Abstract
Wnt/β-catenin signalling controls cell fates in development, tissue homeostasis and cancer. Wnt binding to Frizzled receptors triggers recruitment of Dishevelled to the plasma membrane and formation of a signalosome containing the LRP5/6 co-receptor, whose cytoplasmic tail (ctail) thus becomes phosphorylated at multiple PPP(S/T)Px(S/T) motifs. These then directly inhibit GSK3β, which results in β-catenin accumulation and signalling. Here, we revisit previous epistasis experiments, and show that Dishevelled signals through LRP5/6 in human cells and Drosophila embryos. To recapitulate this signalling event, and to define its functional elements, we fused the Dishevelled DIX domain to the LRP6 ctail, which forms cytoplasmic signalosomes with potent signalling activity mediated by its PPP(S/T)Px(S/T) motifs. Their phosphorylation and activity depends critically on DIX-mediated polymerization, and on multiple stability elements in the LRP6 ctail, including the T1479 epitope upstream of the membrane-proximal PPP(S/T)Px(S/T) motif. Thus, stable polymerization emerges as a key principle underlying the function of Dishevelled-dependent signalosomes.
Keywords: Dishevelled, DIX domain, LRP6 co-receptor
Introduction
The Wnt/β-catenin pathway controls numerous steps during normal animal development and homeostasis of adult tissues, and is also implicated in human disease, most notably in cancer (for reviews, see Clevers, 2006; MacDonald et al., 2009). In the absence of Wnt, the levels of the key effector β-catenin are kept low by a complex containing Axin and the APC tumour suppressor, which promotes phosphorylation of β-catenin by glycogen synthase kinase 3β (GSK3β), to earmark it for ubiquitylation and subsequent proteasomal degradation. Binding of Wnt ligand to the Frizzled transmembrane receptor triggers recruitment of Dishevelled (Dvl) to the plasma membrane (for a review, see Angers and Moon, 2009), probably by direct binding (Wong et al., 2003). In turn, Dvl recruits the Axin complex to the plasma membrane (Cliffe et al., 2003), promoting the formation of a membrane-associated signalosome (Bilic et al., 2007; Schwarz-Romond et al., 2007b; Zeng et al., 2008) that also contains LRP6, the co-receptor for Wnt/β-catenin signalling (Tamai et al., 2000; Wehrli et al., 2000). Dvl thus promotes phosphorylation of the cytoplasmic tail (ctail) of LRP6 at multiple sites, in particular threonine 1479 (T1479) adjacent to a serine/threonine cluster (Ser/Thr cluster) in its membrane-proximal region by casein kinase 1γ (CK1γ) (Bilic et al., 2007; Davidson et al., 2005). T1479 is upstream of five tandem PPP(S/T)Px(S/T) motifs (or PPPSPxS, for simplicity) (Mao et al., 2001b; Tamai et al., 2004). The phosphorylation of the first (S/T) within these motifs [‘sites I’, e.g. T1490 within the membrane-proximal motif (Zeng et al., 2005)] by membrane-associated GSK3β is stimulated by Wnt and Dvl, and serves to prime the CK1-mediated phosphorylation of sites II, the second (S/T) whose phosphorylation is also induced by Wnt and depends on Dvl (Davidson et al., 2005; Zeng et al., 2008; Zeng et al., 2005). Doubly phosphorylated PPPSPxS motifs then recruit Axin and GSK3β (Zeng et al., 2005), and inhibit GSK3β by acting as direct competitive inhibitors of this kinase (Piao et al., 2008; Wu et al., 2009) (see also Cselenyi et al., 2008; Mi et al., 2006). This causes unphosphorylated β-catenin to accumulate in the cytoplasm and translocate to the nucleus, where it associates with TCF transcription factors to operate a transcriptional switch, thereby controlling the fates of normal and diseased cells (Clevers, 2006; MacDonald et al., 2009).
How Dvl facilitates the phosphorylation of LRP6 remains somewhat mysterious, though its function in this process depends on its remarkable ability to self-associate by means of its DIX domain, which undergoes dynamic head-to-tail polymerization (Bilic et al., 2007; Schwarz-Romond et al., 2007a). Bilic et al. proposed that Frizzled-associated Dvl polymerizes to co-cluster the Axin complex with LRP6, whose ctail thus becomes a substrate for phosphorylation – the key step towards GSK3β inactivation and β-catenin signalling (see above). This implies that Dvl functions upstream of and through LRP6, challenging the widely held view that Dvl acts downstream of Frizzled and its co-receptor (e.g. Clevers, 2006; Logan and Nusse, 2004). This view arose partly because Dvl is a cytoplasmic protein, but also owing to an influential epistasis experiment done in Drosophila, indicating that overexpressed Dsh (the only Dvl orthologue in flies) was fully active in the complete absence of Arrow [the only LRP6 orthologue in flies (Wehrli et al., 2000)]. Whether this arrow-independent activity of Dsh reflects an intrinsic difference between flies and vertebrates, or a technical difference between assay conditions, remains unresolved.
To address this, we repeated this epistasis experiment, and found that Dsh has very little signalling activity in arrow mutant Drosophila embryos, similar to Dvl2 whose signalling activity is much reduced in LRP6-depleted mammalian cells. Thus, Dsh and Dvl2 both signal, at least partly, through Arrow and LRP6, respectively. We then went on to test the essence of the signalosome hypothesis, asking whether polymerization of the LRP6 ctail by a linked Dvl DIX domain (DIX>ctail) would be sufficient to trigger signalling. This is the case: DIX>ctail exhibits potent β-catenin-dependent signalling activity in mammalian cells and in Drosophila embryos. We used mutational analysis of DIX>ctail to define its functional elements, which revealed that its DIX-dependent polymerization is essential for both its phosphorylation and signalling activity. We also discovered multiple stabilizing elements in the membrane-proximal region of the LRP6 ctail, including the above-mentioned T1479 epitope (Davidson et al., 2005), which are critical for efficient signalling activity of DIX>ctail. We discuss how stable polymerization of Dvl-dependent signalosomes may confer β-catenin signalling.
Results
Dishevelled function depends on arrow
The original experiment addressing the epistasis between Dishevelled and arrow was done before balancer chromosomes marked with green or red fluorescent protein (GFP or RFP) became widely available (Wehrli et al., 2000). We thus repeated this experiment, using identical strains and conditions (including the prd-GAL4 driver line for mild Dsh overexpression in alternate segments of the early embryo), but marking the arrow null mutant embryos that lack both maternal and zygotic arrow by lack of RFP. We confirmed that prd-GAL4-driven Dsh produces clear expansions of the naked cuticle zones that are interspersed between the ventral denticle belts of wild-type (wt) embryos (Wehrli et al., 2000) (Fig. 1A,B), which signifies ectopic Dsh signalling activity [naked cuticle being the phenotypic Wnt signalling output in this tissue (Logan and Nusse, 2004)]. Note that Dsh, upon overexpression, is capable of triggering Wnt-independent pathway activity, like other Dvl proteins (e.g. Schwarz-Romond et al., 2007a; Smalley et al., 2005) (see also below).
Fig. 1.
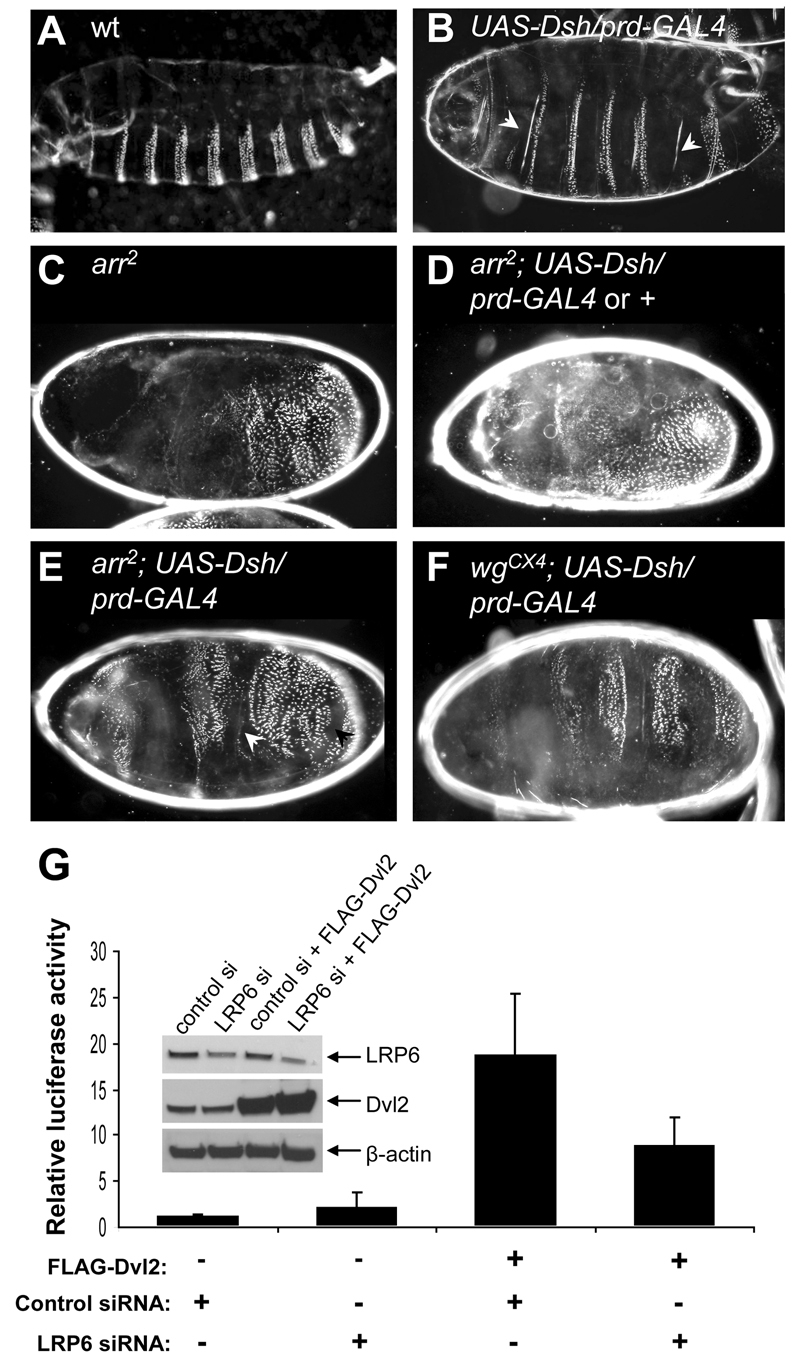
Overexpressed Dvl signals through LRP6/Arrow. (A-F) Ventral cuticles of wt or mutant Drosophila embryos, with or without Dsh overexpressed in alternate segments (by prd-GAL4), as indicated; naked cuticle (arrowheads) reflects the signalling activity of overexpressed Dsh. Note that 50% of the arr2 null mutants (selected by absence of RFP) should express Dsh, although a denticle-lawn phenotype is observed in 62% of the embryos (D), suggesting that this class contains embryos overexpressing Dsh without any activity; occasional naked patches are visible in the remaining 38% (arrowheads in E), reflecting residual activity of the prd-GAL4-driven Dsh (see also text). By contrast, the Dsh-overexpressing embryos are readily identifiable amongst the wgcx4 homozygotes (at the expected frequency of 25%) by their four wide naked zones interspersed between their denticle bands (F), reflecting strong and 100% penetrant activity of overexpressed Dsh. (G) TOPFLASH assays from lysates of LRP6- and mock-depleted HEK293 cells, with or without overexpressed FLAG-Dvl2. Error bars indicate standard deviations from three independent experiments. Inset, relative protein expression levels.
