Summary
A plethora of growth factors regulate keratinocyte proliferation and differentiation that control hair morphogenesis and skin barrier formation. Wavy hair phenotypes in mice result from naturally occurring loss-of-function mutations in the genes for TGF-α and EGFR. Conversely, excessive activities of TGF-α/EGFR result in hairless phenotypes and skin cancers. Unexpectedly, we found that mice lacking the TRPV3 gene also exhibit wavy hair coat and curly whiskers. Here we show that keratinocyte TRPV3, a member of the Transient Receptor Potential (TRP) family of Ca2+-permeant channels, forms a signaling complex with TGF-α/EGFR. Activation of EGFR leads to increased TRPV3 channel activity, which in turn stimulates TGF-α release. TRPV3 is also required for the formation of the skin barrier by regulating the activities of transglutaminases, a family of Ca2+-dependent cross-linking enzymes essential for keratinocyte cornification. Our results show that a TRP channel plays a role in regulating growth factor signaling by direct complex formation.
Introduction
Skin and its appendages provide a protective barrier essential for animal survival. Hair morphogenesis and epidermal development are orchestrated by an array of cytokines and growth factors (Fuchs and Raghavan, 2002). Signaling by these diffusible molecules provides spatially and temporally controlled cellular programs for keratinocyte proliferation, differentiation, migration, and finally, terminal differentiation and cornification. TGF-α and EGF are related autocrine/paracrine growth factors that activate the EGF receptor (EGFR; ErbB1) to regulate the balance between keratinocyte proliferation and differentiation (Schneider et al., 2008). Defective TGF-α/EGFR signaling leads to abnormal hair morphogenesis, manifested by the “wavy hair” and “curly whiskers” phenotype of spontaneous loss-of-function mouse mutations in TGF-α (named waved-1 or wa1) and in the EGFR (named waved-2 or wa2), respectively (Ballaro et al., 2005; Luetteke et al., 1994; Luetteke et al., 1993; Mann et al., 1993; Murillas et al., 1995; Schneider et al., 2008; Sibilia and Wagner, 1995; Threadgill et al., 1995). Excessive activities of TGF-α/EGFR cause a hairless phenotype and skin cancers (Ferby et al., 2006; Schneider et al., 2008). The mechanisms by which TGF-α/EGFR signaling determines cell ate (proliferation versus differentiation) of follicular and interfollicular (epidermal) keratinocytes are not completely understood.
Accumulated evidence suggests that both negative and positive feedback mechanisms coexist in the TGF-α/EGFR signaling axis. EGF binding triggers rapid degradation of the EGFR through endocytic pathways, but also leads to further production and release/shedding of TGF-α/EGF (Coffey et al., 1987; Peschon et al., 1998). This unique auto-induction mechanism may contribute to the effects of TGF-α/EGF on keratinocyte terminal differentiation (Peus et al., 1997; Sakai et al., 1994; Schneider et al., 2008). TGF-α is expressed in both basal (proliferating) and suprabasal (differentiating) layers of epidermis and in the inner root sheath of the hair follicle (Coffey et al., 1987; Luetteke et al., 1993; Mann et al., 1993; Schneider et al., 2008). Although most highly expressed in the basal layer, suprabasal keratinocytes also express EGFR (Luetteke et al., 1993; Mann et al., 1993; Schneider et al., 2008). While induction of differentiation dramatically increases the production of TGF-α/EGF (Denning et al., 2000), these same growth factors promote terminal differentiation (Wakita and Takigawa, 1999).
Previous studies suggest that TGF-α/EGF regulate keratinocyte terminal differentiation likely in a Ca2+-dependent manner (Denning et al., 2000; Sakai et al., 1994). Intracellular Ca2+ regulates both expression and shedding from the membrane-tethered precursors of EGFR ligands (Denning et al., 2000; Horiuchi et al., 2007). Ca2+ ionophores are sufficient to induce both production and release of TGF-α (Horiuchi et al., 2007; Pandiella and Massague, 1991). The Ca2+ influx pathway under physiological conditions, however, has not been identified.
