Abstract
Background
Osteochondral allografts are currently stored at 4°C for 2–6 weeks before implantation. At 4°C, chondrocyte viability, especially in the superficial zone, deteriorates starting at 2 weeks. Alternative storage conditions could maintain chondrocyte viability beyond 2 weeks, and thereby facilitate increased graft availability and enhanced graft quality.
Purpose
Determine effects of prolonged 37°C storage compared to traditional 4°C storage on chondrocyte viability and cartilage matrix content.
Study Design
Controlled Laboratory Study
Methods
Osteochondral samples from humeral heads of adult goats were analyzed (i) fresh, or after storage in medium for (ii) 14d at 4°C including 10% FBS, (iii) 28d at 4°C including 10% FBS, (iv) 28d at 37°C without FBS, (v) 28d at 37°C including 2% FBS, or (vi) 28d at 37°C including 10% FBS. Portions of samples were analyzed by microscopy after LIVE/DEAD® staining to determine chondrocyte viability and density, both en face (to visualize the articular surface) and vertically (overall and in superficial, middle, and deep zones). The remaining cartilage was analyzed for sulfated-glycosaminoglycan and collagen.
Results
37°C storage maintained high chondrocyte viability compared to 4°C storage. Viability of samples after 28d at 37°C was ~80% at the cartilage surface en face, ~65% in the superficial zone, and ~70% in the middle zone, which was much higher than ~45%, ~20%, and ~35%, respectively, in 4°C samples after 28d, and slightly decreased from ~100%, ~85%, and ~95%, respectively, in fresh controls. Cartilage thickness, glycosaminoglycan content, and collagen content were maintained for 37°C and 4°C samples compared to fresh controls.
Conclusion
37°C storage of osteochondral grafts supports long-term chondrocyte viability, especially at the vulnerable surface and superficial zone of cartilage.
Clinical Relevance
Storage of allografts at physiological temperature of 37°C may prolong storage duration, improve graft availability, and improve treatment outcomes.
Keywords: cartilage, chondrocyte viability, osteochondral allograft, storage, temperature
Introduction
Osteochondral allografting is used for the repair of large articular cartilage defects by restoring mature, hyaline cartilage in a biologically, structurally, and functionally appropriate manner. Young, active patients with large (> 2cm2) chondral or osteochondral defects are suitable candidates for osteochondral allografting2,12,25,34. In addition, osteochondral allografting is performed when other surgical techniques, such as marrow stimulation and cell transplantation, have failed previously or are contraindicated12,34. Osteochondral allografts have been used for treatment of focal cartilage defects33,40,59, osteochondritis dissecans19,23, as well as post-traumatic, osteonecrotic, and bipolar lesions in the knee6,11,41. Overall, usage of such allografts has resulted in clinical success rates exceeding 75%6,10,11,14,19,23,41. For transplantation of allografts, as with any allogenic organ or tissue, potential disease transmission and immunogenicity are important considerations 2,14,25,26,34; however, the limited availability of suitable grafts, due in part to storage duration, restricts widespread application25,26.
Currently, fresh graft tissue is stored at 4°C to accommodate federal regulations that require tissue banks to screen and test donor tissue for infectious diseases prior to implantation1. Such testing typically requires 14 days26,33,59; grafts are used after prolonged storage on average 24 days after harvest (range 15–43 days)26,33,40,59. During short-term storage at 4°C, cartilage can be maintained in a biologically viable, but quiescent state, one in which chondrocyte metabolism is lower than normal5,9. However, during prolonged storage at 4°C, chondrocyte viability deteriorates with a substantial decrease by 28 days and with a decrease to as low as 40% by 14 days5,15,17,46,48,53,57,58,61; such deterioration has led to recommended actions for a maximum duration of graft storage (“shelf-life”) and an implantation time within 30 days of harvest33,40. Alternative storage conditions that maintain chondrocyte viability for a longer duration may lead to an increase in “shelf-life”, and in turn, the availability of osteochondral allografts to surgeons and their patients.
Normally, chondrocyte metabolism contributes to cartilage homeostasis, and low chondrocyte viability within allografts is associated with tissue degeneration. Fresh osteochondral allografts, procured within 48 hours of donor death and implanted within 7 days, have high chondrocyte viability; retrieved grafts have contained viable chondrocytes up to 29 years after implantation13,30,39,42,60. Massive, frozen osteochondral allografts have been used in oncologic reconstructions after limb-salvaging resection of joint tissue due to tumors35,37,38. In contrast to fresh allografts, frozen grafts are devoid of viable chondrocytes, and specimens retrieved at 8 months to 5 years after reconstructive surgery generally display degenerate and acellular cartilage20,21. Graft efficacy may be improved by implanting tissue with high chondrocyte viability to better maintain tissue composition, structure, and function16,20,21.
During the routine storage of osteochondral donor tissue at 4°C, death of chondrocytes increases with storage duration, especially in the superficial zone of cartilage. Certain 4°C storage solutions can improve chondrocyte viability, while maintaining cartilage matrix content. Compared to fresh controls, grafts stored at 4°C for 28 days in a variety of storage solutions (i.e. lactated Ringers solution, serum-free culture medium) had lower chondrocyte viability, especially in the superficial zone5,15,46–48,53,57,58,61, while maintaining cartilage glycosaminoglycan (GAG) content5,48. During 4°C storage, supplementation of culture medium with fetal bovine serum (FBS) improved chondrocyte viability in osteochondral grafts, especially in the superficial region, compared to other storage solutions described above, although viability was still lower than that in fresh controls5,46,48,53. In addition, pre-equilibration of medium with 5% CO2, which maintains physiological pH near 7.4, improved chondrocyte viability in grafts compared to equilibration with ambient air, which is traditionally used during 4°C storage17. Since chondrocytes exhibit zone-specific functions,3 assessing depth related variations in chondrocyte viability may help to understand graft performance. The loss of viable chondrocytes, especially in the superficial zone, may contribute to graft degeneration and subsequent failure, since early stage cartilage deterioration is evident at the articular surface. Graft efficacy may benefit from improving chondrocyte viability by modifications to storage conditions.