By contrast, arrow null embryos show lawns of denticles uninterrupted by naked cuticle [signifying loss of Wingless signalling (Logan and Nusse, 2004)] (Fig. 1C). Notably, among the arrow null embryos, of which 50% also express Dsh (see Materials and Methods), we observe a ‘denticle-lawn’ phenotype indistinguishable from that of arrow null mutants (Fig. 1C) in 62% (n=41) of the embryos (Fig. 1D), while the remaining 38% (n=25) show occasional small patches of naked cuticle within their denticle lawns (Fig. 1E, arrowheads). However, none of the arrow null embryos exhibit the broad stripes of excess naked cuticle that were shown previously [in figure 2B of Wehrli et al. (Wehrli et al., 2000)], although we observe these broad naked stripes among wg mutant cuticles at the expected frequency of one in four (Fig. 1F), as previously reported [see figure 2C of Wehrli et al. (Wehrli et al., 2000)]. We therefore conclude that, under the conditions used in this experiment, there is efficient Dsh signalling activity in wg but not in arrow mutants. Thus, whereas Dsh acts entirely downstream of Wingless, it signals at least partly upstream of, and through, Arrow.
LRP5/6 is required for efficient Dvl signalling activity in mammalian cells
Overexpressed Dvl2 exhibits high levels of Wnt-independent signalling activity when measured with a TCF luciferase reporter assay (‘TOPFLASH’) that monitors TCF/β-catenin-dependent gene transcription (Korinek et al., 1997). Importantly, this activity is substantially reduced after RNAi-mediated depletion of LRP6 (Fig. 1G), or after co-depletion of LRP6 and its paralogue LRP5 (Mao et al., 2001b) (supplementary material Fig. S1A). Thus, overexpressed Dvl2 requires, and signals through, LRP5/6 even in the absence of Wnt stimulation. Similarly, Dvl2 activity is abolished by depletion of β-catenin (supplementary material Fig. S1A), indicating that the ability of overexpressed Dvl2 to stimulate TCF-dependent transcription [i.e. the key functional output of the Wnt/β-catenin pathway (Clevers, 2006)] is absolutely dependent on β-catenin. These data support the notion that the Wnt-independent activity of overexpressed Dvl2 mimics the normal function of Dvl in transducing the Wnt signal – which it achieves by promoting LRP6 phosphorylation (Bilic et al., 2007).
We have not been able to detect a physical interaction between Dvl2 and LRP6 by co-immunoprecipitation, possibly because the affinity of Dvl2 for itself and its binding partners is very low, and their associations are highly dynamic (Bilic et al., 2007; Schwarz-Romond et al., 2007a) (see also below). Intriguingly though, we observe strong staining of the cytoplasmic Dvl2 puncta by the phospho-specific Tp1479 LRP6 antibody [which detects the Wnt- and Dvl-dependent phosphorylation of T1479 adjacent to the Ser/Thr cluster (Davidson et al., 2005)] (supplementary material Fig. S1B), and also by the phospho-specific pS1490 LRP6 antibody [which detects phospho-S1490 within the membrane-proximal PPPSPAT motif (Zeng et al., 2005)] (supplementary material Fig. S1C). These signals do not appear to represent cross-reactivities (as neither antiserum detects overexpressed Dvl2 on western blots; not shown), and thus suggest that the Dvl2 puncta may contain phosphorylated LRP6 ctail [as membrane-associated signalosomes do (Bilic et al., 2007)].
DIX-mediated polymerization of the LRP6 ctail triggers Wnt signalling
LRP5/6 truncations lacking their extracellular domain but retaining their single transmembrane domain plus their ctail sequences (LRPΔN) activate β-catenin signalling in a Wnt-independent manner, whereas the ctail sequences on their own have little if any signalling activity (Brennan et al., 2004; Davidson et al., 2005; Mao et al., 2001a; Mao et al., 2001b; Mi and Johnson, 2005; Tamai et al., 2004; Yamamoto et al., 2008) [except if expressed at very high levels, e.g. after injection into Xenopus embryos (Cselenyi et al., 2008)]. These LRPΔN constructs form cytoplasmic speckles when overexpressed, whereas their inactive counterparts tend to be diffuse. Indeed, there is a striking correlation between speckling and signalling activity of various LRP6ΔN constructs described by Yamamoto et al. (Yamamoto et al., 2008), reminiscent of Dvl2, which signals efficiently if punctate (caused by DIX-dependent polymerization), but is inactive if diffuse (Schwarz-Romond et al., 2007a). We therefore wondered whether the DIX-dependent polymerization of the LRP6 ctail might be sufficient to promote its phosphorylation and, consequently, GSK3β inhibition and β-catenin signalling.
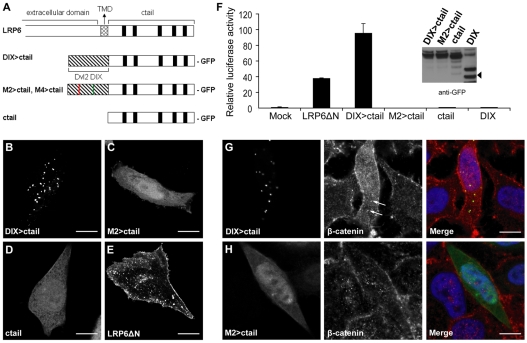
To test this, we fused the DIX domain of human Dvl2 (amino acids 8-95) to the ctail of human LRP6 (amino acids 1396-1613) tagged with GFP at its C-terminus (DIX>ctail; Fig. 2A). As controls, we generated a DIX-less ctail (ctail) and polymerization-defective mutants [M2>ctail, M4>ctail; Fig. 2A; note that the M2 and M4 point mutations are in the two complementary self-interacting surfaces of the DIX domain, abolishing its polymerization in vitro, and puncta formation and signalling activity of Dvl2 in vivo (Schwarz-Romond et al., 2007a)]. Indeed, DIX>ctail forms cytoplasmic puncta in transfected HeLa cells (Fig. 2B), like Dvl2, whereas its controls are diffuse, as expected (Schwarz-Romond et al., 2007a; Yamamoto et al., 2008) (Fig. 2C,D). As in the case of Dvl2 (Schwarz-Romond et al., 2005), the DIX>ctail puncta do not colocalize with caveolin, clathrin or with the endocytic vesicle marker EEA-1 (supplementary material Fig. S2). Furthermore, they are morphologically distinct from the less regular speckles formed by LRP6ΔN (supplementary material Fig. S2E), which tend to be associated with caveolin (Yamamoto et al., 2008).
Fig. 2.
Potent signalling activity of a minimal DIX>ctail module in human cell lines. (A) Schematic representation of LRP6, wt and mutant DIX>ctail derivatives; red and green bars within the DIX domain (hatched) indicate positions of M4 and M2 point mutations, respectively, in its complementary self-interacting surfaces; black bars mark PPPSPxS motifs. (B-E) Confocal images of fixed HeLa cells overexpressing various GFP-tagged constructs, as indicated. (F) TOPFLASH assays (as in Fig. 1G), monitoring the signalling activity of DIX>ctail compared with its controls in HEK293 cells; inset, relative expression levels (arrowheads point to breakdown products of the DIX domain, which in some constructs appears prone to degradation during cell lysis). (G,H) Immunostaining of endogenous β-catenin (red) in HeLa cells transfected with DIX>ctail or M2>ctail (green), co-stained with DAPI (blue) to mark the nuclei. Note the increased level of cytoplasmic and nuclear β-catenin in the DIX>ctail-expressing cell, compared with the non-transfected or M2>ctail-expressing cells; arrows indicate recruitment of β-catenin into DIX>ctail puncta. Scale bars: 10 μm.
