Abstract
Recent results showing that the binding characteristics of 33 steroids for human membrane progesterone receptor alpha (hu-mPRα) differ from those for the nuclear progesterone receptor (nPR) suggest that hu-mPRα-specific agonists can be identified for investigating its physiological functions. The binding affinities of an additional 21 steroids for hu-mPRα were determined to explore the structure-activity relationships in more detail and to identify potent, specific mPRα agonists. Four synthetic progesterone derivatives with methyl or methylene groups on positions 18 or 19, 18a-methylprogesterone (18-CH3P4, Org OE 64-0), 13-ethenyl-18-norprogesterone (18-CH2P4, Org 33663-0), 19a-methylprogesterone (19-CH3P4, Org OD 13-0) and 10-ethenyl-19-norprogesterone (19-CH2P4, Org OD 02-0), showed similar or higher affinities than progesterone for hu-mPRα and displayed mPRα agonist activities in G-protein and MAP kinase activation assays. All four steroids also bound to the nPR in cytosolic fractions of MCF-7 cells. However, two compounds, 19-CH2P4 and 19-CH3P4, showed no nPR agonist activity in a nPR reporter assay and therefore are selective mPRα agonists suitable for physiological investigations. The structure-binding relationships of the combined series of 54 steroids for hu-mPRα deviated strikingly from those of a published set of 60 3-keto or 3-desoxy steroids for nPR. Close correlations were observed between the receptor binding affinities of the steroids and their physicochemical properties calculated by comparative molecular field analysis (CoMFA) for both hu-mPRα and nPR. A comparison of the CoMFA field graphs for the two receptors revealed several differences in the structural features required for binding to hu-mPRα and nPR which could be exploited to develop additional mPR-specific ligands.
Keywords: membrane progesterone receptor alpha, mPRα, nuclear progesterone receptor, nPR, CoMFA, mPR-specific ligands, specific progesterone receptor agonists
1. Introduction
The number of steroid binding proteins proposed to mediate steroid actions has increased dramatically over the past decade. Although the existence of multiple receptors for each steroid hormone in target cells may help explain the pleiotropic actions of steroids, it complicates investigations of the distinct physiological roles of the individual receptors. Structure-activity studies are a powerful tool for determining the structural requirements for binding of steroids to receptor ligand binding pockets [1]. Comparative molecular field analysis (CoMFA) has also been shown to be valuable for predicting the physicochemical properties of steroids required for binding to receptors and for the molecular design of selective steroid receptor modulators [2–4]. These approaches combined with receptor activation assays were used in the present study to compare the binding and agonist activities of a large series of progestin compounds to two human progesterone receptors, the novel membrane progestin receptor alpha, hu-mPRα [5, 6], and the nuclear progesterone receptor, PR-B, in order to identify selective mPRα agonists for physiological studies.
The membrane progestin receptor (mPR) was discovered in spotted seatrout ovaries in 2002 [5] and three related mPRs were subsequently identified in humans and other vertebrates which were named mPRα, mPRβ and mPRγ [6]. Recently, two additional related proteins, named mPRδ and mPRε, have also been shown to bind progesterone [7]. These receptors have no apparent homologies with known G protein –coupled receptors (GPCRs) or nuclear progesterone receptors, but belong with the adiponectin receptors to the progesterone and adipoQ receptor (PAQR) family [8], which has 11 members in humans [9] and has ancient roots in the Eubacteria. All the PAQR family members display a predicted seven-transmembrane (7TM) region, but the membrane topologies of the mPRs and adipoQRs probably differ [10]. Moreover, the mPRs bind small steroid molecules resulting in G- protein activation [10], whereas the adipoQRs bind the large adiponectin molecule and have not been shown to activate G-proteins.
The steroid binding characteristics of recombinant hu-PRα over-expressed in MDA-MB-231 cell membranes are strikingly different from those of nPR [10]. For example, the potent nPR agonists Org 2058 (16α-ethyl-21-hydroxy-19-norpregn-4-ene-3,20-dione) and R5020 (promegestone) have poor binding affinities for hu-mPRα and the nPR antagonist RU486 (mifepristone) does not bind to the receptor. Also, steroidal contraceptives widely used currently such as norgestrel, or previously such as norethisterone, show no detectable binding at 10−5 M for hu-mPRα. Testosterone has higher affinity for hu-mPRα (RBA 22.4 %) than 19-nortestosterone (nandrolone) (RBA 7.4 %), whereas an opposite relationship is observed with the nPR [10–12]. These marked differences in the binding characteristics of steroids for hu-mPRα and the nPR suggest that selective mPRα ligands can be developed.
In the present study the binding affinities of 21 additional steroids for hu-mPRα were investigated to identify potential mPRα-selective ligands. Progestins with high binding affinities for hu-mPRα were evaluated for their agonist activities through the receptor in G-protein and MAPkinase activation assays. Binding of these progestins to nPR was also investigated and they were also screened for their agonist and antagonist activities through the nPR in a PR transactivation assay. Two progestins, 10-ethenyl-19-norprogesterone (19-CH2P4, Org OD 02-0) and 19a-methylprogesterone (19-CH3P4, Org OD 13-0), were identified as selective mPR agonists in the assays. Finally, the physicochemical requirements for progestin binding to hu-mPRα and nPR were compared by comparative molecular field analysis (CoMFA).
2. Experimental procedures
Chemicals
Progesterone and estradiol-17β were purchased from Steraloids (Newport, RI). The other natural and synthetic steroids were obtained from N.V. Organon (Oss, The Netherlands). [2,4,6,7-3H]-progesterone, activity 102 Ci/mmol, was purchased from Amersham (Piscataway, NJ). The compounds are listed in Table 1 and the structures of the four most potent mPRα ligands are depicted in Figure 1B. All other chemicals, buffers and media were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
Table 1.
Rank order of binding affinities of natural and synthetic steroids to plasma membranes prepared from MDA-231 cells transfected with human mPRα
| Compounds | IC50 (mean) | RBA |
|---|---|---|
| 18a-Methylprogesterone (Org OE 64-0) | 4.35 | 2009 |
| 10-Ethenyl-19-norprogesterone (Org OD 02-0) | 33.9 | 257.7 |
| 13-Ethenyl-18-norprogesterone (Org 33663-0) | 52.0 | 168.2 |
| Progesterone | 87.4 | 100.0 |
| Pregna-4,6-diene-3,20-dione | 88.4 | 98.9 |
| Pregna-1,4-diene-3,20-dione | 93.5 | 93.5 |
| 19a-Methylprogesterone (Org OD 13-0) | 94.3 | 92.7 |
| 13-Acetyl-18-norprogesterone (Org OE 62-0) | 175.0 | 49.9 |
| 3α-Hydroxy-5α-pregnan-20-one (allopregnanolone) | 1150 | 7.6 |
| 17-Caproylprogesterone | 1970 | 4.4 |
| 17β-Hydroxy-7α-methylandrost-5-en-3-one (RMI 12936) | 2884 | 3.0 |
| 17-Epitestosterone | 3136 | 2.8 |
| 3,20-dioxopregn-4-ene-18-carbonitrile | 4753 | 1.8 |
| Cortexolone (11-deoxycortisol) | 4853 | 1.8 |
| 19-Hydroxyprogesterone | 11880 | 0.7 |
| 13-Acetyl-18-norpregn-4-en-3-one (Org OD 62-0) | <1% | |
| 3,20-Dioxopregn-4-ene-2α-carbonitrile | <1% | |
| 7α-Acetylthioprogesterone (Org 33718-0) | <1% | |
| Cortisol (hydrocortisone) | NB | |
| 3β-Hydroxy-5α-pregnan-20-one | NB | |
| Androst-4-ene-3,17-dione (androstenedione) | NB | |
| Estradiol-17β | NB |
Each value is the mean of three separate competitive binding assays. IC50 is the competitor concentration (nM) that causes 50% displacement of [3H] progesterone. RBA (Relative Binding Affinity), RBA (%) compared with that of progesterone; NB, no binding at 10−5 M.