Incubation of osteochondral samples at physiological temperature may prevent storage-associated chondrocyte death and also maintain GAG content in the cartilage. In osteochondral cultures retained on their natural bone support, the chondrocytes remained viable, and the cartilage showed no signs of degeneration, even after prolonged storage (3–6 wks) at 37°C in medium with various concentrations of serum18,32. Cartilage explants and osteochondral fragments incubated at 37°C in medium with different serum concentrations resulted in varying amounts of GAG deposition7,27,28,32,54,55; immature cartilage explants in medium with 10–20% FBS incubated for 2–3 weeks maintained GAG content28,43. Thus, the objectives of this study were to determine the effects of prolonged 37°C storage compared to traditional 4°C storage on the (1) chondrocyte viability, especially in the superficial zone, and (2) cartilage matrix content in fresh, goat osteochondral grafts.
Methods
Osteochondral Harvest
Osteochondral cores (n=65) were harvested from seven humeral heads of four mature (3–4 yr) male Boer goats. Caprine shoulders with intact joint capsules were obtained from a USDA licensed vendor and used within 48 hours of sacrifice. Under sterile conditions, joints were isolated and osteochondral cores were harvested by, first, scoring the cartilage surface with a 3.0 mm dermal punch and, second, coring with a custom 3.2 mm bit. The cartilage was kept hydrated throughout the procedure with copious amounts of phosphate-buffered saline (PBS) supplemented with antibiotics-antimycotic, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL fungizone (PSF). Prior to storage, each sample was thoroughly rinsed with PBS+PSF, and the bone was trimmed to a 4:1 bone to cartilage thickness ratio. For all samples, the subchondral bone was not analyzed quantitatively by LIVE/DEAD® staining because qualitatively it contained only dead cells and no viable cells, and this subchondral bone viability did not vary with storage condition.
Storage Conditions
Six storage conditions were analyzed (Table 1). Samples were either analyzed fresh (group i) or after storage for 14 or 28 days (groups ii–vi). Storage medium consisted of minimum essential medium Eagle with additives (10 μg/mL ascorbic acid, 2 mM L-glutamine, and PSF) with 0% FBS, but including 0.01% bovine serum albumin, 2% FBS, or 10% FBS. Samples stored at 4°C with 10% FBS for 14 or 28 days (groups ii and iii) were placed in glass vials with medium pre-equilibrated to 5% CO2 and sealed tightly. Samples stored at 37°C with 0%, 2%, or 10% FBS (groups iv, v, and vi) were incubated for 28 days in 24-well plates in an atmosphere with 5% CO2. At the end of storage, 4°C samples were cut in half with a single edged razor blade (using a custom stainless steel jig that was pre-cooled on ice), gradually warmed at room temperature for 30 minutes, and then incubated at 37°C for an additional 48 hours to bring samples to standard culture conditions. At the end of storage, 37°C samples were cut in half (using a jig warmed at 37°C), and then incubated for an additional 48 hours. Medium, 1.5 mL/sample, was changed three times per week, a relative medium volume and replenishing frequency that were sufficient to keep cartilage viable during prolonged 37°C storage of osteochondral fragments32.
Table 1.
Storage conditions
| Storage Condition | Temperature [°C] | FBS [%] | Duration [days] |
|---|---|---|---|
| i | FRESH CONTROL | 0 | |
| ii | 4 | 10 | 14 |
| iii | 4 | 10 | 28 |
| iv | 37 | 0 | 28 |
| v | 37 | 2 | 28 |
| vi | 37 | 10 | 28 |
Chondrocyte Viability
All samples were analyzed for live and dead cells using LIVE/DEAD® (Molecular Probes, Inc., Eugene, OR) staining, fluorescence microscopy, and image processing. Each half-core sample was stained by incubation in medium containing 2.7 μM calcein AM and 5.0 μM ethidium homodimer-1 for 15 minutes at 37°C. Metabolically active cells permit calcein AM to enter through the intact plasma membrane where the dye is cleaved by cytoplasmic esterases yielding green fluorescence56. In contrast, ethidium homodimer-1, which is membrane impermeable, binds to DNA of membrane-compromised cells yielding red fluorescence45. Samples were fluorescently imaged along a vertical profile using a 10x Plan Fluor objective lens (NA = 0.3; Nikon), a microscope (Eclipse TE300, Nikon, Melville, NY), an arc lamp, a G-2A (for “dead” images; Nikon) or B-2A (for “live” images; Nikon) filter cube, and a SPOT RT camera (Diagnostic Instruments, Sterling Heights, MI) to obtain images indicating live and dead cells. The articular cartilage with the surface intact was then removed from the bone, and the articular surface was imaged en face. For each 8-bit grayscale image with 1600 × 1200 pixels, the field of view was 1.2 × 0.9 mm2.
The images were processed to determine the cartilage thickness and to count live and dead cells using a custom routine (MATLAB® v7.5 with Image Processing Toolkit, Mathworks, Natick, MA) adapted from previous studies4. Briefly, images were processed24 by spatial filtering (5 × 5 Laplacian of Gaussian filter) to accentuate regions representative of cells, median filtering to suppress noise, and thresholding with a size criteria (greater than 40 or 15 μm2 for live or dead cells, respectively) to localize cells. This method had ~90% sensitivity for both live and dead cells as determined from twelve images of six samples by manual counting of randomly cropped regions containing >100 cells. Chondrocyte viability was calculated as the percentage of live cells relative to the total number of cells. Cell density, the number of cells divided by the imaged cartilage area (cells/mm2), was also determined for live, dead, and total cells. For vertical profiles, viability and cell densities were calculated for the overall cartilage thickness as well as zones of cartilage; superficial, middle, and deep zones were defined as the top 15%, the next 35%, and the remaining 50% of the cartilage thickness, respectively, based on pilot studies to identify zones of cartilage based on regional differences in cell morphology.
Cartilage Matrix Content
Some of the remaining half-cores (n=31) were analyzed for sulfated-glycosaminoglycan (GAG) and collagen (COL) content in the cartilage of samples, fresh after harvest or following storage (n=4–9). Full thickness cartilage was removed from the bone, weighed wet, and solubilized with proteinase K at 60°C for 16 h62. Portions of the digest were analyzed to quantify the content of GAG by the dimethylmethylene blue dye binding assay22 and COL by p-dimethyalaminobenzaldehyde binding for hydroxyproline63. The hydroxyproline content was converted to COL content using a mass ratio of 7.25 collagen to hydroxyproline29, 44. All biochemical measures were normalized to wet weight.
