Summary
Background
African Americans with hypertension have higher cardiovascular morbidity and mortality than other ethnic groups. Plasma D-dimer, a fragment generated from fibrin during lysis of mature clot in vivo, is a predictor of adverse cardiovascular events.
Objective
We investigated whether plasma levels of D-dimer differ between African American (AA) and non-Hispanic white (NHW) adults with hypertension.
Methods
Participants included 933 AA (65±9 years, 72% women) and 821 NHW (61±9 years, 56% women) from the community. D-dimer was measured by an immunoturbidimetric assay. Multivariable regression analyses, stratified by sex, were performed to assess whether AA ethnicity was associated with D-dimer levels after adjustment for age, body mass index, total and high-density lipoprotein cholesterol, systolic blood pressure, diabetes, history of smoking, medication (statin and aspirin) use, lifestyle variables (physical activity, alcohol intake, and education), estimated glomerular filtration rate (eGFR), and a marker of inflammation, C-reactive protein (CRP).
Results
D-dimer levels were higher in AA men and women than in their NHW counterparts (mean±SD; men 255±199 vs. 190±182 ng/mL, P <0.001; women, 289±233 vs. 225±195 ng/mL, P <0.001). In both sexes, after adjustment for age, conventional risk factors, medication use, and lifestyle variables, AA ethnicity remained associated with higher D-dimer levels (P = 0.002 in men, P =0.006 in women). These associations remained significant after additional adjustment for eGFR and plasma CRP (P =0.003 in men, P <0.0001 in women).
Conclusions
Among adults with hypertension, AA ethnicity was independently associated with higher plasma levels of D-dimer.
Keywords: ethnicity, hypertension, D-dimer, risk factors
Introduction
Disparity in cardiovascular health is among the most serious public health problems in the United States today. The basis for racial/ethnic disparities in cardiovascular disease appears to be complex and multifactorial and is not completely understood, although data from the Centers for Disease Control and Prevention [1] indicate a significantly higher prevalence of conventional cardiovascular risk factors in minority populations. African Americans with hypertension, especially, have higher cardiovascular morbidity and mortality than other ethnic groups [2]. There is considerable interest in indentifying biomarkers that may explain ethnic variation in susceptibility to cardiovascular diseases.
Plasma D-dimer, a fragment generated from fibrin during lysis of mature clot in vivo, is a sensitive marker of thrombus formation and its subsequent fibrinolysis [3, 4]. Higher baseline levels of D-dimer may be indicative of a procoagulant state favoring progression of atherosclerosis, and are associated with increased risk of myocardial infarction (MI), coronary death, ischemic stroke, and peripheral arterial disease (PAD) [5–17]. For example, in a large prospective cohort of 5201 men and women age 65 years and older, the relative risk of MI or coronary death was 2.5 fold higher for D-dimer values above the median. This relationship was independent of hypertension, diabetes, smoking status, ethnicity, body mass index (BMI), levels of plasminogen-activator inhibitor 1, and C-reactive protein (CRP) [17].
Although ethnic differences in plasma D-dimer levels have been previously described [18, 19], adjustment has been variably reported for potential confounders, particularly renal function and systemic inflammation. The aim of the present study was to investigate ethnic differences in plasma D-dimer levels between hypertensive African American and non-Hispanic white adults, and, if present, whether such a difference could be explained by age, conventional risk factors, medication use, lifestyle variables, and renal function. In addition, because D-dimer levels are associated with an acute phase response, we also adjusted for plasma levels for an established marker of inflammation, CRP.
Methods
Study population
The study was part of the Proteomic Markers of Arteriosclerosis Study which is investigating the association of multiple markers in various etiologic pathway of vascular disease with several phenotypes of arteriosclerosis [20, 21]. Participants included hypertensive adults participating in a multicenter community-based study of hypertensive sibships that aims to identify genes influencing blood pressure (BP) levels and development of target organ damage due to hypertension. Sibships with at least two members diagnosed with hypertension before age 60 years were included. Recruitment and subject characteristics in the initial phase of the study (September 1995 to June 2001) have been described previously. For the present study, we excluded normotensive participants (n = 311 in Jackson and n = 305 in Rochester) and 15 participants with D-dimer levels >1500 ng/mL, 55 participants who were on warfarin and 1 participant who had estimated glomerular filtration rate (eGFR) >300 mg/dL. We also excluded 125 participants with missing D-dimer level, leaving a final study sample of 1749 subjects. The study was approved by the Institutional Review Boards of the Mayo Clinic, Rochester, MN and University of Mississippi Medical Center, Jackson, MS. Written informed consent was obtained from each participant.