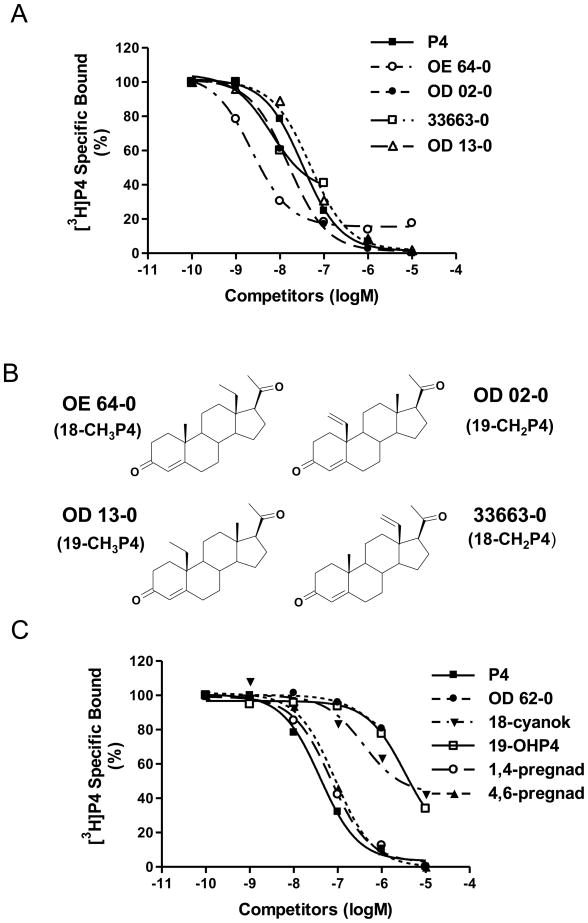
Figure 1.
A and C, Competition curves of ligand binding to recombinant human mPRα on plasma membranes of transfected MDA-231 cells expressed as a percentage of maximum specific [3H]P4 binding. P4, progesterone; 18-CNP4, 3,20-dioxopregn-4-ene-18-carbonitrile; 19-OHP4, 19-hydroxyprogesterone; 1,4-pregnad, pregna-1,4-diene-3,20-dione; 4,6-pregnad, 4,6-pregna-4,6-diene-3,20-dione. For key to other steroid abbreviations see Table 1. Assays were repeated 3 times and similar results were obtained in each assay. B, Molecular structures of four synthetic progestins with high affinities for mPRα.
Culture of MDA-MB-231 cells stably expressing hu-mPRα
MDA-MB-231 cells stably transfected with the hu-mPRα and selectively maintained with 700 μg/ml geneticin [10] were cultured in DMEM/Ham’s F-12 medium supplemented with 5 % charcoal-stripped (cs) fetal bovine serum (FBS) and 100 μg/ml of gentamicin as described previously [10]. Fresh medium without phenol red and csFBS was added to the cell cultures one day (~16 hrs) before experimentation.
hu-mPRα steroid binding assay
Competitive binding of steroids was assayed following procedures described previously for measuring binding of [2,4,6,7-3H]-progesterone ([3H]-P4, 102.1 Ci/mmol) to the recombinant hu-mPRα in plasma membrane fractions [10]. One set of tubes contained 2 nM [3H]-P4 alone (total binding), another set also contained 1 μM progesterone to measure non-specific binding (NSB), and a third set of tubes contained [3H]-P4 and 4–6 different concentrations of the steroid (range 1 nM – 10 μM) competitors. After a 30-min incubation at 4°C with the membrane fractions, the reaction was stopped by filtration with Whatman GF/B filters (presoaked in assay buffer) in a 36-well cell harvester (Brandel, Gaithersburg, MD) at 4°C. The displacement of the radiolabeled progesterone binding by the steroid competitors was expressed as a percentage of the maximum specific binding of progesterone to the receptor.
G-protein activation assay
G-protein activation after progestin treatment was determined by measuring the increase in specific [35S]GTPγ-S binding to plasma membranes of MDA-MB-231 cells stably transfected with hu-mPRα as described previously [10]. Membranes (~ 100 μg/ml protein) were incubated in the presence of 100 nM progestins with 10 μM GDP and 0.25 nM [35S]GTPγ-S (~ 12,000 cpm, 1 Ci/mol, Amersham) in the absence (total binding) or presence of 100 μM GTPγ-S (non-specific binding) for 20 min at 25°C. Bound [35S]GTPγ-S was separated from free by filtration of the incubation mixture through Whatman GF/B glass fiber filters with 36-well cell harvester, followed by several washes.
MAPK activation assay
MAPK activation after progestin treatment of MDA-MB-231 cells stably transfected with hu-mPRα was measured by Western blotting using rabbit monoclonal antibodies directed against total p42/44 (ERK) and phosphor-p42/44 (phosphor-ERK) (Cell Signaling, Danvers MA) as described previously [5]. Control and human mPRα-transfected MDA-MB-231cells were incubated in 6-well plates and were serum starved for 5–6 days to reduce basal MAPK activity. Cells were treated with EGF (positive control) and progestins for 10 min before they were lysed with 1× RIPA buffer (Pierce) supplemented with protease inhibitors. The proteins from each treatment group were mixed with 5× non-reducing loading buffer (Pierce) and loaded (10 μg/lane) onto two SDS gels and separated by PAGE prior to transfer to nitrocellulose membranes following standard Western blot procedures [10]. One membrane was hybridized with p42/44 antibody and the other membrane with phosphor-p42/44 antibody. A HRP-conjugated anti rabbit antibody was used as secondary antibody and was incubated with the membranes in PBS for 1 hour at room temperature. The blots then were treated with SuperSignal® West Pico Chemiluminescent substrate (Pierce) and exposed to X-ray film (Amersham) to visualize the specific bands.