Statistical Analysis
Data are presented as mean ± SEM. The effects of storage condition on chondrocyte viability, live, dead, and total cell density, in addition to cartilage thickness, GAG and COL content, were determined by a one-way ANOVA, with joint as a random factor. Where significant differences were detected, Tukey post hoc comparisons were performed to determine the effects of (1) storage duration at 4°C (conditions i, ii, iii) and (2) storage condition at day 28 (conditions iii, iv, v, vi). For viability and density data, zone (superficial, middle, and deep) was considered a repeated measure, and a two-way repeated measures ANOVA was performed. Since percentages form binomial rather than normal distributions, an arcsine transformation was applied to normalize viability data before the statistical analyses52. Significance was set at α=0.05 and all statistical analyses were performed using Systat 10.2 (Systat Software Inc., Richmond, CA).
Results
Qualitative analysis indicated that osteochondral samples stored at 37°C had higher chondrocyte viability than samples stored at 4°C, especially at the articular surface. En face (Fig. 1A–C) and vertical (Fig. 2A–C) profiles showed that fresh controls, immediately following harvest, contained primarily live cells and relatively few dead cells. After 28 days of storage, 4°C samples stored in medium with 10% FBS had a large proportion of dead cells, especially at the articular surface (Figs. 1G–I and 2G–I), whereas 37°C samples stored in medium with 0%, 2%, or 10% FBS showed a relatively large proportion of live cells at the articular surface (Figs. 1J–R and 2J–R). Vertical profiles also showed scattered cell death in deeper regions of the tissue with similar patterns for all storage conditions (Fig. 2D–R).
Figure 1.
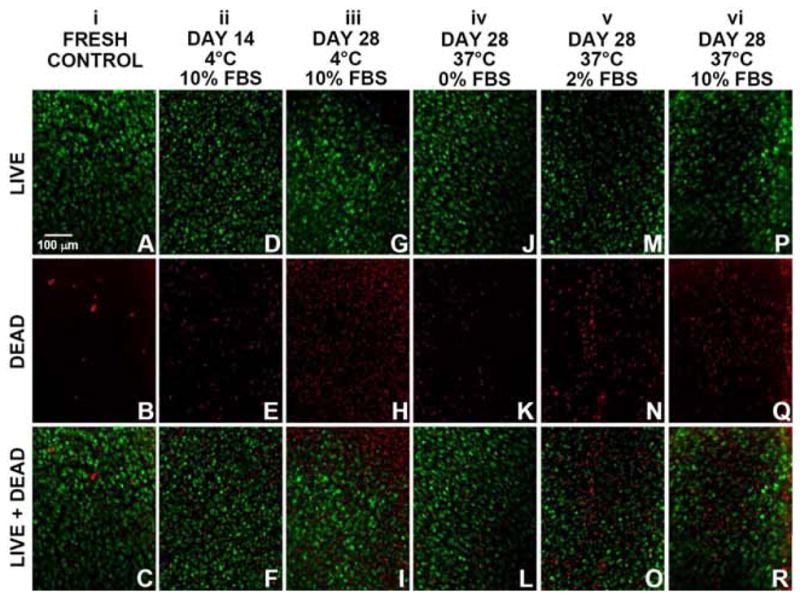
En face view of chondrocyte viability at the articular surface in goat osteochondral samples, as analyzed by LIVE/DEAD® fluorescence staining. (A–C) Fresh controls. Samples stored at 4°C in medium including 10% FBS for (D–F) 14 days and (G–I) 28 days, as well samples stored at 37°C for 28 days in medium including (J–L) 0% FBS, (M–O) 2% FBS, or (P–R) 10% FBS. (A,D,G,J,M,P) Images of live cells (green fluorescence). (B,E,H,K,N,Q) Images of dead cells (red fluorescence). (C,F,I,L,O,R): Merged live+dead images.
Figure 2.
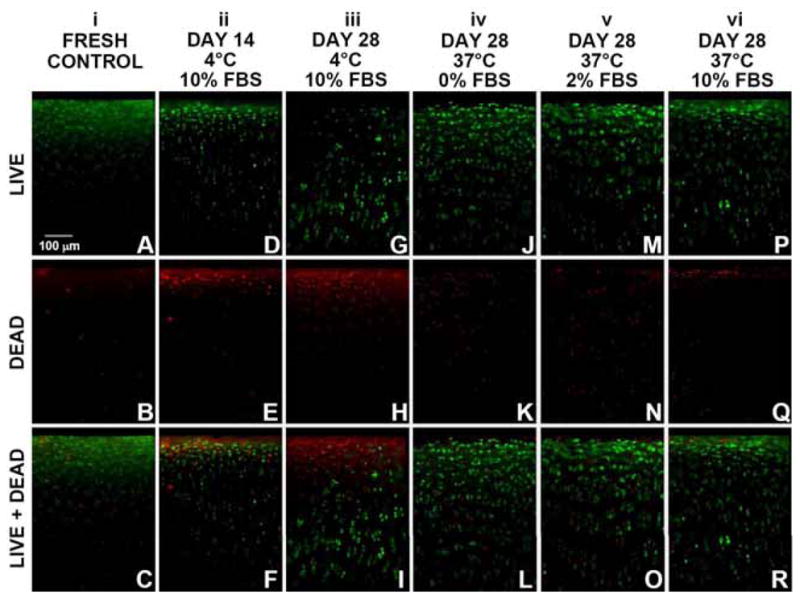
Vertical profile view of chondrocyte viability throughout the depth of articular cartilage in goat osteochondral samples, as analyzed by LIVE/DEAD® fluorescence staining. (A–C) Fresh controls. Samples stored at 4°C in medium including 10% FBS for (D–F) 14 days and (G–I) 28 days, as well samples stored at 37°C for 28 days in medium including (J–L) 0% FBS, (M–O) 2% FBS, or (P–R) 10% FBS. (A,D,G,J,M,P) Images of live cells (green fluorescence). (B,E,H,K,N,Q) Images of dead cells (red fluorescence). (C,F,I,L,O,R): Merged live+dead images.