The cornified cell envelope (CE) is a protein-lipid layer that replaces the plasma membrane of terminally-differentiated keratinocytes (corneocytes), and is crucial for the stratum corneum epidermal barrier (Lorand and Graham, 2003). The CE is a complex layer of lipids attached to a layer of cross-linked proteins. The transglutaminases (TGases) primarily form ε-(γ-glutamyl) lysine isopeptide bonds between proteins and their activities strongly depend on intracellular Ca2+ levels (Lorand and Graham, 2003). EGF can acutely activate TGases to induce CE formation and keratinocyte terminal differentiation (Lorand and Graham, 2003). Cornification-promoting cellular cues may activate an unidentified Ca2+ influx channel to induce TGase activity and subsequent CE formation.
Transient Receptor Potential (TRP) proteins are a large family of Ca2+-permeable channels with diverse functions (Montell, 2005; Nilius et al., 2007; Ramsey et al., 2006). Among these, TRPV3 and TRPV4 are functionally expressed in keratinocytes (Chung et al., 2004; Moqrich et al., 2005). These channels detect ambient temperature changes and are activated by various plant-derived and synthetic compounds (Moqrich et al., 2005; Xu et al., 2006). In this study, we find that TRPV3-deficient mice exhibit hair phenotypes similar to wa1 and wa2. Molecular and biochemical analyses of TRPV3-deficient mice and isolated keratinocytes reveal defective TGF-α/EGFR signaling. We propose that TRPV3 is a Ca2+ entry pathway tightly associated with the TGF-α/EGFR signaling complex orchestrating keratinocyte terminal differentiation.
Results
Whole-animal and keratinocyte-specific disruption of mouse Trpv3 gene
Using a recombineering method, we inserted two loxP sites to flank exon 13 of mouse Trpv3 (see Fig. S1) and obtained mice with homozygous floxed (fl) alleles. To investigate the in vivo function(s) of TRPV3, we generated TRPV3 global knockout (KO) using Sox2-Cre transgenic mice (details described in Experimental Procedures). To elucidate the role of TRPV3 in the skin, we also bred the fl/fl animals to mice harboring a K14-Cre recombinase transgene, which efficiently expresses Cre throughout the epidermis by embryonic day 15.5 (E15.5) (Wang et al., 1997). Mouse genotypes from both TRPV3 global KO (Trpv3−/−; abbreviated as V3 KO) and K14-specific conditional KO (V3 fl/fl: K14 Cre; abbreviated as V3 cKO) were confirmed by PCR (Fig. 1A, also see Fig. S1). No TRPV3 full-length transcript was detected from V3 KO skin tissues using RT-PCR analysis (Fig. S1). No full- length TRPV3 protein was detected by Western blot in V3 KO skin lysates (Fig. 1B) or cultured primary keratinocytes (data not shown). Thus, the mice generated completely lack TRPV3 in their skin.
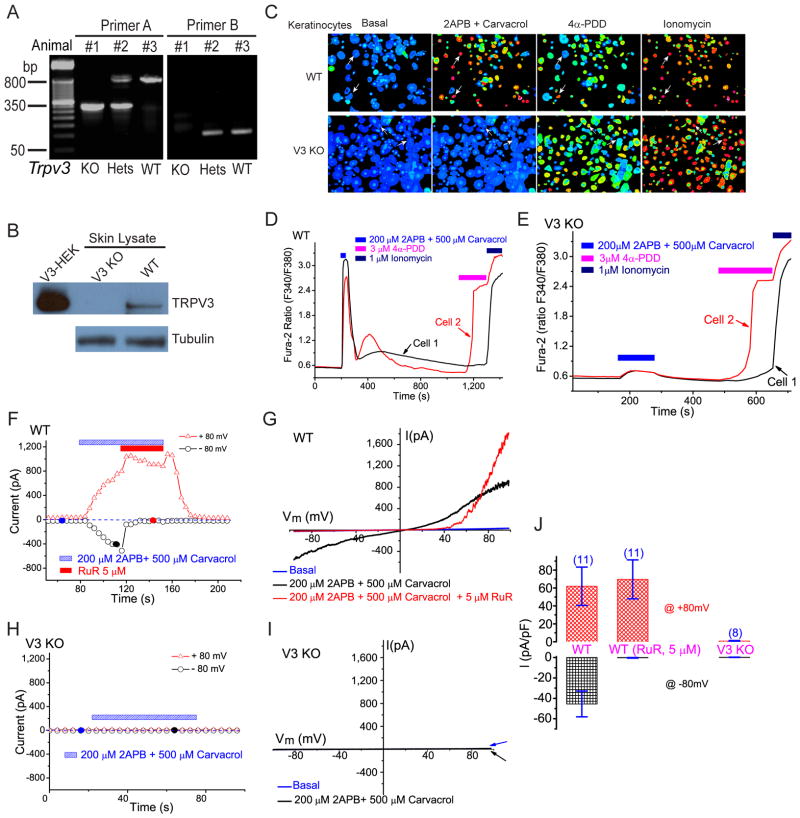
Figure 1. Targeted deletion of mouse Trpv3 abolishes the response of keratinocytes to TRPV3 activators.
