Abstract
Podosomes are spot-like actin-rich structures formed at the ventral surface of monocytic and haematopoietic cells. Podosomes degrade extracellular matrix and are proposed to be involved in cell migration. A key question is whether podosomes form protrusions similar to the invadopodia of cancer cells. We characterised podosomes of immature dendritic cells using electron microscopy combined with both conventional and novel high-resolution structured illumination light microscopy. Dendritic cell podosomes are composed of actin foci surrounded by a specialised ring region that is rich in material containing paxillin. We found that podosomes were preferential sites for protrusion into polycarbonate filters impregnated with crosslinked gelatin, degrading up to 2 μm of matrix in 24 hours. Podosome-associated uptake of colloidal gold-labelled gelatin matrix appeared to occur via large phagosome-like structures or narrow tubular invaginations. The motor protein myosin-II was excluded from ring or core regions but was concentrated around them and the myosin-II inhibitor Blebbistatin reduced the length of podosome protrusions. Finally, we found that degradation, protrusion and endocytosis in this system are dependent on the matrix metalloproteinase MMP-14. We propose that podosomes mediate migration of dendritic cells through tissues by means of myosin-II-dependent protrusion coupled to MMP-14-dependent degradation and endocytosis.
Keywords: Podosome, Electron microscopy, Quantification
Introduction
Podosomes are dynamic assemblies of actin found on the ventral surfaces of monocytic/haematopoietic cells (Marchisio et al., 1984; Marchisio et al., 1987; Zallone et al., 1983). Structurally, podosomes comprise a concentrated core of actin and actin regulatory proteins (about 1 μm across), which turns over continually with a half-life of minutes (Destaing et al., 2003). The central mass of actin filaments displays radial connections to neighbouring podosomes or to adjacent stress fibres (Akisaka et al., 2008; Evans et al., 2003; Luxenburg et al., 2007). Surrounding the core is a ring region that contains scaffolding, signalling and integrin-binding proteins, including the typical focal adhesion proteins FAK, vinculin and paxillin (Block et al., 2008). Non muscle myosins are also associated with podosomes (Kopp et al., 2006; van Helden et al., 2008). Together, the core and the ring contain an extraordinary array of molecular components some of which are essential for podosome assembly (Gimona et al., 2008). Recent work has provided initial clues to the mechanisms of podosome formation via signalling complexes generated at the plasma membrane (Oikawa et al., 2008).
The function of podosomes is unclear. They have been proposed as cellular adhesion structures, e.g. RSV-transformed cells (Tarone et al., 1985) or sealing zones, e.g. osteoclasts (Luxenburg et al., 2007). More recently they have been implicated in matrix degradation and, in this regard, display similarities to surface-associated structures found in invasive cancer cells called invadopodia (Gimona et al., 2008). Invadopodia degrade the extracellular matrix but also produce cellular protrusions that are proposed to mediate invasion of tissues (Bowden et al., 1999; Weaver, 2008). Whether podosomes produce similar cellular protrusions is currently unresolved (Gimona et al., 2008; Linder, 2009), although some data suggest that podosomes could conceivably promote invasion by forming cell extensions (Carman et al., 2007; Varon et al., 2006).
Dendritic cells (DCs) are antigen-presenting cells of the immune system that, when immature, readily form actin-rich podosomes in vitro. The podosome regions of DCs degrade extracellular matrices in vitro (West et al., 2008), and in vivo this could play a role in migration of DC through tissues. Degradation appears to start at discrete points immediately below the podosome cores, and is dependent on the matrix metalloproteinase (MMP) MMP-14 (West et al., 2008). During their lifetime as functioning antigen-presenting cells, DCs move between their tissues of origin (bone marrow and spleen), peripheral tissues and the lymph nodes, where they interact and activate T cells (Alvarez et al., 2008). We reasoned that movement of DCs through tissues cannot occur via degradation alone and some form of cellular protrusion must be involved.
To better characterise podosomes of immature DCs and investigate possible podosome protrusion we used correlated light and electron microscopy (LM and EM, respectively), high-resolution LM, and quantitative conventional and immuno-EM. We demonstrate that DC podosomes are preferential sites for the formation of protrusions and degrade matrix that is removed via endocytosis. Degradation and endocytosis of a gelatin matrix are dependent on metalloproteinase MMP-14, and podosome protrusion is partially sensitive to the myosin-II inhibitor Blebbistatin. We propose that matrix invasion at podosomes is mediated by coordination of myosin-II-driven actin-based protrusion combined with degradation that is mediated locally by metalloproteinases (MMP-14) and removal of matrix components via endocytotic mechanisms.
Results
Correlative LM and EM reveals podosome ultrastructure to be dome shaped
There are no quantitative ultrastructural studies of podosomes. On glass coverslips DCs expanded from mouse spleen, precursors produced podosomes that containing actin-rich foci (Fig. 1A,C,D; TRITC-phalloidin labelling) surrounded by a region rich in paxillin (Fig. 1B,C,F) and vinculin (see also Linder and Aepfelbacher, 2003; West et al., 2008). Most of the cells (~79%) generated podosome regions that were composed of 50-100 discrete units (n=362) covering an average 56 μm2 (n=20). To reveal podosome ultrastructure, the same podosomes from the same cell were correlated using confocal microscopy (paxillin and actin; Fig. 1A-C,D,F) and then conventional epoxy resin transmission electron microscopy (TEM) (Fig. 1E). Cell borders were used as reference structures by each imaging method.
Fig. 1.
Correlative LM and EM of the DC podosomes. (A-C) Low-magnification fluorescent micrographs of a DC podosome region stained with TRITC-phalloidin (A) or paxillin (B); merged image (C). (D-F) The boxed area from C is magnified in (D) (arrows indicate actin-rich foci) and (F) (asterisks indicate interconnected paxillin-rich rings). (E) The correlative EM image (epoxy resin section). Discrete electron lucent regions (arrows) correlate with the actin-rich cores in E (arrows). The electron lucent areas are surrounded by an interconnected electron-dense reticular profiles (asterisks in E) that correlate with paxillin-rich ring (asterisks in F). Arrowhead in E shows irregular border between core and ring regions. (G-I) Micrographs are alternate epoxy resin sections (90 nm thick) through correlated podosome regions (LM not shown). Image G is closest to the membrane and I furthest inside the cell. Dots in G-I indicate positions of actin-rich cores and asterisks electron-dense rings. (J) Reconstruction of podosomes using six successive 90-nm sections from series G-I (see Materials and Methods). Darkest green is closest to the membrane and lightest green furthest inside the cell. The reconstructed podosome cores (dots) are dome shaped and, here, extend at least 450 nm into the cell. Bars (A-C) 2 μm; (D-J) 1 μm.
In EM sections prepared ‘en-face’ to the substratum TRITC-phalloidin-labelled actin-rich cores (Fig. 1D, arrows) appeared as electron lucent patches (Fig. 1E, arrows) lacking recognizable cytoplasmic organelles. Surrounding this core was a more electron-dense region, which corresponded to areas of paxillin fluorescence (asterisks in Fig. 1E,F). The electron densities were arranged in patches or reticulated assemblies with electron-lucent spaces in between (Fig. 1E). The border between central and peripheral regions was irregular and electron densities often extended into the core (Fig. 1E, arrowhead). In this report we will refer to the electron-lucent region as the ‘core’ and the electron-dense periphery as the ‘ring’. 3D topology was revealed using serial TEM sections prepared en-face to the substratum (Fig. 1G-J). In sections situated closer to the plasma membrane (Fig. 1H) both core and ring regions were clearly visible. In sections located deeper in the cell (Fig. 1I), the ring region was indistinct and replaced by cytoplasmic vesiculo-tubular structures (Fig. 1I asterisks). The core (arrowheads in Fig. 1G-I) extended up to 400 nm from the membrane and appeared dome shaped in serial section reconstructions (dots in Fig. 1J).
