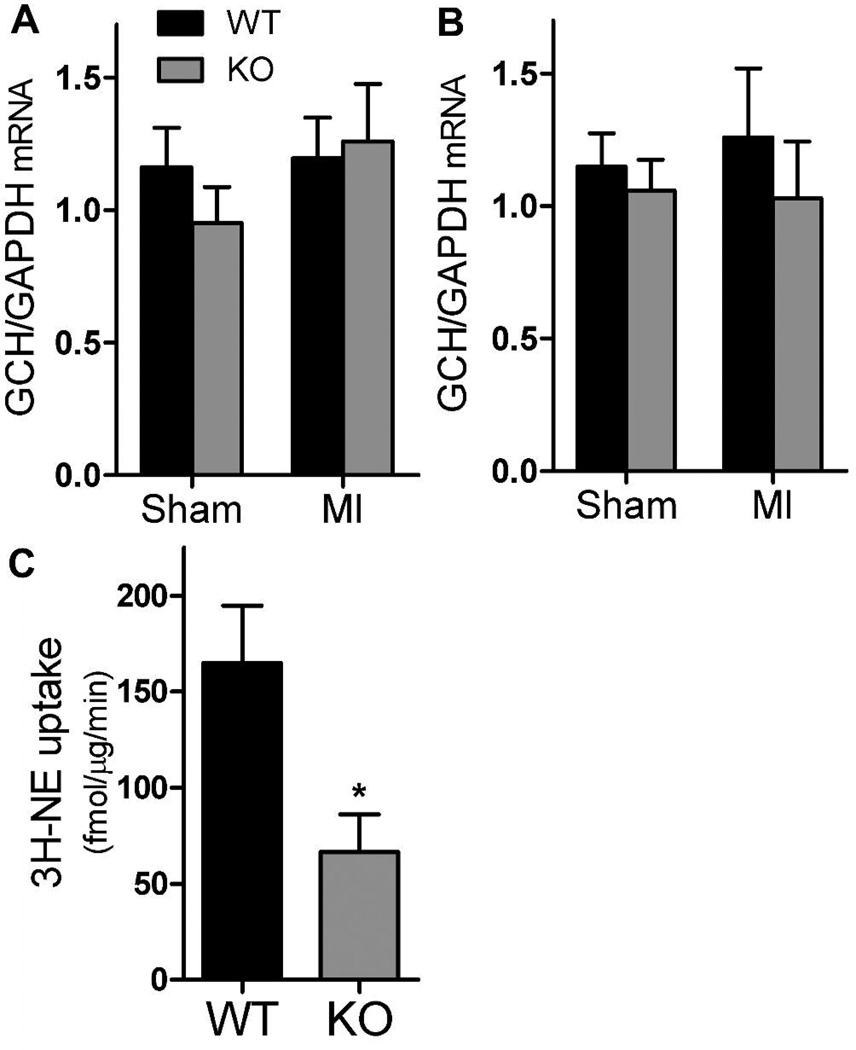
Tyrosine hydroxylase requires the cofactor tetrahydrobiopterin (BH4) in order to general l-DOPA from tyrosine. GTP cyclohydrolase (GCH) is the rate-limiting enzyme in the synthesis of BH4, and GCH expression can be suppressed in sympathetic neurons by gp130 cytokines (Habecker et al., 2002). Cardiac myocytes also express GCH and produce BH4, so to selectively examine neuronal expression we quantified GCH mRNA in the stellate ganglia. GCH mRNA levels were indistinguishable between the genotypes, and were not altered by ischemia-reperfusion (Fig. 5A, B). This suggests that changes in neuronal BH4 do not explain the gap between TH levels and NE content in cardiac sympathetic neurons.
Figure 5.
GTP cyclohydrolase mRNA and NE uptake. Black bars are WT, grey bars are KO mice. A, B) GCH mRNA normalized to GAPDH in stellate ganglia 3 days (A) and 7 days (B) after sham or ischemia-reperfusion surgery. Mean±SEM, n=4. C) Specific 3H-NE uptake into 1 mm sections of WT or KO ventricle reveal decreased NET activity in KO hearts. * p< 0.03; Mean±SEM, n=5.
Neuronal NE content is reflects uptake of released NE in addition to synthesis. NE transport via NET is regulated not only by changes in NET expression but also by changes in NET activity and cellular localization. We examined NE uptake in cardiac sympathetic nerves of WT and KO mice using 3H-NE. Although NET mRNA was identical in both genotypes (Fig. 3), NE uptake was significantly lower in gp130 KO hearts than wild type hearts (Fig. 5C). Thus, lack of NE reuptake may contribute to the discrepancy between TH levels and NE content in the gp130 ventricle.
