Abstract
The myoepithelial sheath of the Caenorhabditis elegans somatic gonad has non-striated actomyosin networks that provide contractile forces during ovulation, a process in which a mature oocyte is expelled from the ovary. Troponin T and troponin C are known regulators of contraction of the myoepithelial sheath. These are two of the three components of the troponin complex that is generally considered as a striated-muscle-specific regulator of actomyosin contraction. Here, we report identification of troponin I as the third component of the troponin complex that regulates ovulatory contraction of the myoepithelial sheath. C. elegans has four genes encoding troponin-I isoforms. We found that tni-1 and unc-27 (also known as tni-2) encode two major troponin-I isoforms in the myoepithelial sheath. Combination of RNA interference and mutation of tni-1 and unc-27 resulted in loss of the troponin-I protein in the gonad and caused sterility due to defective contraction of the myoepithelial sheath. Troponin-I-depleted gonads were hypercontracted, which is consistent with the function of troponin I as an inhibitor of actomyosin contraction. Troponin I was associated with non-striated actin networks in a tropomyosin-dependent manner. Our results demonstrate that troponin I regulates contraction of non-striated actomyosin networks and is an essential cytoskeletal component of the C. elegans reproductive system.
Keywords: Troponin, Contraction, Ovulation, Myoepithelial cells, Actin, Myosin
Introduction
A wide variety of muscle systems occur in different tissues and function as force-generating organs. Muscle is classified into striated and non-striated (or smooth) muscles on the basis of the organization of the actin and myosin filaments. Striated muscle has sarcomeric organization of actin and myosin filaments, and repeats rapid cycles of contraction and relaxation. Non-striated muscle has a lattice-like network of actin and myosin filaments, and generally contracts and relaxes slowly. The difference in their contractile properties can partly be explained by different regulatory systems for actin-myosin interaction. In striated muscle, the actin-myosin interaction is inhibited at low Ca2+ concentrations by troponin that is linked to actin filaments, but the inhibition is released by binding of Ca2+ to troponin (Squire, 1997). However, in smooth muscle, contraction is activated by phosphorylation of myosin regulatory light chain (Gerthoffer, 2005; Somlyo and Somlyo, 1994). Although troponin is predominantly expressed in striated muscle, ascidian smooth muscle expresses troponin (Endo and Obinata, 1981) and a recent study has demonstrated that troponin is also expressed in mammalian vascular smooth muscle (Moran et al., 2008). Non-striated muscle and other non-striated-muscle-like cells, such as myoepithelial cells, myofibroblasts and pericytes, are quite diverse in morphology and function. Therefore, the regulatory mechanism of contractility is also likely to be diverse but has been studied only in a few representative systems.
The nematode Caenorhabditis elegans is a powerful model organism to study the structure and function of striated muscle, because worms have well-developed body-wall muscle with an organized sarcomeric structure that contains conserved myofibrillar proteins (Moerman and Fire, 1997; Waterston, 1988). C. elegans also has several non-striated muscles. In particular, the myoepithelial sheath of the somatic gonad is an interesting system, in which actin and myosin are organized in non-striated networks (Ardizzi and Epstein, 1987; Hall et al., 1999; Strome, 1986). The myoepithelial sheath surrounds oocytes, and its contraction is tightly coupled with oocyte maturation and is essential for ovulation (McCarter et al., 1997; McCarter et al., 1999). However, the regulatory mechanism of ovulatory contraction of the myoepithelial sheath is poorly understood. Despite the fact that the myoepithelial sheath has non-striated actomyosin networks, many of the structural components are expressed from the same genes that are also expressed in the striated body-wall muscle (Ardizzi and Epstein, 1987; Ono and Ono, 2004; Ono et al., 2006; Ono et al., 2007; Rose et al., 1997). The unique feature of the myoepithelial sheath is that components of the troponin complex play major roles in regulating contractility. Troponin is a complex of troponin T (TNT; tropomyosin-binding), troponin I (TNI; inhibitory) and troponin C (TNC; Ca2+-binding), and regulates actomyosin contraction in an actin-linked and Ca2+-dependent manner generally in striated muscle (Ebashi, 1984; Gomes et al., 2002). Mutation of mup-2 (which encodes TNT) (Myers et al., 1996) or RNA interference of pat-10 (which encodes TNC) (Ono and Ono, 2004) causes sterility due to defective contraction of the myoepithelial sheath during ovulation. These observations suggest that a conventional troponin complex is an actin-linked regulator of contraction of non-striated actomyosin networks in the myoepithelial sheath. However, a TNIcomponent has not been identified in the C. elegans myoepithelial sheath.
TNI is the central component of the troponin complex (Perry, 1999). At low Ca2+ concentrations, TNI binds to actin filament and inhibits its interaction with myosin. At high Ca2+ concentrations, binding of Ca2+ to TNC induces dissociation of TNI from actin and allows actomyosin contraction. C. elegans has four TNI-encoding genes: tni-1, unc-27 (also known as tni-2), tni-3 and tni-4. Mutation in unc-27 causes hypercontraction of the body-wall muscle (Burkeen et al., 2004; Ruksana et al., 2005), indicating that UNC-27 is a major TNI isoform in the body-wall muscle. Biochemical analysis of troponin from the giant nematode Ascaris showed that nematode TNI strongly inhibits ATPase activity of actomyosin (Kimura et al., 1987), suggesting that C. elegans TNI behaves in a similar manner. Therefore, when TNI is defective, actin and myosin are allowed to interact without regulation, which will lead to continuous muscle contraction. However, unc-27-null mutants are fertile and do not show a defect in the reproductive system. In this study, we aimed to identify TNI in the myoepithelial sheath and found that two TNI isoforms, TNI-1 and UNC-27, have partially redundant and essential functions in the myoepithelial sheath. This is the first in vivo demonstration that TNI is essential for reproduction and for regulation of contractility of non-striated muscle.