TOPFLASH assays in HEK293 cells confirmed that LRP6ΔN stimulates reporter activity by approx. 40× over mock-transfected cells (Yamamoto et al., 2008) (Fig. 2F). Strikingly, DIX>ctail is even more active, stimulating TOPFLASH values >100×, whereas its controls (ctail, M2>ctail, M4>ctail) are totally inactive, as is the DIX domain on its own (Schwarz-Romond et al., 2005; Yamamoto et al., 2008), despite being expressed at comparable levels (Fig. 2F, inset). As expected, DIX>ctail increases the levels of cytoplasmic and nuclear β-catenin significantly, whereas its non-polymerizing control fails to do so (Fig. 2G,H). Notably, we observe faint β-catenin staining of the DIX>ctail puncta (Fig. 2G, arrows), reflecting recruitment of endogenous β-catenin into these puncta, presumably via the endogenous Axin complex (see also below). We conclude that DIX>ctail is a potent Wnt signalling module whose activity absolutely depends on its DIX-mediated polymerization.
dDIX>ctail has potent Wnt signalling activity in Drosophila embryos
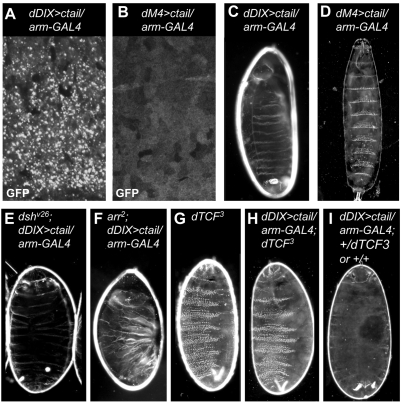
We were concerned that the high signalling activity of DIX>ctail would simply reflect the activity of endogenous LRP5/6 and/or Dvl, with which it could interact through DIX-mediated polymerization. siRNA depletion experiments do not support this (not shown), but are inconclusive since depletion by this method tends to be incomplete and fails to achieve a ‘null’ condition. To test this more rigorously, we used Drosophila: we examined whether a Drosophila DIX>ctail (dDIX>ctail) construct from equivalent fragments of Dsh and Arrow has signalling activity during normal development and, if so, whether it functions in the absence of endogenous Dsh or Arrow. As expected, dDIX>ctail form abundant cytoplasmic puncta upon ubiquitous expression throughout the embryonic epidermis (Fig. 3A), whereas a matched polymerization-defective control (dM4>ctail) merely generates diffuse GFP fluorescence (Fig. 3B; the expression levels of these two chimeras are comparable; not shown).
Fig. 3.
Potent signalling activity of dDIX>ctail in Drosophila embryos. (A,B) Confocal sections of the lateral epidermis of 5- to 7-hour-old fixed wt and mutant embryos, ubiquitously expressing dDIX>ctail or the non-polymerizing M4 mutant, respectively, and (C,D) resulting ventral cuticles (as in Fig. 1). (E-I) Ventral cuticles of dDIX>ctail-expressing wt and mutant embryos, as indicated; null mutants (E,F) were selected by absence of RFP-marked chromosomes. Naked cuticles (C,E,F,I) reflect strong signalling activity of dDIX>ctail, independent of (E) dsh and (F) arrow, while dM4>ctail is completely inactive, allowing 100% of the dM4>ctail-expressing embryos to hatch into normal larvae (D). The denticle-lawn phenotype in H was observed at the frequency expected for dTCF3 homozygotes (in 25% of the dDIX>ctail-expressing embryos), and is indistinguishable from that of the dTCF3 mutants (G), indicating that dDIX>ctail is completely inactive in dTCF3 mutants.
Strikingly, dDIX>ctail proved to be highly active in Drosophila embryos, generating completely naked cuticles (Fig. 3C), i.e. constitutive Wnt pathway activity throughout the embryonic epidermis. By contrast, dM4>ctail has no signalling activity whatsoever since it allows normal embryogenesis and hatching of larvae with wild-type cuticles (Fig. 3D). This is consistent with the results from a membrane-tethered Arrow ctail, which also proved to be inactive in the embryo (Tolwinski et al., 2003).
Next, we overexpressed dDIX>ctail in dsh or arrow null mutant embryos, whose embryonic cuticles show denticle lawns, reflecting complete failure to transduce the Wingless signal (see above, Fig. 1D; not shown). In both cases, amongst the null mutants (selected by absence of RFP), all GFP-positive embryos (which therefore overexpress dDIX>tail) have totally naked cuticles (Fig. 3E,F). This demonstrates that dDIX>tail is fully active in both types of null mutants. To confirm that this activity reflects signalling through the normal Wnt/β-catenin pathway, we also tested dDIX>ctail in dTCF (also known as pangolin; pan) mutants, which exhibit a denticle lawn phenotype (Fig. 3G) similar to that of the dsh and arrow mutants, since they cannot support Wingless-induced transcription (van de Wetering et al., 1997). In this case, 25% of the dDIX>ctail-expressing embryos exhibited denticle lawns indistinguishable from those of the dTCF mutants (Fig. 3H), which must represent the dTCF homozygotes (as these are expected at this frequency), while the remaining 75% GFP-positive embryos (representing wt and dTCF heterozygotes) were completely naked (Fig. 3I). We conclude that dDIX>ctail is highly and ubiquitously active in the absence of endogenous Dsh or Arrow, but that its activity is totally dependent on dTCF, suggesting that this minimal module signals through the same Wnt/β-catenin/dTCF pathway in the Drosophila embryo as Dsh and Arrow themselves.
DIX>ctail activity depends on ctail sequences outside of its PPPSPxS motifs
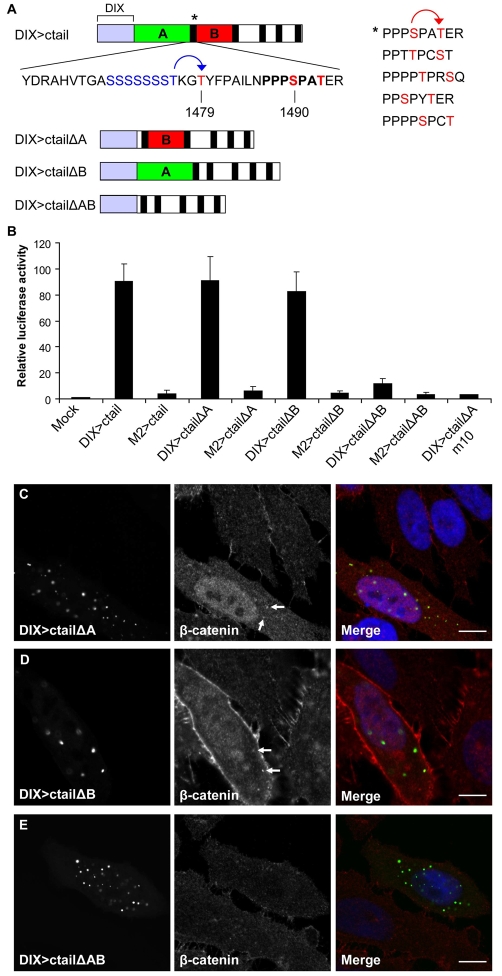
We took advantage of DIX>ctail to define the functional elements within this minimal signalling module that are required for its activity. We first confirmed that this activity depends on its PPPSPxS motifs (Fig. 4A), by showing that an m10 mutant version [bearing double-mutant PPPAPxA motifs (Zeng et al., 2005)] is completely inactive (Fig. 4B). This is entirely as expected since these double-mutant motifs cannot be phosphorylated and thus fail to recruit Axin (Zeng et al., 2005) and to inhibit GSK3β (Cselenyi et al., 2008; Piao et al., 2008; Wu et al., 2009).
Fig. 4.
Elements flanking the membrane-proximal PPPSPAT motif are required for full DIX>ctail activity. (A) Key features of the LRP6 ctail (top) in relation to DIX>ctail deletion mutants (underneath), with sequences of PPPSPxS motifs (vertical black bars) shown on the right (*, membrane-proximal motif; see also Fig. 2A); blue, Ser/Thr cluster; red, phosphorylation sites; red arrow points to GSK3β-mediated priming at the −3 positions for site II phosphorylation by CK1, and blue arrow to putative −3 priming for phospho-T1479 (see also text). (B) TOPFLASH assays in HEK293 cells, revealing loss of activity of m10 and polymerization-defective mutants, and residual activity of the ΔAB double deletion. (C-E) Confocal images of HeLa cells expressing deletion mutants, as indicated, immunostained for endogenous β-catenin. Cytoplasmic and nuclear accumulation of β-catenin, and recruitment into the puncta (marked by arrows), are observed in cells expressing the single mutants, but not in those expressing the double-deletion DIX>ctailΔAB. Scale bars: 10 μm.