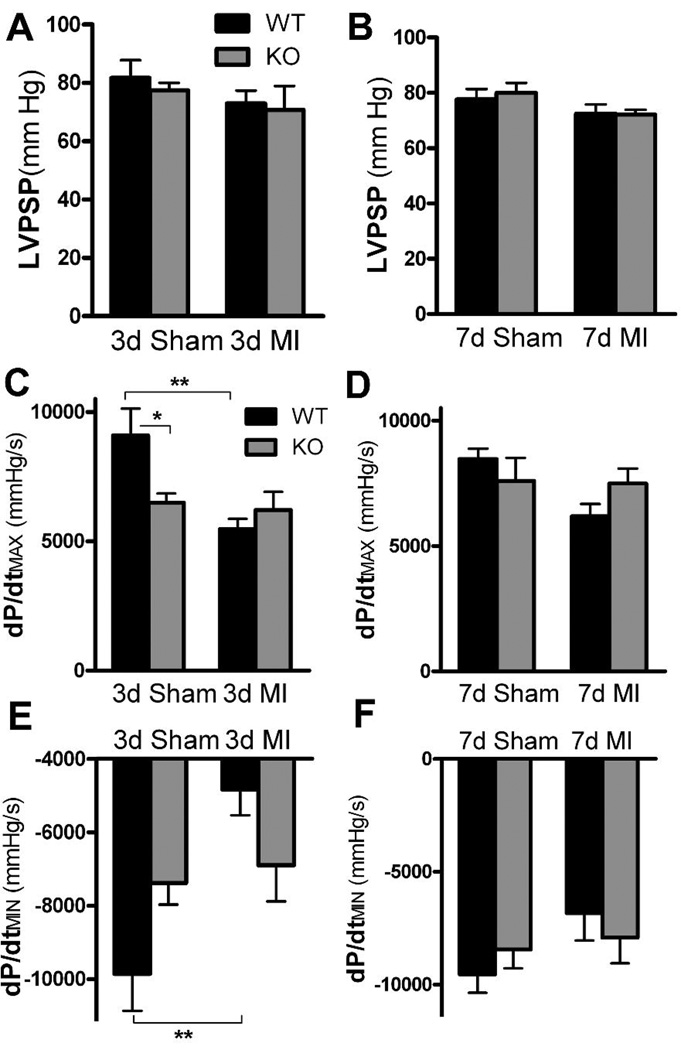
Cardiac function was monitored using a Millar (1.0F) pressure transducer to determine if the changes in TH content observed following MI resulted in altered cardiac function. Left ventricular pressure was not altered significantly following myocardial 10 infarction in either genotype. In contrast, dP/dtMAX and dP/dtMIN were decreased significantly in WT mice 3 days after ischemia-reperfusion compared to WT shams (Fig. 6). Neuronal gp130 KO sham mice exhibited significantly lower dP/dtMAX than WT shams 3 days post-surgery, and there was no further reduction in KO dP/dtMAX after ischemiareperfusion (Fig. 6C). Likewise, dP/dtMIN trended lower in KO shams compared to WT three days after surgery, but there was no further drop in dP/dtMIN after ischemia-reperfusion. One week after ischemia-reperfusion or sham surgery LVP, dP/dtMAX and dP/dtMIN were identical across genotypes and surgical groups (Fig. 6).
Figure 6.
Basal hemodynamics. Black bars are WT, grey bars are KO mice. A, B) Basal left ventricular peak systolic pressure in sham and post-MI animals 3 days (A) and 7 days (B) after surgery. Data are Mean±SEM, n=5–6. C, D) Basal dP/dtMAX in sham and post-MI animals 3 days (C) and 7 days (D) after surgery. Mean±SEM, n=5–6, * p< 0.05, ** p< 0.01. E, F) Basal dP/dtMIN in sham and post-MI animals 3 days (E) and 7 days (F) after surgery. Mean±SEM, n=5–6, ** p< 0.01.