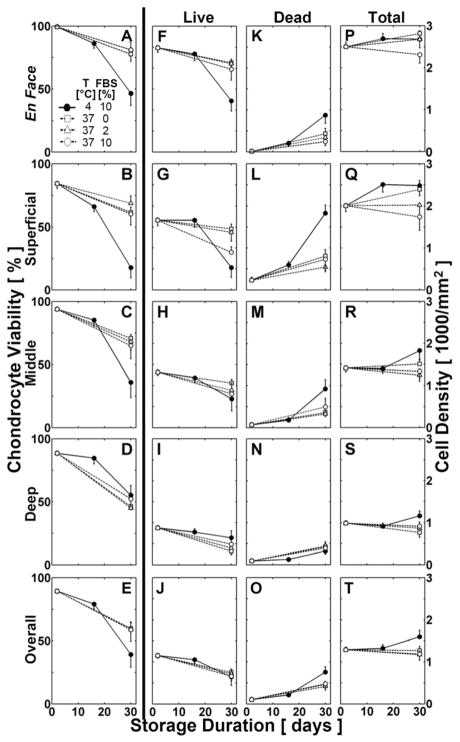
Quantitative analysis, for both en face and vertical profiles, indicated that chondrocyte viability varied significantly with storage condition (p<0.001, Fig. 3A–E). For vertical profiles, chondrocyte viability also varied with zone (p<0.001), with a significant interaction between zone and storage condition (p<0.001). Consistent with these findings, live and dead cell densities in en face and vertical images varied with storage condition (p<0.001, except p<0.01 for overall vertical), without effects on total cell density (p=0.1, Fig. 3F–T). For vertical profiles, live, dead, and total cell density varied with zone (p<0.001), with a significant interaction between zone and storage condition (p<0.05).
Figure 3.
Effects of storage condition on chondrocyte viability, live, dead, and total cell density throughout the depth of cartilage from osteochondral samples over a storage duration of 28 days. Chondrocyte viability and cell densities at (A,F,K,P) the cartilage surface en face, by region for the (B,G,L,Q) superficial [15%], (C,H,M,R) middle [35%], and (D,I,N,S) deep [50%] zones, and (E,J,O,T) in the overall vertical profile.
Effects of Storage Duration at 4°C
During 4°C storage, quantitative analysis at the cartilage surface en face indicated that chondrocyte viability decreased markedly with increasing storage duration (Fig. 3A). En face, chondrocyte viability was initially high for fresh controls at the cartilage surface, nearing ~100%. After 14 days of 4°C storage, viability tended to decrease by ~15% (p=0.2) compared to fresh controls. During 4°C storage, viability decreased by ~40% (between days 14 and 28, p<0.001), and by ~55% after 28 days (vs. fresh controls, p<0.001).
During 4°C storage, quantitative analysis of the vertical profile indicated that chondrocyte viability markedly decreased with increasing storage duration, most notably in the superficial zone, and to a lesser extent in the middle and deep zones (Fig. 3B–E). Chondrocyte viability was initially high for fresh controls in the superficial, middle, and deep zones, averaging 84%, 94%, and 88%, respectively. In the superficial zone, viability decreased by ~25% after 14 days (vs. fresh controls, p<0.05), by ~55% (between days 14–28, p<0.001), and by ~80% after 28 days (vs. fresh controls, p<0.001). In the middle and deep zones, viability was not significantly lower after 14 days (decreased by ~10% and ~5% vs. fresh controls, p=0.7 and p=1.0, respectively), but was markedly decreased by ~50% and ~30% (between days 14–28, p<0.01), and by ~60% and ~35% after 28 days (vs. fresh controls, p<0.001, p<0.01, respectively). Due to the similarity in chondrocyte viability between 4°C storage after 14 days and fresh controls, the effects of storage condition (37°C vs 4°C) on chondrocyte viability was examined after 28 days of storage.
Effects of 37°C vs 4°C Storage at Day 28
After 28 days of storage, quantitative analysis at the articular surface en face was consistent with the qualitative results described above and indicated that 37°C storage maintained chondrocyte viability more effectively than 4°C storage (Fig. 3A). After 28 days, en face chondrocyte viability was ~35% higher following 37°C storage compared to 4°C storage (p<0.001). At 37°C, en face viability among 0–10% FBS samples did not vary significantly (p=1.0).
After 28 days of storage, quantitative analysis of the vertical profile indicated that the superficial and middle zones had chondrocyte viability patterns similar to the surface viewed en face, while deep zone chondrocyte viability was not significantly different between 37°C and 4°C storage. At 37°C, chondrocyte viability among 0–10% FBS samples was not significantly different in any zone (p=1.0). For 0–10% FBS samples stored at 37°C, viability was higher by ~45% in the superficial zone (p<0.01), and by ~35% in the middle zone (p<0.05), compared to samples stored at 4°C (Fig. 3B,C). In the deep zone, viability of 37°C and 4°C stored samples was not significantly different, decreasing to ~45–55% after 28 days of storage (p=1.0) (Fig. 3D). Overall, chondrocyte viability throughout the vertical profile after 28 days fell to ~40% after 4°C storage, but only to ~60% after 37°C storage (p=0.1, Fig. 3E).
After 28 days of storage, live and dead cell densities reflected the relative percentages above, while total cell density was not significantly different between 37°C and 4°C storage. Total cell density was highest at the articular surface en face (2,400 cells/mm2) and in the superficial zone (2,200 cells/mm2), moderate in the middle zone (1,500 cells/mm2) and in the overall vertical profile (1,300 cells/mm2), and lowest in the deep zone (1,000 cells/mm2) (Fig. 3P-T). At the articular surface en face, fresh controls had a high live cell density of 2,400 cells/mm2, and a low dead cell density of <100 cells/mm2 (Fig. 3F,K). For 4°C samples, en face live cell density fell to 1,200 cells/mm2, and dead cell density rose to 1,200 cells/mm2 after 28 days, indicating ~50% less live cells in 4°C samples compared to fresh controls (p<0.001). For 37°C samples, live and dead cell densities at the articular surface en face were moderately affected by 28-day storage compared to fresh controls, averaging a live cell density of 2,000 cells/mm2 and a dead cell density of 400 cells/mm2 (for live: p=0.7, for dead: p<0.05).