Next, we asked whether the sequences outside the PPPSPxS motifs contribute to the signalling activity of DIX>ctail, focusing on the relatively long sequence stretches flanking the membrane-proximal PPPSPAT motif. We thus tested DIX>ctailΔA lacking its upstream sequence [i.e. element A, comprising the membrane-adjacent 88 residues upstream of the motif, including the Ser/Thr cluster and T1479 (Davidson et al., 2005)], and DIX>ctailΔB lacking its downstream element (i.e. element B, comprising the 25 residues between the first two motifs; Fig. 4A), but both of these are as active as DIX>ctail itself (Fig. 4B) and cause readily detectable accumulation of β-catenin in the cytoplasm and nucleus (Fig. 4C,D). As with the wt DIX>ctail, we note faint β-catenin staining of the puncta (Fig. 4C,D, arrows), indicating recruitment of endogenous β-catenin. By contrast, the double-deletion mutant, DIX>ctailΔAB, retains only residual activity (<15% of the wt), despite being expressed at comparable levels (Fig. 4B). As expected from this, we do not detect any β-catenin stabilization in the DIX>ctailΔAB-expressing cells, nor any β-catenin staining of the DIX>ctailΔAB puncta (Fig. 4E). The activities of all three mutants are strictly polymerization dependent since their M2-mutant versions are inactive (Fig. 4B).
To test the function of the two flanking elements (A and B) within the full-length protein, we introduced the same double deletion into LRP6 (LRP6ΔAB), and measured the signalling activity of this mutant in the absence or presence of Wnt. Indeed, LRP6ΔAB has much reduced activity (<15% of the wt) in both settings, despite being expressed at somewhat higher levels than the wt protein (supplementary material Fig. S3). We conclude that the two sequence elements flanking the membrane-proximal PPPSPAT motif are essential for efficient signalling function of LRP6.
LRP6 ctail phosphorylation depends on DIX-mediated polymerization
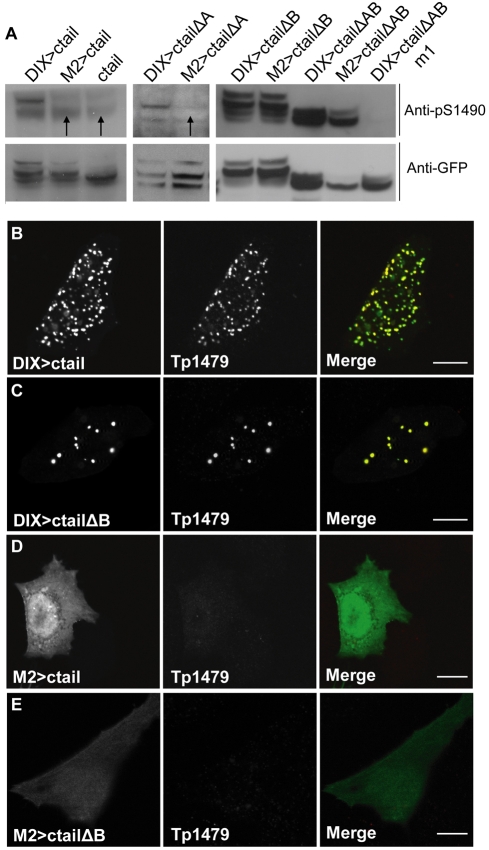
Next, we monitored the phosphorylation of the ctail sequences within wt and mutant DIX>ctail, by using the above-mentioned phospho-specific antibodies that detect the Wnt- and Dvl-dependent phosphorylation of T1479 and S1490. Using western blots analysis, we found that all DIX>ctail constructs show phospho-S1490, with higher levels in the ΔB mutant versions (Fig. 5A; note the complete absence of a signal in m1 which bears an S1490A substitution, demonstrating specificity of the phospho-S1490 signal), which suggests that the B element may be recognized by a factor that antagonizes phosphorylation of the membrane-proximal motif (see Discussion). Notably, phospho-S1490 is much reduced in the polymerization-defective versions of DIX>ctail, and also of the ΔA mutant (Fig. 5A, arrows), indicating that this phosphorylation depends at least partly on DIX-mediated polymerization.
Fig. 5.
Phosphorylation of DIX>ctail depends on a polymerization-competent DIX domain. (A) Western blots of total lysates of HEK293 cells transfected with wt and mutant DIX>ctail, showing phospho-S1490 levels (top) relative to total expression levels of wt and mutant DIX>ctail (bottom panels; re-probing of the blots shown above). Note the reductions of phospho-S1490 (indicated by arrows) in the polymerization-deficient mutants (M4>ctail and M4>ctailΔA behave the same as their M2-mutant counterparts; not shown), and the lack of a signal in m1 (bearing an S1490A substitution). (B-E) HeLa cells expressing DIX>ctail wt and deletion mutants, immunostained with the phospho-specific Tp1479 antibody, revealing strong punctate staining for DIX>ctail and DIX>ctailΔB, but complete absence of staining for M2>ctail and M2>ctailΔB. Scale bars: 10 μm.
Similarly, we found that DIX>ctail and DIX>ctailΔB puncta stain strongly for phospho-T1479 (Fig. 5B,C), whereas phospho-T1479 is undetectable in the polymerization-defective M2>ctail and M2>ctailΔB (Fig. 5D,E). The ΔA mutant versions do not produce any phospho-T1479 staining since they lack the KGTYFP epitope used to generate the Tp1479 antibody (Davidson et al., 2005) (not shown, but see below, Fig. 6). We conclude that the DIX-mediated polymerization of DIX>ctail is absolutely crucial for its phosphorylation at T1479, and also promotes its phosphorylation at S1490 – probably recapitulating the normal activity of Dvl in promoting these phosphorylation events during Wnt-dependent signalling (Bilic et al., 2007; Zeng et al., 2008).
Fig. 6.
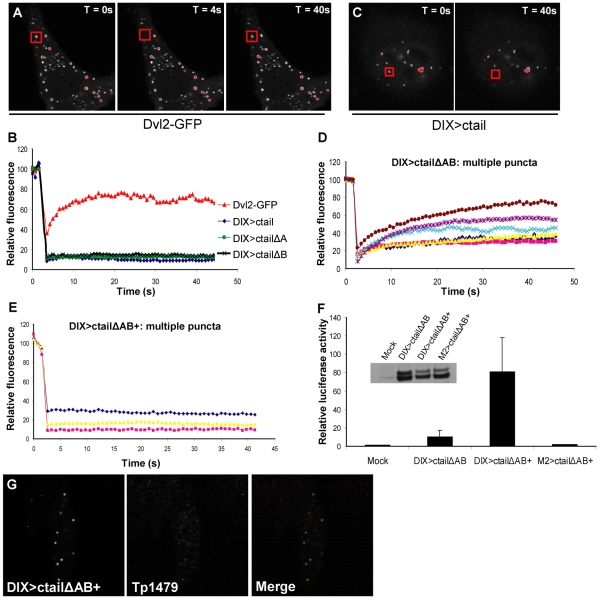
Stability elements in the LRP6 ctail are essential for its efficient signalling activity. (A-E) Live-cell imaging of HeLa cells expressing (A) Dvl2-GFP or (C) DIX>ctail, subjected to FRAP analysis; relative fluorescence intensities were measured after bleaching of >10 single puncta (boxed in red) by short laser pulses (at T=4 seconds), and plotted against time (B,D,E). Fluorescence recovery is observed for every single Dvl2-GFP punctum (A,B), but not for any of the puncta in wt DIX>ctail or single deletion mutants (B,C). (D,E) FRAP analysis of multiple DIX>ctailΔAB puncta (D), exhibiting variable recovery (see also text), and of DIX>ctailΔABplus puncta (E), exhibiting complete stability, restored by the KGTYFP epitope. (F) TOPFLASH assays in HEK293 cells, revealing restoration of signalling activity of the double-deletion by the KGTYFP epitope (values are directly comparable to those in Fig. 2F and Fig. 4B). Inset: protein expression levels. (G) HeLa cells expressing DIX>ctailΔABplus, immunostained with anti-Tp1479, showing that T1479 within the KGTYFP epitope remains unphosphorylated in the DIX>ctailΔABplus puncta (see also Fig. 5B-E), probably because of the lack of a −3 priming residue.
The LRP6 ctail contains stability elements that are critical for its signalling activity
We previously used fluorescence recovery after photobleaching (FRAP) assays to demonstrate that the DIX domain puncta represent highly dynamic and unstable protein assemblies: if an individual DIX-GFP punctum is bleached by a single laser pulse, its fluorescence recovers almost fully with a half-time of 3-5 seconds (Schwarz-Romond et al., 2005). In other words, there is a rapid exchange between the bleached punctate protein pool and the unbleached diffuse cytoplasmic protein pool, which reflects reversible DIX-mediated self-association, or head-to-tail polymerization, as revealed by biochemical and biophysical assays of purified DIX domain (Schwarz-Romond et al., 2007a). We observe essentially the same dynamicity with two different mini DIX>ctail constructs in which the DIX domain is linked to minimal versions of the membrane-proximal motif (mini1 and mini3; supplementary material Fig. S4A,B). Similarly, Dvl2-GFP puncta recover with a half-time of several seconds (Fig. 6A,B), although the maximum recovery in this case tends to be only ~70%, indicating a small fraction of relatively immobile Dvl2 protein within the Dvl2 puncta (Schwarz-Romond et al., 2005). Strikingly however, DIX>ctail puncta exhibit no detectable fluorescence recovery whatsoever, i.e. the bleached punctate protein remains immobile, without taking up any new unbleached protein from the cytoplasmic pool during the time course of the experiment (Fig. 6B,C). We conclude that the DIX>ctail puncta are DIX-mediated self-assemblies that are eminently stable, and that their stability is conferred by LRP6 ctail sequences.