A) PCR genotyping of wild-type (WT), V3 KO and Heterozygous (Hets) mice. Two sets of primers were used as described in Methods. PCR products for primer set A: WT 800 bp, KO 300 bp. For primer set B: WT 130 bp, KO no product. B) Lack of TRPV3 protein expression in the skin of V3 KO mice. TRPV3 was immunoprecipitated and immunoblotted using a TRPV3-specific monoclonal antibody. Cell lysates from HEK293T cells expressing recombinant mouse TRPV3 (V3-HEK) were used as positive controls. γ-tubulin served as a loading control for skin lysates. C–E) Lack of agonist-induced V3-like Ca2+ response in V3 KO primary keratinocytes. C) TRPV3 agonist cocktail (200 μM 2-APB + 500 μM Carvacrol) induced large increases of [Ca2+]i in primary cultured keratinocytes isolated from WT (V3+/+), but not V3 KO (V3−/−) mice. While more than 80% of WT keratinocytes responded strongly to the V3 agonist cocktail, negligible responses were observed for V3 KO keratinocytes. Positive controls: ~ 60–80% keratinocytes from both genotypes (WT and V3 KO) responded to 4α-PDD (3μM; agonist of TRPV4). All cells responded to ionomycin (1μM). D) Ca2+ responses of two representative WT cells from C (arrows; upper panels). One cell responded to both TRPV3 and TRPV4 agonists while the other one only responded to the V3 agonist cocktail. E) Ca2+ responses of two representative V3 KO cells from C (arrows; lower panels). One cell responded to the TRPV4 agonist (cell 2); neither cell responded significantly (< 0.1 fura-2 ratio) to the V3 agonist cocktail. F–J) TRPV3-like currents were completely absent in V3 KO primary keratinocytes. F) Application of the V3 agonist cocktail (200 μM 2-APB + 500 μM Carvacrol) to WT primary keratinocytes induced TRPV3-like (ITRPV3-L) currents. Whole-cell currents were generated in response to 400 ms voltage ramps from −100 to +100 mV, applied every 4 s. Holding potential = 0 mV. Each symbol represents the current amplitude at +80 mV (red triangles) and −80 mV (black circles), respectively. Blue dashed line = zero current. G) Representative ramp current of ITRPV3-L. I-V relations were recorded at time points noted in F (filled circles). ITRPV3-L was doubly rectifying and reversed near 0 mV. Ruthenium red (RuR; 5 μM) selectively blocked inward ITRPV3-L with significant augmentation at very positive potentials. H, I) No significant currents were induced by the V3 agonist cocktail in V3 KO keratinocytes. J) Average ITRPV3-L current densities elicited by the V3 agonist cocktail alone, or with the co-application of RuR (5 μM). At −80 mV, inward ITRPV3-L of WT keratinocytes were −45 ± 13 pA/pF (n=11) and −0.3 ± 0.3 pA/pF (n=11) in the absence or presence of RuR, respectively. At + 80 mV, outward ITRPV3-L of V3+/+ keratinocytes were 62 ± 21 pA/pF (n=11) and 70 ± 21 pA/pF (n=11) in the absence or presence of RuR, respectively. For V3 KO keratinocytes, no significant inward or outward ITRPV3-L was detected: −0.3 ± 0.3 pA/pF at −80mV (n=8) and 0.8 ± 0.5 pA/pF at +80mV (n=8), respectively. See also Figure S1.