nPR steroid binding assay
Competitive binding of steroids was assayed in cytosolic fractions of MCF-7 (PR+) cells using [2,4,6,7-3H]-progesterone ([3H]-P4, 102.1 Ci/mmol) following the nuclear progestin assay procedures described previously [13] with minor modifications. Briefly, MCF-7 cells that had been pretreated with estradiol-17β (40nM) for 72 hrs to upregulate nPR expression were washed with ice-cold PBS and harvested in TEDM buffer (10 mM Tris, 1mM EDTA, 5 mM DTT, 10 mM Na-molybdate). The cells were pelleted by centrifugation at 2000 × g for 5 min, the pellet was homogenized in TEDM buffer and the homogenate was centrifuged at 1000 × g for 7 min to remove nuclei and unbroken cells. The resulting supernatant was centrifuged at 20,000 × g for 20 min to pellet plasma membranes, followed by a final centrifugation at 100,000 × g for 1 hr at 4°C to remove the microsomes. The final supernatant containing the cytosolic fraction was used for the PR assays. Competitive binding assays were conducted with progesterone and the four synthetic progestins over the concentration range of 10−11 to 10−6 M incubated with 4 nM [3H]- progesterone overnight at 4°C. The assay samples were subsequently incubated with dextran-coated charcoal (1% charcoal, 0.5% Dextran T-70 in TEDM buffer) at 4°C for 5 min, followed by centrifugation at 5000 × g for 5 min to separate [3H]- progesterone bound to the receptor from free steroid. Competitor binding was expressed as a percentage of specific binding (total binding minus nonspecific binding in the presence of 100-fold excess progesterone).
nPR luciferase reporter assay
MCF-7 cells were incubated in 12-well plates in DMEM (without phenol red) (1 ml/well) and co-transfected with 0.25 μg of human PR-B expression vector construct (gift of Dr. P. Chambon, Institut National De Sante et de le Recherche Medicale, France), 0.6 μg of MMTV-Luc vector (pHHluc, a gift from Dr. Steve K. Nordeen, UCHSC) [14] and 0.5 μg of the pRL-TK vector (Renilla luciferase, Promega, Madison WI), to correct for transfection efficiency, as described previously [15]. Media were replaced after 6 hrs with fresh culture medium containing various steroids. The cells were grown overnight until 90% confluent. Cell extracts were assayed using a dual-Luciferase reporter assay system (Promega) according to the manufacturer’s instructions. Firefly and Renilla luciferase activities were measured for 10 sec each, respectively, using a FLUOSTAR OPTIMA luminometer (BMG Labtechnologies Inc. Durham, NC). The relative luciferase activity level of each treatment (in triplicate) was expressed as the ratio of Firefly/Renilla luciferase activity value.
Molecular Modeling and 3D QSAR Studies by the CoMFA method
mPRα data set
48 mPRα steroid ligands having a 3-keto or 3-desoxy group were selected from Table 1 in this paper and from Table 1 of reference [10]. The tabulated IC50 values (nM) were transformed to pIC50 values. The compounds with relative binding affinity (RBA) values <1% (progesterone = 100 %) were given a pIC50 value of 5.0 and the compounds with no binding at 10−5 M were given a value of 4.5. The six 3-OH containing steroids from the total set of 54 compounds in these Tables were excluded from the CoMFA analysis since most of them are poor binders to mPRα and are not present in the nPR data set (vide infra).
nPR data set
60 nPR ligands having a 3-keto or 3-desoxy group in the steroid structure were collected from the literature. All selected published binding affinities have been made relative to Org 2058 (RBA = 100%). The competition of 1.95 nM [3H]-Org 2058 is measured in these experiments. The pIC50 of Org 2058 is therefore 8.71. The binding affinities of a set of 44 steroids relative to Org 2058 for human nPR in MCF-7 cells were used [16]. These published log RBA values were transformed to pIC50 values by adding 8.71 – 2.00 = 6.71 to the tabulated values in Table 1 of this paper. The data for 16 other steroids were collected from several papers [17–19] and their extracted pIC50 values are shown in Table 2. The structures of the entire mPRα data set (48 steroids) and nPR data set (60 steroids) are shown in Supplementary Table 1.
Table 2.
Rank order of binding affinities of natural and synthetic steroids to CHO and MCF-7 cells transfected with human nPR
| Compounds | pIC50 (mean) | logRBA |
|---|---|---|
| Promegestone | 8.90 | 2.18 |
| 16α-ethyl-21-hydoxy-19-norpregn-4-ene-3,20-dione (Org 2058) | 8.72 | 2.00 |
| 19-Norprogesterone | 8.53 | 1.81 |
| Mifepristone | 8.26 | 1.54 |
| 17-Hydroxy-19-nor-17α-pregn-4-en-3-one | 8.24 | 1.52 |
| Progesterone | 7.87 | 1.15 |
| Desogestrel | 7.80 | 1.08 |
| 17β-Hydroxy-16α-isopropylestr-4-en-3-one | 7.72 | 1.00 |
| 17β-Hydroxy-17α-methylestr-4-en-3-one | 7.66 | 0.94 |
| Ethylestrenol | 7.21 | 0.49 |
| Nandrolone | 7.20 | 0.48 |
| Allylestrenol | 7.04 | 0.32 |
| Ethisterone | 6.99 | 0.27 |
| Lynestrenol | 6.95 | 0.23 |
| 5α-Dihydronandrolone | 6.50 | −0.22 |
| 5α-Dihydrotestosterone (DHT) | 6.16 | −0.56 |
| Androst-4-ene-3,17-dione (androstenedione) | 6.02 | −0.70 |
| Testosterone | 6.02 | −0.70 |
Each value is the mean of at least three separate competitive binding assays. IC50 is the competitor concentration (nM) that causes 50% displacement of [3H] Org 2058. RBA (Relative Binding Affinity), RBA (%) compared with that of Org 2058.
3D structures of the steroids were modelled with SYBYL 7.3 (Tripos Associates, St. Louis, MO), starting from accurate X-ray structures from the CSD (Conquest version 1.10) as much as possible. Low-energy conformations were generated and atomic charges were calculated using the Gasteiger method. The steroids were aligned by fitting the C- and the D-ring. The CoMFA option in SYBYL was used to develop 3D QSARs for the set of 48 mPRα ligands and the set of 60 nPR ligands. The standard CoMFA grid spacing of 2 Å was used for the aligned steroids. At each grid point steric energy (Lennard-Jones potential) and electrostatic (coulombic) energy were calculated for each molecule experienced by a probe atom (sp3-hybridized carbon with +1 charge). Each CoMFA descriptor column contains the magnitude of either the steric or electrostatic potential, exerted by the atoms in each molecule at the grid points in the Cartesian space surrounding the aligned molecules. To minimize the domination by large steric and electrostatic energies, all energies that exceeded the default 30 kcal/mole value were set to this cutoff value. The two data sets that were generated in this manner were combined with their respective pIC50 values and analysed by the partial least squares (PLS) method in SYBYL. The cross-validation technique was used to validate the generated model equations which correlate the pIC50 values with the physical parameters of the ligands, and was also used to determine the optimal number of components for the ultimate PLS analyses.