Results
Troponin I is expressed in the C. elegans somatic gonad and colocalizes with non-striated actin networks
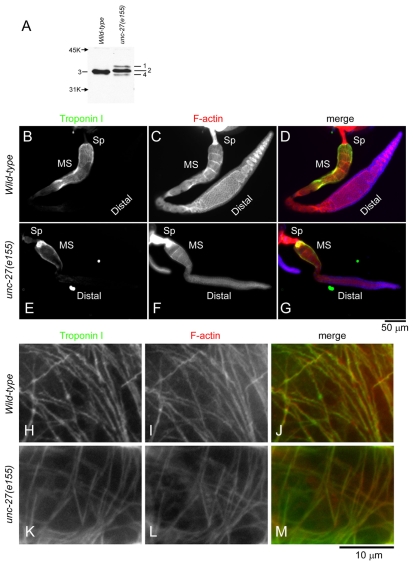
As a tool to detect C. elegans TNI, we re-evaluated the rabbit polyclonal antibody that was raised against Ascaris TNI (Kimura et al., 1987). A previous study has shown that this antibody reacts with C. elegans TNI (Nakae and Obinata, 1993), but this was before C. elegans TNI was molecularly characterized. C. elegans has four TNI genes, tni-1, unc-27, tni-3 and tni-4 (Burkeen et al., 2004; Ruksana et al., 2005). By western blot, we confirmed that this antibody reacted with a major band of TNI in wild-type worms (Fig. 1A). In addition, this antibody also recognized TNI isoforms in an unc-27(e155) (unc-27-null) mutant that lacks the major TNI isoform UNC-27 (Burkeen et al., 2004) (Fig. 1A). By comparing the bands of TNI in wild-type and unc-27-mutant worms, we detected at least four bands (Fig. 1A, bands 1-4). In wild-type worms, band 3 was predominant. Bands 2 and 4 were occasionally resolved from band 3 but these bands were often too minor to be separated clearly from band 3. In unc-27-null mutants, band 3 was absent, indicating that it corresponded to UNC-27 (Fig. 1A). Band 2 was the most intense band in unc-27-null mutants. Because band 2 migrated slower than band 3, band 4 was clearly separated from band 2 in unc-27-null mutants. In addition, band 1 was specifically detected in unc-27-null mutants (Fig. 1A). Therefore, UNC-27 is the major TNI isoform in wild-type worms. However, bands 1 and 2 in unc-27-null mutants are apparently increased as compared with those in wild type, suggesting that there was compensatory upregulation or stabilization of the second and third most abundant TNI isoforms in the absence of UNC-27. Our attempts to resolve TNI proteins by two-dimensional electrophoresis were not successful owing to inconsistent appearance of multiple spots that reacted with anti-TNI antibody, suggesting that TNI proteins are post-translationally modified.
Fig. 1.
Expression and localization of TNI in the myoepithelial sheath. (A) Western blot analysis of TNI in total lysates from wild-type or unc-27(e155) (unc-27-null) worms. Positions of molecular-weight markers are indicated by arrows on the left. Bands of TNI are designated as bands 1, 2, 3 and 4. (B-G) Dissected gonads from wild-type (B-D) or unc-27(e155) (E-G) worms were stained for TNI (B and E) and F-actin (C and F). Merged images are shown in D and G (TNI in green and F-actin in red). Locations of the spermatheca (Sp), myoepithelial sheath (MS) and distal arm (Distal) are indicated. (H-M) High-magnification images of the localization of TNI (H and K) and F-actin (I and L) in the myoepithelial sheath of wild-type (H-J) and unc-27(e155) (K-M) gonads. Merged images are shown in J and M (TNI in green and F-actin in red).
By immunofluorescent staining of the C. elegans gonad using the anti-Ascaris-TNI antibody, we found that TNI is expressed in the myoepithelial sheath (Fig. 1B-G) and that it localized to non-striated actin networks (Fig. 1H-M). In dissected gonads from wild-type worms, TNI was specifically expressed in the myoepithelial sheath that surrounds oocytes in the proximal gonad (Fig. 1B) but not in the spermatheca, oocytes or distal arm containing non-myoepithelial sheath, germline cells and distal-tip cell (Fig. 1B-D). TNI colocalized to non-striated networks of actin filaments in the myoepithelial sheath (Fig. 1H-J). In an unc-27-mutant gonad, TNI was also specifically expressed in the myoepithelial sheath (Fig. 1E) and colocalized with actin filaments (Fig. 1K-M). These results indicate that TNI is a component of non-striated actin networks in the myoepithelial sheath, and a TNI isoform(s) other than UNC-27/TNI-2 is expressed in the gonad.
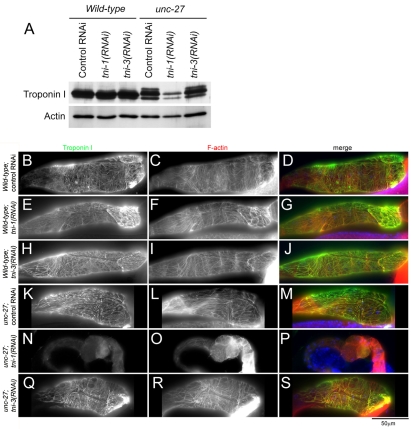
To determine which TNI isoforms are expressed in the myoepithelial sheath, we knocked down each TNI isoform in wild-type worms or unc-27 mutants and examined TNI expression by western blot and immunofluorescence microscopy. Previous studies have demonstrated that tni-4 is specifically expressed in the pharyngeal muscle (Burkeen et al., 2004; Ruksana et al., 2005). Therefore, we focused on tni-1, unc-27 and tni-3 in this study. In wild-type worms, neither tni-1(RNAi) nor tni-3(RNAi) had a detectable effect on the total TNI level as compared with control RNAi (Fig. 2A). By immunofluorescent staining of dissected wild-type gonads, it was observed that neither tni-1(RNAi) nor tni-3(RNAi) caused a detectable change in intensity and localization patterns of TNI (Fig. 2E,H) as compared with control-RNAi-treated gonads (Fig. 2B), suggesting that UNC-27 is a major TNI isoform in the myoepithelial sheath. In unc-27-null-mutant worms, tni-1(RNAi) greatly reduced the TNI protein level, whereas tni-3(RNAi) caused no detectable change in the TNI level (Fig. 2A). In tni-1(RNAi)-treated unc-27 mutants, the top band of TNI was not detectable, and the most abundant middle band was significantly reduced (Fig. 2A), suggesting that the TNI-1 protein is resolved in these two bands. By immunofluorescent staining of dissected unc-27 gonads, tni-1(RNAi) resulted in a marked reduction of TNI in the myoepithelial sheath (Fig. 2N), whereas tni-3(RNAi) did not affect the staining patterns of TNI (Fig. 2Q) as compared with control-RNAi-treated gonads (Fig. 2K). Therefore, in the absence of UNC-27, TNI-1 is the major isoform in the myoepithelial sheath. Taken together, these results indicate that TNI-1 and UNC-27 are the two major TNI isoforms in the myoepithelial sheath.
Fig. 2.
Identification of TNI-1 and UNC-27 as the major TNI isoforms in the myoepithelial sheath. (A) Western blot analysis of TNI and actin in total lysates from wild-type or unc-27-null worms that were treated with control RNAi, tni-1(RNAi) or tni-3(RNAi). (B-S) Wild-type (B-J) or unc-27-null (K-S) worms were treated with control RNAi (B-D and K-M), tni-1(RNAi) (E-G and N-P) or tni-3(RNAi) (H-J and Q-S), and dissected gonads were stained for TNI (left column) and F-actin (middle column). Merged images are shown in the right column (TNI in green and F-actin in red).