To identify these stability elements, we examined the mutant DIX>ctail constructs. Intriguingly, while DIX>ctailΔA and DIX>ctailΔB are as stable as the wt (Fig. 6B), the functionally compromised double deletion is far more dynamic: we observe a range of recovery dynamics of individual DIX>ctailΔAB puncta, whereby some puncta recover ~80% of the initial fluorescence, whereas others recover only ~25% (Fig. 6D). Importantly, all DIX>ctailΔAB puncta display some degree of recovery, whereas none of the puncta from the fully functional DIX>ctail constructs ever recover during the time course of our experiments. Note that there is no detectable difference between the stable puncta (from DIX>ctailΔA and DIX>ctailΔB) and the dynamic DIX>ctailΔAB puncta with regard to their morphology or size range (supplementary material Fig. S5). We conclude that the sequence stretches at either side of the membrane-proximal PPPSPAT motif contain stability elements that are functionally redundant with one another. Furthermore, the stability of DIX>ctail constructs correlates with their signalling function.
The T1479 epitope confers both stability and activity
To test the functional importance of the DIX>ctail stability experimentally, we asked whether we could restore the signalling activity of DIX>ctailΔAB by inserting a short and well-defined sequence motif that may represent a functional ligand binding site. We chose the KGTYFP epitope (in which the bold residue is T1479) because (1) this epitope is highly conserved amongst vertebrate LRP5/6 orthologues (supplementary material Fig. S4C), (2) its phosphorylation is Wnt and Dvl2 dependent (Bilic et al., 2007; Davidson et al., 2005), and (3) a mini DIX>ctail construct containing this epitope is completely stable (mini2; supplementary material Fig. S4B).
We thus inserted the KGTYFP epitope into the unstable double deletion (DIX>ctailΔABplus), and found that this restored complete stability of every single punctum analyzed (Fig. 6E). Again, these puncta were indistinguishable from the others in terms of morphology and size range (supplementary material Fig. S5). Strikingly, the KGTYFP epitope also restored efficient signalling activity (Fig. 6F). Indeed, DIX>ctailΔABplus is similarly active as wt DIX>ctail and the single deletions (ΔA and ΔB; see Fig. 4B), and is expressed at the same level as its matched controls (Fig. 6F, inset). As in the case of all other functional constructs, its activity is completely dependent on a polymerization-competent DIX domain (Fig. 6F). Intriguingly though, the T1479 residue within DIX>ctailΔABplus is not phosphorylated (Fig. 6G), most likely because the KGTYFP epitope lacks its normal phospho-priming residue upstream (namely S1476, the last residue of the Ser/Thr cluster; Fig. 4A): phosphorylation of T1479 is normally mediated by CKIγ – a kinase requiring an upstream phospho-priming event at the −3 position of its target residue (Davidson et al., 2005). Indeed, a T1479A-substituted version of DIX>ctailΔABplus is equally stable and active (not shown), confirming that the phosphorylation of T1479 within this construct is not required for its function. In summary, the unphosphorylated KGTYFP epitope is a key functional element within the LRP6 ctail that can confer both stability and signalling activity.
Axin is recruited into DIX>ctail signalosomes
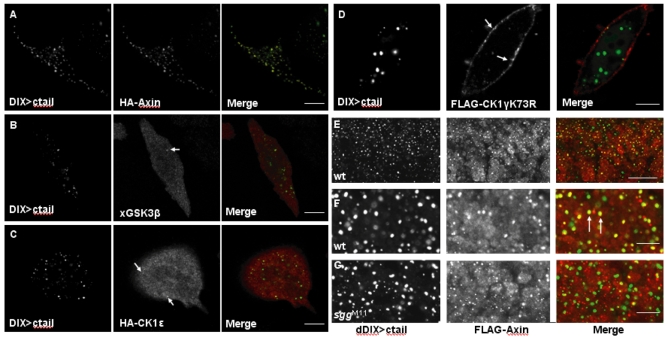
Two proteins were found to interact directly with the LRP5/6 or Arrow ctail, based on yeast two-hybrid screens or assays conducted by different groups – namely Axin (Mao et al., 2001b; Tolwinski et al., 2003) and GSK3β (Mi et al., 2006; Zeng et al., 2005). Direct in vitro binding has also been shown between recombinant GSK3β and LRP6 ctail (Mi et al., 2006; Piao et al., 2008). Notably, the in vivo association of Axin with LRP6 ctail depends on GSK3β-mediated phosphorylation of its PPPSPxS motifs (Zeng et al., 2005), and that of GSK3β with LRP6 ctail on CK1-mediated phosphorylation of the Ser/Thr cluster (Yum et al., 2009), which itself appears to be a docking site for GSK3β (Piao et al., 2008). Recall that both these sites are phosphorylated in DIX>ctail (Fig. 5), so we tested whether Axin and GSK3β are recruited to DIX>ctail puncta.
Endogenous Axin is very difficult to detect since its levels are exceedingly low (Lee et al., 2003), so we co-expressed HA-Axin with DIX>ctail to monitor their interaction. We found that virtually all DIX>tail puncta strongly stain for HA-Axin, suggesting a robust and apparently stoichiometric interaction between the two proteins (Fig. 7A). By contrast, if we co-overexpress GSK3β, we only observe weak staining of the DIX>ctail puncta, while most of the GSK3β remains diffuse (Fig. 7B, arrows). This resembles the staining pattern with endogenous β-catenin – mostly diffuse, and weak staining of the DIX>ctail puncta (see above, Fig. 4C). A similarly weak staining of the DIX>ctail puncta is seen if we monitor endogenous GSK3β (not shown), or co-expressed CKIε, the cytoplasmic isoform of CKIγ (which is recruited into Dvl2 puncta and synergizes with Dvl2) (Schwarz-Romond et al., 2007b) (Fig. 7C, arrows), or co-expressed CKIγ, but since this isoform is membrane-associated, we only observe staining of occasional DIX>ctail puncta that are near the plasma membrane (Fig. 7D, arrows). These staining patterns are consistent with direct binding between Axin and DIX>ctail, but do not support direct binding between GSK3β and DIX>ctail. Rather, it appears that GSK3β, like β-catenin and CK1 kinases, is recruited to DIX>ctail through endogenous proteins, possibly the endogenous Axin complex.
Fig. 7.
Efficient recruitment of Axin into DIX>ctail puncta. (A-D) Confocal images of HeLa cells, transfected and immunostained as indicated (a catalytically inactive mutant was used to monitor CK1γ). Note the apparent stoichiomeric recruitment of HA-Axin into DIX>ctail puncta (A), in contrast to the kinase recruitment (arrows in B-D) resembling the sub-stoichiomeric recruitment of β-catenin (Fig. 2G). (E-G) Confocal images of 5- to 7-hour-old wt and sggM11 null mutant embryos, coexpressing dDIX>ctail and FLAG-Axin, fixed and stained as indicated. (E) Low-magnification overview of multiple parasegments of the lateral epidermis (note that arm-GAL4-mediated expression is somewhat stochastic, with individual cells expressing variable levels of FLAG-Axin and/or dDIX>ctail). (F,G) High-magnification views, revealing a high incidence of dDIX>ctail-associated FLAG-Axin puncta (arrows in F) in wt cells that coexpress both proteins, but largely separate dDIX>ctail and FLAG-Axin puncta in the sgg null mutants (G). Scale bars: 10 μm (A-D), 25 μm (E), and 5 μm (F,G).
We also observe a robust interaction between dDIX>ctail and FLAG-Axin (Hamada et al., 1999) upon coexpression in Drosophila embryos, with many dDIX>ctail puncta coinciding with FLAG-Axin puncta (Fig. 7E,F, arrows), although some of the dDIX>ctail puncta do not stain for FLAG-Axin, and vice versa (Fig. 7F), possibly because their relative expression levels vary in individual cells because of the arm-GAL4 driver. Importantly, the colocalization between dDIX>ctail and FLAG-Axin is essentially abolished in the absence of GSK3β (i.e. in sgg null mutants), and we only observe very occasional ‘off-set’ colocalizations in these mutants (Fig. 7G). This is consistent with earlier findings that GSK3β-mediated phosphorylation of the LRP5/6 ctail is required for its interaction with Axin (Mao et al., 2001b; Zeng et al., 2005).
Discussion
Our experiments in Drosophila and mammalian cells have clarified the functional relationship between Dvl and LRP/Arrow, by establishing that overexpressed Dvl depends at least partly on LRP/Arrow for signalling activity. Our results consolidate the signalosome hypothesis (Bilic et al., 2007) according to which Dvl clusters LRP6 through DIX-mediated polymerization, thus promoting the phosphorylation of its ctail, in particular of its PPPSPxS motifs; these in turn directly inhibit GSK3β, thereby promoting β-catenin accumulation and β-catenin-dependent transcription. However, our work extends this hypothesis by the discovery of key functional elements in the membrane-proximal LRP6 ctail, outside its PPPSPxS motifs, that confer stability on the DIX-dependent signalosomes, thus enabling them to signal efficiently. Furthermore, our evidence supports the notion that the LRP6 ctail directly recruits Axin, but not GSK3β. Below, we propose that the main function of the stability elements is to promote a productive interaction between the LRP6 ctail and Axin, thus assisting a two-pronged effector function of the ctail which elicits β-catenin signalling.
Dvl signals through LRP/Arrow
We have shown that the Wnt-independent signalling mode of overexpressed Dvl depends at least partly on LRP6/Arrow (Fig. 1) and on β-catenin (supplementary material Fig. S1), suggesting that this mode employs the same, or a similar, molecular mechanism as the normal Wnt-dependent signalling mode of Dvl. Note that Dvl may also signal in a Wnt-independent fashion during normal development in cells with high Dvl expression levels; indeed, this signalling mode could be particularly prevalent in neoplastic cells in which Dvl is overexpressed (Uematsu et al., 2003) (C.M., unpublished data). Evidently, both Wnt-dependent and -independent signalling by Dvl requires an interaction with LRP/Arrow, which is likely to be labile, judging by the results from FRAP and biochemical analysis (Bilic et al., 2007; Schwarz-Romond et al., 2007b) (see also below), and which is almost certainly indirect, given that direct binding between the two proteins has never been reported. Indeed, the LRP5 ctail does not interact with Dvl in yeast two-hybrid assays (Mao et al., 2001b).