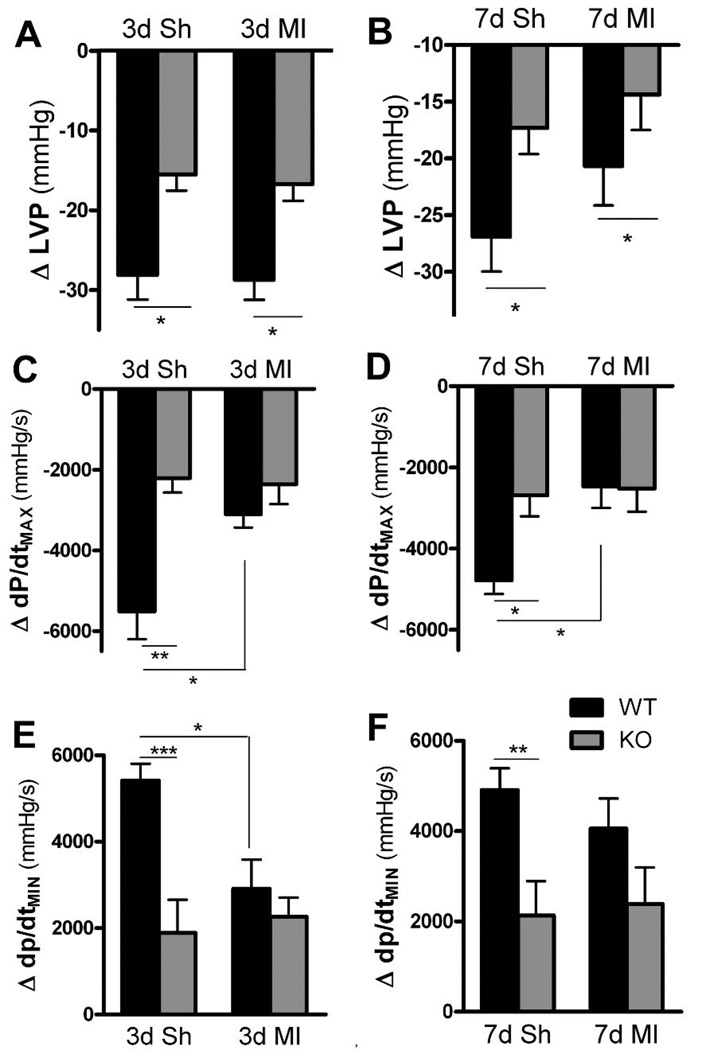
Previous studies identified altered autonomic transmission in the hearts of unoperated control gp130 KO mice (Parrish et al., 2009). In this study of sham and post-MI mice, average LVP, dP/dtMAX, and dP/dtMIN values were not significantly different between the two genotypes before or after ganglionic block. However, WT sham mice exhibited a significantly larger change in LVP, dP/dtMAX, and dP/dtMIN following ganglionic block than the KO sham animals (Fig. 7).
Figure 7.
Changes in cardiac function after ganglionic block. Black bars are WT, grey bars are KO mice. A, B) Blockade of ganglionic transmission caused a decrease in LVP from basal levels 3 days (A) and 7 days (B) after sham or MI surgery. Mean±SEM, n=5–6, * p< 0.05. C, D) Blockade of ganglionic transmission caused a decrease in dP/dtMAX from basal levels 3 days (C) and 7 days (D) after sham or MI surgery. Mean±SEM, n=5–6, * p<0.05, ** p<0.01. E, F) Blockade of ganglionic transmission caused a decrease in dP/dtMIN from basal levels 3 days (E) and 7 days (F) after sham or MI surgery. Mean±SEM, n=5–6, * p<0.05, ** p<0.01, *** p<0.001.
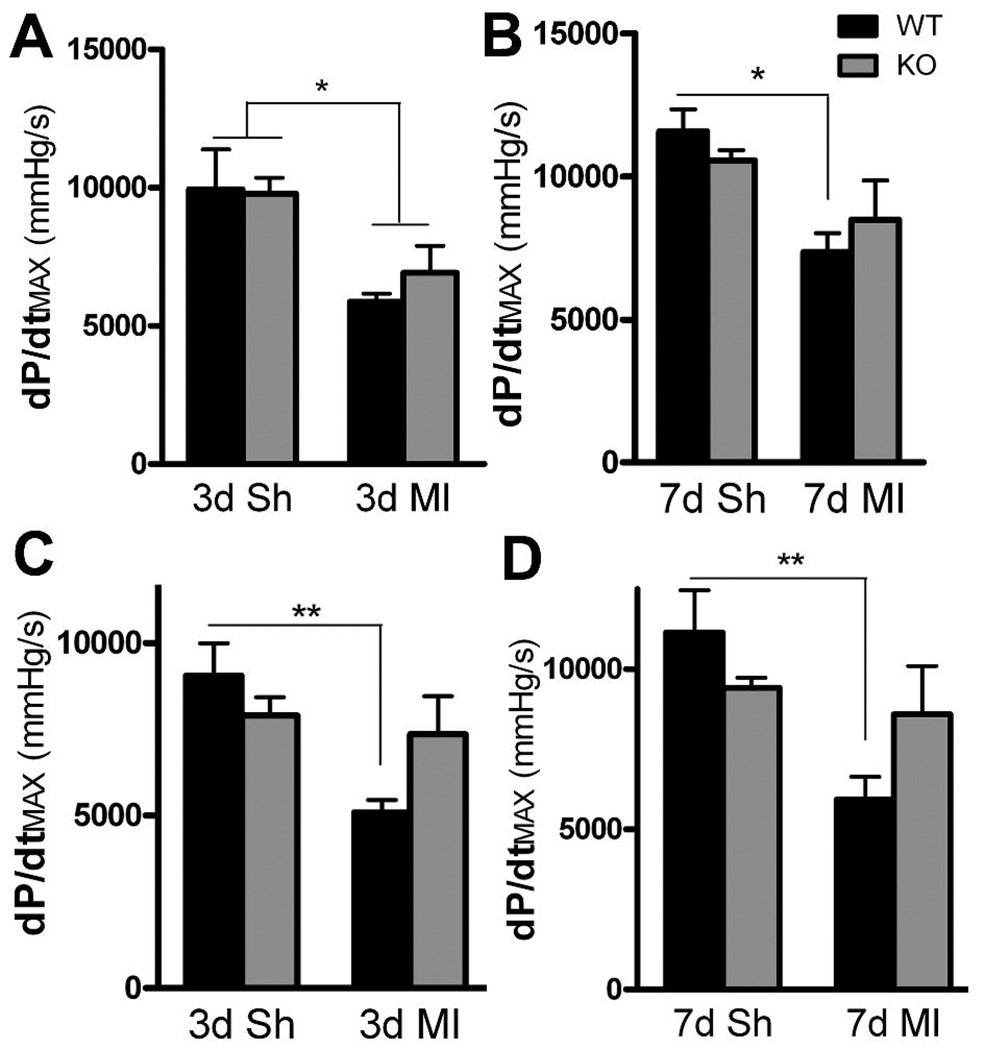
Infusion of a maximally active dose of the beta agonist dobutamine stimulated similar increases in dP/dtMAX in sham and post-MI animals from both genotypes (Fig. 8A, B). The dP/dtMAX was impaired significantly in both genotypes three days after ischemia-reperfusion compared to the sham controls, but a week after MI dP/dtMAX was impaired only in WT mice. LVP and dP/dtMIN were similar across all groups after dobutamine administration and were not changed following MI (data not shown). Stimulating release of endogenous NE with tyramine increased dP/dtMAX in all groups. There were no differences between WT and KO mice in tyramine-stimulated dP/dtMAX within each surgical group (Fig. 8). Post-MI dP/dtMAX was significantly lower in WT mice compared to WT shams, but was not decreased significantly in KO mice compared to KO sham controls. LVP was identical across all groups following tyramine administration (data not shown).