Two TNI isoforms are functionally redundant and essential for ovulatory contraction of the somatic gonad
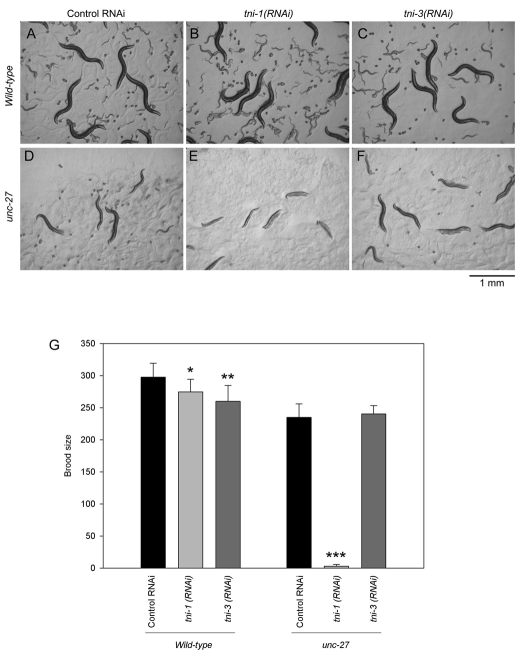
We found that simultaneous depletion of TNI-1 and UNC-27 caused sterility, indicating that these two TNI isoforms are functionally redundant and essential for reproduction in C. elegans. Wild-type worms that were treated with control RNAi (Fig. 3A), tni-1(RNAi) (Fig. 3B) or tni-3(RNAi) (Fig. 3C) produced ~250-300 progeny (brood size) (Fig. 3G). tni-1(RNAi) and tni-3(RNAi) only slightly reduced brood size (Fig. 3G). The unc-27-null mutant with control RNAi was fertile (Fig. 3D) but produced less progeny than wild type (Fig. 3G, compare control RNAi in wild type and unc-27; P<0.001). However, tni-1(RNAi)-treated unc-27 mutants (Fig. 3E) were 33% (5/15) sterile with an average brood size of 3.1±2.6 per worm (n=10) (Fig. 3G). tni-3(RNAi) did not affect brood size in the unc-27 mutant (Fig. 3F,G). These results strongly suggest that TNI-1 and UNC-27 play redundant and major roles in C. elegans reproduction.
Fig. 3.
Requirement of TNI-1 and UNC-27 for C. elegans reproduction. (A-F) Wild-type (A-C) or unc-27-null (D-F) worms were treated with control-RNAi (A and D), tni-1(RNAi) (B and E) or tni-3(RNAi) (C and F), and micrographs of live worms on agar plates are shown. Small worms and embryos are progeny of the adult worms. However, tni-1(RNAi)-treated unc-27-mutant worms produced only a few progeny (E; embryos are not shown in the micrograph). (G) Measurement of brood size (number of progeny per worm) (n=10). A t-test was performed on control RNAi and tni-1(RNAi) or tni-3(RNAi) for each strain. Single, double and triple asterisks indicate 0.01<P<0.05, 0.001<P<0.01 and P<0.001, respectively.
To better understand the role of TNI in reproduction, we analyzed TNI-depleted phenotypes in the hermaphroditic gonad. The myoepithelial sheath, in which TNI is expressed (Fig. 1), provides contractile forces during ovulation to transport a mature oocyte into the spermatheca (McCarter et al., 1997; McCarter et al., 1999). Defective contraction of the myoepithelial sheath often causes accumulation of endomitotic oocytes in the proximal ovary because DNA replication repeatedly occurs in the absence of fertilization, and this phenotype is called Emo phenotype (endomitotic oocytes in the ovary) (Iwasaki et al., 1996). Indeed, Emo phenotype was observed when tropomyosin or TNC was depleted (Ono and Ono, 2004).
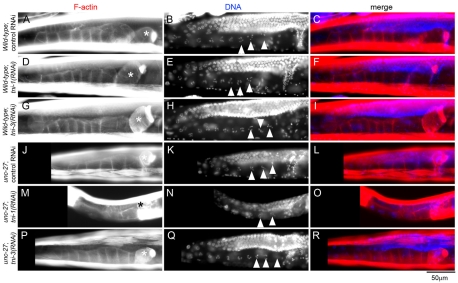
However, Emo phenotype was not detected after depletion of tni-1 or tni-3 in wild-type or unc-27 worms (Fig. 4). In wild-type worms with control RNAi, oocytes with condensed chromosomes were linearly organized in the proximal ovary (Fig. 4A-C, arrowheads in B indicate oocyte nuclei), and tni-1(RNAi) or tni-3(RNAi) did not affect this morphology (Fig. 4D-I). The unc-27-null mutant with control RNAi also had normal oocytes with condensed chromosomes in a linear arrangement (Fig. 4J-L). The tni-1(RNAi)-treated unc-27 mutant had oocytes with condensed chromosomes in the proximal ovary (Fig. 4M-O). We did not detect any abnormalities in the morphology of the nucleus and chromosomes (our unpublished data). In addition, the presence of some embryos in the uterus suggests that successful ovulation and fertilization took place (our unpublished data). The tni-3(RNAi)-treated unc-27 mutant showed no detectable difference in the morphology of the gonad from the unc-27 mutant with control RNAi (Fig. 4P-R).
Fig. 4.
Depletion of TNI does not cause Emo phenotype. Wild-type (A-I) or unc-27-null (J-R) worms were treated with control RNAi (A-C and J-L), tni-1(RNAi) (D-F and M-O) or tni-3(RNAi) (G-I and P-R), and fluorescently stained for F-actin (left column) and DNA (middle column). Merged images are shown in the right column (F-actin in red and DNA in blue). Asterisks indicate the spermatheca, and arrowheads point to nuclei of proximal oocytes. tni-1(RNAi)-treated unc-27 worms were nearly sterile but did not have endomitotic oocytes in the proximal ovary (M-O), which is typically associated with ovulation defects.
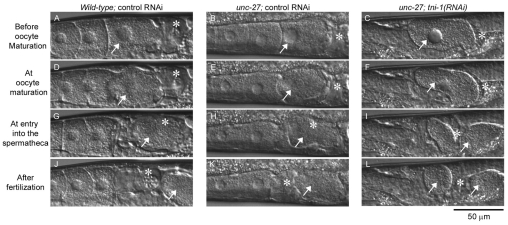
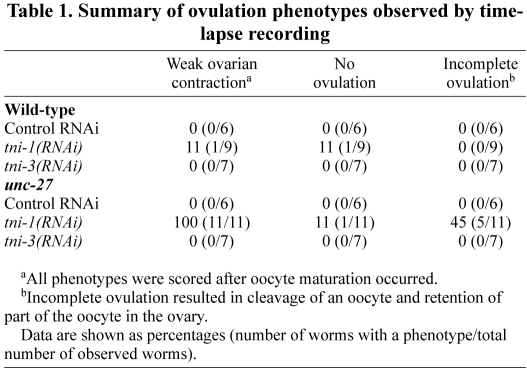
Further analysis of the process of ovulation in live worms revealed that ovulatory contraction of the myoepithelial sheath was greatly weakened by depletion of TNI (Fig. 5). In wild-type worms with control RNAi (supplementary material Movie 1), upon oocyte maturation, which is characterized by nuclear-envelope breakdown (Fig. 5A,D), the proximal ovary vigorously contracted and pushed the oocyte into the spermatheca (Fig. 5G). Wild-type worms with tni-1(RNAi) or tni-3(RNAi) showed normal ovulation (Table 1, supplementary material Movie 2), although one case out of nine observed tni-1(RNAi)-treated wild-type worms exhibited weak ovarian contraction and failed ovulation (Table 1). unc-27-null-mutant worms with control RNAi also executed normal ovulation (Fig. 5B,E,H,K, supplementary material Movie 3). However, tni-1(RNAi)-treated unc-27 worms showed abnormal ovulation processes (Table 1). All of the observed worms (n=11) did not exhibit vigorous ovarian contraction after oocyte maturation (Fig. 5F,I, supplementary material Movie 4). One observed worm did not ovulate at all (Table 1). In most cases, an oocyte was apparently pushed into the spermatheca by tonic contraction and weak twitching of the proximal ovary (Fig. 5F,I, supplementary material Movie 4). Furthermore, in 45% (5/11) of the observed events, ovulation was incomplete and a portion of an oocyte was pinched off and retained in the ovary (Fig. 5L), indicating that contraction and relaxation of the myoepithelial sheath and dilation of the spermatheca were uncoordinated. unc-27 worms with tni-3(RNAi) showed normal ovulation (Table 1). These live observations indicate that TNI is required for vigorous ovarian contraction after oocyte maturation.