In orthogonal (vertical) sections (Fig. 2A) the dome-shaped profiles of podosomes was clearly evident and in HM23 resin sections the core region of these structures was gold labelled for actin using immuno-EM (Fig. 2B). Serial sections were then used to confirm the dome shape and central actin distribution in 3D (data not shown; correlative microscopy was not attainable in this orientation using this system). The actin-rich core was flanked by zones of puckered plasma membrane (Fig. 2C), which were associated with electron-dense material containing striations that extended perpendicular to the substratum (Fig. 2C,D and insert) and were immunogold labelled for paxillin (Fig. 2D). These peripheral densities probably contribute to the patchy electron density of the ring seen in en-face sections. Three-dimensional structured illumination microscopy (SIM) (Schermelleh et al., 2008) is a new approach for higher resolution immunofluorescence microscopy. This confirmed the dome-shaped distribution of actin and revealed a patchy distribution of another ring component, vinculin (Fig. 2E-H). In z-axis projections (Fig. 2H), vinculin staining appeared as linear ‘streaks’ in similar position and orientation to the electron-dense paxillin-rich structures observed by EM (Figs 1 and 2; supplementary material Fig. S1). In en-face view, bundles of actin radiated from the actin-rich cores with vinculin staining interspersed between the bundles (Fig. 2E,F; arrowheads in insert in Fig. 2E). These features were not visible using conventional LM techniques.
Fig. 2.
EM and SIM of DC podosomes. (A-D) EM sections of DC podosomes orientated orthogonal to the substratum. (A) Epoxy resin section in which the dome shape is evident (arrows) and the peripheral ring regions (asterisks) lie adjacent to dome-shaped core. (B) HM23 resin section in which gold labelling for actin is most intense towards the core centre. The matrix has slightly greater electron density compared with the less intensely labelled periphery (arrows). (C) Epoxy resin section. The core regions of three dome-shaped podosomes (arrowed) are flanked by adjacent ring regions (bars). The ring regions display puckered membrane profiles, and in HM23 section displayed in D the gold labelling for paxillin is located along striations extending orthogonally from the plasma membrane (arrows). The insert illustrates the striations as they appear in optimally contrasted epoxy resin sections extending from puckered membrane regions of the ring. In these orthogonal sections, podosome height was 685 nm and width at the membrane was 1245 nm (see Materials and Methods; n=96, s.d.=0.22 μm for each, respectively). (E-H) SIM. Image E shows actin (phalloidin labelling; red) concentrated in the core regions that have irregular borders, whereas ring regions contain actin filaments (insert; arrowheads). In F the vinculin ring (using Alexa-Flour 488 secondary antibody; green) is distributed in patches often situated between the actin filaments (arrows in E and F). Image H is a Z-projection through the same podosome region as in E-G, taken along the white line in G. Arrows in G and H indicate corresponding actin-rich podosome cores, and white arrowheads in H indicates vinculin staining in linear arrays extending away from the membrane. Bars (A-D) 200 nm, insert in (D) 100 nm. (E-H) 2 μm and insert in (E) 1 μm.
Cells cultured on glass coverslips showed no evidence of podosome-related tubules that had been described previously (Nermut et al., 1991; Nitsch et al., 1989; Ochoa et al., 2000); 1100 podosome profiles examined). However tubules did form on 2-μm thick gelatin layers crosslinked using 0.5% Glutaraldehyde (supplementary material Fig. S1C) and on porous filters coated with crosslinked gelatin (see below and Fig. 7 and supplementary material Fig. S7).
Fig. 7.
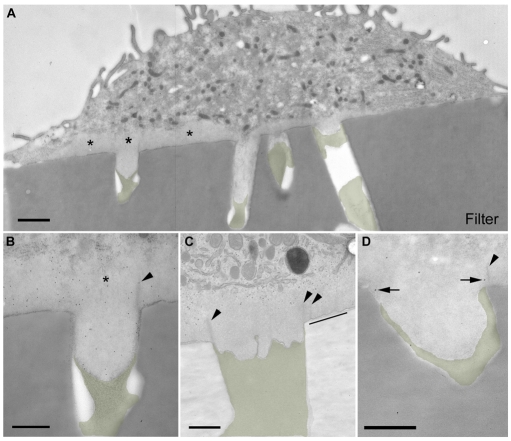
Gold-particle uptake is associated with podosomes. DCs grown on crosslinked gelatin-containing colloidal gold-gelatin complexes were processed for epoxy resin TEM. (A,B) Low-power micrographs. Non-podosome containing cells (A) lie on a smooth-surfaced unlabelled gelatin layer that is superficial to a gold-labelled zone lying on the filter surface (arrowhead). In podosome-containing (B, asterisk) cells, the smooth unlabelled gelatin surface is absent and the podosome displays an irregular plasma membrane inside the gold-labelled gelatin. Insets show lysosome-like profiles that are label free in A (non-podosome cell profiles) and gold-labelled in B (podosome cell profiles). (C,D) Higher magnification views of podosome regions (C) and podosome processes (D) show invagination of cell surface (arrowheads) and compaction of gold-gelatin complexes close to the membrane (arrows). (E) Canaliculus (arrowhead) in a podosome region that contains gold particles (arrows). (F) Canaliculi (arrows) extend into a podosome ring region from groups of compacted gelatin-gold particles that were surrounded by plasma membrane invaginations. Bars, (A,B) 1 μm, inserts 200 nm; (C,D) 500 nm; (E,F) 300 nm.
Podosome protrusion occurs on porous polycarbonate filters impregnated with crosslinked gelatin
A central issue in podosome biology is whether podosomes form protrusions (Gimona et al., 2008; Linder, 2009). To investigate this, we used polycarbonate filters with pores partially or completely filled with gelatin as a degradable matrix (Fig. 3A). DCs seeded onto these filters formed podosomes indistinguishable in form from those found on plastic or ‘thick’ gelatin (Fig. 2, supplementary material Figs S1 and S2). Accordingly, on non-porous regions of the filters coated with gelatin, podosomes showed dome-shaped cores (actin-rich by immuno-EM; Fig. 3A,B), peripheral membrane puckering (Fig. 3C) and associated striations (arrowheads in Fig. 3B-D) labelled for paxillin (Fig. 3D).
Fig. 3.
Protrusion and matrix degradation in polycarbonate filters impregnated with crosslinked gelatin. (A) Protrusion from a DC podosome region (*) into pores of a polycarbonate filter in collage of EM micrographs. (B) Detail of protrusion in A. The protrusion contacts gelatin in the pore (pale green) and is immunogold labelled for actin along its length. The protrusion base is continuous with podosomes above and is also labelled for actin. At the pore rim, striations extend into the podosome (arrow). (C) Pore protrusion shows irregular close contact with gelatin. Electron-dense striations (arrows) extend into the podosome close to the pore-aperture and extend from puckered plasma membrane profiles typical of a podosome ring region (line). (D) Representative paxillin distribution (HM23 section). Gold particles label electron-dense areas (arrows) that flank the protrusion base and a striation is indicated (arrowhead). Gelatin is coloured pale green in A-D. Bars in (A) 1 μm, (B-D) 500 nm.
Remarkably, where a podosome region covered a filter pore, protrusions were frequently observed extending into the pores and these filled the width of each pore completely, whether the pore diameter was 1 μm or 2 μm (data not shown). By TEM the protrusions appeared homogeneous and were continuous with podosome cores situated above the filter (Fig. 3A asterisks). Immunogold labelling showed actin distributed throughout the protrusion (Fig. 3B) and at the pore-aperture electron-dense striations labelled for paxillin (Fig. 3B-D), and these striations extended away from the membrane.