Height was measured by stadiometer, weight by electronic balance, and BMI was calculated as weight in kilograms divided by the square of height in meters. Resting systolic BP and diastolic BP were measured by trained study coordinators using random zero sphygmomanometer and cuffs appropriate for arm size. Three readings were taken in the right arm after the subject had rested in the sitting position for at least 5 minutes; the last two readings were averaged for the analyses. Diagnosis of hypertension was established based on BP levels measured at the study visit (≥140/90 mmHg) or a prior diagnosis of hypertension and current treatment with antihypertensive medications. Diabetes was considered present if the subject was being treated with insulin or oral agents or had a fasting glucose level ≥126 mg/dL. “Ever” smoking was defined as having smoked more than 100 cigarettes. Each prescription drug recorded at the study visit was assigned a code number corresponding to the first six digits of the Medi-Span Generic Product Identifier. This number identifies pharmacologically equivalent drug products and was used to categorize agents with a similar therapeutic action.
Blood was drawn by venipuncture after an overnight fast. Serum total cholesterol and high-density lipoprotein (HDL) cholesterol were measured by standard enzymatic methods. eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) equation as previously described [22].
Lifestyle variables
We constructed a physical activity scale by considering the responses to questions on how many hours per day of heavy activity, moderate activity, slight activity, and sedentary activity the participant engaged in. Specifically, the physical activity score was derived as follows: 3*heavy + 2*moderate + light (hours). Alcohol intake was quantified as ounces of alcohol per month and was estimated from responses to questions on the frequency (servings per week or servings per month), and ounces per serving of beer, wine, hard liquor, wine cooler, and sake. The ounces per month of each of these categories were then multiplied by 5%, 12%, 40%, 8%, and 15%, respectively, and summed to give ounces of alcohol per month. This variable was highly skewed and was transformed to a log scale after adding 1, for analysis purposes.
Plasma levels of D-dimer and CRP
D-dimer was measured immunoturbidimetrically using STA-Liatest D-Dimer reagents (Diagnostica Stago, Asnieres, France) on a STA Compact (Diagnostica Stago, Asnieres, France). Intra-assay coefficients of variation (CVs) were 24% and 3% at 100 and 1071 ng/mL, and inter-assay CVs were 15 and 4% at 245 and 2060 ng/mL. Plasma CRP levels were measured by a highly sensitive immunoturbidimetric assay. Inter-assay CVs were: 14%, 3.2%, 3.4%, and 3.6% at 0.33, 1.05, 9.07, and 23.8 mg/dL, respectively.
Statistical methods
Statistical analyses were carried out using SAS v 9.1 (SAS Institute, Cary NC) software package. Descriptive statistics are given as mean ± standard deviation or frequency and percent, separately in men and women in each ethnic group. Alcohol intake, eGFR, CRP, and D-dimer levels were log transformed due to their skewed distribution. In each sex and ethnic group, we assessed the correlation of D-dimer with the following: 1) age and conventional risk factors (total and HDL cholesterol, systolic BP, smoking, diabetes, and BMI); 2) use of medications (statins and aspirin); 3) lifestyle variables (physical activity, alcohol intake, and education); 4) a measure of renal function, eGFR, and a marker of systemic inflammation, CRP.
In each sex, we constructed stepwise multivariable linear regression models that adjusted for conventional risk factors and other potential confounding variables to assess whether African American ethnicity was independently associated with plasma D-dimer levels. Adjustments were performed for a) age; b) + BMI, systolic BP, smoking history, diabetes, total and HDL cholesterol, medications (statins and aspirin) use, previous history of MI or stroke, and lifestyle variables (physical activity, alcohol intake, and education); c) + renal function (eGFR); and finally d) + plasma CRP. Because of the presence of sibships in the sample, population-averaged generalized estimating equations were used to assess the possible impact of familial correlations on the relationships between predictor and outcome variables [23]. A two-sided P-value of <0.05 was deemed statistically significant.
Results
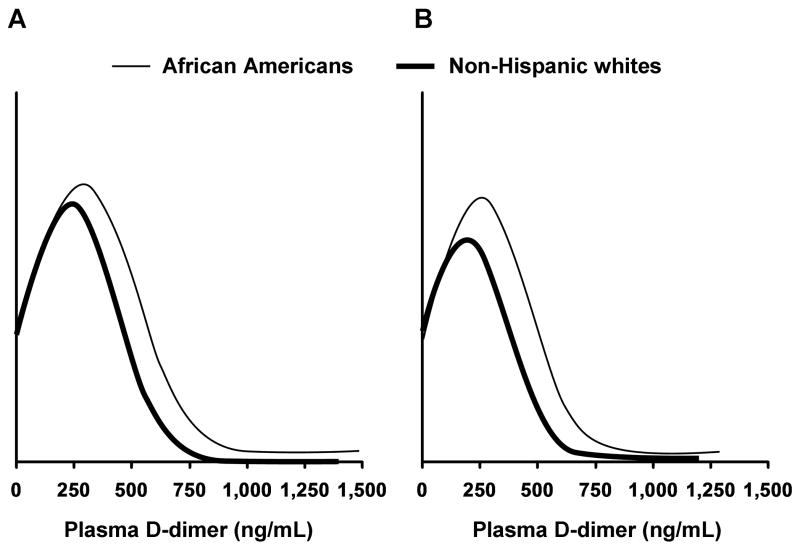
Subject characteristics are shown in Table 1. Significant ethnic differences were present in both men and women for conventional risk factors, medication use, and lifestyle variables. Plasma D-dimer levels were higher in African American men and women than in their non-Hispanic white counterparts (mean±SD; men 255±199 vs. 190±182 ng/mL, P <0.001; women, 289±233 vs. 225±195 ng/mL, P <0.001) (Fig 1) as the distribution of D-dimer levels was different with a significant shift in African Americans (Fig 2).