Fig. 5.
Depletion of TNI weakens ovarian contraction during ovulation. Ovulation processes of wild-type with control RNAi (left column), unc-27 with control RNAi (middle column) and unc-27 with tni-1(RNAi) (right column) worms were recorded by time-lapse microscopy (also see supplementary material Movies 1, 3 and 4). Selected images of gonads before oocyte maturation (A-C), at oocyte maturation (nuclear-envelope breakdown) (D-F), at entry of an oocyte into the spermatheca (G-I) and after fertilization (J-L) are shown. Asterisks indicate positions of the spermatheca. Arrows indicate oocytes being ovulated. In unc-27 worms with tni-1(RNAi), a portion of the oocyte was pinched off by uncoordinated activity of the spermatheca and retained in the ovary (arrows in L). Ovulation processes of wild-type with tni-1(RNAi) are only shown in supplementary material Movie 2.
Table 1.
Summary of ovulation phenotypes observed by time-lapse recording
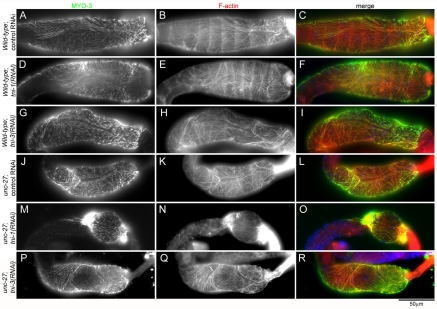
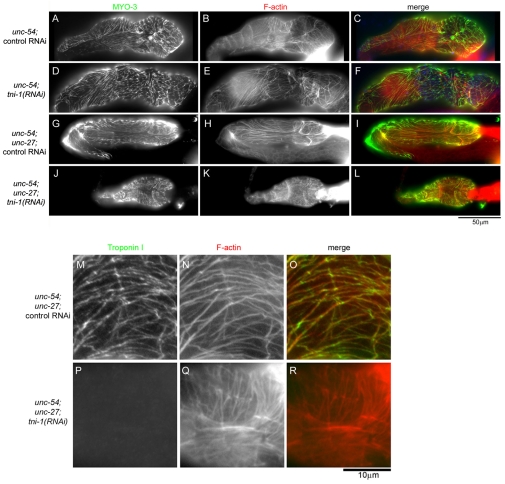
Localization of a myosin heavy chain in the myoepithelial sheath revealed that the sheath was hypercontracted in TNI-depleted gonads (Fig. 6). MYO-3 is a myosin heavy chain that is expressed in the myoepithelial sheath and localizes to the contractile apparatuses (Ardizzi and Epstein, 1987). In wild-type gonads with control RNAi, thick filaments containing MYO-3 were distributed throughout the myoepithelial sheath, which covered ~ten oocytes in the proximal ovary (Fig. 6A-C). tni-1(RNAi) or tni-3(RNAi) in wild-type gonads did not alter this morphology (Fig. 6D-I). In unc-27-null gonads with control RNAi (Fig. 6J-L), the myoepithelial sheaths were shorter than those in wild-type gonads with control RNAi (Fig. 6, compare A and J). This indicates that the myoepithelial sheath was partially contracted by deficiency of UNC-27 alone, although ovulation was apparently normal (Fig. 5, supplementary material Movie 3). More strikingly, tni-1(RNAi)-treated unc-27 gonads showed much greater hypercontraction of the myoepithelial sheath (Fig. 6M-O). The myoepithelial sheath was so contracted that it covered only one to three oocytes (our unpublished data). By contrast, tni-3(RNAi) did not induce further contraction in the unc-27 mutant (Fig. 6P-R). These results indicate that TNI-1 and UNC-27 play major roles in inhibiting contraction of the myoepithelial sheath.
Fig. 6.
Depletion of TNI causes hypercontraction of the myoepithelial sheath. Wild-type (A-I) or unc-27-null (J-R) worms were treated with control-RNAi (A-C and J-L), tni-1(RNAi) (D-F and M-O) or tni-3(RNAi) (G-I and P-R) and dissected gonads were stained for MYO-3 myosin heavy chain (left column), F-actin (middle column) and DNA (only shown in merged images). Merged images are shown in the right column (MYO-3 in green, F-actin in red and DNA in blue). MYO-3 is a marker of the contractile apparatuses in the myoepithelial sheath and shows partially contracted sheaths in unc-27 mutants treated with control RNAi (J) or tni-3(RNAi) (P) and a hypercontracted sheath in unc-27 mutants treated with tni-1(RNAi) (M) as compared with relaxed sheaths in wild-type (A, D and G).
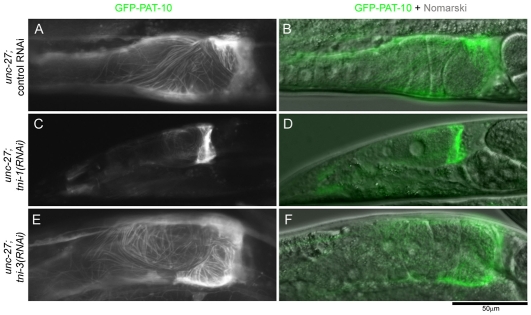
To exclude the possibility that the hypercontraction phenotype of the myoepithelial sheath is due to an artifact of dissection, fixation and/or immunostaining, we observed myoepithelial sheath in living worms using a fluorescent marker (Fig. 7). GFP-tagged PAT-10 (TNC) was expressed by the promoter of ppk-1, which is active in the myoepithelial sheath (Xu et al., 2007), and the morphology of the intact proximal gonad was observed by fluorescence microscopy without fixation. In unc-27 worms with control RNAi (Fig. 7A,B) or tni-3(RNAi) (Fig. 7E,F), the myoepithelial sheath was in elongated normal morphology. By contrast, unc-27 worms with tni-1(RNAi) had a shrunk myoepithelial sheath adjacent to the spermatheca (Fig. 7C,D), indicating that the hypercontraction phenotype of the myoepithelial sheath in TNI-depleted worms is not an artifact of immunostaining procedures.