Protrusions emanating from podosomes consistently penetrated deep into the pore, making contact with the gelatin matrix (Fig. 3B-D). The interface often appeared irregular (Fig. 3C), consistent with localised degradation (gelatin was reproducibly smooth in the absence of protrusions). By contrast, pores below regions of the cell that lacked podosomes (see Fig. 5B) were not filled with protrusions and the cell membrane failed to make contact with gelatin in the pore. When cells were seeded onto filters whose pores had been completely filled with gelatin, podosome protrusions still entered the pores (supplementary material Fig. S3A,B), whereas non-podosomal cell regions did not (supplementary material Fig. S3A,C). Taken together, these data indicated that podosome regions preferentially generate protrusions in regions where extracellular matrix degradation takes place. We next used rigorous quantitative EM techniques to test these possibilities.
Fig. 5.
Quantitative analysis of podosome protrusion and degradation in wild-type and MMP-14−/− DCs. (A) Fractional area of pore occupied by protrusions, quantified using intersection counting of podosome and non-podosome regions (see Materials and Methods); Wt, wild-type; MMP-14−/−, MMP-14 knockout in (A,C-E). (B) Representative diagram of the measurements and terminology used in text and (C-E). (C) Measurement of processes extending into pores beneath podosome and non-podosome regions (Non-podo). (D) The pore-aperture to gelatin distance (Pore depth) was measured at podosome regions, non-podosome areas (Non-podo) and cell free areas of the filter (Non-cell). (E) The fraction of protrusions contacting gelatin directly (defined in Materials and Methods) as counted in podosome and non-podosome regions. Data in A,C-E were obtained from three independent experiments. Error bars represent ± s.e.m.
Immuno-EM reveals podosome components in podosome-related protrusions
To investigate the relationship between protrusions and podosomes further, we mapped characteristic proteins on podosomes using quantitative immuno-EM as illustrated in (Fig. 4 and supplementary material Fig. S4). In podosomes formed on plastic, actin labelling was most concentrated within an electron-dense sub-region of the core (see Fig. 2B). Paxillin labelling was most concentrated in the ring region (Fig. 4B, see supplementary material Fig. S4B for quantification), which contained the electron-dense striations extending from the membrane indentations (arrows in Fig. 2D). The quantitative distribution map of these core and ring components therefore appears very similar to the qualitative data obtained using SIM. Two additional podosome components, namely phosphorylated tyrosine (Tyr-P) residues (Linder, 2007) and gelsolin (Chellaiah et al., 2000; Marchisio et al., 1987; West et al., 1999) were also mapped using this method. Tyr-P labelling was concentrated centrally adjacent to the plasma membrane at the podosome core (Fig. 4C-E) and was reduced by 96% after incubation of sections with phosphatase 1B catalytic domains, indicating specificity (Fig. 4E). Without affecting podosome numbers, podosome labelling for gelsolin (Fig. 4F-H) was reduced by 75% in gelsolin-knockout cells compared with wild type, and was concentrated in two distinct domains: one near the peak of the podosome core and the other over the centre of the core, close to the plasma membrane (Fig. 4F). The lack of effect on podosome number is consistent with our previous data in DCs (West et al., 1999). Distributions of actin, paxillin, Tyr-P and gelsolin labelling are summarised in Fig. 4N.
Fig. 4.
Mapping of podosome components using quantitative immuno-EM. (A-H) Immunogold labelling over podosomes. (A-C,F) Gold labelling quantified in standardised fractions of the total podosome profile(s) as described in Materials and Methods. Black outlines represent the average location of the cytoplasmic border and the grey line the plasma membrane. Examples of labelling for actin and paxillin are shown in Fig. 2, and here for Tyr-P in panel D and gelsolin in panel G. Bar charts in panels E (Tyr-P) and H (gelsolin) show the fraction of specific label over different compartments estimated from control experiments. Cyt, cytosol; Nuc, Nucleus; ER, endoplasmic reticulum; End, endosome; Ruff, ruffles; PM, plasma membrane; Pod, podosome. See Materials and Methods for further details. For each protein n=5-28 podosomes, >200 total gold particles from three spleen preparations were analysed. (I-K) Immunogold labelling over protrusions; quantification as described in Materials and Methods. Black dashed line represents the average location of the podosome-cytoplasm junction; grey line represents the average location of plasma membrane. For each protein n=5-32 podosomes, 200 gold particles and three spleen preparations were analysed. (L,M) Localisation of Tyr-P (arrows) in the tip (L) and ring region at the base of a protrusion (M). Bars in density maps (A-C and F; I-K) represent 200 nm, in D and G 200 nm, in L and M 500 nm. (N) Overlay of peak intensities from distributions shown in (A-C,F) and (I-K) in podosomes and protrusions. The actin-rich core is partially obscured by the paxillin and Tyr-P peak intensities.
In protrusions, quantification of labelling for podosome components (Fig. 4I-M; supplementary material Fig. S5) revealed the focus of actin labelling being now close to the protrusion tip (compare Fig. 4A with 4I). Paxillin labelling was most concentrated in the peripheral zone close to the pore aperture, in which electron-dense striations were found (Fig. 4B and Fig. 3D). Interestingly, labelling for Tyr-P was found at the protrusion tip (Fig. 4K,L) and also over the paxillin-rich electron-dense ring regions (arrowheads Fig. 4M) surrounding at the pore-aperture. These distributions are summarised in Fig. 4N. From these data we conclude that the protrusions possess typical podosome core and ring components in similar distributions to those found in podosomes. In addition, structural similarities, such as electron-dense striations found at similar locations, strongly suggest that protrusions derive from podosomes.
Quantitative evidence of matrix degradation at podosome protrusions
95% of pores beneath podosomes were filled with protrusions (Fig. 5A), compared with 29% in non-podosomal areas, confirming that podosome regions are hot-spots for protrusion into the pores. None of the protrusions found in the non-podosome areas resembled the large homogeneous actin-rich protrusions associated with podosomes. Rather, they were thinner and more irregular, containing a variety of cytosolic components (not shown). Since protrusions formed from podosomes (Fig. 5C) and were 3.67 times longer than those formed in non-podosome regions (2.72 μm and 0.74 μm, respectively), we calculated that 100 μm2 of podosome region produces 70.72 μm of protrusion, compared with 5.92 μm in the non-podosome region (Fig. 7A; see footnote1). Therefore, podosomes are 11.95 times more active in producing protrusions compared with the rest of the cell. Protrusions were only rarely observed over gelatin layers found above the filter (up to 300 nm thick; supplementary material Fig. S3D-G). These protrusions were broad (up to 2 μm across) and only occasionally penetrated the gelatin to make contact with the filter surface (supplementary material Fig. S3D-G). Therefore, podosome protrusions form preferentially at filter-pore apertures. The data in Fig. 5A and C formed the basis of a model shown in Fig. 8A, which illustrates to-scale the increased frequency of filled pores and increased process length over the podosomes compared with non-podosomal areas, and preferential formation at pores versus the filter surface.
Fig. 8.
Models of protrusive podosomes from quantitative EM data. (A) Representative model drawn to scale to illustrate the data shown in Fig. 5. The podosome region is 50 μm2 and pores measure 1 μm across. The panel illustrates the increased frequency of pore occupancy and increased length of protrusions below podosomes. (B) Model of protruding podosome proposed from the data shown in Figs 1, 2, 3, 4, 5, 6 and 7. In the periphery, recruitment of paxillin and vinculin at sites of integrin binding (not shown) promotes assembly of the ring structure, which has a fibrillar composition. The inner surfaces of the ring provides an interaction domain for actin filaments, vinculin and paxillin, allowing for stabilisation of the interaction, possible force generation and membrane puckering. Centrally, close to the plasma membrane, podosome assembly occurs via phosphotyrosine signalling and the phosphorylation of actin regulatory proteins. Protrusion at the membrane is driven by actin treadmilling activity that is associated with gelsolin capping and cleavage. Without progressive protrusion the podosome rapidly disassembles. Actin assembly, therefore, occurs centrally in the developing protrusion while mechanical tension is generated in the more stable periphery. (C) Protrusion is coupled to degradation by membrane located MMP-14 and protrusion extends into space generated by the degradation. Degraded matrix is removed at the periphery of the protrusion by tubules or larger endocytic carriers (not shown).