3. Results
Binding of progesterone derivatives and other steroids to mPRα
Three of the synthetic progestins, 18a-methylprogesterone (18-CH3P4, Org OE 64-0), 10-ethenyl-19-norprogesterone (19-CH2P4, Org OD 02-0), and 13-ethenyl-18-norprogesterone (18-CH2P4, Org 33663-0) bound to recombinant hu-mPRα with affinities higher than that of progesterone (Fig. 1A, Table 1). The structurally-related 19a-methylprogesterone (19-CH3P4, Org OD 13-0) also displayed relatively high affinity for this receptor (Fig. 1A). Their structures, depicted in Fig. 1B, have small lipophilic substitutions at position 18 or 19 of progesterone in common. Substitutions at these positions with polar groups are unfavourable for binding to mPRα: 3,20-dioxopregn-4-ene-18-carbonitrile, Org OE 62-0 (13-acetyl-18-norprogesterone) and 19-hydroxyprogesterone have low binding affinities for this receptor (Fig. 1C, Table 1).
The extra double bond at position 1 or position 6 in pregna-1,4-diene-3,20-dione or in pregna-4,6-diene-3,20-dione has little or no influence relative to the progesterone response (Fig. 1C, Table 1). The acetylthio substitution at the 7α position leads to a complete loss of binding affinity for Org 33718-0 (Table 1). The 7α-methyl substituent in RMI 12936 (17β-hydroxy-7α-methylandrost-5-en-3-one) also leads to a 10-fold decrease in binding relative to the most structurally-related steroid molecule testosterone, that was investigated earlier [10]. The inactivity of 3,20-dioxopregn-4-ene-2α-carbonitrile is in line with the observed poor binding of 2α-hydroxyprogesterone in the earlier series of compounds evaluated [10]. Cortexolone (11-deoxycortisol) is less potent than deoxycorticosterone investigated earlier as predicted since this compound is substituted with a 17α-hydroxy group which has been shown to markedly decrease affinities of steroids for mPRα. The complete loss of affinity of cortisol for mPRα in comparison to cortexolone is likely due to the presence of the extra 11β-hydroxy substituent in cortisol since it was observed earlier that this modification of progesterone in 11β-hydroxyprogesterone resulted in a 10 fold lower binding affinity for the receptor.
The weak binding of 3α-hydroxy-5α-pregnan-20-one (allopregnanolone) (RBA 7.6%) or the absence of binding of 3β-hydroxy-5α-pregnan-20-one demonstrate that a 3α-hydroxy or a 3β-hydroxy group cannot substitute for the 3-keto group in progesterone, as has been observed previously with the binding affinities of pregnenolone (RBA 4%) and 4-androstenediol (RBA < 1%) [10]. As expected estradiol-17β has no binding affinity for mPRα. 13-acetyl-18-norpregn-4-en-3-one (Org OD 62-0) that lacks the 20-keto group of progesterone lost all affinity for mPRα. This phenomenon was not observed with nPRs [20]. Org OD 62-0 retains in comparison with progesterone 1/4 of the progestational activity in the s.c. Clauberg (McPhail) assay in female rabbits [20–21], and is surprisingly almost equipotent to 13-acetyl-18-norprogesterone (Org OE 62.0) that has 1/3 of the potency of progesterone [20]. 17-Epitestosterone (RBA 2.8%) has lower affinity for mPRα than testosterone (RBA 22.4%). 17-caproyloxyprogesterone- has only poor affinity for mPRα (RBA 4.4%) due to its 17α-substitution, while it is a potent progestin through nPR [10,11]. The 17-keto group in androst-4-ene-3,17-dione (androstenedione) makes this steroid unable to bind to mPRα.
mPRα agonist activities of progesterone derivatives
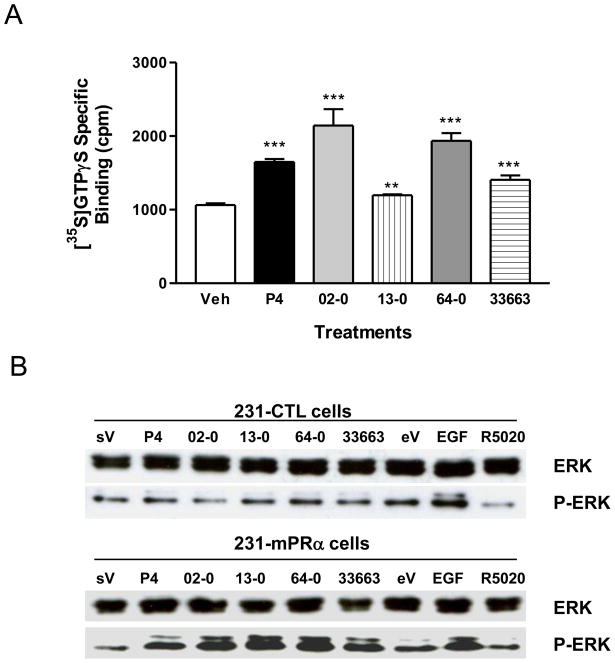
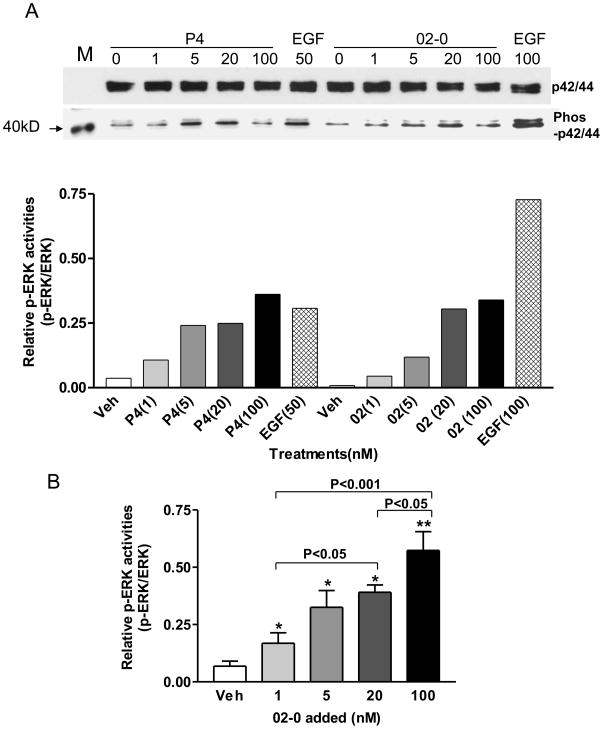
Specific binding of [35S]GTPγS to cell membranes of MDA-MB-231cells transfected with human mPRα was significantly increased after treatment with 100 nM progesterone (P4), 19-CH2P4 (Org OD 02-0), 19-CH3P4 (Org OD 13-0), 18-CH3P4 (Org OE 64-0), and 18-CH2P4 (Org 33663-0), indicating that all of them are mPRα agonists (Figure 2A). Overall there was a positive relationship between the activities of the progestins in the G protein activation assay and their binding to the receptor. The two progestins with highest affinities for mPRα, 19-CH2P4 and 18-CH3P4, displayed greater agonist activities than progesterone and the two other progestins at the single concentration tested of 100 nM. Progestin hormones have previously been shown to activate MAPK in cells transfected with mPRα [5]. The four synthetic progestins at concentrations of 100 nM also activated the second messenger MAPK in mPRα-transfected MDA-MB-231 cells, but not in untransfected controls, resulting in increased phosphorylation of ERK (Figure 2B). Thus the MAPK assay results confirm those obtained with the G protein activation assay demonstrating that 19-CH2P4, 19-CH3P4, 18-CH3P4, and 18-CH2P4 are mPRα agonists. MAPK activity was significantly stimulated in a concentration-dependent manner by 19-CH2P4 (Org OD 02-0) over the concentration range of 1nM to 100 nM (Figure 3A,B).