Stored osteochondral samples, at both 37°C and 4°C for 28 days, had similar cartilage matrix content, as determined by GAG and COL, compared to fresh controls (Table 2). Cartilage thickness, GAG content, and COL content did not vary with storage condition (p=0.2, p=0.2, p=0.7, respectively). GAG content of samples for all storage conditions and durations was maintained within ~20% of fresh controls (35 mg/g). COL content of 4°C samples stored for 14d and 28d varied by ~15% compared to fresh controls (118 mg/g); but COL content of 37°C samples stored for 28d remained within ~5% compared to fresh controls.
Table 2.
Cartilage thickness, GAG and COL content of fresh and stored goat osteochondral samples. Data are mean ± SEM.
| Storage Condition | n | Thickness [mm] | n | GAG [mg/g] | COL [mg/g] |
|---|---|---|---|---|---|
| i | 18 | 0.71 ± 0.20 | 9 | 34.7 ± 3.1 | 117.6 ± 8.4 |
| ii | 13 | 0.71 ± 0.20 | 4 | 41.7 ± 8.6 | 118.2 ± 8.0 |
| iii | 10 | 0.68 ± 0.19 | 4 | 40.9 ± 14.1 | 101.6 ± 7.5 |
| iv | 7 | 0.83 ± 0.13 | 7 | 27.4 ± 3.7 | 120.8 ± 8.2 |
| v | 7 | 0.85 ± 0.09 | 7 | 27.9 ± 3.8 | 114.8 ± 5.2 |
| vi | 10 | 0.68 ± 0.24 | 4 | 23.2 ± 5.5 | 121.5 ± 9.8 |
| p-values | 0.2 | 0.2 | 0.7 | ||
Discussion
This study examined the effects of 37°C and 4°C storage on chondrocyte viability and cartilage matrix content of fresh goat osteochondral samples. After 28 days, 37°C samples had higher chondrocyte viability compared to 4°C samples, specifically at the articular surface. 4°C samples had a significant decline in chondrocyte viability to ~50% at the cartilage surface en face, to ~20% in the superficial zone, and to ~40% in the middle zone (Figs. 1G–I, 2G–I, and 3A–C), whereas 37°C samples had higher viability in those regions, averaging ~80%, ~65%, and ~70%, respectively (Figs. 1J–R, 2J–R, and 3A–C). In the deep zone, viability was similar at ~45–55% following 37°C and 4°C storage (Figs. 1, 2, and 3D). Additionally, there were no significant differences in cartilage thickness and matrix (GAG, COL) content for 37°C samples, 4°C samples, and fresh controls (Table 2). These results indicate 37°C storage of osteochondral grafts supports long-term viability of chondrocytes, especially at the articular surface.
The use of goat osteochondral samples for studying allograft storage conditions involved consideration of a number of issues. The small size of cores allowed many samples to be harvested from each joint, with the cores still having a sample radius (r=1.6 mm) that was greater than the cartilage thickness (t=0.5–1.0 mm). Therefore, the shortest path of diffusion, through the cartilage thickness, would be the same for these small cores or larger samples. For this study, storage of osteochondral samples in 1.5 mL of medium provided a medium volume that was ~50 times the total volume of the sample (cartilage+bone), while previous studies stored donor tissue in medium ~10 times the total volume of the sample48,61.
Viability data of caprine tissue from this study were similar to viability data of human tissue following clinically established 4°C storage conditions. Consistent with the results presented here, 4°C storage of human osteochondral grafts resulted in high chondrocyte death, especially at the articular surface46,48. The metabolic properties of adult goat cartilage during storage have not been previously investigated, and may differ compared to human cartilage, especially at physiological temperature. However, adult goats can provide consistent normal cartilage compared to humans, where aging and degeneration can often be a factor. Therefore, viability data suggests that storage of adult goat osteochondral samples can be a reasonable model for adult human osteochondral samples for up to one month. However, differences in cartilage thickness between caprine and human tissue may affect the diffusion of nutrients to the deeper regions of cartilage. Thus, studies with human tissue are needed to confirm the present findings.
Chondrocyte viability results presented here after 28-day 4°C storage were consistent with previous studies. Overall full-thickness viability averaged 45–78% in medium including FBS for human46,48, bovine53, canine57, and ovine58 models. Average viability through the vertical profile, determined by fluorescent microscopy of thin sections53 (~45%) or by confocal microscopy57 (~65%), were more similar to the results here (~40%). Results reported by flow cytometry46,58, which required digestion of the cartilage matrix, or averaging images at random locations parallel to the articular surface48, were at the high end of the range above (67–78%). However, these methods do not account for regional variations and may not provide an accurate representation of viability; cells may be lost during digestion, and averages may be skewed by ineffective sampling (i.e. inconsistent locations between samples and greater frequency in less affected regions).
The results presented here extend the past work on chondrocyte viability after 4°C storage by assessing quantitative estimates of chondrocyte viability at the articular surface en face and in morphologically analogous cartilage zones. Previously, cartilage was analyzed as three zones of equal thickness, an assumption that would overestimate the cellularity of the true superficial zone (33% vs. 15% of cartilage thickness), and reported lower viability in the top-third (~50%) compared to overall viability (~67%) after 28-day 4°C storage48. Division of the cartilage into superficial, middle, and deep zones makes it difficult to directly compare viability to those of past studies. In addition, previous studies reported high overall viability (averaging ~80–95%) after 14-day 4°C storage5,53,57,58,61 and qualitatively described preferential cell death at the cartilage surface with increasing storage duration5,17,47,58. In the present study, viability after 14-day 4°C storage (~80%) was consistent with previously reported values of overall viability. Previous studies may not have been sensitive to declines in morphologically defined superficial zone, which occurred in this study as early as 14 days after 4°C storage.
Long-term storage of osteochondral grafts at 37°C and 4°C may have opposing effects on chondrocyte viability and GAG metabolism; at 37°C, most chondrocytes are viable and matrix content can be modulated whereas at 4°C, most chondrocytes are dead and metabolically inactive. Following 28-day 4°C storage, osteochondral grafts and cartilage explants had no change in GAG content compared to baseline controls; however, chondrocytes exhibited reduced GAG metabolism following 4°C storage compared to baseline controls when GAG synthesis was measured at physiological temperature5,9,47,61. During cartilage explant culture, cellular outgrowth has been observed during incubation for 4–6 weeks at 37°C in the presence of 10–20% FBS, particularly from deep zone chondrocytes;8,36 such outgrowth may also occur during 37°C storage of osteochondral grafts and affect subsequent in vivo performance. In addition, following 28-day 37°C storage, osteochondral fragments and cartilage explants could have a variety of changes in matrix content (GAG and COL) depending on the culture conditions7,9,27,28,32,49,54,55, whereas 37°C samples containing viable chondrocytes may be able to maintain cartilage homeostasis and prevent tissue degeneration following implantation. The results in this study reported that GAG and COL could be maintained for osteochondral samples stored at 37°C for 28 days. While matrix content measurements are not as sensitive to changes as synthesis and degradation rates, they can typically be correlated with mechanical properties of the tissue.