To analyse degradation at protrusive podosomes we measured the distance from pore-aperture to gelatin within the pore (1) below podosomes (3.17 μm), (2) over non-podosomal areas (1.62 μm) or (3) over filter-regions where no cell was found (1.61 μm; n=3 independent experiments) (see Fig. 5C and D). The extent of gelatin removal compared with areas where no cell was found was 1.55 μm (3.17-1.61; over 24 hours) at podosomes and only 0.01 μm (1.62-1.61) over non-podosomal cell regions.
Knockout of MMP-14 abrogates degradation and limits protrusion
Our previous work indicated that MMP-14 is required for the degradation of crosslinked gelatin by podosomes (West et al., 2008). This raised the question of whether podosome protrusion depends on MMP-14. DCs from MMP-knockout (MMP-14−/−) mice were cultured on filters in parallel to DCs from paired wild-type littermates and fixed for EM analysis 24 hours later. Podosome protrusions from MMP-14−/− DCs were similar to those in wild type cells. They occupied a similar fraction of pore apertures (Fig. 5A) and showed no reduction in the frequency of contact with gelatin (Fig. 5E), indicating that the biogenesis of individual protrusions is not affected in MMP-14−/− cells. However, in MMP-14−/− DCs the length of podosome protrusions was substantially reduced to levels found at non-podosomal cell regions in the wild-type cells (Fig. 5C). In addition, the distance from the pore aperture to the gelatin was also substantially reduced compared with wild type (Fig. 5D), indicating that MMP14−/− cells were unable to degrade gelatin within the pores.
The myosin-II inhibitor Blebbistatin decreases the length of podosome protrusions
Non-muscle myosin-II has been shown to localise to podosome regions (Chabadel et al., 2007; van Helden et al., 2008). Myosin-IIa was localised using confocal microscopy to determine whether it was associated with DC podosomes. Myosin-IIa staining was distributed on stress fibres and in cortical actin close to the plasma membrane (supplementary material Fig. S6), and was also concentrated in a region juxtaposed to podosomes in a peripheral ring-like distribution (Fig. 6A). The staining was clearly distinct from, and did not overlap with, the podosome core (phalloidin) or the ring (vinculin staining; 1381 podosome regions observed; Fig. 6A lower panel). This indicated that myosin-II is not an integral DC podosome component.
Fig. 6.
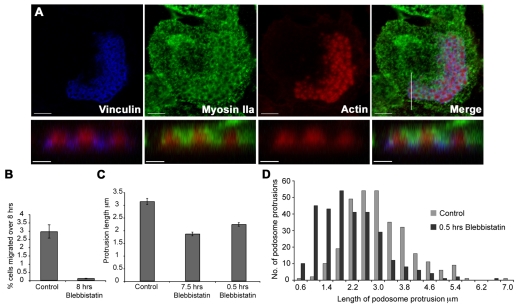
Myosin-IIa distribution and quantitative effects of Blebbistatin on DC podosomes. (A) Myosin-IIa distribution in podosome regions. Upper panels show overviews of a podosome region stained for vinculin, myosin-IIa and actin. Lower panels show Z-reconstructions from the same cell. Podosomes in lower panels are from the white line of the merged image in the upper panel. Myosin-IIa is distributed to the periphery of the actin staining of the podosome (see upper panel) but does not overlap with actin (core) or vinculin staining (ring) (B) Migration of SDCs through a 3-μm filter pore in the absence (Control) and presence of 10 μM Blebbistatin over 8 hours. (C) Podosome protrusion length in the control after 7.5 hours or 0.5 hours of treatment with 10 μM Blebbistatin. (D) Frequency distribution of podosome protrusion lengths. For clarity, categories (0.39 μm) are labelled alternately with only the lower size limit included. Data in B and C were obtained from from three independent experiments; data in D were obtained from 300 measurements per experimental condition. Error bars represent ± s.e.m. Bars (A) upper panels 5 μm; lower panels 1 μm.
To assess the role of myosin-II in podosome-based protrusion we used the myosin-II inhibitor Blebbistatin (Straight et al., 2003). Over an 8-hour exposure, Blebbistatin (10 μM) almost completely inhibited migration through 3-μm pores in a transwell filter assay (see Materials and Methods; Fig. 6B). We next investigated whether Blebbistatin treatment affected podosome formation or protrusion in DCs cultured on gelatin impregnated porous filters. Addition of Blebbistatin to DCs seeded on filters for either 0.5 or 7.5 hours did not change the average number of podosomes per cell [podosomes per cell for control 15 (s.e.m. ±0.8), for 0.5 hours 14 (s.e.m. ±2.0) and 7.5 hours 18 (s.e.m. ±2.2), n=3 in each case]. Additionally, there was only a minor increase in the percentage of cells lacking podosomes (means 25% and 29% at 0.5 hours and 7.5 hours, respectively, n=3) indicating that Blebbistatin does not grossly affect podosome levels. Crucially, we found a reduction in mean podosome protrusion length of 0.9 μm and 1.28 μm at 0.5 hours and 7.5 hours, respectively (Fig. 6C), whereas a more detailed analysis showed that the reduction in length occurred over the whole population of protrusions rather than in a subset of these structures (Fig. 6D). The shortening of the podosome protrusions was not due to cells lifting from the substratum as these remained closely associated with it. These data suggest that protrusion of podosomes into filter pores is regulated by myosin-II.
Podosome- and/or protrusion-associated degradation and endocytosis of a gelatin-gold matrix
We sought more direct evidence for degradation and removal of the matrix using a gelatin-labelled colloidal gold complex. After injection into the filter, these preparations were characterised by patches of gold-labelled gelatin covered by more superficial areas of gold-free gelatin (Fig. 7A). After 24 hours of culture, the appearance of cell regions in contact with the gelatin surface (observed by TEM) was of two types. When podosomes were absent, a smooth superficial layer of gold-free gelatin was invariably present (Fig. 7A, arrowhead) with accumulations of gold deeper in the gelatin. When podosomes were present, the superficial gelatin layer was now absent and the plasma membrane was in contact with gold-labelled gelatin, forming irregular profiles associated with invaginations containing μm-sized gold-gelatin spurs (Fig. 7B). These effects were also observed within the pores and were consistent with matrix degradation at both the filter surface and within the pores themselves (Fig. 7C,D).
The invaginations were putative sites of matrix internalisation, and where podosomes were in contact with gold-gelatin, gold particles were found within membrane-bound organelles resembling endosomes or lysosomes (Fig. 7B, insert). In fact such cells contained nearly tenfold (9.96 times) more gold in their endosomes or lysosomes (0.184 gold particles per lysosome, s.e.m. ±0.0066; n=3) compared with cells lacking podosome profiles (0.0185 gold particles/lysosome, s.e.m.=0.0084; n=3). Uptake of gold particles was, therefore, strongly associated with the presence of podosomes. Serial reconstructions of podosomes further indicated that protrusions and podosomes are sites where uptake occurs (supplementary material Fig. S7A-H). In these preparations, we also observed narrow 20-30 nm tubules (arrows in Fig. 7E,F; average neck width 30.79 nm, s.e.m. ±2.96 nm, n=31), which formed at sites of clustered gold particles, representing another potential conduit of internalisation (supplementary material Fig. S7B,C,H). Critical examination of gold aggregates adjacent to plasma membrane showed that the component particles were more closely packed than further away from the membrane (supplementary material Fig. S7J), which is consistent with membrane-associated degradation (see Discussion). This effect was most striking close to the mouth of narrow tubules and within larger invaginations (arrows Fig. 7C and D; supplementary material Fig. S7B,C). MMP-14−/− cells appeared incapable of degrading gelatin-gold matrices and lacked gold particles in their lysosomes, clearly linking matrix endocytosis to MMP-14-dependant matrix degradation. There were ninefold more gold particles per endosome or lysosome profile in wild type compared with MMP-14−/− cells (0.097 per profile and 0.011 gold particles per profile, respectively; n=200 cells counted in each case).