Figure 8.
Dobutamine and tyramine stimulation of cardiac function. Black bars are WT, grey bars are KO mice. A, B) The beta agonist dobutamine stimulates dP/dtMAX to similar extent in both genotype 3 days (A) and 7 days (B) after surgery. dP/dtMAX is decreased compared to sham in both genotypes 3 days after MI, but is decreased only in WT mice 7 days after MI. Mean±SEM, n=5–6, * p<0.05. C, D) Tyramine induced NE release stimulates dP/dtMAX to similar extent in both genotype 3 days (C) and 7 days (D) after surgery. dP/dtMAX is decreased significantly after MI only in WT mice. Mean±SEM, n=5–6, ** p<0.01.
Discussion
Heterogeneity of sympathetic innervation in the heart following ischemia-reperfusion is thought to be a major contributor to arrhythmias and sudden cardiac death. One cause of heterogeneity is depletion of tyrosine hydroxylase enzyme from sympathetic nerve fibers in the damaged left ventricle (Li et al., 2004). The local depletion of TH in the left ventricle is correlated with decreased NE content in the peri-infarct ventricle compared to the rest of the heart (Li et al., 2004;Parrish et al., 2008). Indirect evidence from tissue culture studies suggested that gp130 cytokines had a direct effect on TH and DBH protein levels in addition to their well-known effect on the expression of noradrenergic genes (Cervini et al., 1994;Dziennis & Habecker, 2003). We used mice whose sympathetic neurons lacked gp130 to test the role of these cytokines in the loss of TH enzyme nearest the site of injury. Tyrosine hydroxylase levels were decreased in both genotypes three days after injury, but neuronal TH enzyme levels had returned to normal a week after ischemia-reperfusion in the mice whose sympathetic neurons lacked gp130. In contrast, TH levels were further decreased a week after injury in the WT mice. Thus, cytokines acting through gp130 are responsible for the prolonged depletion of TH in peri-infarct cardiac sympathetic neurons following ischemia-reperfusion.