Table 1.
Participants characteristics and ethnic differences
| Women |
Men |
|||||
|---|---|---|---|---|---|---|
| AA (n =679) | NHW (n =455) | P | AA (n =254) | NHW (n =357) | P | |
| Age, years | 64.7±8.8 | 61.0±9.2 | <.0001 | 65.4±8.5 | 60.8±9.3 | <.0001 |
| BMI, kg/m2 | 32.7±7.0 | 31.7±6.9 | 0.002 | 29.5±4.8 | 31.1±5.1 | 0.003 |
| Total cholesterol, mg/dL | 205.9±42.1 | 203.2±33.0 | 0.17 | 191.8±42.0 | 187.4±30.5 | 0.22 |
| HDL cholesterol, mg/dL | 60.6±17.6 | 55.7±14.8 | <.0001 | 50.1±16.4 | 44.7±11.7 | <.0001 |
| Systolic BP, mm Hg | 143.6±21.3 | 136.6±17.8 | <.0001 | 140.4±20.4 | 133.4±15.6 | <.0001 |
| Diastolic BP, mm Hg | 79.2±11.3 | 73.6±9.5 | <.0001 | 82.5±11.7 | 76.0±9.6 | <.0001 |
| Ever smoker, n (%) | 208 (30.6) | 177 (38.9) | 0.001 | 166 (65.4) | 212 (59.4) | 0.16 |
| Diabetes mellitus, n (%) | 231 (34.0) | 80 (17.1) | <.0001 | 82 (32.3) | 67 (18.8) | 0.0005 |
| Previous history of MI or stroke, n (%) | 66 (9.7) | 41 (9.0) | 0.59 | 44 (17.3) | 62 (17.4) | 0.70 |
| Statin use, n (%) | 134 (19.7) | 123 (27.0) | 0.001 | 60 (23.6) | 151 (42.3) | <.0001 |
| Aspirin use, n (%) | 227 (33.4) | 200 (44.0) | 0.0004 | 115 (45.3) | 207 (58.0) | 0.0009 |
| Physical activity score | 9.5±3.2 | 12.5±4.8 | <.0001 | 10.0±4.2 | 13.8±5.3 | <.0001 |
| Alcohol intake, oz/month | 0.8±4.0 | 2.9±6.3 | <.0001 | 3.2±7.9 | 9.8±15.0 | <.0001 |
| Education, years | 12.1±3.4 | 13.3±2.0 | <.0001 | 11.5±4.0 | 13.4±2.6 | <.0001 |
| eGFR, mg/dL | 100.4±32.5 | 84.4±24.3 | <.0001 | 90.4±28.3 | 82.1±21.3 | <.0001 |
| C-reactive protein, mg/dL | 7.4±13.3 | 5.8±7.7 | <.0001 | 5.0±7.6 | 2.8±3.5 | <.0001 |
| D-dimer, ng/mL | 289.7±233.0 | 224.7±195.3 | <.0001 | 252.7±197.1 | 190.3±182.6 | 0.0004 |
AA, African American; NHW, non-Hispanic white; BMI, body mass index; HDL, high density lipoprotein; BP, blood pressure; MI, myocardial infarction; eGFR, estimated glomerular filtration rate.
Figure 1.

Mean±SD of plasma D-dimer levels in each gender/ethnic group.
*P value <0.001
Figure 2.
Distribution of plasma D-dimer levels in each gender/ethnic group. A. Women; B. Men.
Spearman rank correlations between D-dimer and select variables are shown in Table 2. Among women, older age, higher BMI, higher systolic BP, lower diastolic BP, lower physical activity score, lower level of education, lower eGFR, and higher CRP levels, correlated with higher D-dimer levels (Table 2). Higher levels of serum cholesterol in African American women and the presence of diabetes in non-Hispanic white women were also correlated with higher D-dimer levels. Among men, older age, higher systolic BP, lower diastolic BP, the presence of diabetes, Previous history of MI or stroke, use of aspirin, lower level of education, lower eGFR, and higher CRP levels, correlated with higher D-dimer levels (Table 2). Lower physical activity score in African American men and lower levels of serum cholesterol and smoking history in non-Hispanic white men were also correlated with higher D-dimer levels.