Discussion
Our results reveal new structural, molecular and functional features of podosomes. They show that, at high resolution, podosomes are dome-shaped accumulations of actin – as was previously indicated using LM (Kaverina et al., 2003). An actin-rich core is surrounded by an electron-dense region, containing ring-associated proteins. Within the podosome ring novel striations are associated with localised membrane puckering (Nermut et al., 1991; Nitsch et al., 1989; Ochoa et al., 2000). The striated material had no clear membrane profiles and was, therefore, distinct from tubules found at the base of podosome protrusions (Fig. 7; supplementary material Fig. S7). The striations contained paxillin and corresponded to elongated structures visualised by vinculin staining in SIM. Thus vinculin and paxillin appear to be concentrated in the same elongated structures, revealed as a discontinuous sheath around the podosome core by SIM. Up to now vinculin and paxillin have not been described in linear structures but are known to bind actin and a number of podosome proteins (Gimona et al., 2008; Harburger and Calderwood, 2009). Vinculin appears to contribute to force transfer between integrins and actin, and may stabilise focal adhesions (Ziegler et al., 2006), paxillin could cooperate with vinculin in this force-transfer process through its ability to bind the tail region of vinculin and to transmit signals through FAK/Pyk2 to the actomyosin complex (Mierke et al., 2008). Interestingly, the ring region has a distinct similarity to focal adhesions (Block et al., 2008). We propose that the striated electron densities of the ring represent actin filaments ‘decorated’ with vinculin-paxillin complexes that link integrin function to actin of the podosome (see Fig. 8B). We observed puckered membrane at the periphery of podosome in our TEM investigations (modelled in Fig. 8B). This membrane curvature is suggestive of mechanical force occurring at the ring region. The force-generating protein myosin-IIa was not localised to the ring region directly but was concentrated close to the core and the ring (see below).
An open question is whether podosomes are protrusive structures (Linder, 2009). This is supported by a number of our observations. First, we found that protrusions had a very similar structural and molecular organisation to podosomes formed on gelatin and glass (i.e. had an actin-rich core, a ring region containing vinculin-paxillin-rich striations, around the rim of the pore and a central focus of phosphotyrosine). Second, we observed that pore-filling actin-rich protrusions only formed at podosomes and were continuous with them – again indicating that protrusions were derived directly from podosome structures. The few protrusion that entered pores in non-podosome regions were not filled with actin and appear to contain various cytosolic structures. Previous studies described cellular protrusions extending from lymphocytes and/or myelocytes into underlying endothelial cells (Carman et al., 2007). However, clear evidence for podosome components was not provided since the structures illustrated in their figures (by EM) were not filled completely with actin filaments, were not associated with adjacent actin-rich podosomes and appeared to contain cytoplasmic components. In addition (Varon et al., 2006) reported actin-rich protrusions from aortic endothelial cells, extending into pores of a transwell filter. It is unclear whether these protrusions were preferentially formed at podosome regions, and whether they penetrated the underlying substratum. In the case of DCs, rigorous quantitative EM techniques have been invaluable in characterising the relationship of protrusive structures to podosomes.
The precise relationship between podosome protrusions and invadopodia is still unclear. Invadopodia clearly protrude into and degrade extracellular matrix by a metalloproteinase-dependent mechanism (Buccione et al., 2004) as we have described for podosome protrusions (see below). There are very few published transverse EM-views of invadopodia and they measure significantly less than 200 nm in diameter (Bowden et al., 1999; Mueller and Chen, 1991). Furthermore they appear actin-poor when compared with DC podosome-protrusions. Actin-rich structures described in A375 MM cells (Baldassarre et al., 2006; Baldassarre et al., 2003) in published micrographs do not give exact morphology of protrusions within the matrix and the precise relationship to protrusive podosomes remains unclear. Our data suggest closer parallels between podosomes and invadopodia, and further rigorous correlative and immuno-EM analysis of these structures are now required.
We show that DC podosome protrusions degrade substantial gelatin barriers, a process more marked and frequent within the pores compared with the gelatin surface. Degradation was dependent on the membrane-anchored metalloproteinase MMP-14. This protein activates the soluble metalloproteinases MMP-2, MMP-8 and MMP-13, which can also degrade gelatin (Poincloux et al., 2009); previous studies ruled out a requirement for MMP-2 and MMP-9 in degradation of thin gelatin films by DCs (West et al., 2008). Our data are indicative of membrane-based degradation because degradation was only seen when protrusions contacted gelatin and compaction of gelatin-gold was most marked near to the plasma membrane and/or invaginations. This suggests podosome related domains containing MMP-14. Interestingly, in endothelial cells MMP-14 generates guidance tunnels within collagen gels, and cancer cells use a similar mechanism to protrude and degrade as they move through matrices (Poincloux et al., 2009; Stratman et al., 2009). Thus, MMP-14 domains might enable DC protrusions to tunnel through extracellular matrices as cells move through tissues. This issue awaits high-resolution localisation of MMP-14 to podosomes using immunofluorescence or immuno-EM – so far unsuccessful in our hands by using an array of available antibodies.
How is matrix removed? There are at least two possibilities, namely diffusion and bulk removal via endocytosis. We have not investigated solubilisation of gelatin in our system but our data do indicate that podosome protrusions remove matrix via endocytotic mechanisms. Previously, a number of proteins associated with endocytic functions had been found in podosomes, e.g. dynamin and endophilin 2 (Ochoa et al., 2000), but there was no direct evidence for internalisation at these structures. Here, we observed large invaginations containing fragments of gold-labelled matrix suggesting DCs can phagocytose fragments of collagenous extracellular matrix by phagocytic mechanisms (Jiang and Grinnell, 2005). We also observed gold-labelled tubules of 20-30 nm in diameter emanating from puckered membrane in the ring-like regions close to the pore aperture (Fig. 8C). Canaliculi have been observed previously in podosomes of Src-transformed cells and osteoclasts (Nermut et al., 1991; Nitsch et al., 1989; Ochoa et al., 2000). The close association of the membrane bending and/or sensing proteins CIP-4, ASAP-1 and FBP-17 (Albiges-Rizo et al., 2009) could be relevant to the formation of the membrane curvature seen in podosomes on glass and thick gelatin. Irrespective of the mode of uptake, we found evidence for matrix internalisation in cells possessing podosomes. Future studies will determine which of these structures mediates podosome-associated endocytosis.
How might protrusions form at podosomes? We have not studied the detailed dynamics of protrusion formation in real time but we believe our data are consistent with the following model (see Fig. 8C). We suggest that podosomes represent transient probes of the extracellular space anchored by a focal adhesion-like rim (the ring). Myosin-II interaction at the ring and/or core periphery could then exert a traction force on the core, which manifests as the bulge in non-protrusive podosomes, with the consequent tension at the ring inducing membrane puckering. These forces would rely on the presence of anti-parallel actin filaments inserting into a vinculin-paxillin-rich ring in a similar fashion to focal adhesions (Block et al., 2008). In the case that no protrusion ensues, podosome disassembly would occur, consistent with the rapid podosome turnover of DC podosomes observed on non-degradable substrates such as glass (West et al., 2008). If, however, protrusion is allowed by means of matrix degradation or the availability of a pore, the developing podosome protrusions and/or ring interactions might become more stable, with active actin assembly occurring at the protrusion tip (this is consistent with our observations of foci of actin and tyrosine phosphorylation, which are necessary for correct podosome dynamics) (Dovas et al., 2009; Luxenburg et al., 2006). We suggest that protrusion is coupled to degradation and removal of substrate by means of membrane-localised proteases, such as MMP-14, and endocytosis. Proteases and membrane would be delivered to the growing process by as-yet-uncharacterised exocytic vesicles.