The mechanisms by which cytokines stimulate the degradation of TH protein in sympathetic neurons are unknown. Our preliminary data indicate that stimulation of cultured sympathetic neurons with cytokines leads to ubiquitination and proteasomal degradation of TH (Shi & Habecker, 2009). Cytokines stimulate several signaling pathways in sympathetic neurons including the Jak/STAT3 (Signal Transducers and Activators of Transcription) pathway (Boulton et al., 1995), and an ERK 1&2 (Extracellular Signal-Regulated Kinases) pathway (Dziennis & Habecker, 2003). Further studies are underway to identify the signaling within sympathetic neurons responsible for stimulating the ubiquitination and degradation of tyrosine hydroxylase.
Surprisingly, the recovery of TH enzyme in the left ventricle of neuronal gp130 KO mice did not result in increased NE content. The quantity of NE stored within sympathetic axons reflects synthesis, release, and reuptake. Tyrosine hydroxylase enzyme activity is tightly regulated (Kumer & Vrana, 1996;Vrana et al., 1981), and changes in enzyme activity might explain the discrepancy between enzyme levels and NE content. For example, if cytokines stimulate TH activity in WT mice, the absence of cytokine signaling would result in lower enzyme activity in gp130 KO mice even though TH levels are higher. Previous studies using conditioned medium suggest that enzyme activity is not altered by gp130 cytokines (Raynaud et al., 1987), but further experiments are required to determine conclusively if cytokines regulate TH activity. NE reuptake is mediated by the NE transporter, and NET mRNA was unchanged in both genotypes following myocardial infarction. In addition, our previous studies indicate that NET content is unchanged in the rat left ventricle following ischemia-reperfusion when normalized to PGP to control for innervation density (Parrish et al., 2008). NET activity can be decreased in certain forms of heart failure (Backs et al., 2001;Kristen et al., 2006), and our new data indicate that NE uptake is decreased in gp130 KO mice even in the absence of lower gene expression. Thus, altered NE uptake may contribute to the discrepancy between TH levels and neuronal NE content after in the gp130 KO ventricle.
Neuronal NE content is also regulated by the rate of NE release, and indirect evidence suggests that NE release may be altered in the neuronal gp130 KO mice. Our previous studies in unoperated control mice showed that basal heart rate, LVP, dP/dtMAX, dP/dtMIN, and NE content were identical in WT and gp130 KO mice, even though β1 adrenergic receptor (β1AR) expression was low in gp130 KO hearts (Parrish et al., 2009). Dobutamine dose-response curves were shifted to the right at lower doses in the knockouts, but blockade of β receptors caused identical bradycardia in both genotypes. Furthermore, 25% of gp130 KO mice developed ventricular tachycardia during reperfusion, compared to 2% of WT mice. Frequent reperfusion arrhythmias in mice with low β1AR levels was very unexpected because β1AR antagonists protect against reperfusion arrhythmias in animals and in humans who have undergone cardiac ischemia and reperfusion (Billman et al., 1997;Du et al., 1999;Haverkamp et al., 1990;Lopez-Sendon et al., 2004). One possible explanation for all of these results is that although NE content is normal in gp130 KO, NE release is elevated. This would contribute to arrhythmias, would compensate for the low β1AR in basal conditions, and would provide at least a partial explanation for the lack of accumulation of neuronal NE in the gp130 KO left ventricle after MI. Pilot studies of NE turnover suggest that NE release is elevated in sham gp130 KO mice compare to wild type, but additional studies are required to confirm this and to determine if post-infarct regulation of NE release differs between the two genotypes.
Given the similar levels of functional noradrenergic transmission in both genotypes, it is not surprising that cardiac function as measured by LVP, dP/dtMAX, and dP/dtMIN were similar in both genotypes following MI. The biggest functional differences that we observed took place following ganglionic block. WT sham mice consistently exhibited larger changes in LVP, dP/dtMAX, and dP/dtMIN than gp130 KO shams. This is consistent with our observation of autonomic imbalance in neuronal gp130 KO control mice (Parrish et al., 2009). The other consistent functional change was a decrease in functional parameters three days after MI in the wild type mice. Cardiac function in gp130 KO mice was not consistently impaired after ischemia-reperfusion, but this was due to a trend toward lower basal function in the KO sham group compared to WT shams. Overall, cardiac function was similar in both genotypes.