Table 2.
Spearman correlations between plasma D-dimer levels and select variables
| Women |
Men |
|||
|---|---|---|---|---|
| AA (n =679) | NHW (n =455) | AA (n =254) | NHW (n =357) | |
| Risk Factors |
r P value |
r P value |
r P value |
r P value |
| Age, years | 0.32 <.0001 |
0.31 <.0001 |
0.45 <.0001 |
0.34 <.0001 |
| BMI, kg/m2 | 0.08 0.040 |
0.23 <.0001 |
−0.06 0.30 |
0.04 0.43 |
| Total cholesterol, mg/dL | 0.13 0.0009 |
−0.06 0.24 |
0.03 0.68 |
−0.15 0.005 |
| HDL cholesterol, mg/dL | 0.04 0.31 |
−0.09 0.044 |
−0.03 0.65 |
−0.05 0.32 |
| Systolic BP, mm Hg | 0.08 0.028 |
0.13 0.007 |
0.15 0.016 |
0.18 0.0006 |
| Diastolic BP, mm Hg | −0.08 0.043 |
−0.11 0.018 |
−0.12 0.047 |
−0.11 0.036 |
| Ever smoker | −0.03 0.41 |
−0.04 0.35 |
0.03 0.61 |
0.12 0.028 |
| Diabetes mellitus | −0.03 0.40 |
0.16 0.0009 |
0.13 0.037 |
0.17 0.001 |
| Previous history of MI or stroke | 0.05 0.20 |
0.05 0.25 |
0.13 0.046 |
0.16 0.002 |
| Statin use | −0.01 0.89 |
0.03 0.46 |
−0.08 0.22 |
0.04 0.47 |
| Aspirin use | 0.04 0.36 |
0.09 0.047 |
0.15 0.018 |
0.15 0.004 |
| Physical activity score | −0.22 <.0001 |
−0.10 0.037 |
−0.28 <.0001 |
−0.04 0.42 |
| Alcohol intake, oz/month | −0.06 0.079 |
−0.06 0.18 |
−0.03 0.68 |
−0.08 0.10 |
| Education, years | −0.16 <.0001 |
−0.11 0.019 |
−0.25 <.0001 |
−0.19 0.0002 |
| eGFR, mg/dL | −0.23 <.0001 |
−0.27 <.0001 |
−0.16 0.010 |
−0.15 0.008 |
| C-reactive protein, mg/dL | 0.11 0.003 |
0.19 <.0001 |
0.28 <.0001 |
0.15 0.005 |
AA, African American; NHW, non-Hispanic white; BMI, body mass index; HDL, high density lipoprotein; BP, blood pressure; MI, myocardial infarction; eGFR, estimated glomerular filtration rate.
After adjustment for age, conventional risk factors, medication use, and lifestyle variables, African American ethnicity was associated with higher D-dimer levels in both men (P =0.002) and women (P =0.006). Additional adjustment for eGFR, accentuated this association in both men (P =0.0002) and women (P <0.0001) (Table 3). Further adjustment for CRP levels slightly attenuated the association in men (P =0.003), but not in women (P <0.0001). In addition, in men, older age, higher systolic BP, the presence of diabetes, lower eGFR, and higher CRP were associated with higher plasma D-dimer levels. In women, older age, higher BMI, higher systolic BP, lower eGFR, and higher CRP were associated with a higher plasma D-dimer (Table 4). Neither lifestyle variables nor smoking were independently associated with plasma levels of D-dimer in men or women, in the multiple regression models.
Table 3.
Association between African American ethnicity with plasma D-dimer levels
| Women, n = 1134 | Men, n = 611 | |||||
|---|---|---|---|---|---|---|
| Estimate | SE | P | Estimate | SE | P | |
| Model 1 | 0.18 | 0.04 | <.0001 | 0.20 | 0.05 | 0.0002 |
| Model 2 | 0.12 | 0.04 | 0.006 | 0.17 | 0.05 | 0.002 |
| Model 3 | 0.22 | 0.05 | <.0001 | 0.20 | 0.05 | 0.0002 |
| Model 4 | 0.21 | 0.05 | <.0001 | 0.16 | 0.05 | 0.003 |
eGFR, CRP, and D-dimer were natural log-transformed.
The co-efficients represent the change in log D-dimer for each unit change in the continuous predictor variable or for the presence of the specified condition of the categorical predictor variable.
Model 1: Adjusted for age
Model 2: Adjusted for age, BMI, total and HDL cholesterol, systolic BP, diabetes, history of smoking, medication (statin and aspirin) use, previous history of MI or stroke, and lifestyle variables (physical activity, alcohol intake, and education)
Model 3: Adjusted for above variables and eGFR
Model 4: Adjusted for above variables and CRP
BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; MI, myocardial infarction; eGFR, estimated glomerular filtration rate; CRP, C-reactive protein.