In conclusion, our observations indicate DCs can remodel and invade extracellular matrix using podosomes and podosome protrusions. DC podosomes might form as transient degradative ‘probes’ for areas of weakness in the extracellular matrix, which then mature into penetrative protrusions that function as pathfinders for cells as they progress on their way through the extracellular matrix toward the target tissue.
Materials and Methods
Chemicals, antibodies, cell culture
Mouse anti-phosphotyrosine and mouse anti-paxillin antibodies (for LM) were from (Millipore, Hertfordshire, UK), mouse anti-paxillin (for EM) was from (BD Biosciences, Erembodegem, Belgium), mouse anti-vinculin was from (Sigma, Poole, UK) Rabbit anti-myosin IIa antibody was from (Biomedical Technologies, Stoughton, MA), rabbit anti-actin antibody was kindly provided by Giuilo Gabbiani (The University of Geneva, Geneva, Switzerland), rabbit anti-gelsolin was a kind gift from David J. Kwiatkowski (Harvard Medical School, Boston, MA). Eight and 10 nm Protein-A–gold was prepared according to (Lucocq, 1993). Spleens from B6 mice and paired littermates of MMP-14 wild-type and MMP-14−/− mice (Zhou et al., 2000) were processed and DCs prepared, cultured and immunofluorescence carried out as described by West et al. (West et al., 2008).
Structured illumination protocol for image acquisitions
The protocol was essentially as described previously (Schermelleh et al., 2008). Images were acquired using the OMX Version 2 (Applied Precision, Issaquah, WA). Samples were illuminated by a coherent scrambled laser to generate structured illumination (SIM) with lateral stripes approximately 0.2 μm apart. The pattern was shifted laterally through five phases and through three angular rotations of 60° for each z-section, separated by 0.125 μm. Exposure times were 200-500 mseconds and laser power and/or intensities were selected to minimise photo bleaching. Frame acquisitions were separated by a 300-msecond pause. Raw images were processed and reconstructed to reveal structures with greater resolution (Gustafsson et al., 2008). The channels were then aligned in x, y and rotationally by using predetermined shifts as measured using a target lens and the Softworx alignment tool (Applied Precision). After correction for image shifts due to differences in emission wavelengths, data sets were transferred to Imaris (Bitplane, Zurich, Switzerland) for visualisation and generation of xz plane images. For comparison wide-field image stacks of the same regions were also collected and subjected to constrained iterative deconvolution to remove out-of-focus blur (Schermelleh et al., 2008).
Correlated LM and EM
Cells were plated onto photo-etched coverslips (Bellco Glass, SciQuip, Shropshire, UK) and fixed for paxillin immunofluorescence (see West et al., 2008). Labelled cells were located using differential interference contrast, then post-fixed and embedded in epoxy resin as described in (Lucocq et al., 1989). Cells were sectioned serially (90 nm) en-face to the monolayer and stained with lead citrate. Images were recorded on a JEOL 1200 EX (80 kV) with phospho-imaging plates (DITABIS, Pforzheim, Germany) and viewed in Photoshop (Adobe Systems). Reconstructions of serial sections were generated in Photoshop by aligning sequential images of membrane-bound structures, and then drawing around the core and superimposing images from each section colour coded according to the position in the z-direction.
Conventional and immuno-EM
Conventional EM was carried out according to Lucocq et al. (Lucocq et al., 1989). For immuno-EM, SDCs were embedded in Lowicryl HM23 resin by progressively lowering the temperature (see Lucocq, 2003). 80-nm sections were incubated on 2% BSA/PBS, followed by antibodies in 2% BSA/PBS. Rabbit primary antibodies were located using 8-nm protein-A–gold followed by washes in PBS (×5) and distilled water (×3). For mouse primary antibodies, an intermediate rabbit anti-mouse secondary antibody was used for 30 minutes (Southern Biotech, Birmingham, AL). Sections were contrasted using 3% uranyl acetate and lead citrate, and viewed in a Tecnai 12 transmission electron microscope (FEI, Eindhoven, The Netherlands).
Quantification of immunolabeling
Cell profiles were selected using systematic random scans (1,050×). Near orthogonal podosome profiles (dome-shaped peak and clearly imaged plasma membrane) were recorded (see Fig. 2). Because of profile size variation, we located gold particles on a normalised podosome map. Images of podosomes (20,500×) were displayed in Adobe Photoshop C2 and a line drawn to bisect the podosome from the peak of the dome to the membrane (Z direction). The length from podosome centre (at the membrane) to the point at which the domed profile flattened off laterally was designated Y′ on one side and Y″ on the other. The location of gold particles was recorded as a fraction of the distance along each z and y axis (at least 200 gold particles were counted per experiment). The mean height (Z) was 0.685 μm and the standard deviation (s.d.) was 0.21 μm. For Y′ and Y″ combined, the mean length was 0.690 μm and s.d. 0.22 μm (n=96 podosomes). The distribution of gold particles over the podosome was plotted on a scatter graph using Excel (Microsoft) and the frequency over normalized areas of 0.1 μm2 counted (applying unbiased counting rules to the quadrats (Lucocq, 1994). Densities were represented using the Excel surface contour function.
Specific label was estimated as follows: Raw counts over organelles and/or compartments were converted to a ‘density’ by dividing by the number of intersection counts of a scanning line with the plasma membrane. Gelsolin-knockout cells and sections pre-incubated with commercial tyrosine phosphatase were used as controls. For each condition >200 gold particles were counted (n=3). The fractional reduction in gold-labelling density over cell compartments of interest was then calculated.
Gelatin filters
Polycarbonate filters (Sterlitech, Kent, WA) were washed in acetic acid (80 mM) at 50°C (Sigma, Paisley, UK) for 20 minutes. 2.5% pigskin gelatin (Sigma, Paisley, UK) was dissolved in 2.5% w/v sucrose in RPMI ‘complete’ at 50°C. Filters were placed in a 25-mm filter holder (Millipore, Herts, UK) and gelatin was injected through the filter at 37°C. Further steps were as described in Chen et al. (Chen et al., 1984). Cells were cultured on filters at approximately 2×105 cells for 24 hours and fixed in either 4% PFA or 4% PFA/0.1% GA for 1 hour at 37°C. Filters were either labelled with phalloidin as described above or prepared for epoxy resin embedding avoiding propylene oxide (see Lucocq, 2003).
Gold-gelatin conjugation
20-nm gold particles (British Biocell Int., Cardiff, UK) were conjugated to gelatin dissolved in pyrogen free water (Sigma, Poole, UK) at a final concentration of 0.12 mg/ml as described (Lucocq, 1993). Conjugates were centrifuged for 1 hour at 8000 g. Pellets were resuspended in pyrogen-free water at 1 mg/ml and mixed with a gelatin/sucrose solution [final concentration 2.5% gelatin (w/v), 2.5% sucrose (w/v)] and injected into filters.