Functional characterization of TRPV3 global and keratinocyte-specific KO mice
We performed functional studies in keratinocytes isolated from V3 KO and control (WT, V3+/+; heterozygous or Hets, V3+/−) mice. Fura-2 Ca2+ imaging was employed to study the response of keratinocytes to TRPV3 chemical agonists (Xu et al., 2006). Application of most TRPV3 agonists alone, for example, 2-APB (200 μM) or Carvacrol (500 μM), induced a small increase in intracellular Ca2+ ([Ca2+]i) in a subset of cells (30% to 80% of cells; data not shown). However, co-application of two agonists, for example, 200 μM 2-APB + 500 μM Carvacrol (TRPV3 agonist cocktail), reliably induced a dramatic (ΔF340/F380 >1) increase of [Ca2+]i in the majority (> 80%) of keratinocytes isolated from WT mice (WT keratinocytes; Fig. 1C, 1D). Similar results were obtained for other combinations of TRPV3 agonists such as 200 μM 2-APB + 5 mM Camphor; removal of external Ca2+ abolished most of the agonist-induced Ca2+ responses. No significant increase (ΔF340/F380 < 0.1) in [Ca2+]i was seen in keratinocytes isolated from V3 KO mice (V3 KO keratinocytes; Fig. 1C, 1E). In contrast, in both WT and V3 KO cells, large [Ca2+]i increases were evoked by 4α-PDD (1μM), an agonist of TRPV4 (Watanabe et al., 2002) that is also expressed in keratinocytes. Similar results were obtained in keratinocytes from V3 cKO mice. These results demonstrate that TRPV3 KO mouse keratinocytes completely lack TRPV3-mediated Ca2+ responses.
Consistent with the Ca2+ imaging results, TRPV3 agonist cocktail evoked a slowly developing, large TRPV3-like current (ITRPV3-L) in most WT keratinocytes (Fig. 1F, 1G, 1J). Ruthenium red (RuR, 5 μM), a non-specific voltage-dependent blocker of TRPV1-4 channels (Chung et al., 2004; Hu et al., 2004; Ramsey et al., 2006; Xu et al., 2006), almost completely (> 99%) inhibited agonist-activated inward ITRPV3-L. Similar RuR-sensitive ITRPV3-L was also evoked by other TRPV3 agonists (200 μM 2-APB + heat or 200 μM 2-APB + 5 mM Camphor) in the same cells. In V3 KO keratinocytes, in contrast, no significant current was evoked by the V3 agonist cocktail (Fig. 1H, 1I, 1J). Similar results were obtained in keratinocytes from V3 cKO mice.
TRPV3-deficient mice exhibit curly whiskers and wavy hair
Although previous animal studies identified TRPV3’s function in temperature sensation (Moqrich et al., 2005), the most obvious phenotypic changes we observed from our V3 KO mice are abnormalities in skin, hair and whiskers. In contrast with littermate controls (WT and heterozygotes (Hets)), most whiskers of V3 KO mice were characteristically curled or hooked (Fig. S2). The curly morphology of whiskers was apparent at birth (Fig. S2); newborn V3 KO mice could be identified based on whisker morphology alone. Keratinocyte-specific V3 cKO mice exhibited similar curly whiskers (Fig. 2A, 2B), suggesting that the phenotype was caused by specific V3-deficiency in keratinocytes. Whisker curliness grew more obvious with age (Fig. 2B & Fig. S2). Both the dorsal and ventral coat fur, as well as the tail hair of V3 KO and cKO mice was wavy (Fig. 2B & Fig. S2) beginning 1 week after birth, but was most apparent once the hair was well formed (~ 3–4 weeks postnatal), gradually reducing with age. In contrast to a previous study reporting abnormality of ventral hairs in a subset of V3 KO mice (Moqrich et al., 2005), curly whiskers and wavy hair was present throughout the coat at all ages with 100% penetrance for both V3 KO and cKO mice that we have generated, as well as the V3 KO mice reported by Moqrich et. al (S.M.H. and M.C., unpublished data). In comparison, TRPV1 KO mice hair shape and distribution is normal (Fig. S2). Consistent with the expression of TRPV3 in follicular keratinocytes of mouse (Peier et al., 2002) and human (Xu et al., 2002), the wavy phenotype correlated with V3-deficiency in keratinocytes.