Noradrenergic transmission in the heart is critical for proper control of cardiac function, and sympathetic dysfunction contributes to several cardiac pathologies. We identified a local depletion of tyrosine hydroxylase in the peri-infarct left ventricle after ischemia-reperfusion. We asked if the absence of gp130 signaling in sympathetic neurons prevented the loss of TH, and found that it did prevent the depletion of tyrosine hydroxylase in peri-infarct axons a week after ischemia-reperfusion. However, restoring TH content in the damaged left ventricle did not increase NE content or lead to significant differences in ventricular function compared to WT mice. Further studies are underway to identify the signaling pathways involved in regulation of TH, and its connection to functional sympathetic transmission.
Acknowledgements
This work was supported by NIH R01 HL068231.
References
- Aoyama T, Takimoto Y, Pennica D, Inoue R, Shinoda E, Hattori R, Yui Y, Sasayama S. Augmented expression of cardiotrophin-1 and its receptor component, gp130, in both left and right ventricles after myocardial infarction in the rat. J Mol Cell Cardiol. 2000;32:1821–1830. doi: 10.1006/jmcc.2000.1218. [DOI] [PubMed] [Google Scholar]
- Backs J, Haunstetter A, Gerber SH, Metz J, Borst MM, Strasser RH, Kubler W, Haass M. The neuronal norepinephrine transporter in experimental heart failure: evidence for a posttranscriptional downregulation. J Mol Cell Cardiol. 2001;33:461–472. doi: 10.1006/jmcc.2000.1319. [DOI] [PubMed] [Google Scholar]
- Barber MJ, Mueller TM, Henry DP, Felten SY, Zipes DP. Transmural myocardial infarction in the dog produces sympathectomy in noninfarcted myocardium. Circulation. 1983;67:787–796. doi: 10.1161/01.cir.67.4.787. [DOI] [PubMed] [Google Scholar]
- Billman GE, Castillo LC, Hensley J, Hohl CM, Altschuld RA. Beta2-adrenergic receptor antagonists protect against ventricular fibrillation: in vivo and in vitro evidence for enhanced sensitivity to beta2-adrenergic stimulation in animals susceptible to sudden death. Circulation. 1997;96:1914–1922. doi: 10.1161/01.cir.96.6.1914. [DOI] [PubMed] [Google Scholar]
- Boulton TG, Zhong Z, Wen Z, Darnell JEJ, Stahl N, Yancopoulos GD. STAT3 activation by cytokines utilizing gp130 and related transducers involves a secondary modification requiring an H7-sensitive kinase. Proc Natl Acad Sci U S A. 1995;92:6915–6919. doi: 10.1073/pnas.92.15.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brar BK, Stephanou A, Liao Z, O'Leary RM, Pennica D, Yellon DM, Latchman DS. Cardiotrophin-1 can protect cardiac myocytes from injury when added both prior to simulated ischaemia and at reoxygenation. Cardiovasc Res. 2001;51:265–274. doi: 10.1016/s0008-6363(01)00294-2. [DOI] [PubMed] [Google Scholar]
- Cao JM, Chen LS, KenKnight BH, Ohara T, Lee MH, Tsai J, Lai WW, Karagueuzian HS, Wolf PL, Fishbein MC, Chen PS. Nerve sprouting and sudden cardiac death. Circ Res. 2000a;86:816–821. doi: 10.1161/01.res.86.7.816. [DOI] [PubMed] [Google Scholar]
- Cao JM, Fishbein MC, Han JB, Lai WW, Lai AC, Wu TJ, Czer L, Wolf PL, Denton TA, Shintaku IP, Chen PS, Chen LS. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation. 2000b;101:1960–1969. doi: 10.1161/01.cir.101.16.1960. [DOI] [PubMed] [Google Scholar]
- Cervini R, Berrard S, Bejanin S, Mallet J. Regulation by CDF/LIF and retinoic acid of multiple ChAT mRNAs produced from distinct promoters. NeuroReport. 1994;5:1346–1348. [PubMed] [Google Scholar]
- Cheng JG, Pennica D, Patterson PH. Cardiotrophin-1 induces the same neuropeptides in sympathetic neurons as do neuropoietic cytokines. J Neurochem. 1997;69:2278–2284. doi: 10.1046/j.1471-4159.1997.69062278.x. [DOI] [PubMed] [Google Scholar]
- Dae MW, Lee RJ, Ursell PC, Chin MC, Stillson CA, Moise NS. Heterogeneous sympathetic innervation in German shepherd dogs with inherited ventricular arrhythmia and sudden cardiac death. Circulation. 1997;96:1337–1342. doi: 10.1161/01.cir.96.4.1337. [DOI] [PubMed] [Google Scholar]