Fig. 7.
The hypercontraction phenotype of the myoepithelial sheath observed in live worms. GFP–PAT-10 was transgenically expressed in the myoepithelial sheath of unc-27 mutants and treated with control RNAi (A and B), tni-1(RNAi) (C and D), or tni-3(RNAi) (E and F). Morphology of the proximal gonads was observed by the GFP fluorescence (A, C and E) in live worms without fixation. B, D and F are overlayed images of the GFP fluorescence and Nomarski micrographs.
The hypercontraction phenotype of the myoepithelial sheath in TNI-depleted worms was partially suppressed by a mutation in the UNC-54 myosin heavy chain. UNC-54 is a major myosin heavy chain of the thick filaments in the myoepithelial sheath (Rose et al., 1997). An unc-54(s95) mutation impairs muscle contractility without altering myofibril organization (Moerman et al., 1982). In unc-54(s95) worms with control RNAi (Fig. 8A-C) or tni-1(RNAi) (Fig. 8D-F), morphology of the myoepithelial sheath as shown by localization of the MYO-3 myosin heavy chain (Fig. 8A,D) and actin filaments (Fig. 8B,E) appeared normal. unc-54(s95);unc-27(e155) double-mutant worms with control RNAi had a nearly normal myoepithelial sheath (Fig. 8G-I) with normal expression and localization of TNI (Fig. 8M-O). unc-54(s95);unc-27(e155) double-mutant worms with tni-1(RNAi) had a partially contracted but significantly more elongated myoepithelial sheath (Fig. 8J-L) than unc-27(e155) with tni-1(RNAi) (Fig. 6M-O). The TNI protein was undetectable in the myoepithelial sheath of these worms (Fig. 8P-R). Three pairs of the myoepithelial-sheath cells are distinguishable in unc-54(s95);unc-27(e155) with tni-1(RNAi) gonads (Fig. 8J), indicating that TNI depletion did not disturb overall organization of the myoepithelial sheath. Importantly, the partial suppression of the hypercontraction phenotype in TNI-depleted gonad by a myosin mutation strongly suggests that the phenotype is due to unregulated actin-myosin interaction in the absence of inhibitory function of TNI.
Fig. 8.
Partial suppression of the TNI-depletion phenotype by a mutation in the UNC-54 myosin heavy chain. (A-L) unc-54 single mutant (A-F) or unc-54;unc-27 double mutant (G-L) worms were treated with control RNAi (A-C and G-I) or tni-1(RNAi) (D-F and J-L), and dissected gonads were stained for MYO-3 myosin heavy chain (left column), F-actin (middle column) and DNA (only shown in merged images). Merged images are shown in the right column (MYO-3 in green, F-actin in red and DNA in blue). (M-R) unc-54;unc-27 double-mutant worms were treated with control RNAi (M-O) or tni-1(RNAi) (P-R), and dissected gonads were stained for TNI (left column) and F-actin (middle column). Merged images are shown in the right column (TNI in green and F-actin in red).
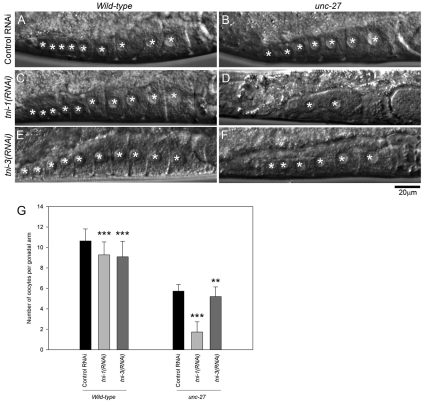
Furthermore, we found that the contraction states of the myoepithelial sheath were correlated with a reduction in the number of oocytes in the proximal gonad in TNI-depleted worms. Wild-type worms with control-RNAi treatment had 9 to 11 oocytes per gonadal arm (Fig. 9A,G), and tni-1(RNAi) or tni-3(RNAi) only slightly reduced the number of oocytes (Fig. 9C,E,G). unc-27-mutant worms with control RNAi treatment had five to seven oocytes per gonadal arm (Fig. 9B,G). Thus, loss of UNC-27 is sufficient to cause a reduction of oocytes. Furthermore, unc-27 mutant worms with tni-1(RNAi) had only one to three oocytes per arm (Fig. 9D,G), whereas tni-3(RNAi) in the unc-27 background did not further reduce the number of oocytes (Fig. 9F,G). Thus, hypercontraction of the myoepithelial sheath causes a negative impact on oocyte production, suggesting that maintaining a relaxed state of the myoepithelial sheath is important for hosting an environment for proper oocyte development.
Fig. 9.
Depletion of TNI reduces the number of oocytes in the proximal gonad. Wild-type (A, C and E) or unc-27-null (B, D and F) worms were treated with control-RNAi (A and B), tni-1(RNAi) (C and D) or tni-3(RNAi) (E and F), and Nomarski micrographs of the proximal gonads are shown. Asterisks indicate oocyte nuclei. (G) Number of oocytes per gonadal arm (n=50). A t-test was performed on control RNAi and tni-1(RNAi) or tni-3(RNAi) for each strain. Double and triple asterisks indicate 0.001<P<0.01 and P<0.001, respectively.
Association of TNI with thin filaments depends on tropomyosin but not on TNC
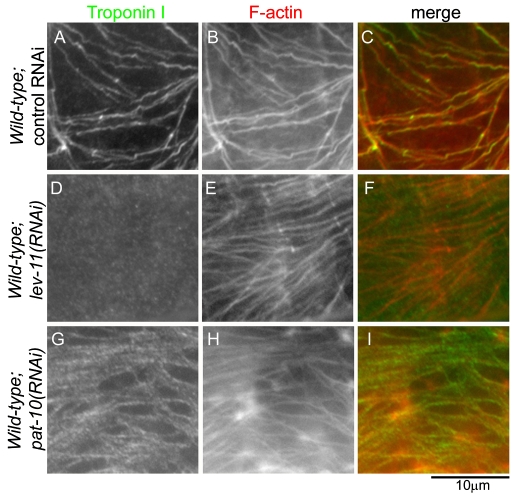
Tropomyosin mediates association of the troponin complex with actin filaments (Ebashi and Kodama, 1966). We previously showed that PAT-10 requires tropomyosin for it to localize to actin filaments in the myoepithelial sheath (Ono and Ono, 2004). However, TNI can directly bind to actin filaments in vitro (Perry, 1999), and it is not clear whether this direct binding is sufficient for TNI to localize to actin filaments in vivo. We found that tropomyosin was required for association of TNI with actin filaments in the myoepithelial sheath (Fig. 10). When tropomyosin was depleted by RNAi, TNI was detected in the diffuse cytoplasm and was no longer associated with filamentous actin (Fig. 10D-F), as compared with clear filamentous localization of TNI in control-RNAi-treated gonad (Fig. 10A-C). In addition, when PAT-10 was depleted, filamentous staining of TNI was slightly weaker than that in control-RNAi-treated gonad (Fig. 10G-I), indicating that PAT-10 is not required for association of TNI with actin filaments in vivo but might enhance or stabilize binding of TNI to the filaments. These results suggest that TNI functions as a component of the troponin complex that is anchored to actin filaments via tropomyosin.