Figure 2.
G protein (A) and MAPK (B) activation by various progestins in MDA-231 cells transfected with human mPRα. A, Specific [35S]GTPγS binding to plasma membranes was measured after 100 nM progestin treatment for 20 min. CTL, vehicle control; P4, progesterone; 02-0, 19-CH2P4 (Org OD 02-0); 13-0, 19-CH3P4 (Org OD 13-0); 64-0, 18-CH3P4 (Org OE 64-0); 33663, 18-CH2P4 (Org 33663-0); ***: P<0.0001, **: P<0.001 compared to Veh. B, MAPK activation in human mPRα-transfected MDA-231 cells (231-mPRα cells) compared to untransfected controls (231-CTL cells) after 100 nM progestin treatment for 10 min. sV, steroid vehicle control; eV, EGF vehicle control; EGF, epidermal growth factor positive control; R5020, promegestone; ERK, total ERK; P-ERK, active phosphorylated ERK. Both assays were repeated 3 times and similar results were obtained in each assay.
Figure 3.
Concentration-response relationship of MAPK activation by 19-CH2P4 (Org OD 02-0) and progesterone in MDA-231 cells transfected with human mPRα over the range of 1nM to 100 nM.. A, Representative results from a single MAPK activation assay. B, Combined results of response to 19-CH2P4 from four separate experiments. * P < 0.05, ** P<0.001 compared to Veh.
Binding of progesterone derivatives to nPR and their nPR agonist and antagonist activities
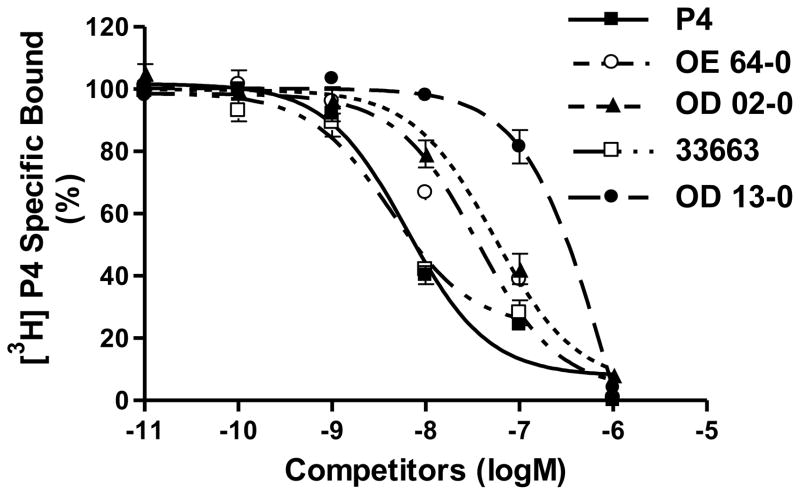
The four synthetic progestins also bound to the nPR in cytosolic fractions of MCF-7 cells but the rank order of their binding affinities for the nPR and mPRα differed (Figure 4). 18-CH2P4 (Org 33663-0) displayed a binding affinity (IC50 7.69 nM) very similar to that of progesterone (IC50 7.74 nM), whereas the other three compounds had lower binding affinities than progesterone for the receptor. The relative binding affinities of Org OE 64-0, Org OD 02-0 and Org OD 13-0 were 20.8% (IC50 37.2 nM), 12.9% (IC50 60.1 nM) and 2.3% (IC50 335 nM) that of progesterone, respectively.
Figure 4.
Competition curves of binding by progesterone and various progestins to human nPR in cytosolic fractions of MCF-7 cells expressed as a percentage of maximum specific [3H]P4 binding. Cells were pretreated with estradiol-17β for 72 hrs prior to the assay to upregulate nPR expression. For key to steroid abbreviations see legend for Figure 2. Assays were repeated 3 times and similar results were obtained in each assay.
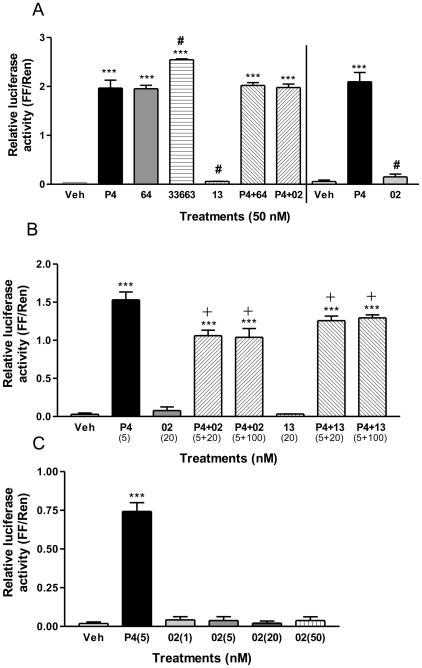
Nuclear PR agonist and antagonist activities of the four progestins were evaluated in MCF-7 cells co-transfected with PR-B and a PRE-luciferase reporter system. Both 18-CH3P4 (Org OE 64-0) and 18-CH2P4 (Org 33663-0) mimicked the action of progesterone in this nPR activity assay, i.e. they showed nPR agonist activity (Figure 5A). In contrast, 19-CH2P4 (Org OD-02) and 19-CH3P4 (Org OD 13-0) showed no significant nPR activity (Figure 5A, 5C). These two progestins also showed weak antagonist activity in the assay in the presence of a low concentration of progesterone (5 nM, Figure 5B), but 19-CH2P4 showed no such activity at a higher progesterone concentration (50 nM, Fig. 5A), suggesting these compounds may act as nPR antagonists under certain conditions (Figure 5B).
Figure 5.
Effects of treatment with various progestins on transactivation of human PR-B using a PRE luciferase reporter system in PR-B –transfected MCF-7 cells. A, Agonist activities of the progestins in the PR transactivation assay at a concentration of 50 nM or 50 nM P4 + 50 nM 64 (or 50 nM 02). Veh, vehicle control; P4, progesterone; 64, 18-CH3P4 (Org OE 64-0); 02, 19-CH2P4 (Org OD 02-0); 13, 19-CH3P4 (Org OD 13-0); 33663, 18-CH2P4 (Org 33663-0).***: P<0.0001 compared to vehicle (Veh), #: P<0.05 compared to P4; B, Agonist and antagonist activities of 19-CH2P4 and 19-CH3P4 in the PR transactivation assay at concentrations of 20 and 100 nM in response to treatment with 5 nM progesterone. ***: P<0.0001 compared to Veh, +: P<0.05 compared to P4. C Agonist activities of 19-CH2P4 (OD-02-0) over the concentration range 1–50 nM.**: P<0.001 compared to Veh. Assays were repeated 3 times and similar results were obtained each time.