Table 4.
Variables associated with plasma D-dimer levels: multi-variable regression models.
| Women, n =1134 | Men, n =611 | |||||
|---|---|---|---|---|---|---|
| Estimate | SE | P | Estimate | SE | P | |
| Age, years | 0.02 | 0.002 | <.0001 | 0.02 | 0.003 | <.0001 |
| BMI, kg/m2 | 0.01 | 0.003 | <.0001 | - | - | - |
| Systolic BP, mm Hg | 0.002 | 0.001 | 0.059 | 0.003 | 0.001 | 0.040 |
| Diabetes | −0.09 | 0.04 | 0.041 | 0.16 | 0.06 | 0.008 |
| Physical activity score | −0.01 | 0.005 | 0.087 | - | - | - |
| Log eGFR | −0.45 | 0.07 | <.0001 | −0.22 | 0.10 | 0.031 |
| Log C-reactive protein | 0.08 | 0.02 | 0.0001 | 0.12 | 0.03 | <.0001 |
| African American ethnicity | 0.21 | 0.05 | <.0001 | 0.16 | 0.05 | 0.003 |
eGFR, C-reactive protein, and D-dimer were natural log-transformed.
The co-efficients represent the change in log D-dimer for each unit change in the continuous predictor variable or for the presence of the specified condition of the categorical predictor variable.
BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate.
Discussion
The main finding of this study is that among adults with hypertension, plasma D-dimer levels are significantly higher in African American men and women than in their non-Hispanic white counterparts. The differences could not be explained by age, conventional risk factors, medication use, lifestyle factors, renal function, and systemic inflammation, suggesting that unmeasured environmental and genetic factors may contribute. These results suggest that fibrin turnover is higher in hypertensive African Americans than in their non-Hispanic white counterparts and may be a possible explanation for increased cardiovascular morbidity and mortality among African Americans with hypertension.
Cushman et al [18] reported higher D-dimer levels in non-whites (most non-whites were African Americans) than in whites in a nested case control study of 923 participants (78% were whites) from the Longitudinal Investigation of Thrombosis Etiology (LITE) study. However the authors did not report whether the association was independent of conventional cardiovascular risk factors. In a random sample of 1727 community-dwelling elderly persons (age >72 years), Pieper et al [19] reported that average plasma D-dimer level was nearly 40% higher in African Americans than in white participants. This difference was not substantially attenuated in multivariable analyses adjusting for demographic and socioeconomic variables. Our study shows higher D-dimer levels in hypertensive African Americans than in their non-Hispanic white counterparts. In addition, we report that this association was independent of conventional risk factors, medication use, lifestyle factors, renal function, and systemic inflammation.
We found D-dimer levels increased with age likely due to higher fibrinogen concentrations in the elderly, reduced renal function, increased fibrin generation, occult disease, higher prevalence of risk factors, and increased inflammation [19, 24]. We also observed that female sex was independently associated with higher D-dimer levels (analyses not shown). This is consistent with the results of a prospective study of 1000 healthy adults by Hughes et al [25] that found age, female sex (independent of hormone therapy), the use of hormone therapy, thrombophilic state, presence of co-morbid conditions, and BMI, as the major predictors of plasma D-dimer levels. However, in the present study, we did not find estrogen use to be associated with D-dimer levels (analyses not shown). Higher D-dimer levels were independently associated with lower eGFR in both men and women, likely due to decreased clearance by the kidney [26, 27]. Renal insufficiency is associated with increased levels of inflammatory and procoagulant biomarkers, including CRP, interleukin-6 (IL-6), fibrinogen, and D-dimer [28]. In the present study, in spite of higher eGFR in African Americans than in non-Hispanic whites, D-dimer levels were higher in former.
The association between D-dimer with plasma CRP was significant and independent of potential confounders. Local fibrin formation and lysis are part of the inflammatory response and it has been shown that D-dimer levels increase during an acute phase response [29, 30]. Systemic inflammation by causing low-grade activation of coagulation cascade may therefore increase levels of D-dimer. Multiple inflammatory cytokines such as tumor necrosis factor (TNF)- α and IL-6 may directly activate the coagulation system [31, 32]. However, the association may be bi-directional as D-dimer has been shown to stimulate neutrophil and monocyte activation, secretion of cytokines, including IL-6 and interleukin-1, and thus indirectly stimulate the production of acute phase proteins [33–36].
A strength of the present study is the use of a uniform protocol in the two ethnic groups, including, questionnaires, anthropometric measurements, and D-dimer measurements that were made in a single core laboratory. The gold standard for the measurement of the plasma D-dimer tests is an enzyme-linked immunosorbent assay. In the present study, we used the STA Liatest D-Dimer reagents on a STA Compact. Waser et al [37] showed good agreement (kappa >0.81) and correlation (r >0.95, P <0.0001) between the STA Liatest and the classical enzyme-linked immunosorbent assays.