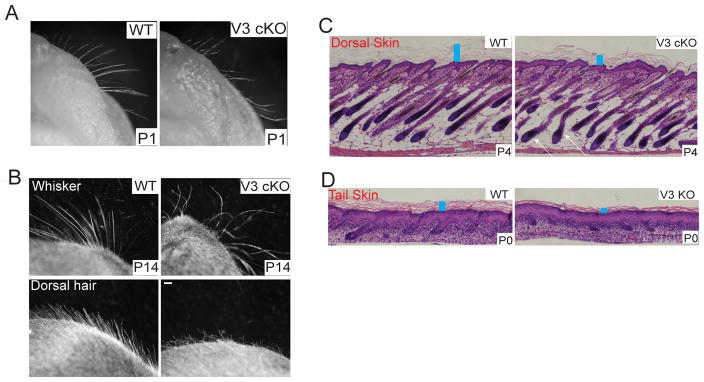
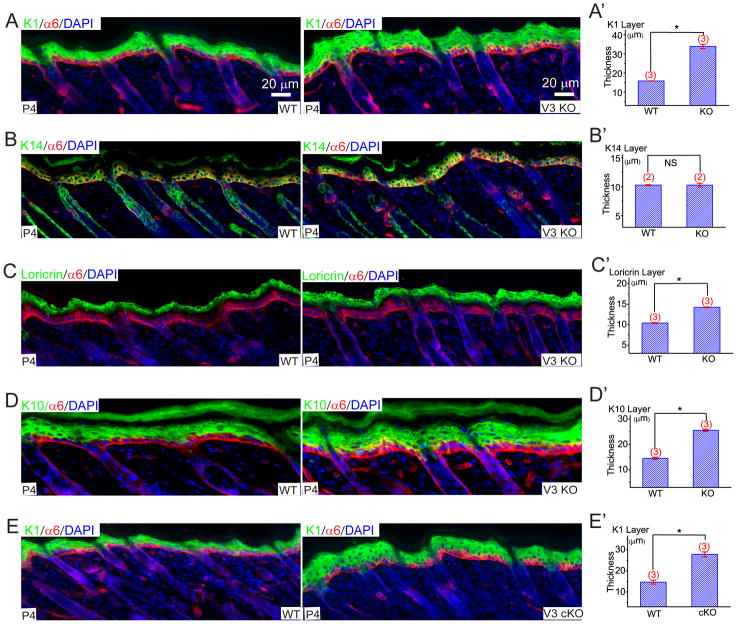
Figure 2. TRPV3-deficient mice exhibit curly whiskers, wavy hair, misaligned hair follicles, and a thin stratum corneum.
A) Newborn (P1) V3 cKO (fl/fl: K14 Cre) mice; curly whiskers: littermate WT (V3 fl/fl) animals whiskers; straight. B) Whiskers in an adult WT mouse (P14) were straight; the whiskers of littermate V3 cKO mice were distinctively curly and hooked (upper panels). V3 cKO mice also exhibited wavy dorsal coats (lower panels). C & D) Skin and hair follicle abnormalities of V3-deficient mice revealed by H&E staining of dorsal (C) and tail (D) skin sections from WT and KO or cKO mice. In the back skin of WT pups (P4), all hair follicles lay parallel in an anterior to posterior direction with an angle of ~ 45° (left panel). In contrast, hairs of littermate cKO mice angled in different directions (right panel). Arrows indicated two misaligned horizontally-oriented hair follicles. In addition to the hair follicle abnormality, the stratum corneum (SC) layer (denoted by blue rectangle bars) of the V3 KO or cKO mice was significantly thinner but more compact than that of the WT mice. See also Figure S2.
Skin contains many cell types, including sensory nerves. However, since the K14 promoter drives the expression of Cre recombinase specifically in all keratinocytes (both follicular and inter-follicular) in the skin (Coulombe et al., 1989), the results obtained from V3 cKO, V3 fl/fl: K14 Cre mice, suggested that the defect in hair morphogenesis was due to the lack of TRPV3 expression in keratinocytes in a cell autonomous manner. Both hair and whisker phenotypes were independent of pigmentation of the hair and genetic background, as black-coated (C57BL/6) back-crossed (> 6 generations) V3 KO mice displayed similar phenotypes as mice with mixed genetic background (BL6 and 129sv) (Fig. S2). V3 KO pups were born with the expected Mendelian ratio and body weight was comparable to control mice (Fig. S2).