CoMFA analyses of steroid binding to mPRα and nPR
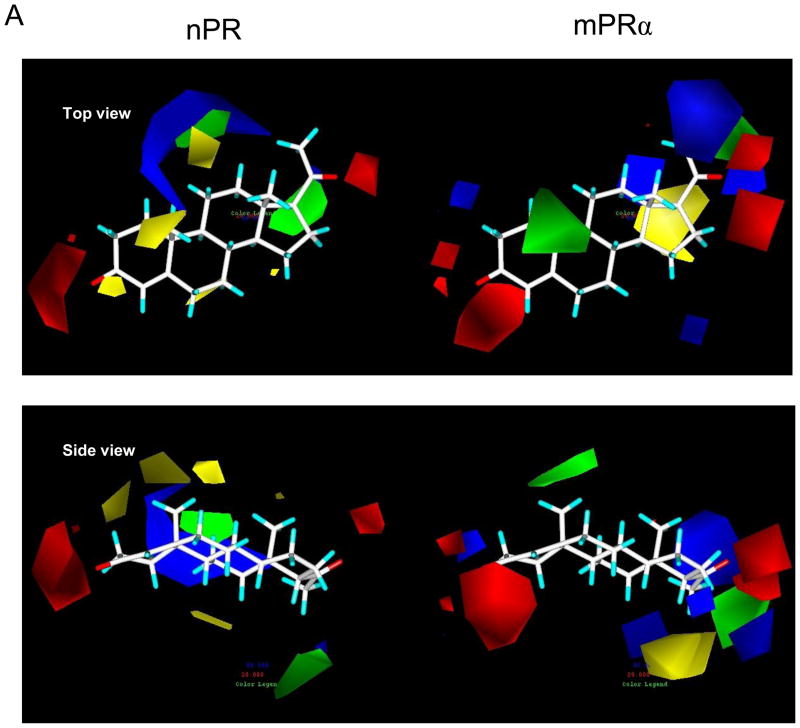
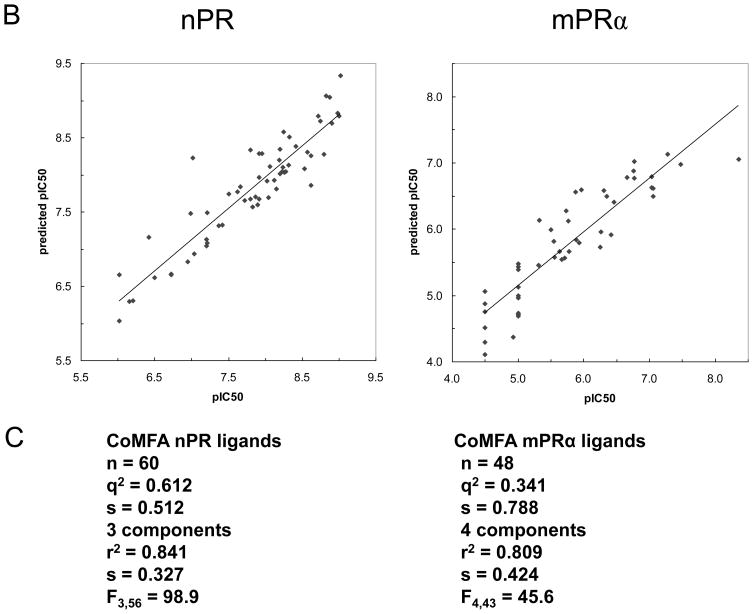
The results of the CoMFA studies are summarized in Figures 6A, 6B and 6C. The analyses based on CoMFA steric and electrostatic fields produced significant correlations between predicted and measured pIC50 values of the set of 48 steroids (Figure 6B, 6C) tested for hu-mPRα binding and for the set of 60 steroids tested for nPR binding. When the CoMFA expressions are iso-contoured, surfaces become visible surrounding areas where the coefficients have similar values. In the case of the CoMFA steric and electrostatic field graphs in Figure 6A regions in space around the aligned steroid molecules are shown as iso-contoured volumes, where specific steric or electrostatic properties are most highly associated with differences in target property, thus enhancing or detracting from the affinity of the compounds for both receptors. The presence of steric bulk in the green regions in Figure 6A contributes positively to the affinity for mPRα or nPR, while the yellow region decreases the affinity for these receptors. Opposite steric effects of steroids are observed at positions 10 and 17α on binding to the two receptors. Substitution with small alkyl groups is favourable for binding to hu-mPRα and unfavourable for binding to nPR. Substitutions at 17α are favourable for binding to nPR, while they are not allowed for steric reasons at hu-mPRα. The red areas suggest regions where the molecule could benefit from hydrogen bond acceptors in the steroid skeleton or from electronegative atoms. The 3-keto and 20-keto positions seem to be essential for binding at both receptors. The blue areas point to positions where electropositive substituents might enhance the affinity for the two receptors.
Figure 6.
Physicochemical properties of steroid binding to mPRα (on right) and nPR (on left) assessed by comparative molecular field analyses (CoMFA) A. CoMFA steric and electrostatic field contour plots for the mPRα and nPR models. Progesterone is shown as the reference compound in the top view and the side view. The green volumes indicate favourable steric interactions and the yellow volumes represent unfavourable steric interactions. The red volumes indicate regions where the presence of negative potential contributes to the affinity. The blue volumes indicate regions where the presence of positive potential increases binding affinity. B. Plots of CoMFA predicted vs measured pIC50 values of 48 mPRα ligands and 60 nPR ligands. C. Details of the two CoMFA analyses using the pIC50 values of 48 mPRα ligands and of 60 nPR ligands. The structures of all the ligands are shown in Supplementary Table 1.
4. Discussion
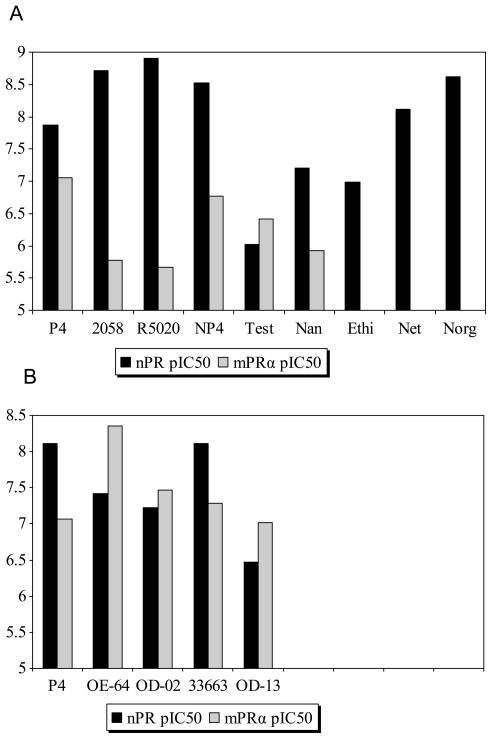
The co-expression of mPRs with the nPRs in a wide variety of reproductive tissues, including the myometrium, gonads, gametes, and breast [6, 15, 22–24], has complicated investigations on their distinct physiological functions. The finding that both the potent nPR agonist R5020 (promegestone) and antagonist RU486 (mifepristone) show little or no affinity for recombinant human mPRα has been exploited to distinguish nPR-mediated progestin actions from mPR-mediated ones in a variety of target cells [10, 15, 25] Several other steroids including Org 2058, 19-norprogesterone, ethisterone, norethisterone, and norgestrel also display greater binding affinity for nPR than mPRα [10] (Figure 7). However, until now specific mPR modulators had not been identified for conducting complementary studies to examine mPR-mediated progestin effects.