Clinical implications
Mortality rates in United States differ substantially according to ethnicity [2]. Hypertension mediated mortality from target organ damage and stroke is higher in African Americans and this is not fully explained by higher burden of conventional cardiovascular risk factors, including diabetes and hypertension in this ethnic group [38]. D-dimer levels have been consistently associated with presence and severity of cardiovascular diseases [39–42]. Whether plasma D-dimer contributes to the ethnic difference in cardiovascular disease needs further investigation. Measurement of D-dimer levels may be helpful in assessing the risk of cardiovascular disease, especially in African Americans. Whether lowering the elevated levels of D-dimer in susceptible population groups by lifestyle/pharmacologic means will lower cardiovascular morbidity and mortality will need confirmation by randomized controlled trials.
Conclusion
In conclusion, our results indicate that plasma levels of D-dimer are higher in hypertensive African Americans than in their non-Hispanic white counterparts. The ethnic differences are independent of age, conventional risk factors, medication use, lifestyle factors, renal function, and systemic inflammation. Unmeasured environmental and genetic factors may partially contribute to the ethnic difference in plasma D-dimer levels. Further studies are needed to elucidate the pathophysiological basis for this observation. The higher D-dimer in hypertensive African Americans than in their non-Hispanic white counterparts may be a potential mechanism of increased cardiovascular risk in the former. Further investigation is needed to confirm this hypothesis.
Acknowledgments
This work was supported by grant HL-81331 from the National Institutes of Health.
Footnotes
Disclosure of Conflict of Interests: None
References
- 1.Mensah GA. Eliminating disparities in cardiovascular health: six strategic imperatives and a framework for action. Circulation. 2005;111:1332–6. doi: 10.1161/01.CIR.0000158134.24860.91. [DOI] [PubMed] [Google Scholar]
- 2.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–41. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 3.Kearon C, Ginsberg JS, Douketis J, Crowther M, Brill-Edwards P, Weitz JI, Hirsh J. Management of suspected deep venous thrombosis in outpatients by using clinical assessment and D-dimer testing. Ann Intern Med. 2001;135:108–11. doi: 10.7326/0003-4819-135-2-200107170-00011. [DOI] [PubMed] [Google Scholar]
- 4.Kelly J, Hunt BJ. Role of D-dimers in diagnosis of venous thromboembolism. Lancet. 2002;359:456–8. doi: 10.1016/s0140-6736(02)07669-9. [DOI] [PubMed] [Google Scholar]
- 5.Cassar K, Bachoo P, Ford I, Greaves M, Brittenden J. Markers of coagulation activation, endothelial stimulation and inflammation in patients with peripheral arterial disease. Eur J Vasc Endovasc Surg. 2005;29:171–6. doi: 10.1016/j.ejvs.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 6.De Buyzere M, Philippe J, Duprez D, Baele G, Clement DL. Coagulation system activation and increase of D-dimer levels in peripheral arterial occlusive disease. Am J Hematol. 1993;43:91–4. doi: 10.1002/ajh.2830430204. [DOI] [PubMed] [Google Scholar]
- 7.Fowkes FG, Lowe GD, Housley E, Rattray A, Rumley A, Elton RA, MacGregor IR, Dawes J. Cross-linked fibrin degradation products, progression of peripheral arterial disease, and risk of coronary heart disease. Lancet. 1993;342:84–6. doi: 10.1016/0140-6736(93)91288-w. [DOI] [PubMed] [Google Scholar]
- 8.Lee AJ, Fowkes FG, Lowe GD, Rumley A. Fibrin D-dimer, haemostatic factors and peripheral arterial disease. Thromb Haemost. 1995;74:828–32. [PubMed] [Google Scholar]
- 9.Lowe GD. Fibrin D-dimer and cardiovascular risk. Semin Vasc Med. 2005;5:387–98. doi: 10.1055/s-2005-922485. [DOI] [PubMed] [Google Scholar]
- 10.McDermott MM, Greenland P, Green D, Guralnik JM, Criqui MH, Liu K, Chan C, Pearce WH, Taylor L, Ridker PM, Schneider JR, Martin G, Rifai N, Quann M, Fornage M. D-dimer, inflammatory markers, and lower extremity functioning in patients with and without peripheral arterial disease. Circulation. 2003;107:3191–8. doi: 10.1161/01.CIR.0000074227.53616.CC. [DOI] [PubMed] [Google Scholar]