The results of the present study suggest that osteochondral grafts stored at 37°C for 2 to 4 weeks have better biological performance than grafts stored at 4°C for the same duration. Cell death in the superficial zone may have deleterious effects after implantation, as chondrocytes in this zone normally secrete a lubricant molecule proteoglycan-4 (PRG4)51. PRG4 has a boundary-lubricating ability50, and has been shown to protect the cartilage surface from wear by reducing friction when compared to PRG4 deficient joints31. During storage, reduction of viable chondrocytes in the superficial zone and their secreted lubricants may be key factors in graft failure. Studies to investigate more closely the relationship between chondrocyte viability and function through the production of zonal-specific molecules may provide additional insight into the factors necessary for graft success. Storage alternatives, including physiological temperature, could increase the storage duration window for suitable donor tissue and provide higher quality graft tissue.
The present study also suggests that storage at 37°C, compared to traditional 4°C, may allow extended storage duration of grafts, during which chondrocyte viability and cartilage matrix content are maintained. 37°C storage maintained chondrocyte viability, especially in the superficial zone, although the mechanism of cell death and its variation within the zones of cartilage are still topics of speculation. Chondrocytes at the surface may be more susceptible to storage-associated death because their native physical and chemical environment is disrupted, whereas chondrocytes in the deeper regions of cartilage are in a matrix-filled environment that more closely resembles the native form. Preserving chondrocyte viability during prolonged storage intervals could facilitate increased graft shelf-life and availability of osteochondral allografts. However, grafts stored at 37°C rather than 4°C may have a higher incidence of infection and spoilage because microorganisms typically grow faster at such elevated temperatures. In addition, conditions that maintain viability in the superficial zone, in particular, may improve clinical performance by providing lubricating molecules, such as PRG4, to maintain a low-friction articulating surface. The relationship among overall chondrocyte viability, superficial zone viability, and subsequent success after osteochondral allografting remains to be elucidated.
Acknowledgments
This work was supported by grants from the National Institutes of Health and an award to UCSD under the Howard Hughes Medical Institute Professors Program.
We thank William McCarty and Rebecca Rone for technical support in harvesting tissue used for this study. This work was supported by grants from the National Institutes of Health and an award to UCSD for R.L.S. under the Howard Hughes Medical Institute Professors Program.
References
- 1.Services USDoHaH. Guidance for Industry: Screening and testing of human tissue intended for transplantation. Food and Drug Administration Center for Biologics Evaluation and Research; 2002. [Google Scholar]
- 2.Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33(2):295–306. doi: 10.1177/0363546504273510. [DOI] [PubMed] [Google Scholar]
- 3.Aydelotte MB, Kuettner KE. Differences between sub-populations of cultured bovine articular chondrocytes. I. morphology and cartilage matrix production. Connect Tissue Res. 1988;18:205–222. doi: 10.3109/03008208809016808. [DOI] [PubMed] [Google Scholar]
- 4.Bae WC, Schumacher BL, Sah RL. Indentation probing of human articular cartilage: effect on chondrocyte viability. Osteoarthritis Cartilage. 2007;15:9–18. doi: 10.1016/j.joca.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Ball ST, Amiel D, Williams SK, et al. The effects of storage media on fresh human osteochondral allografts. Clin Orthop Relat Res. 2004;418:246–252. doi: 10.1097/00003086-200401000-00043. [DOI] [PubMed] [Google Scholar]
- 6.Beaver RJ, Mahomed M, Backstein D, Davis A, Zukor DJ, Gross AE. Fresh osteochondral allografts for post-traumatic defects in the knee. J Bone Joint Surg Br. 1992;74-B:105–110. doi: 10.1302/0301-620X.74B1.1732235. [DOI] [PubMed] [Google Scholar]
- 7.Bian L, Lima EG, Angione SL, et al. Mechanical and biochemical characterization of cartilage explants in serum-free culture. J Biomech. 2008;41(6):1153–1159. doi: 10.1016/j.jbiomech.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bos PK, Kops N, Verhaar JAN, van Osch GJVM. Cellular origin of neocartilage formed at wound edges of articular cartilage in a tissue culture experiment. Osteoarthritis Cartilage. 2008;16(2):204–211. doi: 10.1016/j.joca.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Brighton CT, Shadle CA, Jimenez SA, Irwin JT, Lane JM, Lipton M. Articular cartilage preservation and storage. I. Application of tissue culture techniques to the storage of viable articular cartilage. Arthritis Rheum. 1979;22(10):1093–1101. doi: 10.1002/art.1780221008. [DOI] [PubMed] [Google Scholar]
- 10.Bugbee WD. Fresh osteochondral allografts. J Knee Surg. 2002;15(3):191–195. [PubMed] [Google Scholar]
- 11.Chu CR, Convery FR, Akeson WH, Meyers M, Amiel D. Articular cartilage transplantation: clinical results in the knee. Clin Orthop Rel Res. 1999;360:159–168. [PubMed] [Google Scholar]