Figure 7.
A, Receptor binding profiles of nine steroids for nPR and mPRα expressed as pIC50 values. Org 2058 and P4 were used as reference compounds for the nPR and mPRα receptors, respectively. P4, progesterone; 2058, Org 2058; R5020, promegestone; NP4, 19-norprogesterone; Test, testosterone; Nan, nandrolone; Ethi, ethisterone; Net, norethisterone, Norg, norgestrel. B, Receptor binding profiles of five steroids for nPR and mPRα expressed as pIC50. P4 was used as reference compound for both the nPR and mPRα. P4, progesterone; OE-64, 18-CH3P4; OD-02, 19-CH2P4; 33663, 18-CH2P4; OD-13, 19-CH3P4.
Although 18-CH3P4 (Org OE 64-0) showed the highest binding affinity for mPRα, it had similar efficacy to progesterone in the nPR transactivation assay and therefore is not a selective mPRα agonist. On the other hand 19-CH2P4 (Org OD 02-0), which has the second highest binding affinity for mPRα, is a much more selective mPRα agonist, since it displayed high activity in the G-protein activation and MAPkinase assays and no agonist activity in the nPR transactivation assay. 19a-Methylprogesterone (19-CH3P4, Org OD 13-0) also showed no nPR agonist activity, consistent with the prediction from the structure-binding relationship analysis that the introduction of small lipophilic substituents at position 19 of progesterone leads to a decrease in affinity for nPR. 19-CH3P4 was also identified as a selective mPRα agonist, although it has a lower binding affinity than 19-CH2P4 (Org OD 02-0) and appears to have a lower efficacy in the G protein activation assay. It is concluded therefore that 19-CH2P4 (Org OD 02-0) is the best compound available at present to study the role of mPRα in reproductive processes, pharmacological studies essential for establishing that mPRs mediate distinct physiological functions as progesterone receptors. For example, the important role of mPRα but not nPR as the intermediary in progestin induction of oocyte meiotic maturation in zebrafish has recently been confirmed using 19-CH2P4 [26].
The possible therapeutic applications of 19-CH2P4 (Org OD 02-2) remain unclear at present because additional research is required to determine the precise role of mPRα in many reproductive processes. However, some recent findings on mPRα indicate several potential uses of the compound. The mPRα protein is localized on the midpieces of fish and human sperm and is the likely intermediary in progestin upregulation of motility and fertility [27], suggesting a potential use for the compound in the treatment of male subfertility. Progestin signaling through mPRα has also been demonstrated in human myometrial cells at term [15], in human T lymphocytes during the menstrual cycle [25], and in rodent gonadotropin releasing hormone (GnRH)–secreting neurons during progestin down-regulation of GnRH secretion [28]. Thus there is a growing number of mPRα targets in reproductive and non reproductive tissues for possible intervention with 19-CH2P4.
Although it has been demonstrated by several research groups that both wildtype and recombinant mPRs have the ligand binding characteristics of membrane progesterone receptors [5–7, 10, 29, 30], it is also necessary to develop molecular models that describe the interactions of steroids with mPRs in order to establish that they are true steroid receptors. The CoMFA conducted with hu-mPRα ligands has permitted the development of the first molecular model describing the structural requirements for steroid binding for a novel steroid receptor. The correlation between the measured binding affinities of steroids for mPRα and their predicted affinities by CoMFA (Fig. 6B) demonstrates that ligand binding to mPRα can be effectively modelled with almost similar performance characteristics to those of the nPR model, thereby providing strong additional evidence that mPRα functions as a steroid receptor with a well defined single binding site. The development of a testable model for the steroid binding site of mPRα provides a framework for evaluating the binding affinities of additional steroids as well predicting novel steroids likely to be selective mPRα agonists.
The physicochemical characteristics of steroid binding to two distinct classes of steroid receptors were compared for the first time in the present study. Negative potentials (charges) in the vicinity of positions C3 and C17 on the steroid nucleus, critically important for binding to nPR [31], were also shown by CoMFA analysis to be involved in binding to mPRα. In contrast, the molecular modeling results indicate that positive potentials in different positions around the steroid nucleus are involved in ligand binding to the two receptors. The positions of both favourable and unfavourable steric interactions also appear to differ between the two receptors and are shown as green and yellow shaded areas in Figure 6A. The mPRs are structurally unrelated to nPRs so it is not surprising that the electrostatic and steric affinity maps of the steroid binding pockets of the two receptors show marked differences. Parallel studies using site-directed mutagenesis to identify the critical amino acid residues of mPRα for steroid binding and the structure of the ligand binding pocket are currently underway. The development of both structural and CoMFA models of the mPRα ligand pocket would satisfy the major remaining criteria for designation of this novel seven-transmembrane protein as a steroid receptor. In addition, a knowledge of the molecular basis for ligand/receptor interactions would advance our understanding of this alternative steroid mechanism that evolved in vertebrates enabling target cells to respond to progestins secreted by the reproductive system.