- 11.McDermott MM, Greenland P, Guralnik JM, Ferrucci L, Green D, Liu K, Criqui MH, Schneider JR, Chan C, Ridker P, Pearce WH, Martin G, Clark E, Taylor L. Inflammatory markers, D-dimer, pro-thrombotic factors, and physical activity levels in patients with peripheral arterial disease. Vasc Med. 2004;9:107–15. doi: 10.1191/1358863x04vm525oa. [DOI] [PubMed] [Google Scholar]
- 12.Moresco RN, Silla L. D-dimer and inflammatory markers in the peripheral arterial disease. Thromb Res. 2007;119:797–8. doi: 10.1016/j.thromres.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Musicant SE, Taylor LM, Jr, Peters D, Schuff RA, Urankar R, Landry GJ, Moneta GL. Prospective evaluation of the relationship between C-reactive protein, D-dimer and progression of peripheral arterial disease. J Vasc Surg. 2006;43:772–80. doi: 10.1016/j.jvs.2005.12.051. discussion 80. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Hennekens CH, Cerskus A, Stampfer MJ. Plasma concentration of cross-linked fibrin degradation product (D-dimer) and the risk of future myocardial infarction among apparently healthy men. Circulation. 1994;90:2236–40. doi: 10.1161/01.cir.90.5.2236. [DOI] [PubMed] [Google Scholar]
- 15.Smith FB, Lee AJ, Fowkes FG, Price JF, Rumley A, Lowe GD. Hemostatic factors as predictors of ischemic heart disease and stroke in the Edinburgh Artery Study. Arterioscler Thromb Vasc Biol. 1997;17:3321–5. doi: 10.1161/01.atv.17.11.3321. [DOI] [PubMed] [Google Scholar]
- 16.Smith FB, Rumley A, Lee AJ, Leng GC, Fowkes FG, Lowe GD. Haemostatic factors and prediction of ischaemic heart disease and stroke in claudicants. Br J Haematol. 1998;100:758–63. doi: 10.1046/j.1365-2141.1998.00626.x. [DOI] [PubMed] [Google Scholar]
- 17.Cushman M, Lemaitre RN, Kuller LH, Psaty BM, Macy EM, Sharrett AR, Tracy RP. Fibrinolytic activation markers predict myocardial infarction in the elderly. The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1999;19:493–8. doi: 10.1161/01.atv.19.3.493. [DOI] [PubMed] [Google Scholar]
- 18.Cushman M, Folsom AR, Wang L, Aleksic N, Rosamond WD, Tracy RP, Heckbert SR. Fibrin fragment D-dimer and the risk of future venous thrombosis. Blood. 2003;101:1243–8. doi: 10.1182/blood-2002-05-1416. [DOI] [PubMed] [Google Scholar]
- 19.Pieper CF, Rao KM, Currie MS, Harris TB, Chen HJ. Age, functional status, and racial differences in plasma D-dimer levels in community-dwelling elderly persons. J Gerontol A Biol Sci Med Sci. 2000;55:M649–57. doi: 10.1093/gerona/55.11.m649. [DOI] [PubMed] [Google Scholar]
- 20.Gerszten RE, Accurso F, Bernard GR, Caprioli RM, Klee EW, Klee GG, Kullo I, Laguna TA, Roth FP, Sabatine M, Srinivas P, Wang TJ, Ware LB. Challenges in translating plasma proteomics from bench to bedside: update from the NHLBI Clinical Proteomics Programs. Am J Physiol Lung Cell Mol Physiol. 2008;295:L16–22. doi: 10.1152/ajplung.00044.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Granger CB, Van Eyk JE, Mockrin SC, Anderson NL. National Heart, Lung, And Blood Institute Clinical Proteomics Working Group report. Circulation. 2004;109:1697–703. doi: 10.1161/01.CIR.0000121563.47232.2A. [DOI] [PubMed] [Google Scholar]
- 22.Ellington AA, Malik AR, Klee GG, Turner ST, Rule AD, Mosley TH, Jr, Kullo IJ. Association of plasma resistin with glomerular filtration rate and albuminuria in hypertensive adults. Hypertension. 2007;50:708–14. doi: 10.1161/HYPERTENSIONAHA.107.095257. [DOI] [PubMed] [Google Scholar]
- 23.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 24.Hager K, Platt D. Fibrin degeneration product concentrations (D-dimers) in the course of ageing. Gerontology. 1995;41:159–65. doi: 10.1159/000213677. [DOI] [PubMed] [Google Scholar]
- 25.Hughes R, Thomson K, Hopkins R, Weatherall M, Wiltshire C, Wilsher M, Beasley R. Determinants of plasma D-dimer levels in a traveling population. J Thromb Haemost. 2005;3:2445–8. doi: 10.1111/j.1538-7836.2005.01568.x. [DOI] [PubMed] [Google Scholar]
- 26.Gordge MP, Faint RW, Rylance PB, Ireland H, Lane DA, Neild GH. Plasma D dimer: a useful marker of fibrin breakdown in renal failure. Thromb Haemost. 1989;61:522–5. [PubMed] [Google Scholar]