In histological examinations of skin from the mid-dorsal region of mice of different ages, we found that a subset of hair follicles exhibited an obvious but gentle curvature (Fig. S2). In Haematoxylin & Eosin (H&E) stained skin sections from control mice (P4), hair follicle shafts were parallel and posed roughly at a 45° angle to the subcutaneous muscle layer (Fig. 2C). In contrast, individual hair follicles of V3 cKO mice were, in many cases, gently curved and pointed in different directions with variable angles (Fig. 2C). Several hair follicles even grew horizontally to the subcutaneous muscle layer. Similar follicular derangement was obvious for V3 KO mice (Fig. S2). Notably, similar alterations in hair follicle morphology have been reported in EGFR-deficient mice (Threadgill et al., 1995). These results suggest that the wavy hair phenotype of V3 KO mice was due to a defect in follicle formation, and that mouse TRPV3 was required for normal morphogenesis of hair and whiskers. In addition to follicular abnormalities, V3 KO and cKO mice also exhibited abnormalities in the epidermal stratum corneum (Fig. 2C, 2D; Fig. S2; details see below). The hair cycle, however, was not significantly altered (Fig. S2).
Defective TGF-α/EGFR signaling in TRPV3-deficient mice
The hair and whisker phenotype of V3 KO mice resembled largely those of wa1 and wa2 mice, as well as other mouse mutations with reduced expression/release/activity in TGF-α and/or EGFR (Ballaro et al., 2005; Du et al., 2004; Luetteke et al., 1994; Luetteke et al., 1993; Mann et al., 1993; Miettinen et al., 1995; Murillas et al., 1995; Peschon et al., 1998; Schneider et al., 2008; Sibilia and Wagner, 1995; Threadgill et al., 1995) (see Fig. S3). Thus we investigated whether TGF-α and/or EGFR signaling was altered in V3 KO mice. Real time semi-quantitative PCR (q-PCR) analysis of the skin of V3 KO pups revealed that the mRNA expression level of TGF-α was half that of WT (P4; Fig. 3A). TGF-α mRNA levels of newborn (P0) V3 KO animals, though significantly less than that of the P4 mice, was comparable to that of the littermate controls (P0). Expression (mRNA) levels of several other EGFR ligands and EGFR (Fig. S3), however, were not significantly altered in the skin of V3 KO mice. These results suggest that TRPV3 affects the expression level of TGF-α in postnatal skin in vivo.
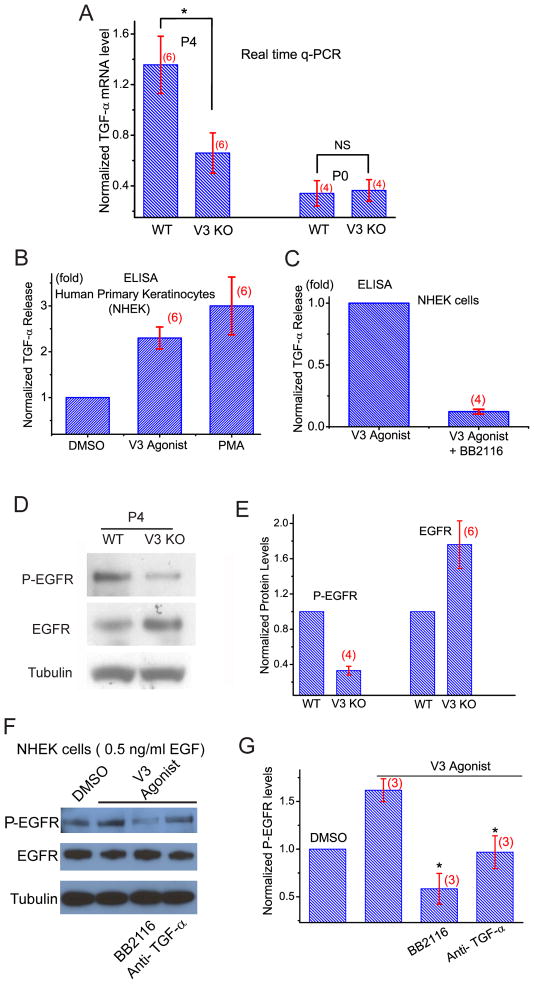
Figure 3. Reduced levels of TGF-α and decreased activity of EGFR in the skin of TRPV3-deficient mice.