Quantification
Filters were embedded flat or rolled for sectioning vertical to the filter surface. 90-nm sections were mounted on pioloform-coated slot-grids (Agar Scientific, Essex, UK). DC profiles were recorded at a magnification of 1250× and protrusions recorded at 4000×. Podosomes were defined as an exclusion zone for organelles close to the plasma membrane. Individual podosomes had a dome shape with a diameter of up to ~1 μm, whereas podosome regions were more extensive with irregular borders on their cytoplasmic aspect. Protrusions were defined as any membrane-bound structure that encroached below the filter-pore aperture. Gelatin appeared as non-cellular electron-dense material that either partially or completely filled each pore. The fractional area of structures at the filter or pore aperture was estimated using line intersection counting (Lucocq, 2008; Weibel, 1979). Quantitative analysis showed the fraction of filter occupied by pores was 0.216 (n=4692, total intersections). Random position and orientation around the vertical axis was assumed. The length of protrusions was measured from the central point of the pore aperture to the central point of the protrusion tip (Fig. 6B). Contact between gelatin and protrusion was defined as lack of electron-lucent space between the protrusion membrane and gelatin across the protrusion tip.
Blebbistatin experiments
Transwell migration was assessed essentially as described by West et al. with a few changes (West et al., 2008). Cells were not mixed or stained with cell trackers or CD11c and 3-μm-pore transwell inserts (Costar, Corning, NY) were used. Wells contained MIP1α at 50 ng/ml (Peprotech, London, UK) in 2% FCS, RPMI (controls) or MIP1α 10 μM Blebbistatin in 2% FCS. The migration time was 8 hours at 37°C.
To analyse podosome and podosome protrusions cells were allowed to attach to gelatin filters for 30 minutes. Cells were treated with 10 μM Blebbistatin (Merck, Nottingham, UK) for 7.5 hours or remained on the filters for 7.5 hours before adding 10 μM Blebbistatin for 30 minutes. An 8-hour untreated control was also generated. Samples were processed for fluorescent microscopy then Z-stacks were taken from ten discrete locations for each sample using a Zeiss 700 LSM microscope (Carl Zeiss Ltd, UK). One-hundred protrusions, selected by systematic scanning were evaluated per repeat. Measurements of protrusion lengths were made from the top of the podosome to the protrusion tip using the Zen software (Carl Zeiss Ltd, UK; minimum length was 0.6 μm).
Nearest-neighbour gold particle quantification
Particles were from randomly selected quadrats (square grid), using the unbiased forbidden-line counting rule (Gundersen, 1977) and categorised as (1) particles within three gold-particle widths of the podosome-related membrane and (2) particles more distant from the membrane. Nearest neighbour centre-centre distances between gold particles were measured in Adobe Photoshop (n=566 gold particles from three separate cultures).
Supplementary Material
Acknowledgments
We thank John James (University of Dundee, UK) for EM advice, Michele West (University of Dundee, UK) for discussions and Andreas Elkeries (DITABIS, Pforzheim, Germany) for technical advice. Giuilo Gabbiani, David J. Kwiatkowski and Robert Wallin (Karolinska Institutet, Stockholm, Sweden) kindly provided gelsolin-knockout cells. This research was funded by the MRC capacity studentship grant, MRC programme grant MRC (to C.W.), Hong Kong Research Grant Council GRF HKU 781808M (to Z.Z.). Deposited in PMC for release after 6 months.
Footnotes
By stereology pores covered 21.6 μm2. 100 μm2 podosome region had 21.6÷0.785 μm2 =27.5 pores (0.785 μm2 is the cross sectional area of a 1 μm diameter pore). The fraction of pores occupied by protrusion under podosomes was 0.95 (Fig. 5A). 100 μm2 podosome region produce 27.5×0.95=26 protrusions with average length 26×2.72=70.72 μm. In 100 μm2 of non-podosome region the number of protrusions was 27.5×0.29=8 with length of 8×0.74=5.92 μm.
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/9/1427/DC1
References
- Akisaka T., Yoshida H., Suzuki R., Takama K. (2008). Adhesion structures and their cytoskeleton-membrane interactions at podosomes of osteoclasts in culture. Cell Tissue Res. 331, 625-641 [DOI] [PubMed] [Google Scholar]
- Albiges-Rizo C., Destaing O., Fourcade B., Planus E., Block M. R. (2009). Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions. J. Cell Sci. 122, 3037-3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez D., Vollmann E. H., von Andrian U. H. (2008). Mechanisms and consequences of dendritic cell migration. Immunity 29, 325-342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassarre M., Pompeo A., Beznoussenko G., Castaldi C., Cortellino S., McNiven M. A., Luini A., Buccione R. (2003). Dynamin participates in focal extracellular matrix degradation by invasive cells. Mol. Biol. Cell 14, 1074-1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassarre M., Ayala I., Beznoussenko G., Giacchetti G., Machesky L. M., Luini A., Buccione R. (2006). Actin dynamics at sites of extracellular matrix degradation. Eur. J. Cell Biol. 85, 1217-1231 [DOI] [PubMed] [Google Scholar]
- Block M. R., Badowski C., Millon-Fremillon A., Bouvard D., Bouin A. P., Faurobert E., Gerber-Scokaert D., Planus E., Albiges-Rizo C. (2008). Podosome-type adhesions and focal adhesions, so alike yet so different. Eur. J. Cell Biol. 87, 491-506 [DOI] [PubMed] [Google Scholar]
- Bowden E. T., Barth M., Thomas D., Glazer R. I., Mueller S. C. (1999). An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene 18, 4440-4449 [DOI] [PubMed] [Google Scholar]
- Buccione R., Orth J. D., McNiven M. A. (2004). Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat. Rev. Mol. Cell Biol. 5, 647-657 [DOI] [PubMed] [Google Scholar]
- Carman C. V., Sage P. T., Sciuto T. E., de la Fuente M. A., Geha R. S., Ochs H. D., Dvorak H. F., Dvorak A. M., Springer T. A. (2007). Transcellular diapedesis is initiated by invasive podosomes. Immunity 26, 784-797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabadel A., Banon-Rodriguez I., Cluet D., Rudkin B. B., Wehrle-Haller B., Genot E., Jurdic P., Anton I. M., Saltel F. (2007). CD44 and beta3 integrin organize two functionally distinct actin-based domains in osteoclasts. Mol. Biol. Cell 18, 4899-4910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellaiah M., Kizer N., Silva M., Alvarez U., Kwiatkowski D., Hruska K. A. (2000). Gelsolin deficiency blocks podosome assembly and produces increased bone mass and strength. J. Cell Biol. 148, 665-678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. T., Olden K., Bernard B. A., Chu F. F. (1984). Expression of transformation-associated protease(s) that degrade fibronectin at cell contact sites. J. Cell Biol. 98, 1546-1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destaing O., Saltel F., Geminard J. C., Jurdic P., Bard F. (2003). Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol. Biol. Cell 14, 407-416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovas A., Gevrey J. C., Grossi A., Park H., Abou-Kheir W., Cox D. (2009). Regulation of podosome dynamics by WASp phosphorylation: implication in matrix degradation and chemotaxis in macrophages. J. Cell Sci. 122, 3873-3882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. G., Correia I., Krasavina O., Watson N., Matsudaira P. (2003). Macrophage podosomes assemble at the leading lamella by growth and fragmentation. J. Cell Biol. 161, 697-705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimona M., Buccione R., Courtneidge S. A., Linder S. (2008). Assembly and biological role of podosomes and invadopodia. Curr. Opin. Cell Biol. 20, 235-241 [DOI] [PubMed] [Google Scholar]
- Gundersen H. J. G. (1977). Notes on the estimation of the numerical density of arbitrary profiles: The edge effect. J. Microsc. 111, 21-23 [Google Scholar]