Fig. 10.
Association of TNI with actin filaments is dependent on tropomyosin in the myoepithelial sheath. (A-I) Wild-type worms were treated with control RNAi (A-C), lev-11(RNAi) (RNAi for tropomyosin) (D-F) or pat-10(RNAi) (RNAi for TNC) (G-I), and dissected gonads were stained for TNI (A, D, and G) and F-actin (B, E, and H). Merged images are shown in C, F and I (TNI in green and F-actin in red)
Discussion
In this study, we identified TNI genes that control contraction and relaxation of non-striated muscle (myoepithelial sheath) in the C. elegans somatic gonad. Among the four TNI genes, two of them, tni-1 and unc-27, play major roles in the gonad. TNI was associated with non-striated networks of actin filaments in the myoepithelial sheath, and this association was dependent on tropomyosin. Depletion of the two TNI isoforms resulted in sterility with excessive contraction of the proximal ovary. These results demonstrate that two TNI isoforms have partially redundant functions and are crucial for regulation of muscle contraction during ovulation.
Identification of TNI in the C. elegans gonad and previous studies on TNT (Myers et al., 1996) and TNC (Ono and Ono, 2004) strongly suggest that the conventional troponin complex consisting of TNT, TNI and TNC regulates contraction of the myoepithelial sheath during ovulation by an actin-linked mechanism. Each component of the troponin complex is essential for C. elegans reproduction. TNC and TNI are expressed in the myoepithelial sheath and localize to the actin thin filaments, although TNT has not been characterized at a protein level. However, the TNI-depletion phenotype was different from the TNT-mutant and TNC-RNAi phenotypes. When TNT or TNC is defective, ovulation fails, and endomitotic oocytes are accumulated in the proximal ovary (Myers et al., 1996; Ono and Ono, 2004). However, when TNI is defective, mature oocytes are often incompletely ovulated, and the myoepithelial sheath is constantly hypercontracted. TNI directly interacts with actin and inhibits its interaction with the myosin head at low Ca2+ concentrations, and Ca2+-bound TNC releases its inhibitory action and allows actin-myosin interaction (Ebashi, 1984). Because we observed that TNI was still associated with actin filaments in TNC-depleted gonads, TNI could still inhibit actomyosin contraction in the absence of TNC. However, when TNI was depleted, actin and myosin could interact regardless of changes in Ca2+ concentrations, thereby allowing constant contraction of the myoepithelial sheath. RNAi does not generally deplete 100% of a target protein, and residual TNI in tni-1(RNAi)-treated unc-27 gonads might be sufficient to support cycles of contraction and relaxation during ovulation. Thus, the phenotypic consequences of depletion of troponin components are consistent with known biochemical properties of the conventional troponin complex.
Decrease in the number of oocytes in the proximal ovary by depletion of TNI was an unexpected phenotype. Because ovulation was incomplete but partially successful in TNI-depleted worms, insufficient supply of oocytes seemed to be the major cause of sterility. In wild-type worms, the proximal ovary normally holds 10-12 oocytes, which can keep up with ovulation that occurs every 20-30 minutes (McCarter et al., 1997; McCarter et al., 1999). In unc-27-null-mutant worms, the number of oocytes was decreased to five to seven, but brood size was only slightly less than that of wild type under the same culture conditions. However, tni-1(RNAi)-treated unc-27 mutants had only one to three oocytes in the ovary and, after ovulation of an oocyte, the next oocyte was often absent or immature. Interestingly, the decrease in oocytes was correlated with the extent of contraction of the myoepithelial sheath. unc-27 mutants with control RNAi or tni-3(RNAi) showed a partially contracted myoepithelial sheath and a decreased number of oocytes, and unc-27 mutants with tni-1(RNAi) showed further hypercontraction and decrease in the number of oocytes. These observations suggest that maintaining a relaxed state of the myoepithelial sheath is important for oocyte development. Gonadal sheath cells play important roles in germline development. In particular, distal sheath cells promote proliferation of the germline in the distal gonad (Killian and Hubbard, 2005). Contraction of the myoepithelial sheath might affect the function of distal sheath cells, which might result in a reduction in the number of germ cells. In addition, the proximal ovary is an important organ for oogenesis; it is here that oocytes gain cytoplasmic components from the distal gonad through the rachis (Wolke et al., 2007) and yolk proteins from the body cavity by endocytosis (Grant and Hirsh, 1999). Therefore, excessive contraction of the myoepithelial sheath might physically limit the number of residing oocytes and prevent these crucial processes in oogenesis.
To our knowledge, the C. elegans myoepithelial sheath is the only non-striated muscle in which the function of troponin is genetically demonstrated. Ascidian smooth muscle also expresses a functional troponin complex (Endo and Obinata, 1981), but it has not been characterized by a genetic approach. Otherwise, troponin is predominantly expressed in striated muscle and has been widely accepted as a striated-muscle-specific regulator of contraction (Ebashi, 1984; Squire and Morris, 1998). However, recently, expression of troponin in mouse vascular smooth muscle has been reported (Moran et al., 2008), although its function is still unclear. Furthermore, TNI is involved in the regulation of chromosomal stability and cell polarity in early Drosophila embryos (Sahota et al., 2009). Thus, additional cell-biological and genetic investigation on troponin in non-striated muscle and non-muscle cells might reveal unknown functions of the troponin complex or individual troponin components.
Cell contractility is also implicated in mammalian ovulation. In mammalian ovaries, each oocyte is surrounded by a follicle that contains an external layer of smooth-muscle-like cells (Espey, 1978). These cells express markers of smooth muscle, including smooth-muscle α-actin and desmin, but the function of these cells is not understood. A recent report suggests that these smooth-muscle-like cells are indeed contractile and contribute to rupture of a follicle at the late step of ovulation in the mouse ovary (Ko et al., 2006). Therefore, although the structure of the gonads and the process of ovulation are different between nematodes and mammals, there might be a similarity in the mechanisms of regulation of cell contractility during ovulation. Involvement of troponin in mammalian ovulation is unknown, but there is a possibility that cell contraction is regulated by an actin-linked mechanism during ovulation. Further analysis of the mechanisms of ovulation in different species might reveal evolutionarily conserved roles of actin-associated proteins in reproductive systems.
Materials and Methods
Nematode strains
Wild-type strain N2, an unc-27-null mutant CB155 unc-27(e155) and an unc-54 mutant RW5008 unc-54(s95) were obtained from the Caenorhabditis Genetic Center (Minneapolis, MN). An unc-54(s95);unc-27(e155) double mutant was generated by standard crosses. A transgenic line carrying an extrachromosomal array kqEx61[ppk-1::pat-10::gfp, pRF4] (Xu et al., 2007) was provided by Myeongwoo Lee (Baylor University, Waco, TX). The transgene was placed in the unc-27(e155) background by standard crosses. Nematodes were grown under standard conditions at 20°C as described previously (Brenner, 1974).