- Du XJ, Cox HS, Dart AM, Esler MD. Sympathetic activation triggers ventricular arrhythmias in rat heart with chronic infarction and failure. Cardiovasc Res. 1999;43:919–929. doi: 10.1016/s0008-6363(99)00139-x. [DOI] [PubMed] [Google Scholar]
- Dziennis S, Habecker BA. Cytokine Suppression of Dopamine-beta -hydroxylase by Extracellular Signal-regulated Kinase-dependent and -independent Pathways. J Biol Chem. 2003;278:15897–15904. doi: 10.1074/jbc.M212480200. [DOI] [PubMed] [Google Scholar]
- Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- Gritman K, Van Winkle DM, Lorentz CU, Pennica D, Habecker BA. The lack of cardiotrophin-1 alters expression of interleukin-6 and leukemia inhibitory factor mRNA but does not impair cardiac injury response. Cytokine. 2006;36:9–16. doi: 10.1016/j.cyto.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwechenberger M, Mendoza LH, Youker KA, Frangogiannis NG, Smith CW, Michael LH, Entman ML. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation. 1999;99:546–551. doi: 10.1161/01.cir.99.4.546. [DOI] [PubMed] [Google Scholar]
- Habecker BA, Klein MG, Sundgren NC, Li W, Woodward WR. Developmental regulation of neurotransmitter phenotype through tetrahydrobiopterin. J Neurosci. 2002;22:9445–9452. doi: 10.1523/JNEUROSCI.22-21-09445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habecker BA, Sachs HH, Rohrer H, Zigmond RE. The dependence on gp130 cytokines of axotomy induced neuropeptide expression in adult sympathetic neurons. Dev Neurobiol. 2009;69:392–400. doi: 10.1002/dneu.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp W, Hindricks G, Gulker H. Antiarrhythmic properties of beta-blockers. J Cardiovasc Pharmacol. 1990;16 Suppl 5:S29–S32. S29-S32. [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota H, Chen J, Betz UA, Rajewsky K, Gu Y, Ross J, Jr, Muller W, Chien KR. Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell. 1999;97:189–198. doi: 10.1016/s0092-8674(00)80729-1. [DOI] [PubMed] [Google Scholar]
- Kreusser MM, Buss SJ, Krebs J, Kinscherf R, Metz J, Katus HA, Haass M, Backs J. Differential expression of cardiac neurotrophic factors and sympathetic nerve ending abnormalities within the failing heart. J Mol Cell Cardiol. 2008;44:380–387. doi: 10.1016/j.yjmcc.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Kristen AV, Kreusser MM, Lehmann L, Kinscherf R, Katus HA, Haass M, Backs J. Preserved norepinephrine reuptake but reduced sympathetic nerve endings in hypertrophic volume-overloaded rat hearts. J Card Fail. 2006;12:577–583. doi: 10.1016/j.cardfail.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Kumer SC, Vrana KE. Intricate regulation of tyrosine hydroxylase activity and gene expression. J Neurochem. 1996;67:443–462. doi: 10.1046/j.1471-4159.1996.67020443.x. [DOI] [PubMed] [Google Scholar]
- Lewis SE, Rao MS, Symes AJ, Dauer WT, Fink JS, Landis SC, Hyman SE. Coordinate regulation of choline acetyltransferase, tyrosine hydroxylase, and neuropeptide mRNAs by ciliary neurotrophic factor and leukemia inhibitory factor in cultured sympathetic neurons. J Neurochem. 1994;63:429–438. doi: 10.1046/j.1471-4159.1994.63020429.x. [DOI] [PubMed] [Google Scholar]
- Li W, Knowlton D, Van Winkle DM, Habecker BA. Infarction alters both the distribution and noradrenergic properties of cardiac sympathetic neurons. Am J Physiol Heart Circ Physiol. 2004;286:H2229–H2236. doi: 10.1152/ajpheart.00768.2003. [DOI] [PubMed] [Google Scholar]
- Li W, Knowlton D, Woodward WR, Habecker BA. Regulation of noradrenergic function by inflammatory cytokines and depolarization. J Neurochem. 2003;86:774–783. doi: 10.1046/j.1471-4159.2003.01890.x. [DOI] [PubMed] [Google Scholar]