- 12.Cole BJ, Lee SJ. Complex knee reconstruction: articular cartilage treatment options. Arthroscopy. 2003;19(Suppl 1):1–10. doi: 10.1016/j.arthro.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Convery FR, Akeson WH, Amiel D, Meyers MH, Monosov A. Long-term survival of chondrocytes in an osteochondral articular cartilage allograft. A case report. J Bone Joint Surg Am. 1996;78-A:1082–1088. doi: 10.2106/00004623-199607000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Convery FR, Meyers MH, Akeson WH. Fresh osteochondral allografting of the femoral condyle. Clin Orthop Rel Res. 1991;273:139–145. [PubMed] [Google Scholar]
- 15.Csonge L, Bravo D, Newman-Gage H, et al. Banking of osteochondral allografts, Part II. Preservation of chondrocyte viability during long-term storage. Cell Tissue Bank. 2002;3:161–168. doi: 10.1023/A:1023687419152. [DOI] [PubMed] [Google Scholar]
- 16.Czitrom AA, Keating S, Gross AE. The viability of articular cartilage in fresh osteochondral allografts after clinical transplantation. J Bone Joint Surg. 1990;72-A:574–581. [PubMed] [Google Scholar]
- 17.Dontchos BN, Coyle CH, Izzo NJ, et al. Optimizing CO2 normalizes pH and enhances chondrocyte viability during cold storage. J Orthop Res. 2008;26(5):643–650. doi: 10.1002/jor.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dumont J, Ionescu M, Reiner A, et al. Mature full-thickness articular cartilage explants attached to bone are physiologically stable over long-term culture in serum-free media. Connect Tissue Res. 1999;40(4):259–272. doi: 10.3109/03008209909000704. [DOI] [PubMed] [Google Scholar]
- 19.Emmerson BC, Gortz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35(6):907–914. doi: 10.1177/0363546507299932. [DOI] [PubMed] [Google Scholar]
- 20.Enneking WF, Campanacci DA. Retrieved human allografts: a clinicopathological study. J Bone Joint Surg Am. 2001;83-A(7):971–986. [PubMed] [Google Scholar]
- 21.Enneking WF, Mindell ER. Observations on massive retrieved human allografts. J Bone Joint Surg Am. 1991;73-A:1123–1142. [PubMed] [Google Scholar]
- 22.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 23.Garrett JC. Fresh osteochondral allografts for treatment of articular defects in osteochondritis dissecans of the lateral femoral condyle in adults. Clin Orthop Rel Res. 1994;303:33–37. [PubMed] [Google Scholar]
- 24.Gonzalez RC, Woods RE, Eddins SL. Digital Image Processing Using MATLAB. Upper Saddle River, N. J: Pearson Prentice Hall; 2004. [Google Scholar]
- 25.Gortz S, Bugbee WD. Allografts in articular cartilage repair. J Bone Joint Surg Am. 2006;88(6):1374–1384. doi: 10.2106/00004623-200606000-00030. [DOI] [PubMed] [Google Scholar]
- 26.Gortz S, Bugbee WD. Fresh osteochondral allografts: graft processing and clinical applications. J Knee Surg. 2006;19(3):231–240. doi: 10.1055/s-0030-1248112. [DOI] [PubMed] [Google Scholar]
- 27.Handley CJ, McQuillan DJ, Campbell MA, Bolis S. Steady-state metabolism in cartilage explants. In: Kuettner K, Schleyerbach R, Hascall VC, editors. Articular Cartilage Biochemistry. New York: Raven Press; 1986. pp. 163–179. [Google Scholar]
- 28.Hascall VC, Handley CJ, McQuillan DJ, Hascall GK, Robinson HC, Lowther DA. The effect of serum on biosynthesis of proteoglycans by bovine articular cartilage in culture. Arch Biochem Biophys. 1983;224:206–223. doi: 10.1016/0003-9861(83)90205-9. [DOI] [PubMed] [Google Scholar]
- 29.Herbage D, Bouillet J, Bernengo J-C. Biochemical and physicochemical characterization of pepsin-solubilized type-II collagen from bovine articular cartilage. Biochem J. 1977;161:303–312. doi: 10.1042/bj1610303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jamali AA, Hatcher SL, You Z. Donor cell survival in a fresh osteochondral allograft at twenty-nine years. A case report. J Bone Joint Surg Am. 2007;89:166–169. doi: 10.2106/JBJS.F.00618. [DOI] [PubMed] [Google Scholar]
- 31.Jay GD, Torres JR, Rhee DK, et al. Association between friction and wear in diarthrodial joints lacking lubricin. Arthritis Rheum. 2007;56(11):3662–3669. doi: 10.1002/art.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korver GHV, van de Stadt RJ, van Kampen GPJ, Kiljan E, van der Korst JK. Bovine sesamoid bones: a culture system for anatomically intact articular cartilage. In Vitro Cell Dev Biol. 1989;25:1099–1106. doi: 10.1007/BF02621260. [DOI] [PubMed] [Google Scholar]
- 33.LaPrade RF, Botker J, Herzog M, Agel J. Refrigerated osteoarticular allografts to treat articular cartilage defects of the femoral condyles. A prospective outcomes study. J Bone Joint Surg Am. 2009;91(4):805–811. doi: 10.2106/JBJS.H.00703. [DOI] [PubMed] [Google Scholar]
- 34.Lattermann C, Romine SE. Osteochondral allografts: state of the art. Clin Sports Med. 2009;28(2):285–301. ix. doi: 10.1016/j.csm.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Lexer E. Joint transplantation. Clin Orthop Relat Res. 1985;197:4–10. [PubMed] [Google Scholar]
- 36.Luyten FP, Hascall VC, Nissley SP, Morales TI, Reddi AH. Insulin-like growth factors maintain steady-state metabolism of proteoglycans in bovine articular cartilage explants. Arch Biochem Biophys. 1988;267:416–425. doi: 10.1016/0003-9861(88)90047-1. [DOI] [PubMed] [Google Scholar]
- 37.Mankin HJ, Doppelt S, Tomford W. Clinical experience with allograft implantation: the first ten years. Clin Orthop Rel Res. 1983;174:69–86. [PubMed] [Google Scholar]
- 38.Mankin HJ, Gebhardt MC, Tomford WW. The use of frozen cadaveric allografts in the management of patients with bone tumors of the extremities. Orthop Clin North Am. 1987;18(2):275–289. [PubMed] [Google Scholar]
- 39.Maury AC, Safir O, Heras FL, Pritzker KP, Gross AE. Twenty-five-year chondrocyte viability in fresh osteochondral allograft. A case report. J Bone Joint Surg Am. 2007;89:159–165. doi: 10.2106/JBJS.E.00815. [DOI] [PubMed] [Google Scholar]