A) mRNA expression levels (qPCR) of TGF-α were significantly (P < 0.05) lower in V3 KO skin tissues from P4 but not P0 mice. B) Short application of V3 agonist cocktail (100 μM 2-APB + 250 μM Carvacrol; 30 min) significantly increased TGF-α release into the culture medium from primary human keratinocytes (NHEK). TGF-α was measured with ELISA; PMA was used as a positive control. C) V3 agonist-induced TGF-α release was diminished in the presence of BB2116 (20 μM), an inhibitor of ADAM17 required for the shedding of TGF-α. D) Immunoblotting analysis of phosphorylated (active; P-EGFR) and total EGFR expression levels of WT and V3 KO skin lysates. Compared to WT mice, the level of P-EGFR was significantly decreased in V3 KO skin lysates. In contrast, the expression level of total EGFR was slightly but significantly increased in V3 KO skin lysates. E) Statistical analyses of EGFR and P-EGFR expression levels. F, G) EGFR activity (P-EGFR) was enhanced by V3 agonist cocktail (100 μM 2-APB + 250 μM Carvacrol) for 30 min. The basal activation of EGFR was induced by a minimal concentration of EGF (0.5 ng/ml). The enhancement was abolished in the presence of BB2116 (20 μM) or a neutralizing antibody against TGF-α (1 μg/ml). See also Figure S3.
Both expression and proteolytic shedding of the membrane-tethered TGF-α are known to be Ca2+ dependent (Denning et al., 2000; Horiuchi et al., 2007). We investigated the role of TRPV3 in TGF-α shedding/release using an ELISA assay optimized for human TGF-α. In the presence of TRPV3 agonist cocktail (100 μM 2-APB + 250 μM Carvacrol; 30 min), normal human epidermal keratinocytes (NHEK) released more than twice the amount of TGF-α into the culture medium. This is comparable to the effect of PMA, a stimulus well known to induce release and expression of TGF-α (Fig. 3B). ADAM17, the principal sheddase required for TGF-α release (Peschon et al., 1998), was required for V3 agonist-induced TGF-α release/shedding (Fig. 3C).
Since the level of TGF-α was reduced in V3 KO skin, one would expect that its activated receptor, phosphorylated EGFR (P-EGFR), might also be reduced. By immunoblot analysis of P-EGFR in skin lysates of V3 KO skin, EGFR activity was only about one third of that of WT controls (Fig. 3D & E). Interestingly, the expression level of total EGFR was slightly but significantly increased in V3 KO skin (1.8 ± 0.3 fold, n=6), so that the ratio of P-EGFR/total EGFR was about 5-fold less (0.20 ± 0.03, n=4) in V3 KO skin. This level of the reduction was comparable to those of the hypo-functional EGFR mutations causing “wavy” phenotypes (Du et al., 2004). Consistent with biochemical results, EGFR staining was more prominent in V3 KO frozen skin sections (Fig. S3), while P-EGFR immunostaining was weaker. These results are consistent with dramatically reduced EGFR activity in V3 KO mice and suggest that the level of total EGFR was increased as a consequence of reduced activity, tyrosine phosphorylation-dependent endocytic degradation (Schneider et al., 2008), or other compensatory mechanisms. The reduction of EGFR activity in V3 KO mice was probably due to the loss of the TRPV3 channel activity, as activation of TRPV3 in cultured keratinocytes using TRPV3 agonists resulted in increases in both TGF-α release (Fig. 3B, C) and EGFR activity (Fig. 3F & Fig. S3). Notably, the TRPV3-induced increase of EGFR activity in keratinocytes was abolished by a neutralizing TGF-α antibody or ADAM 17 sheddase inhibitor (Fig. 3F, G), suggesting that activation of TRPV3 led to an increase in TGF-α release and subsequent EGFR activation.
Regulation of TRPV3 channel activity by TGF-α/EGFR signaling
Several in vitro studies provided evidence that TRP channels are regulated by members of receptor tyrosine kinase (RTK) family (Li et al., 1999; Ramsey et al., 2006). We hypothesized that TRPV3 channel activity could also be up-regulated by EGFR signaling. In serum-starved primary keratinocytes cultured in the absence of TGF-α, Ca2+ responses could be induced by a high concentration (100 μM 2-APB + 250 μM Carvacrol), but not by a lower concentration (50 μM 2-APB + 125 μM Carvacrol), of V3 agonist cocktail (Fig. 4A, C). In the presence of TGF-α (100 ng/ml for 3–5 h), however, large [Ca2+]i increases were recorded even with a low concentration (50 μM 2-APB + 125 μM Carvacrol) of V3 agonist cocktail; responses to a high concentration of V3 agonists were comparable to those without TGF-α treatment (Fig. 4B, C). Similar results were seen with EGF (100 ng/ml) pre-treatment (Fig. S4). TGF-α/EGF treatment altered the sensitivity of TRPV3 in keratinocytes to V3 agonists, suggesting that the increased activity was at least partially mediated by increased channel gating, rather than the expression or surface expression of TRPV3 proteins. Consistent with this interpretation, the temperature–induced response (from 22 to 41°C) was also significantly larger in TGF-α-treated keratinocytes (Fig. S4). The sensitizing effect of TGF-α was most likely mediated by EGFR, as an EGFR inhibitor (AG1478) or shRNA knockdown of EGFR expression (Fig. S4), completely or largely eliminated its potentiation (Fig. 4D, 4E). Because inhibitors of PLC (U73122) and ERK (PD98059) completely or partially blocked potentiation (Fig. 4D), these pathways may underlie the sensitizing effect downstream of EGFR receptor activation. In support of this finding, shRNA knockdown of PLC-γ1 expression (Fig. S4) significantly decreased the sensitizing effect of TGF-α (Fig. 4E). Consistent with our [Ca2+] measurements, ITRPV3-L exhibited a similar dependence on TGF-α (Fig. 4F).