- Liang CS, Fan TH, Sullebarger JT, Sakamoto S. Decreased adrenergic neuronal uptake activity in experimental right heart failure. A chamber-specific contributor to betaadrenoceptor downregulation. J Clin Invest. 1989;84:1267–1275. doi: 10.1172/JCI114294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Sendon J, Swedberg K, McMurray J, Tamargo J, Maggioni AP, Dargie H, Tendera M, Waagstein F, Kjekshus J, Lechat P, Torp-Pedersen C. Expert consensus document on beta-adrenergic receptor blockers. Eur Heart J. 2004;25:1341–1362. doi: 10.1016/j.ehj.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Parrish DC, Alston EN, Rohrer H, Hermes SM, Aicher SA, Nkadi P, Woodward WR, Stubbusch J, Gardner RT, Habecker BA. The absence of gp130 in dopamine {beta} hydroxylase-expressing neurons leads to autonomic imbalance and increased reperfusion arrhythmias. Am J Physiol Heart Circ Physiol. 2009;297:H960–H967. doi: 10.1152/ajpheart.00409.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish DC, Gritman K, Van Winkle DM, Woodward WR, Bader M, Habecker BA. Postinfarct sympathetic hyperactivity differentially stimulates expression of tyrosine hydroxylase and norepinephrine transporter. Am J Physiol Heart Circ Physiol. 2008;294:H99–H106. doi: 10.1152/ajpheart.00533.2007. [DOI] [PubMed] [Google Scholar]
- Rao MS, Sun Y, Escary JL, Perreau J, Tresser S, Patterson PH, Zigmond RE, Brulet P, Landis SC. Leukemia inhibitory factor mediates an injury response but not a target-directed developmental transmitter switch in sympathetic neurons. Neuron. 1993;11:1175–1185. doi: 10.1016/0896-6273(93)90229-k. [DOI] [PubMed] [Google Scholar]
- Raynaud B, Faucon-Biguet N, Vidal S, Mallet J, Weber MJ. The use of a tyrosine-hydroxylase cDNA probe to study the neurotransmitter plasticity of rat sympathetic neurons in culture. Dev Biol. 1987;119:305–312. doi: 10.1016/0012-1606(87)90036-4. [DOI] [PubMed] [Google Scholar]
- Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J Clin Invest. 2005;115:2305–2315. doi: 10.1172/JCI26381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma VK, Banerjee SP. Inhibition of [3H]norepinephrine uptake in peripheral organs of some mammalian species by ouabain. Eur J Pharmacol. 1977;41:417–429. doi: 10.1016/0014-2999(77)90262-x. [DOI] [PubMed] [Google Scholar]
- Shi X, Habecker BA. Gp130 cytokines stimulate proteasomal degradation of tyrosine hydroxylase in sympathetic neurons. The FASEB Journal. 2009;23:576. doi: 10.1111/j.1471-4159.2011.07539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke M, Duong CV, Pape M, Geissen M, Burbach G, Deller T, Gascan H, Otto C, Parlato R, Schutz G, Rohrer H. Target-dependent specification of the neurotransmitter phenotype: cholinergic differentiation of sympathetic neurons is mediated in vivo by gp 130 signaling. Development. 2006;133:141–150. doi: 10.1242/dev.02189. [DOI] [PubMed] [Google Scholar]
- Taga T. gp130, a shared signal transducing receptor component for hematopoietic and neuropoietic cytokines. J Neurochem. 1996;67:1–10. doi: 10.1046/j.1471-4159.1996.67010001.x. [DOI] [PubMed] [Google Scholar]
- Vrana KE, Allhiser CL, Roskoski RJ. Tyrosine hydroxylase activation and inactivation by protein phosphorylation conditions. J Neurochem. 1981;36:92–100. doi: 10.1111/j.1471-4159.1981.tb02382.x. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Taga T, Saito M, Suematsu S, Kumanogoh A, Tanaka T, Fujiwara H, Hirata M, Yamagami T, Nakahata T, Hirabayashi T, Yoneda Y, Tanaka K, Wang WZ, Mori C, Shiota K, Yoshida N, Kishimoto T. Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc Natl Acad Sci U S A. 1996;93:407–411. doi: 10.1073/pnas.93.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipes DP. Influence of myocardial ischemia and infarction on autonomic innervation of heart. Circulation. 1990;82:1095–1105. doi: 10.1161/01.cir.82.4.1095. [DOI] [PubMed] [Google Scholar]
- Zou Y, Takano H, Mizukami M, Akazawa H, Qin Y, Toko H, Sakamoto M, Minamino T, Nagai T, Komuro I. Leukemia Inhibitory Factor Enhances Survival of Cardiomyocytes and Induces Regeneration of Myocardium After Myocardial Infarction. Circulation. 2003;108:748–753. doi: 10.1161/01.CIR.0000081773.76337.44. [DOI] [PubMed] [Google Scholar]