- 27.Shibata T, Magari Y, Perparim K, Sumie A, Ishii T, Tomo T, Sato J, Yasumori R, Nasu M. Significance of urinary fibrin/fibrinogen degradation products in renal diseases measured by a highly sensitive ELISA. Nephron. 1995;69:54–8. doi: 10.1159/000188360. [DOI] [PubMed] [Google Scholar]
- 28.Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, Furberg CD, Psaty BM. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107:87–92. doi: 10.1161/01.cir.0000042700.48769.59. [DOI] [PubMed] [Google Scholar]
- 29.Kollef MH, Eisenberg PR, Shannon W. A rapid assay for the detection of circulating D-dimer is associated with clinical outcomes among critically ill patients. Crit Care Med. 1998;26:1054–60. doi: 10.1097/00003246-199806000-00027. [DOI] [PubMed] [Google Scholar]
- 30.Shorr AF, Thomas SJ, Alkins SA, Fitzpatrick TM, Ling GS. D-dimer correlates with proinflammatory cytokine levels and outcomes in critically ill patients. Chest. 2002;121:1262–8. doi: 10.1378/chest.121.4.1262. [DOI] [PubMed] [Google Scholar]
- 31.Esmon CT, Taylor FB, Jr, Snow TR. Inflammation and coagulation: linked processes potentially regulated through a common pathway mediated by protein C. Thromb Haemost. 1991;66:160–5. [PubMed] [Google Scholar]
- 32.Stouthard JM, Levi M, Hack CE, Veenhof CH, Romijn HA, Sauerwein HP, van der Poll T. Interleukin-6 stimulates coagulation, not fibrinolysis, in humans. Thromb Haemost. 1996;76:738–42. [PubMed] [Google Scholar]
- 33.Ritchie DG, Levy BA, Adams MA, Fuller GM. Regulation of fibrinogen synthesis by plasmin-derived fragments of fibrinogen and fibrin: an indirect feedback pathway. Proc Natl Acad Sci U S A. 1982;79:1530–4. doi: 10.1073/pnas.79.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gauldie J, Northemann W, Fey GH. IL-6 functions as an exocrine hormone in inflammation. Hepatocytes undergoing acute phase responses require exogenous IL-6. J Immunol. 1990;144:3804–8. [PubMed] [Google Scholar]
- 35.Robson SC, Shephard EG, Kirsch RE. Fibrin degradation product D-dimer induces the synthesis and release of biologically active IL-1 beta, IL-6 and plasminogen activator inhibitors from monocytes in vitro. Br J Haematol. 1994;86:322–6. doi: 10.1111/j.1365-2141.1994.tb04733.x. [DOI] [PubMed] [Google Scholar]
- 36.Mandl J, Csala M, Lerant I, Banhegyi G, Biro J, Machovich R, Falus A. Enhancement of interleukin-6 production by fibrinogen degradation product D in human peripheral monocytes and perfused murine liver. Scand J Immunol. 1995;42:175–8. doi: 10.1111/j.1365-3083.1995.tb03642.x. [DOI] [PubMed] [Google Scholar]
- 37.Waser G, Kathriner S, Wuillemin WA. Performance of the automated and rapid STA Liatest D-dimer on the STA-R analyzer. Thromb Res. 2005;116:165–70. doi: 10.1016/j.thromres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Flack JM, Ferdinand KC, Nasser SA. Epidemiology of hypertension and cardiovascular disease in African Americans. J Clin Hypertens (Greenwich) 2003;5:5–11. doi: 10.1111/j.1524-6175.2003.02152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reich LM, Heiss G, Boland LL, Hirsch AT, Wu K, Folsom AR. Ankle-brachial index and hemostatic markers in the Atherosclerosis Risk in Communities (ARIC) study cohort. Vasc Med. 2007;12:267–73. doi: 10.1177/1358863X07082767. [DOI] [PubMed] [Google Scholar]
- 40.McDermott MM, Green D, Greenland P, Liu K, Criqui MH, Chan C, Guralnik JM, Pearce WH, Ridker PM, Taylor L, Rifai N, Schneider JR. Relation of levels of hemostatic factors and inflammatory markers to the ankle brachial index. Am J Cardiol. 2003;92:194–9. doi: 10.1016/s0002-9149(03)00537-x. [DOI] [PubMed] [Google Scholar]
- 41.Morange PE, Bickel C, Nicaud V, Schnabel R, Rupprecht HJ, Peetz D, Lackner KJ, Cambien F, Blankenberg S, Tiret L. Haemostatic factors and the risk of cardiovascular death in patients with coronary artery disease: the AtheroGene study. Arterioscler Thromb Vasc Biol. 2006;26:2793–9. doi: 10.1161/01.ATV.0000249406.92992.0d. [DOI] [PubMed] [Google Scholar]
- 42.Barber M, Langhorne P, Rumley A, Lowe GD, Stott DJ. D-dimer predicts early clinical progression in ischemic stroke: confirmation using routine clinical assays. Stroke. 2006;37:1113–5. doi: 10.1161/01.STR.0000209240.63821.1a. [DOI] [PubMed] [Google Scholar]