- Gustafsson M. G., Shao L., Carlton P. M., Wang C. J., Golubovskaya I. N., Cande W. Z., Agard D. A., Sedat J. W. (2008). Three-dimensional resolution doubling in wide-field fluorescence microscopy by structured illumination. Biophys. J. 94, 4957-4970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburger D. S., Calderwood D. A. (2009). Integrin signalling at a glance. J. Cell Sci. 122, 159-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Grinnell F. (2005). Cell-matrix entanglement and mechanical anchorage of fibroblasts in three-dimensional collagen matrices. Mol. Biol. Cell 16, 5070-5076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverina I., Stradal T. E., Gimona M. (2003). Podosome formation in cultured A7r5 vascular smooth muscle cells requires Arp2/3-dependent de-novo actin polymerization at discrete microdomains. J. Cell Sci. 116, 4915-4924 [DOI] [PubMed] [Google Scholar]
- Kopp P., Lammers R., Aepfelbacher M., Woehlke G., Rudel T., Machuy N., Steffen W., Linder S. (2006). The kinesin KIF1C and microtubule plus ends regulate podosome dynamics in macrophages. Mol. Biol. Cell 17, 2811-2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S. (2007). The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell. Biol. 17, 107-117 [DOI] [PubMed] [Google Scholar]
- Linder S. (2009). Invadosomes at a glance. J. Cell Sci. 122, 3009-3013 [DOI] [PubMed] [Google Scholar]
- Linder S., Aepfelbacher M. (2003). Podosomes: adhesion hot-spots of invasive cells. Trends Cell. Biol. 13, 376-385 [DOI] [PubMed] [Google Scholar]
- Lucocq J. (1993). Particulate markers for immunoelectron microscopy. In Fine Structure Immunocytochemistry (ed. Griffiths G.), pp. 279-302 Berlin: Springer-Verlag; [Google Scholar]
- Lucocq J. (1994). Quantitation of gold labelling and antigens in immunolabelled ultrathin sections. J. Anat. 184, 1-13 [PMC free article] [PubMed] [Google Scholar]
- Lucocq J. (2003). Electron microscopy in cell biology. In Essential Cell Biology – A Practical Approach (ed. Davey J., Lord M.), pp. 53-112Oxford: Oxford University Press; [Google Scholar]
- Lucocq J. (2008). Quantification of structures and gold labeling in transmission electron microscopy. Methods Cell Biol. 88, 59-82 [DOI] [PubMed] [Google Scholar]
- Lucocq J. M., Berger E. G., Warren G. (1989). Mitotic Golgi fragments in HeLa cells and their role in the reassembly pathway. J. Cell Biol. 109, 463-474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxenburg C., Parsons J. T., Addadi L., Geiger B. (2006). Involvement of the Src-cortactin pathway in podosome formation and turnover during polarization of cultured osteoclasts. J. Cell Sci. 119, 4878-4888 [DOI] [PubMed] [Google Scholar]
- Luxenburg C., Geblinger D., Klein E., Anderson K., Hanein D., Geiger B., Addadi L. (2007). The architecture of the adhesive apparatus of cultured osteoclasts: from podosome formation to sealing zone assembly. PLoS One 2, e179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchisio P. C., Cirillo D., Naldini L., Primavera M. V., Teti A., Zambonin-Zallone A. (1984). Cell-substratum interaction of cultured avian osteoclasts is mediated by specific adhesion structures. J. Cell Biol. 99, 1696-1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchisio P. C., Cirillo D., Teti A., Zambonin-Zallone A., Tarone G. (1987). Rous sarcoma virus-transformed fibroblasts and cells of monocytic origin display a peculiar dot-like organization of cytoskeletal proteins involved in microfilament-membrane interactions. Exp. Cell Res. 169, 202-214 [DOI] [PubMed] [Google Scholar]
- Mierke C. T., Kollmannsberger P., Zitterbart D. P., Smith J., Fabry B., Goldmann W. H. (2008). Mechano-coupling and regulation of contractility by the vinculin tail domain. Biophys. J. 94, 661-670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S. C., Chen W. T. (1991). Cellular invasion into matrix beads: localization of beta 1 integrins and fibronectin to the invadopodia. J. Cell Sci. 99, 213-225 [DOI] [PubMed] [Google Scholar]
- Nermut M. V., Eason P., Hirst E. M., Kellie S. (1991). Cell/substratum adhesions in RSV-transformed rat fibroblasts. Exp. Cell Res. 193, 382-397 [DOI] [PubMed] [Google Scholar]
- Nitsch L., Gionti E., Cancedda R., Marchisio P. C. (1989). The podosomes of Rous sarcoma virus transformed chondrocytes show a peculiar ultrastructural organization. Cell Biol. Int. Rep. 13, 919-926 [DOI] [PubMed] [Google Scholar]
- Ochoa G. C., Slepnev V. I., Neff L., Ringstad N., Takei K., Daniell L., Kim W., Cao H., McNiven M., Baron R., et al. (2000). A functional link between dynamin and the actin cytoskeleton at podosomes. J. Cell Biol. 150, 377-389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa T., Itoh T., Takenawa T. (2008). Sequential signals toward podosome formation in NIH-src cells. J. Cell Biol. 182, 157-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poincloux R., Lizarraga F., Chavrier P. (2009). Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J. Cell Sci. 122, 3015-3024 [DOI] [PubMed] [Google Scholar]
- Schermelleh L., Carlton P. M., Haase S., Shao L., Winoto L., Kner P., Burke B., Cardoso M. C., Agard D. A., Gustafsson M. G., et al. (2008). Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science 320, 1332-1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight A. F., Cheung A., Limouze J., Chen I., Westwood N. J., Sellers J. R., Mitchison T. J. (2003). Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science 299, 1743-1747 [DOI] [PubMed] [Google Scholar]
- Stratman A. N., Saunders W. B., Sacharidou A., Koh W., Fisher K. E., Zawieja D. C., Davis M. J., Davis G. E. (2009). Endothelial cell lumen and vascular guidance tunnel formation requires MT1-MMP-dependent proteolysis in 3-dimensional collagen matrices. Blood 114, 237-247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarone G., Cirillo D., Giancotti F. G., Comoglio P. M., Marchisio P. C. (1985). Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp. Cell Res. 159, 141-157 [DOI] [PubMed] [Google Scholar]
- van Helden S. F., Oud M. M., Joosten B., Peterse N., Figdor C. G., van Leeuwen F. N. (2008). PGE2-mediated podosome loss in dendritic cells is dependent on actomyosin contraction downstream of the RhoA-Rho-kinase axis. J. Cell Sci. 121, 1096-1106 [DOI] [PubMed] [Google Scholar]
- Varon C., Tatin F., Moreau V., Van Obberghen-Schilling E., Fernandez-Sauze S., Reuzeau E., Kramer I., Genot E. (2006). Transforming growth factor beta induces rosettes of podosomes in primary aortic endothelial cells. Mol. Cell. Biol. 26, 3582-3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver A. M. (2008). Invadopodia. Curr. Biol. 18, R362-R364 [DOI] [PubMed] [Google Scholar]
- Weibel E. R. (1979). Stereological Methods London, New York, Toronto: Academic Press; [Google Scholar]
- West M. A., Antoniou A. N., Prescott A. R., Azuma T., Kwiatkowski D. J., Watts C. (1999). Membrane ruffling, macropinocytosis and antigen presentation in the absence of gelsolin in murine dendritic cells. Eur J. Immunol. 29, 3450-3455 [DOI] [PubMed] [Google Scholar]
- West M. A., Prescott A. R., Chan K. M., Zhou Z., Rose-John S., Scheller J., Watts C. (2008). TLR ligand-induced podosome disassembly in dendritic cells is ADAM17 dependent. J. Cell Biol. 182, 993-1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallone A. Z., Teti A., Primavera M. V., Naldini L., Marchisio P. C. (1983). Osteoclasts and monocytes have similar cytoskeletal structures and adhesion property in vitro. J. Anat. 137, 57-70 [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Apte S. S., Soininen R., Cao R., Baaklini G. Y., Rauser R. W., Wang J., Cao Y., Tryggvason K. (2000). Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc. Natl. Acad. Sci. USA 97, 4052-4057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler W. H., Liddington R. C., Critchley D. R. (2006). The structure and regulation of vinculin. Trends Cell. Biol. 16, 453-460 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.