During Wnt-dependent signalling, the interaction between Dvl and LRP5/6 could be promoted by proximity, through Wnt-induced Dvl-Frizzled and Frizzled-LRP5/6 associations (Cong et al., 2004; Tamai et al., 2000; Tolwinski et al., 2003; Zeng et al., 2008). How this interaction occurs in the absence of Wnt ligand is less clear: LRP6 normally resides in the plasma membrane, or in internal membranes upon endocytosis by the caveolin pathway (Yamamoto et al., 2006; Yamamoto et al., 2008), but the cytoplasmic Dvl2 puncta are not associated with endocytic membrane markers [e.g. of the clathrin or caveolin pathways (Schwarz-Romond et al., 2005; Smalley et al., 2005)]. Perhaps the dynamic Dvl2 puncta interact with the LRP6 ctail only transiently, upon random collision with the plasma membrane, as can be observed by real-time imaging (Schwarz-Romond et al., 2005; Smalley et al., 2005). Alternatively, the functionally relevant interaction between Dvl2 and LRP6 could be mediated entirely by the Dvl2 sub-pool near the plasma membrane, which can recruit CK1γ (Fig. 7D). However, given that virtually all cytoplasmic Dvl2 puncta stain strongly with both phospho-specific LRP6 antibodies (supplementary material Fig. S1), our preferred idea is that these puncta may contain a proteolytic ctail product, resulting from regulated intramembrane proteolytic cleavage of LRP6 (Mi and Johnson, 2007), as is observed with other LRP family members (May et al., 2002). Our attempts to detect a Dvl2-associated proteolytic ctail product biochemically have been unsuccessful so far (see Results), possibly because of the labile interaction between the two proteins.
Clustering of the LRP6 ctail is critical for its signalling activity
DIX-mediated polymerization of the LRP/Arrow ctail is sufficient to trigger high Wnt pathway activity (Fig. 2, 3), consistent with earlier findings that artificial clustering of LRP5/6 bypasses Dvl in signalling to β-catenin (Cong et al., 2004; Li et al., 2002). Indeed, spontaneous clustering of LRPΔN constructs (Brennan et al., 2004; Davidson et al., 2005; Mao et al., 2001a; Mao et al., 2001b; Mi and Johnson, 2005; Tamai et al., 2004) could account for their constitutive signalling activity, although it was suggested that the signalling function of LRP6 depended on its caveolin-mediated internalization (Yamamoto et al., 2006), possibly explaining why caveolin-ctail chimerae are able to signal (Yamamoto et al., 2008). However, DIX>ctail puncta neither coincide with caveolin nor with other endocytic vesicle markers (supplementary material Fig. S2), and they therefore signal apparently without having to associate with caveolin or endocytic vesicle membranes.
Interestingly, the trans-membrane domain of LRP6 contains a known oligomerization motif, GxxxGxxxT, identified in glycophorin A (Langosch et al., 1996), but known to occur in other trans-membrane proteins, driving their oligomerization (Gurezka et al., 1999). This motif is highly conserved among LRP5/6 orthologues and might explain the spontaneous clustering and signalling activity of LRP6ΔN constructs. Indeed, mutation of this motif to LxxxLxxxT reduces the activity of LRP6ΔN by about 50%, without affecting its subcellular localization (not shown). Normal full-length LRP6 may therefore have a propensity to oligomerize, which is possibly a contributory factor in Wnt signal transduction.
DIX-mediated polymerization of the LRP6 ctail promotes its phosphorylation
The DIX-dependent polymerization of the LRP6 ctail is critical for its phosphorylation at T1479 (a CK1 target), and also stimulates phosphorylation of S1490 (a GSK3β target; Fig. 5). This recapitulates the events observed upon Wnt stimulation, which triggers the recruitment of Dvl to Frizzled receptor, enabling Dvl to promote the phosphorylation of these ctail residues (Bilic et al., 2007; Davidson et al., 2005; Zeng et al., 2008; Zeng et al., 2005). We have previously proposed that the DIX-mediated polymerization of Dvl generates a high local concentration of its protein-interaction domains, enhancing their avidity for low-affinity binding partners [such as Frizzled, Axin and CK1 (Schwarz-Romond et al., 2007a)]. We believe that the same concept applies here: DIX-mediated clustering of the LRP6 ctail generates a high local concentration of low-affinity phosphorylation targets, thus increasing their avidity for kinase recognition. Notably, the Ki for GSK3β inhibition by phospho-PPPSPxS is ~13 μM (Piao et al., 2008), which reflects a relatively weak interaction.
Although phospho-PPPSPxS motifs are clearly crucial for the recruitment of Axin (Zeng et al., 2005) and the inhibition of GSK3β (Piao et al., 2008; Wu et al., 2009), they are not sufficient for signalling activity of DIX>ctail: the double-deletion construct (DIX>ctailΔAB) retains only residual activity, and fails to stabilize β-catenin (Fig. 4), despite containing a normal complement of five PPPSPxS motifs that are likely to be fully phosphorylated (judging by the high level of phospho-T1490; Fig. 5A). Indeed, the cis-regulatory elements flanking the membrane-proximal PPPSPAT motif (Fig. 4A) are required for efficient signalling by DIX>ctail and by full-length LRP6. These elements act redundantly with one another, but exhibit no obvious sequence similarity, suggesting that they are recognized by distinct cognate factors (see below). In fact, the two elements show somewhat distinct activities: for example, phospho-S1490 is elevated upon deletion of element B (Fig. 5A), suggesting that a protein binding to it antagonizes S1490 phosphorylation, perhaps by direct steric hindrance. Indeed, the relatively low level of phospho-S1490 in wt DIX>ctail parallels a similarly low level of phospho-S1490 in LRP6 itself (Davidson et al., 2005; Zeng et al., 2005). Thus, it appears that the membrane-proximal PPPSPAT motif and its flanking elements serve primarily to recruit factors required for signalosome formation or function (see below), rather than mediating the effector function of the ctail in blocking GSK3β, which could be conferred entirely by the distal PPPSPxT motifs.
Stability elements outside the PPPSPxS motifs enable the LRP6 ctail to signal efficiently
Previous analyses by FRAP, and by biochemical and biophysical methods, demonstrated that the polymerization of Dvl2, and of its DIX domain, is highly dynamic and reversible in vivo and in vitro, with an estimated auto-affinity of the DIX domain in the low micromolar range (5-20 μM) (Schwarz-Romond et al., 2007a; Schwarz-Romond et al., 2005; Schwarz-Romond et al., 2007b). Furthermore, Dvl2 and DIX domain polymers are biochemically unstable (Schwarz-Romond et al., 2007a). Taken together, this implies a high off-rate of the DIX-mediated self-interaction. We were therefore surprised to discover that the fusion of the DIX domain to the LRP6 ctail generated utterly stable puncta (Fig. 6). We consider it unlikely that the ctail binds to itself directly: it does not form puncta on its own (Fig. 2C,D), was not found in any of the yeast two-hybrid screens with ctail baits (Hay et al., 2005; Mao et al., 2001b; Mi et al., 2006; Tolwinski et al., 2003), and is predicted to be intrinsically disordered (Piao et al., 2008). Therefore, in all probability, the stability of the DIX>ctail puncta is conferred by ctail-binding factor(s) that function to stabilize (‘cross-link’) the labile DIX-mediated self-interaction, e.g. factors that form stable self-associating complexes themselves.
Our FRAP analysis of DIX>ctail led us to discover the cis-regulatory elements within the LRP6 ctail to which these putative stability factors bind (Fig. 6B-E). These elements could also be responsible for the considerable stability of the Wnt-dependent LRP5/6 signalosomes: note that these can be fractionated on sucrose gradients as stable ribosome-sized complexes (Bilic et al., 2007; Schwarz-Romond et al., 2007b). Importantly, given that they enable DIX>ctail to signal efficiently (Fig. 4B), these stability elements could also be important for the function of the normal Wnt-dependent LRP5/6 signalosomes.
Several conserved sequences within these elements could mediate ligand binding, e.g. the YDRxxxTGASS motif within element A which is invariant in all known LRP5/6 orthologs from Hydra to man, and a highly conserved YRPY motif within element B (Fig. 4A; supplementary material Fig. S4C). We pinpointed one of these conserved motifs as functionally significant, namely the KGTYFP epitope that contains T1479 whose phosphorylation is Wnt and Dvl dependent (Bilic et al., 2007; Davidson et al., 2005). This epitope confers both stability and function, although its phosphorylation is clearly irrelevant for both (at least within the minimal DIX>ctail signalling module; Fig. 6F,G). Although the recognition of this epitope by its cognate factor might be phosphorylation insensitive, we think this unlikely since a phosphate group is expected to contribute a significant fraction of the total binding energy of a short motif. However, it is conceivable that the phosphorylation of T1479 reflects a ‘bystander’ effect (albeit a Wnt-dependent one) of the activity of CK1γ in phosphorylating site II of the PPPSPxS motifs, which is clearly relevant for signalling (Piao et al., 2008; Wu et al., 2009; Zeng et al., 2005). In the light of our results (Fig. 6F,G), we propose that the KGTYFP epitope functions prior to Wnt stimulation, and that its cognate factor predisposes the LRP6 ctail for Wnt-induced signalling, for example by enabling it to interact productively with Axin (see below). It will be interesting to identify this KGTYFP-binding factor.