RNAi experiments
RNAi experiments were performed by feeding with E. coli expressing double-stranded RNA as described previously (Ono and Ono, 2002). RNAi clones for tni-1 (X-5B01) and tni-3 (V-11A06) were obtained from MRC GeneService (Kamath et al., 2003). RNAi clones for C. elegans tropomyosin (lev-11) targeting four isoforms (previously designated as TM2) and pat-10 were described previously (Ono and Ono, 2004; Ono and Ono, 2002). L4 larvae were treated with RNAi, and phenotypes were characterized in the F1 generation.
Western blot
Western blot analysis of TNI was performed essentially as described (Ono and Ono, 2009) with modifications. Briefly, ten adult worms were lysed in SDS-lysis buffer, and subjected to SDS-PAGE (12% acrylamide gel) and blotting. Rabbit anti-Ascaris-TNI antibody (Nakae and Obinata, 1993) was diluted at 1:2000 to 1:8000 in Signal Enhancer HIKARI (Nacalai USA) and used to detect C. elegans TNI. Mouse anti-actin monoclonal antibody (C4; MP Biomedicals) was used to demonstrate equal loading of the samples. Horseradish-peroxidase-labeled secondary antibodies were used to detect primary antibodies. The reactivity was detected with a SuperSignal West Pico chemiluminescence reagent (Pierce Chemical).
Fluorescence microscopy
The gonads were dissected by cutting adult hermaphrodites at the level of pharynx on poly-L-lysine-coated slides as described previously (Rose et al., 1997). Immunostaining of dissected gonads were performed essentially as described (Ono et al., 2008; Ono et al., 2007) with some modifications. The samples were fixed with 4% paraformaldehyde in cytoskeleton buffer (138 mM KCl, 3 mM MgCl2, 2 mM EGTA, and 10 mM MES-KOH, pH 6.1) containing 0.32 M sucrose for 10 minutes at room temperature followed by treatment with phosphate-buffered saline (PBS) containing 0.5% Triton X-100 and 30 mM glycine for 10 minutes. They were incubated with rabbit anti-TNI antibody or mouse anti-MYO-3 monoclonal antibody 5-6 (Miller et al., 1983), 0.2 μg/ml tetramethylrhodamine-phalloidin and 0.2 μg/ml 4′6-diamidino-2-phenylindole (DAPI) in PBS containing 0.5% Triton X-100 and 30 mM glycine for 1 hour and washed with PBS containing 0.5% Triton X-100 and 30 mM glycine. The primary antibodies were visualized by Alexa-Fluor-488-conjugated goat anti-rabbit IgG (Invitrogen) or Alexa-Fluor-488-conjugated goat anti-mouse IgG (Invitrogen).
Staining of whole worms with tetramethylrhodamine-phalloidin was performed as described previously (Ono, 2001).
Samples of fixed worms or tissues were mounted with ProLong Gold (Invitrogen) and observed by epifluorescence using a Nikon Eclipse TE2000 inverted microscope with a CFI Plan Fluor ELWD 40× (dry; NA 0.60) or Plan Apo 60× (oil; NA 1.40) objective. To observe the GFP fluorescence in live worms, worms were anesthetized in 0.1% tricaine, 0.01% tetramisole in M9 buffer for 30 minutes and mounted on 2% agarose pads. Images were captured by a SPOT RT monochrome CCD camera (Diagnostic Instruments) and processed by IPLab imaging software (BD Biosciences) and Adobe Photoshop CS3.
Time-lapse Nomarski microscopy
Worms were anesthetized in 0.1% tricaine, 0.01% tetramisole in M9 buffer for 30 minutes and mounted on 2% agarose pads (McCarter et al., 1997). Tricaine and tetramisole paralyzes body-wall movement but does not block two to three consecutive rounds of oocyte maturation and ovulation. They were set on a Nikon Eclipse TE2000 inverted microscope and observed with a 40× CFI Plan Fluor objective (N.A. 1.4). Images were captured at room temperature by a SPOT Idea CMOS camera (Diagnostic Instruments) and recorded at five frames per second for 30 minutes by the SPOT Imaging Software (Diagnostic Instruments). Movie files were saved in a compressed AVI format at 20 frames per second (four times as fast as real time) and phenotypes analyzed later. Movie files were edited and converted to QuickTime files using Adobe Premiere 6.5.
Supplementary Material
Acknowledgments
Monoclonal antibody 5-6 was developed by Henry Epstein (University of Texas Medical Branch, Galveston), and was obtained from the Developmental Studies Hybridoma Bank, developed under auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA. C. elegans strains were provided by Caenorhabditis Genetics Center, which is supported by the National Institute of Health National Center for Research Resources. We thank Myeongwoo Lee (Baylor University, Waco, TX) for kindly providing us with the GFP–pat-10 transgenic worms. This work was supported by grants from the Ministry of Education, Science and Culture, Japan (Grant number 20570075) to T.O. and the National Institute of Health (R01 AR48615) and University Research Committee of Emory University to S.O. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/9/1557/DC1
References
- Ardizzi J. P., Epstein H. F. (1987). Immunochemical localization of myosin heavy chain isoforms and paramyosin in developmentally and structurally diverse muscle cell types of the nematode Caenorhabditis elegans. J. Cell Biol. 105, 2763-2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkeen A. K., Maday S. L., Rybicka K. K., Sulcove J. A., Ward J., Huang M. M., Barstead R., Franzini-Armstrong C., Allen T. S. (2004). Disruption of Caenorhabditis elegans muscle structure and function caused by mutation of troponin I. Biophys. J. 86, 991-1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S. (1984). Ca2+ and the contractile proteins. J. Mol. Cell Cardiol. 16, 129-136 [DOI] [PubMed] [Google Scholar]
- Ebashi S., Kodama A. (1966). Interaction of troponin with F-actin in the presence of tropomyosin. J. Biochem. 59, 425-426 [DOI] [PubMed] [Google Scholar]
- Endo T., Obinata T. (1981). Troponin and its components from ascidian smooth muscle. J. Biochem. 89, 1599-1608 [DOI] [PubMed] [Google Scholar]
- Espey L. L. (1978). Ovarian contractility and its relationship to ovulation: a review. Biol. Reprod. 19, 540-551 [DOI] [PubMed] [Google Scholar]
- Gerthoffer W. T. (2005). Actin cytoskeletal dynamics in smooth muscle contraction. Can. J. Physiol. Pharmacol. 83, 851-856 [DOI] [PubMed] [Google Scholar]
- Gomes A. V., Potter J. D., Szczesna-Cordary D. (2002). The role of troponins in muscle contraction. IUBMB Life 54, 323-333 [DOI] [PubMed] [Google Scholar]
- Grant B., Hirsh D. (1999). Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol. Biol. Cell 10, 4311-4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. H., Winfrey V. P., Blaeuer G., Hoffman L. H., Furuta T., Rose K. L., Hobert O., Greenstein D. (1999). Ultrastructural features of the adult hermaphrodite gonad of Caenorhabditis elegans: relations between the germ line and soma. Dev. Biol. 212, 101-123 [DOI] [PubMed] [Google Scholar]