- 40.McCulloch PC, Kang RW, Sobhy MH, Hayden JK, Cole BJ. Prospective evaluation of prolonged fresh osteochondral allograft transplantation of the femoral condyle: minimum 2-year follow-up. Am J Sports Med. 2007;35:411–420. doi: 10.1177/0363546506295178. [DOI] [PubMed] [Google Scholar]
- 41.McDermott AG, Langer F, Pritzker KP, Gross AE. Fresh small-fragment osteochondral allografts. Long-term follow-up study on first 100 cases. Clin Orthop Rel Res. 1985;197:96–102. [PubMed] [Google Scholar]
- 42.McGoveran BM, Pritzker KP, Shasha N, Price J, Gross AE. Long-term chondrocyte viability in a fresh osteochondral allograft. J Knee Surg. 2002;15(2):97–100. [PubMed] [Google Scholar]
- 43.Morales TI, Hascall VC. Factors involved in the regulation of proteoglycan metabolism in articular cartilage. Arthritis Rheum. 1989;32:1197–1201. doi: 10.1002/anr.1780321003. [DOI] [PubMed] [Google Scholar]
- 44.Pal S, Tang L-H, Choi H, et al. Structural changes during development in bovine fetal epiphyseal cartilage. Collagen Rel Res. 1981;1:151–176. doi: 10.1016/s0174-173x(81)80017-9. [DOI] [PubMed] [Google Scholar]
- 45.Papadopoulos NG, Dedoussis GV, Spanakos G, Gritzapis AD, Baxevanis CN, Papamichail M. An improved fluorescence assay for the determination of lymphocyte-mediated cytotoxicity using flow cytometry. J Immunol Methods. 1994;177(1–2):101–111. doi: 10.1016/0022-1759(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 46.Pearsall AW, Tucker JA, Hester RB, Heitman RJ. Chondrocyte viability in refrigerated osteochondral allografts used for transplantation within the knee. Am J Sports Med. 2004;32(1):125–131. doi: 10.1177/0095399703258614. [DOI] [PubMed] [Google Scholar]
- 47.Pennock AT, Robertson CM, Wagner F, Harwood FL, Bugbee WD, Amiel D. Does subchondral bone affect the fate of osteochondral allografts during storage? Am J Sports Med. 2006;34(4):586–591. doi: 10.1177/0363546505281815. [DOI] [PubMed] [Google Scholar]
- 48.Pennock AT, Wagner F, Robertson CM, Harwood FL, Bugbee WD, Amiel D. Prolonged storage of osteochondral allografts: does the addition of fetal bovine serum improve chondrocyte viability? J Knee Surg. 2006;19(4):265–272. doi: 10.1055/s-0030-1248117. [DOI] [PubMed] [Google Scholar]
- 49.Sah RL, Trippel SB, Grodzinsky AJ. Differential effects of serum, insulin-like growth factor-I, and fibroblast growth factor-2 on the maintenance of cartilage physical properties during long-term culture. J Orthop Res. 1996;14:44–52. doi: 10.1002/jor.1100140109. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 2007;56:882–891. doi: 10.1002/art.22446. [DOI] [PubMed] [Google Scholar]
- 51.Schumacher BL, Hughes CE, Kuettner KE, Caterson B, Aydelotte MB. Immunodetection and partial cDNA sequence of the proteoglycan, superficial zone protein, synthesized by cells lining synovial joints. J Orthop Res. 1999;17:110–120. doi: 10.1002/jor.1100170117. [DOI] [PubMed] [Google Scholar]
- 52.Sokal RR, Rohlf FJ. Biometry. 3. New York: WH Freeman and Co; 1995. [Google Scholar]
- 53.Teng MS, Yuen AS, Kim HT. Enhancing osteochondral allograft viability: effects of storage media composition. Clin Orthop Relat Res. 2008;466(8):1804–1809. doi: 10.1007/s11999-008-0302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Von den Hoff HW, de Koning MH, van Kampen GP, van der Korst JK. Transforming growth factor-beta stimulates retinoic acid-induced proteoglycan depletion in intact articular cartilage. Arch Biochem Biophys. 1994;313(2):241–247. doi: 10.1006/abbi.1994.1383. [DOI] [PubMed] [Google Scholar]
- 55.von den Hoff JW, van Kampen GPJ, van de Stadt RJ, van der Korst JK. Kinetics of proteoglycan turnover in bovine articular cartilage explants. Matrix. 1993;13:195–201. doi: 10.1016/s0934-8832(11)80003-x. [DOI] [PubMed] [Google Scholar]
- 56.Weston SA, Parish CR. New fluorescent dyes for lymphocyte migration studies. Analysis by flow cytometry and fluorescence microscopy. J Immunol Methods. 1990;133(1):87–97. doi: 10.1016/0022-1759(90)90322-m. [DOI] [PubMed] [Google Scholar]
- 57.Williams JM, Virdi AS, Pylawka TK, Edwards RB, 3rd, Markel MD, Cole BJ. Prolonged-fresh preservation of intact whole canine femoral condyles for the potential use as osteochondral allografts. J Orthop Res. 2005;23(4):831–837. doi: 10.1016/j.orthres.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 58.Williams RJ, 3rd, Dreese JC, Chen CT. Chondrocyte survival and material properties of hypothermically stored cartilage: an evaluation of tissue used for osteochondral allograft transplantation. Am J Sports Med. 2004;32(1):132–139. doi: 10.1177/0095399703258733. [DOI] [PubMed] [Google Scholar]
- 59.Williams RJ, 3rd, Ranawat AS, Potter HG, Carter T, Warren RF. Fresh stored allografts for the treatment of osteochondral defects of the knee. J Bone Joint Surg Am. 2007;89:718–726. doi: 10.2106/JBJS.F.00625. [DOI] [PubMed] [Google Scholar]
- 60.Williams SK, Amiel D, Ball ST, et al. Analysis of cartilage tissue on a cellular level in fresh osteochondral allograft retrievals. Am J Sports Med. 2007;35:2022–2032. doi: 10.1177/0363546507305017. [DOI] [PubMed] [Google Scholar]
- 61.Williams SK, Amiel D, Ball ST, et al. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85-A:2111–2120. doi: 10.2106/00004623-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 62.Williamson AK, Masuda K, Thonar EJ-MA, Sah RL. Growth of immature articular cartilage in vitro: correlated variation in tensile biomechanical and collagen network properties. Tissue Eng. 2003;9:625–634. doi: 10.1089/107632703768247322. [DOI] [PubMed] [Google Scholar]
- 63.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]