Evidence that Axin is a direct ligand of the LRP6 ctail
As mentioned above, the only two proteins known to interact directly with the LRP6 ctail are Axin and GSK3β (Mao et al., 2001b; Mi et al., 2006; Tolwinski et al., 2003; Zeng et al., 2005). Our evidence argues that Axin is a direct ctail ligand because, of all the proteins we have tested (Figs 4, 7), Axin is the only one that displays apparently stoichiometric recruitment to the DIX>ctail puncta. By contrast, the sub-stoichiometric recruitment of GSK3β argues that this kinase (like CK1) is an indirect ligand that is recruited, for example, through its association with endogenous Axin. Notably, Axin interacts with all mutant DIX>ctail modules; however, in the case of the inactive double-deletion DIX>ctailΔAB, the staining pattern suggests that DIX>ctailΔAB is recruited by Axin, rather than the converse (not shown), indicating that DIX>ctailΔAB puncta cannot actively recruit Axin. Regardless, this indicates that Axin binds to an element that is common to all DIX>ctail modules, most probably the phospho-PPPSPxS motifs (Fig. 5A), consistent with the notion that these are docking sites for Axin (Mao et al., 2001b; Piao et al., 2008; Wu et al., 2009; Zeng et al., 2008; Zeng et al., 2005). Another common element is the DIX domain itself, however, the Dvl DIX domain on its own is neither capable of binding to the Axin DIX domain in vitro, nor of recruiting Axin in vivo (Kishida et al., 1999; Schwarz-Romond et al., 2007b).
How does the binding of Axin to the ctail enable LRP6 to signal? Previous evidence suggests that the interaction between Axin and ctail is very weak: it requires both the DIX domain and the β-catenin-binding domain of Axin, but neither domain by itself is sufficient for binding (Mao et al., 2001b; Tolwinski et al., 2003). A possible scenario is that the ctail has a weak binding affinity for the β-catenin-binding region of Axin, and that this weak interaction depends on avidity provided by the polymerization of the Axin DIX domain (see above). If so, the binding of Axin to the LRP6 ctail and to β-catenin might be mutually exclusive, which raises the interesting possibility that the two interactions may compete. Signalling by LRP5/6 might therefore involve a two-pronged effector function, namely a direct inhibition of GSK3β (Piao et al., 2008; Wu et al., 2009), as well as an interference with the Axin-β-catenin interaction. This interference would attenuate further the phosphorylation of β-catenin by GSK3β, which depends on a scaffolding function provided by Axin (Dajani et al., 2003).
In conclusion, we propose that the stability elements within LRP6 discovered by our work predispose its ctail for a productive interaction with Axin, and that their cognate factors help to overcome the weak affinity between ctail and Axin, for example by stabilizing the weak binding between the two. These elements could thus enable both effector functions of the LRP5/6 ctail – inhibiting GSK3β and interfering with the scaffolding function of Axin in promoting the GSK3β-mediated phosphorylation of β-catenin.
Materials and Methods
Plasmids
The following plasmids were used: FLAG-Dvl2, Dvl2-GFP, DIX-GFP, HA-CK1ε, HA-Axin, xGSK3β (Schwarz-Romond et al., 2007a; Schwarz-Romond et al., 2007b); LRP6-GFP (Mao et al., 2001a); LRP6ΔN-GFP (Yamamoto et al., 2008); FLAG-CK1γK73R (Davidson et al., 2005). Fusion PCR was used to generate DIX>ctail (details available on request), which was subcloned into pEGFP-N1 (Clontech). Mutant variants of DIX>ctail were generated by standard mutagenesis techniques, with DIX>ctail as template. dDIX>ctail and dM4>ctail were constructed similarly, with Dsh and Arrow cDNAs as templates (Wehrli et al., 2000), and were subcloned into pUAST, for fly transformation.
Cell transfection and luciferase assays
Human HEK293 and HeLa cells were grown at 10% CO2 in DMEM containing 10% FCS and 1% penicillin/streptomycin. Cell transfection with 1-2 μg plasmid DNA was carried out with Lipofectamine 2000 (Invitrogen). Depletion of endogenous LRP6, LRP5 and β-catenin in HEK293 cells was conducted with 100 nM siRNAs (Smartpool, Dharmacon Inc.), and subsequent cell transfections were done after 24 hours (with Lipofectamine 2000), followed by cell lysis after an additional 24 hours.
For TOPFLASH assays, the highly sensitive SuperTOPFlash plasmid was used (Veeman et al., 2003), and dual-luciferase assays were done 24 hours post-transfection as described previously (Schwarz-Romond et al., 2007b); CMV-Renilla was used as internal control, and values of mock-transfected cells were set to 1.
Immunofluorescence and western blot analysis
Cells were fixed and stained with the following antibodies, as described previously (Schwarz-Romond et al., 2007b): anti-LRP6 (Santa Cruz); anti-Dvl2 (H-75, Santa Cruz; Fig. 1G); anti-pLRP6 Ser1490 (Cell Signalling Technologies) (Zeng et al., 2005); anti-LRP6 Tp1479 (Davidson et al., 2005) (a kind gift from Christof Niehrs); anti-β-catenin (C2206, Sigma); anti-GFP (G1544, Sigma); mouse anti-FLAG (F3165, Sigma); rabbit anti-FLAG (F7425, Sigma; Fig. 7); anti-Wingless, anti-Sxl (Developmental Studies Hybridoma Bank); antibodies against vesicle markers (supplementary material Fig. S2) were previously described (Schwarz-Romond et al., 2005).
Photobleaching experiments
FRAP experiments were carried out essentially as described previously (Schwarz-Romond et al., 2005). Cells were seeded onto Lab-Tek II chambered coverglasses (Nunc), and transfected with GFP-tagged constructs the following day. Photobleaching was conducted 24 hours post transfection. Single small or average-sized GFP puncta were selected for bleaching with five maximum-intensity scans with the 488 and 514 nm lines of a 40 mW argon laser (Zeiss LSM510 inverted confocal microscope). Fluorescence recovery was monitored in images taken during the following 60 seconds, at 1.5-second intervals. The fluorescence intensity was normalized to the mean fluorescence intensity of the whole cell (as a control for non-specific photobleaching during imaging).
Analysis of Drosophila embryos
Multiple transformants of dDIX>ctail and M4>ctail were isolated by standard procedures, and two to three independent lines were each tested for signalling activity. The following mutant and transgenic strains were used (see also FlyBase): wgcx4, dshv26, sggM11, UAS-Axin-GFP, arm-GAL4 (Cliffe et al., 2003); UAS-FLAG-Axin (Hamada et al., 1999); FRT42B arr2, Df(2R)AA1, UAS-Dsh, prd-GAL4 (Wehrli et al., 2000) to generate yw hs-flp/+; FRT42B arr2/FRT42B ovoD; prd-GAL4/+ females and Df(2R)AA1/RFP; UAS-Dsh/GFP males, to produce arr null mutant embryos (from germ-line clone-bearing females, by standard procedures); dTCF3 (van de Wetering et al., 1997). Embryos lacking GFP and RFP were selected from the progeny for analysis (shown in Fig. 1D,E); these are all null mutant for arr (as they lack the RFP-marked chromosome balancing the arr deficiency Df(2R)AA1), and 50% of them overexpress Dsh (as they lack the GFP-marked chromosome balancing UAS.Dsh). The experiment shown in Fig. 1 was done twice, at different temperatures and by different investigators (25°C, J.M.; 29°C, C.M.-T.). To overexpress dDIX>ctail-GFP and M4>ctail-GFP, arm-GAL4 was used (Fig. 3; Fig. 7E-I), and GFP-positive embryos were selected for analysis; null mutant embryos were identified by their lack of RFP (Fig. 3E,F) or lack of anti-Sxl antibody staining (Fig. 7G).
Fixation and antibody staining of embryos was done as described previously (Cliffe et al., 2003). Cuticles were prepared by standard procedures, and inspected under dark-field illumination.