- Iwasaki K., McCarter J., Francis R., Schedl T. (1996). emo-1, a Caenorhabditis elegans Sec61p gamma homologue, is required for oocyte development and ovulation. J. Cell Biol. 134, 699-714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath R. S., Fraser A. G., Dong Y., Poulin G., Durbin R., Gotta M., Kanapin A., Le Bot N., Moreno S., Sohrmann M., et al. (2003). Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421, 231-237 [DOI] [PubMed] [Google Scholar]
- Killian D. J., Hubbard E. J. (2005). Caenorhabditis elegans germline patterning requires coordinated development of the somatic gonadal sheath and the germ line. Dev. Biol. 279, 322-335 [DOI] [PubMed] [Google Scholar]
- Kimura K., Tanaka T., Nakae H., Obinata T. (1987). Troponin from nematode: Purification and characterization of troponin from Ascaris body wall muscle. Comp. Biochem. Physiol. 88B, 399-407 [Google Scholar]
- Ko C., Gieske M. C., Al-Alem L., Hahn Y., Su W., Gong M. C., Iglarz M., Koo Y. (2006). Endothelin-2 in ovarian follicle rupture. Endocrinology 147, 1770-1779 [DOI] [PubMed] [Google Scholar]
- McCarter J., Bartlett B., Dang T., Schedl T. (1997). Soma-germ cell interactions in Caenorhabditis elegans: multiple events of hermaphrodite germline development require the somatic sheath and spermathecal lineages. Dev. Biol. 181, 121-143 [DOI] [PubMed] [Google Scholar]
- McCarter J., Bartlett B., Dang T., Schedl T. (1999). On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev. Biol. 205, 111-128 [DOI] [PubMed] [Google Scholar]
- Miller D. M., Ortiz I., Berliner G. C., Epstein H. F. (1983). Differential localization of two myosins within nematode thick filaments. Cell 34, 477-490 [DOI] [PubMed] [Google Scholar]
- Moerman D. G., Fire A. (1997). Muscle: structure, function, and development. In C. elegans II (ed. Riddle D. L., Blumenthal T., Meyer B. J., Priess J. R.), pp. 417-470 Plainview, NY: Cold Spring Harbor Laboratory Press; [PubMed] [Google Scholar]
- Moerman D. G., Plurad S., Waterston R. H., Baillie D. L. (1982). Mutations in the unc-54 myosin heavy chain gene of Caenorhabditis elegans that alter contractility but not muscle structure. Cell 29, 773-781 [DOI] [PubMed] [Google Scholar]
- Moran C. M., Garriock R. J., Miller M. K., Heimark R. L., Gregorio C. C., Krieg P. A. (2008). Expression of the fast twitch troponin complex, fTnT, fTnI and fTnC, in vascular smooth muscle. Cell Motil. Cytoskeleton 65, 652-661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers C. D., Goh P. Y., Allen T. S., Bucher E. A., Bogaert T. (1996). Developmental genetic analysis of troponin T mutations in striated and nonstriated muscle cells of Caenorhabditis elegans. J. Cell Biol. 132, 1061-1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae H., Obinata T. (1993). Immunochemical localization of troponin I and C in the muscles of Caenorhabditis elegans. Zool. Sci. 10, 375-379 [Google Scholar]
- Ono K., Ono S. (2004). Tropomyosin and troponin are required for ovarian contraction in the Caenorhabditis elegans reproductive system. Mol. Biol. Cell 15, 2782-2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K., Ono S. (2009). Actin-ADF/cofilin rod formation in Caenorhabditis elegans muscle requires a putative F-actin binding site of ADF/cofilin at the C-terminus. Cell Motil. Cytoskeleton 66, 398-408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K., Yu R., Mohri K., Ono S. (2006). Caenorhabditis elegans kettin, a large immunoglobulin-like repeat protein, binds to filamentous actin and provides mechanical stability to the contractile apparatuses in body wall muscle. Mol. Biol. Cell 17, 2722-2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K., Yu R., Ono S. (2007). Structural components of the nonstriated contractile apparatuses in the Caenorhabditis elegans gonadal myoepithelial sheath and their essential roles for ovulation. Dev. Dyn. 236, 1093-1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K., Yamashiro S., Ono S. (2008). Essential role of ADF/cofilin for assembly of contractile actin networks in the C. elegans somatic gonad. J. Cell Sci. 121, 2662-2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S. (2001). The Caenorhabditis elegans unc-78 gene encodes a homologue of actin-interacting protein 1 required for organized assembly of muscle actin filaments. J. Cell Biol. 152, 1313-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S., Ono K. (2002). Tropomyosin inhibits ADF/cofilin-dependent actin filament dynamics. J. Cell Biol. 156, 1065-1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V. (1999). Troponin I: inhibitor or facilitator. Mol. Cell Biochem. 190, 9-32 [PubMed] [Google Scholar]
- Rose K. L., Winfrey V. P., Hoffman L. H., Hall D. H., Furuta T., Greenstein D. (1997). The POU gene ceh-18 promotes gonadal sheath cell differentiation and function required for meiotic maturation and ovulation in Caenorhabditis elegans. Dev. Biol. 192, 59-77 [DOI] [PubMed] [Google Scholar]
- Ruksana R., Kuroda K., Terami H., Bando T., Kitaoka S., Takaya T., Sakube Y., Kagawa H. (2005). Tissue expression of four troponin I genes and their molecular interactions with two troponin C isoforms in Caenorhabditis elegans. Genes Cells 10, 261-276 [DOI] [PubMed] [Google Scholar]
- Sahota V. K., Grau B. F., Mansilla A., Ferrus A. (2009). Troponin I and Tropomyosin regulate chromosomal stability and cell polarity. J. Cell Sci. 122, 2623-2631 [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. (1994). Signal transduction and regulation in smooth muscle. Nature 372, 231-236 [DOI] [PubMed] [Google Scholar]
- Squire J. M. (1997). Architecture and function in the muscle sarcomere. Curr. Opin. Struct. Biol. 7, 247-257 [DOI] [PubMed] [Google Scholar]
- Squire J. M., Morris E. P. (1998). A new look at thin filament regulation in vertebrate skeletal muscle. FASEB J. 12, 761-771 [DOI] [PubMed] [Google Scholar]
- Strome S. (1986). Fluorescence visualization of the distribution of microfilaments in gonads and early embryos of the nematode Caenorhabditis elegans. J. Cell Biol. 103, 2241-2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterston R. H. (1988). Muscle. In The Nematode C. elegans (ed. Wood W. B.), pp. 281-335 New York: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Wolke U., Jezuit E. A., Priess J. R. (2007). Actin-dependent cytoplasmic streaming in C. elegans oogenesis. Development 134, 2227-2236 [DOI] [PubMed] [Google Scholar]
- Xu X., Guo H., Wycuff D. L., Lee M. (2007). Role of phosphatidylinositol-4-phosphate 5′ kinase (ppk-1) in ovulation of Caenorhabditis elegans. Exp. Cell Res. 313, 2465-2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.