Supplementary Material
Acknowledgments
This research was supported by N.V. Organon, a part of Schering-Plough Corporation, and by National Institutes of Health grant ESO 12961 to P.T.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anstead GM, Carlson KE, Katzenellenbogen JA. The estradiol pharmacophore: Ligand structure-estrogen receptor binding affinity relationships and a model for the receptor site. Steroids. 1997;62:268–303. doi: 10.1016/s0039-128x(96)00242-5. [DOI] [PubMed] [Google Scholar]
- 2.Cramer RD, III, Patterson DE, Bunce JD. Comparative molecular field analysis (CoMFA). 1. Effect of shape on binding of steroids to carrier proteins. J Am Chem Soc. 1988;110:5959–67. doi: 10.1021/ja00226a005. [DOI] [PubMed] [Google Scholar]
- 3.Bohl CE, Cheng C, Mohler ML, Chen J, Swann PW, Dalton JT. A ligand-based approach to identify quantitative structure-activity relationships for the androgen receptor. J Med Chem. 2004;47:3765–76. doi: 10.1021/JM0499007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xing L, Welsh WJ, Tong W, Perkins R, Sheehan DM. Comparison of estrogen receptor alpha and beta subtypes based on comparative molecular field analysis (CoMFA) SAR QSAR Environ Res. 1999;10:215–37. doi: 10.1080/10629369908039177. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA. 2003;100(5):2231–6. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci USA. 2003;100(5):2237–42. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith JL, Kupchak BR, Garitaonandia I, Haong LK, Maina AS, Regalla LM, Lyons TJ. Heterologous expression of human mPRalpha, mPRbeta and mPRgamma in yeast confirms their ability to function as membrane progesterone receptors. Steroids. 2008;73(11):1160–73. doi: 10.1016/j.steroids.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamouchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423 (6941):762–9. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 9.Tang YT, Hu T, Arterburn M, Boyle B, Bright JM, Emtage PC, et al. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol. 2005;61(3):372–80. doi: 10.1007/s00239-004-0375-2. [DOI] [PubMed] [Google Scholar]
- 10.Thomas P, Pang Y, Dong J, Groenen P, Kelder J, de Vlieg J, Zhu Y, Tubbs C. Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor alpha subtypes and their evolutionary origins. Endocrinology. 2007;148:705–18. doi: 10.1210/en.2006-0974. [DOI] [PubMed] [Google Scholar]
- 11.Kontula K, Jänne O, Vihko R, de Jager E, de Visser J, Zeelen FJ. Progesterone-binding proteins: In vitro binding and biological activity of different steroidal ligands. Acta Endocrin. 1975;78(3):574–92. doi: 10.1530/acta.0.0780574. [DOI] [PubMed] [Google Scholar]
- 12.Lee DL, Kollman PA, Marsh FJ, Wolff ME. Quantitative relationships between steroid structure and binding to putative progesterone receptors. J Med Chem. 1977;20(9):1139–46. doi: 10.1021/jm00219a006. [DOI] [PubMed] [Google Scholar]
- 13.Pinter J, Thomas P. Characterization of a progestogen receptor in the ovary of the spotted seatrout, Cynoscion nebulosus. Biol Reprod. 1995;52:667–75. doi: 10.1095/biolreprod52.3.667. [DOI] [PubMed] [Google Scholar]
- 14.Nordeen SK. Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques. 1988;6(5):454–8. [PubMed] [Google Scholar]
- 15.Karteris E, Zervou S, Pang YF, Dong J, Hillhouse EW, Randeva HS, et al. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: potential role in functional progesterone withdrawal at term. Mol Endocrinol. 2006;20:1519–34. doi: 10.1210/me.2005-0243. [DOI] [PubMed] [Google Scholar]
- 16.Bursi R, Dao T, van Wijk T, de Gooyer M, Kellenbach E, Verwer P. Comparative spectra analysis (CoSA): spectra as three-dimensional molecular descriptors for the prediction of biological activities. J Chem Inf Comput Sci. 1999;39(5):861–7. doi: 10.1021/ci990038z. [DOI] [PubMed] [Google Scholar]
- 17.Verheul HAM, Tittes EV, Kelder J, Schuurs AHWM. Effects of steroids with different endocrine profiles on the development, morphology and function of the bursa of Fabricius in chickens. J Steroid Biochem. 1986;25(5A):665–75. doi: 10.1016/0022-4731(86)90009-9. [DOI] [PubMed] [Google Scholar]
- 18.Schoonen WGEJ, Joosten JWH, Kloosterboer HJ. Effects of two classes of progestagens, pregnane and 19-nortestosterone derivatives on cell growth of human breast tumor cells: I. MCF-7 cell lines. J Steroid Biochem Molec Biol. 1995;55(3–4):423–37. doi: 10.1016/0960-0760(95)00215-4. [DOI] [PubMed] [Google Scholar]
- 19.Kloosterboer HJ, Deckers GH, de Gooyer ME, Dijkema R, Orlemans EOM, Schoonen WGEJ. Pharmacological properties of a new selective antiprogestagen: Org 33628. Annals NY Acad Sci. 1995;761:192–201. doi: 10.1111/j.1749-6632.1995.tb31379.x. [DOI] [PubMed] [Google Scholar]
- 20.Schönemann KH, van Vliet NP, Zeelen FJ. Structure and progestational activity of 13-substituted-18-norpregn-4-ene-3,20 diones, a pilot study. Steroids. 1985;45(3–4):297–302. doi: 10.1016/0039-128x(85)90078-9. [DOI] [PubMed] [Google Scholar]
- 21.Deckers GH, Schoonen WGEJ, Kloosterboer Influence of the substitution of 11-methylene, Δ15, and/or 18-methyl groups in norethisterone on receptor binding, transactivation assays and biological activities in animals. J Steroid Biochem Molec Biol. 2000;74:83–92. doi: 10.1016/s0960-0760(00)00093-5. [DOI] [PubMed] [Google Scholar]
- 22.Dressing GE, Thomas P. Identification of membrane progestin receptors in human breast cancer cell lines and biopsies and their potential involvement in breast cancer. Steroids. 2007;72(2):111–6. doi: 10.1016/j.steroids.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Cai Z, Stocco C. Expression and regulation of progestin membrane receptors in the rat corpus luteum. Endocrinology. 2005;146(12):5522–32. doi: 10.1210/en.2005-0759. [DOI] [PubMed] [Google Scholar]
- 24.Thomas P. Characteristics of membrane progestin receptor alpha (mPRalpha) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Frontiers in Neuroendocrinology. 2008;29(2):292–312. doi: 10.1016/j.yfrne.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dosiou C, Hamilton AE, Pang Y, Overgaard MT, Tulac S, Dong J, et al. Expression of membrane progesterone receptors on human T lymphocytes and Jurkat cells and activation of G-proteins by progesterone. J Endocrinol. 2008;196(1):67–71. doi: 10.1677/JOE-07-0317. [DOI] [PubMed] [Google Scholar]
- 26.Thomas P, Harris C, Pang Y. The roles of different types of progestin receptors in steroid induction of oocyte maturation in zebrafish. Biol Reprod. 2008;(Sp Iss S1):220. Abs 705. [Google Scholar]
- 27.Thomas P, Tubbs C, Garry VF. Progestin functions in vertebrate gametes mediated by membrane progestin receptors (mPRs): identification of mPRα on human sperm and its association with sperm motility. Steroids. 2009;74:614–21. doi: 10.1016/j.steroids.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Sleiter N, Pang Y, Park C, Horton TH, Dong J, Thomas P, et al. Progesterone receptor A (PRA) and PRB–independent effects of progesterone on GnRH release. Endocrinology. 2009;150:3833–44. doi: 10.1210/en.2008-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashley RL, Clay CM, Farmerie TA, Niswender GD, Nett TM. Cloning and characterization of an ovine intracellular seven transmembrane receptor for progesterone that mediates calcium mobilization. Endocrinology. 2006;147(9):4151–9. doi: 10.1210/en.2006-0002. [DOI] [PubMed] [Google Scholar]
- 30.Josefsberg Ben-Yehoshua LF, Lewellyn AL, Thomas P, Maller JL. The role of Xenopus membrane progesterone receptor beta in mediating the effects of progesterone on oocyte maturation. Mol Endocrinol. 2007;21(3):664–73. doi: 10.1210/me.2006-0256. [DOI] [PubMed] [Google Scholar]
- 31.Tanenbaum DM, Wang Y, Williams SP, Sigler PB. Crystallographic comparison of the estrogen and progesterone receptor’s ligand binding domains. Proc Natl Acad Sci USA. 1998;95(11):5998–6003. doi: 10.1073/pnas.95.11.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.