Supplementary Material
Acknowledgments
We thank Christof Niehrs for the antibody, Akira Kikuchi for plasmids, Marcel Wehrli for fly strains, and Ben Nichols for discussion and comments on the manuscript. This work was supported by the MRC (U.1051.030.20.00001.01). Deposited in PMC for release after 6 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/9/1588/DC1
References
- Angers S., Moon R. T. (2009). Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 10, 468-477 [DOI] [PubMed] [Google Scholar]
- Bilic J., Huang Y. L., Davidson G., Zimmermann T., Cruciat C. M., Bienz M., Niehrs C. (2007). Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 316, 1619-1622 [DOI] [PubMed] [Google Scholar]
- Brennan K., Gonzalez-Sancho J. M., Castelo-Soccio L. A., Howe L. R., Brown A. M. (2004). Truncated mutants of the putative Wnt receptor LRP6/Arrow can stabilize β-catenin independently of Frizzled proteins. Oncogene 23, 4873-4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. (2006). Wnt/β-catenin signalling in development and disease. Cell 127, 469-480 [DOI] [PubMed] [Google Scholar]
- Cliffe A., Hamada F., Bienz M. (2003). A role of Dishevelled in relocating Axin to the plasma membrane during Wingless signalling. Curr. Biol. 13, 960-966 [DOI] [PubMed] [Google Scholar]
- Cong F., Schweizer L., Varmus H. (2004). Wnt signals across the plasma membrane to activate the β-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development 131, 5103-5115 [DOI] [PubMed] [Google Scholar]
- Cselenyi C. S., Jernigan K. K., Tahinci E., Thorne C. A., Lee L. A., Lee E. (2008). LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3's phosphorylation of β-catenin. Proc. Natl. Acad. Sci. USA 105, 8032-8037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajani R., Fraser E., Roe S. M., Yeo M., Good V. M., Thompson V., Dale T. C., Pearl L. H. (2003). Structural basis for recruitment of glycogen synthase kinase 3β to the axin-APC scaffold complex. EMBO J. 22, 494-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson G., Wu W., Shen J., Bilic J., Fenger U., Stannek P., Glinka A., Niehrs C. (2005). Casein kinase 1γ couples Wnt receptor activation to cytoplasmic signal transduction. Nature 438, 867-872 [DOI] [PubMed] [Google Scholar]
- Gurezka R., Laage R., Brosig B., Langosch D. (1999). A heptad motif of leucine residues found in membrane proteins can drive self-assembly of artificial transmembrane segments. J. Biol. Chem. 274, 9265-9270 [DOI] [PubMed] [Google Scholar]
- Hamada F., Tomoyasu Y., Takatsu Y., Nakamura M., Nagai S., Suzuki A., Fujita F., Shibuya H., Toyoshima K., Ueno N., et al. (1999). Negative regulation of Wingless signalling by D-axin, a Drosophila homolog of axin. Science 283, 1739-1742 [DOI] [PubMed] [Google Scholar]
- Hay E., Faucheu C., Suc-Royer I., Touitou R., Stiot V., Vayssiere B., Baron R., Roman-Roman S., Rawadi G. (2005). Interaction between LRP5 and Frat1 mediates the activation of the Wnt canonical pathway. J. Biol. Chem. 280, 13616-13623 [DOI] [PubMed] [Google Scholar]
- Kishida S., Yamamoto H., Hino S., Ikeda S., Kishida M., Kikuchi A. (1999). DIX domains of dvl and axin are necessary for protein interactions and their ability to regulate β-catenin stability. Mol. Cell. Biol. 19, 4414-4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V., Barker N., Morin P. J., van Wichen D., de Weger R., Kinzler K. W., Vogelstein B., Clevers H. (1997). Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 275, 1784-1787 [DOI] [PubMed] [Google Scholar]
- Langosch D., Brosig B., Kolmar H., Fritz H. J. (1996). Dimerisation of the glycophorin A transmembrane segment in membranes probed with the ToxR transcription activator. J. Mol. Biol. 263, 525-530 [DOI] [PubMed] [Google Scholar]
- Lee E., Salic A., Kruger R., Heinrich R., Kirschner M. W. (2003). The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 1, E10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Mao J., Sun L., Liu W., Wu D. (2002). Second cysteine-rich domain of Dickkopf-2 activates canonical Wnt signalling pathway via LRP-6 independently of dishevelled. J. Biol. Chem. 277, 5977-5981 [DOI] [PubMed] [Google Scholar]
- Logan C. Y., Nusse R. (2004). The Wnt signalling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781-810 [DOI] [PubMed] [Google Scholar]
- MacDonald B. T., Tamai K., He X. (2009). Wnt/β-catenin signalling: components, mechanisms, and diseases. Dev. Cell 17, 9-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao B., Wu W., Li Y., Hoppe D., Stannek P., Glinka A., Niehrs C. (2001a). LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature 411, 321-325 [DOI] [PubMed] [Google Scholar]
- Mao J., Wang J., Liu B., Pan W., Farr G. H., 3rd, Flynn C., Yuan H., Takada S., Kimelman D., Li L., et al. (2001b). Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signalling pathway. Mol. Cell 7, 801-809 [DOI] [PubMed] [Google Scholar]
- May P., Reddy Y. K., Herz J. (2002). Proteolytic processing of low density lipoprotein receptor-related protein mediates regulated release of its intracellular domain. J. Biol. Chem. 277, 18736-18743 [DOI] [PubMed] [Google Scholar]
- Mi K., Johnson G. V. (2005). Role of the intracellular domains of LRP5 and LRP6 in activating the Wnt canonical pathway. J. Cell Biochem. 95, 328-338 [DOI] [PubMed] [Google Scholar]
- Mi K., Johnson G. V. (2007). Regulated proteolytic processing of LRP6 results in release of its intracellular domain. J. Neurochem. 101, 517-529 [DOI] [PubMed] [Google Scholar]
- Mi K., Dolan P. J., Johnson G. V. (2006). The low density lipoprotein receptor-related protein 6 interacts with glycogen synthase kinase 3 and attenuates activity. J. Biol. Chem. 281, 4787-4794 [DOI] [PubMed] [Google Scholar]
- Piao S., Lee S. H., Kim H., Yum S., Stamos J. L., Xu Y., Lee S. J., Lee J., Oh S., Han J. K., et al. (2008). Direct inhibition of GSK3β by the phosphorylated cytoplasmic domain of LRP6 in Wnt/β-catenin signalling. PLoS ONE 3, e4046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Romond T., Merrifield C., Nichols B. J., Bienz M. (2005). The Wnt signalling effector Dishevelled forms dynamic protein assemblies rather than stable associations with cytoplasmic vesicles. J. Cell Sci. 118, 5269-5277 [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T., Fiedler M., Shibata N., Butler P. J., Kikuchi A., Higuchi Y., Bienz M. (2007a). The DIX domain of Dishevelled confers Wnt signalling by dynamic polymerization. Nat. Struct. Mol. Biol. 14, 484-492 [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T., Metcalfe C., Bienz M. (2007b). Dynamic recruitment of axin by Dishevelled protein assemblies. J. Cell Sci. 120, 2402-2412 [DOI] [PubMed] [Google Scholar]
- Smalley M. J., Signoret N., Robertson D., Tilley A., Hann A., Ewan K., Ding Y., Paterson H., Dale T. C. (2005). Dishevelled (Dvl-2) activates canonical Wnt signalling in the absence of cytoplasmic puncta. J. Cell Sci. 118, 5279-5289 [DOI] [PubMed] [Google Scholar]
- Tamai K., Semenov M., Kato Y., Spokony R., Liu C., Katsuyama Y., Hess F., Saint-Jeannet J. P., He X. (2000). LDL-receptor-related proteins in Wnt signal transduction. Nature 407, 530-535 [DOI] [PubMed] [Google Scholar]
- Tamai K., Zeng X., Liu C., Zhang X., Harada Y., Chang Z., He X. (2004). A mechanism for Wnt coreceptor activation. Mol. Cell 13, 149-156 [DOI] [PubMed] [Google Scholar]
- Tolwinski N. S., Wehrli M., Rives A., Erdeniz N., DiNardo S., Wieschaus E. (2003). Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3β activity. Dev. Cell 4, 407-418 [DOI] [PubMed] [Google Scholar]
- Uematsu K., Kanazawa S., You L., He B., Xu Z., Li K., Peterlin B. M., McCormick F., Jablons D. M. (2003). Wnt pathway activation in mesothelioma: evidence of Dishevelled overexpression and transcriptional activity of β-catenin. Cancer Res. 63, 4547-4551 [PubMed] [Google Scholar]
- van de Wetering M., Cavallo R., Dooijes D., van Beest M., van Es, J., Loureiro J., Ypma A., Hursh D., Jones T., Bejsovec A., et al. (1997). Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88, 789-799 [DOI] [PubMed] [Google Scholar]
- Veeman M. T., Slusarski D. C., Kaykas A., Louie S. H., Moon R. T. (2003). Zebrafish prickle, a modulator of noncanonical Wnt/Fz signalling, regulates gastrulation movements. Curr. Biol. 13, 680-685 [DOI] [PubMed] [Google Scholar]
- Wehrli M., Dougan S. T., Caldwell K., O'Keefe L., Schwartz S., Vaizel-Ohayon D., Schejter E., Tomlinson A., DiNardo S. (2000). arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature 407, 527-530 [DOI] [PubMed] [Google Scholar]
- Wong H. C., Bourdelas A., Krauss A., Lee H. J., Shao Y., Wu D., Mlodzik M., Shi D. L., Zheng J. (2003). Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol. Cell 12, 1251-1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Huang H., Garcia Abreu J., He X. (2009). Inhibition of GSK3 phosphorylation of β-catenin via phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS ONE 4, e4926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Komekado H., Kikuchi A. (2006). Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of β-catenin. Dev. Cell 11, 213-223 [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Sakane H., Michiue T., Kikuchi A. (2008). Wnt3a and Dkk1 regulate distinct internalization pathways of LRP6 to tune the activation of β-catenin signalling. Dev. Cell 15, 37-48 [DOI] [PubMed] [Google Scholar]
- Yum S., Lee S. J., Piao S., Xu Y., Jung J., Jung Y., Oh S., Lee J., Park B. J., Ha N. C. (2009). The role of the Ser/Thr cluster in the phosphorylation of PPPSP motifs in Wnt coreceptors. Biochem. Biophys. Res. Commun. 381, 345-349 [DOI] [PubMed] [Google Scholar]
- Zeng X., Tamai K., Doble B., Li S., Huang H., Habas R., Okamura H., Woodgett J., He X. (2005). A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 438, 873-877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Huang H., Tamai K., Zhang X., Harada Y., Yokota C., Almeida K., Wang J., Doble B., Woodgett J., et al. (2008). Initiation of Wnt signalling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development 135, 367-375 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.