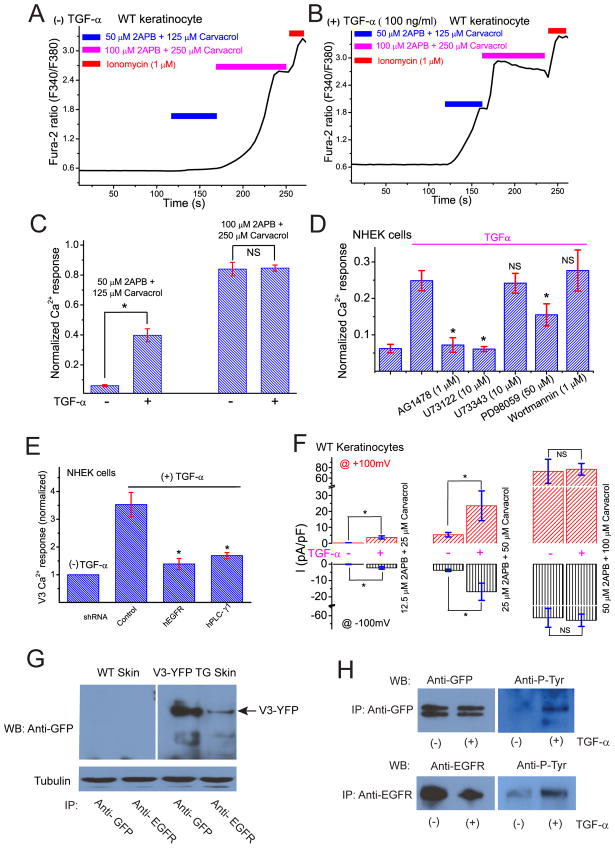
Figure 4. Activation of EGFR increases TRPV3 channel activity in cultured primary keratinocytes.
A) Weak V3 Ca2+ responses were seen in serum-starved keratinocytes without TGF-α treatment. A representative WT keratinocyte failed to respond significantly to a low concentration of V3 agonist cocktail (50 μM 2-APB + 125 μM Carvacrol). A higher concentration of V3 agonist cocktail (100 μM 2-APB + 250 μM Carvacrol), however, induced a significant increase of [Ca2+]i in the same cell. B) A representative keratinocyte that was pre-treated with TGF-α (100 ng/ml) for 3 h showed a significant response to a low concentration of V3 agonist cocktail (50 μM 2-APB + 125 μM Carvacrol). A larger increase in [Ca2+]i was seen with a higher concentration of V3 agonist cocktail (100 μM 2-APB + 250 μM Carvacrol). C) Average V3 Ca2+ responses in mouse keratinocytes with and without TGF-α pre-treatment. D) EGFR mediates the sensitizing effect of TGF-α in human primary keratinocytes in a PLC-dependent manner. TGF-α (100 ng/ml) pre-treatment significantly increased V3 Ca2+ response (by 100 μM 2-APB + 250 μM Carvacrol) in NHEK keratinocytes. In the presence of AG1478 (1 μM; a selective inhibitor of EGFR) or U73122 (10 μM, a PLC inhibitor), TGF-α failed to increase agonist-induced V3 Ca2+ responses. Partial inhibition was seen in cells treated with ERK inhibitors. E) ShRNA-mediated knockdown of EGFR or PLC-γ abrogates the sensitizing effect of TGF-α in human primary keratinocytes. F) TGF-α pre-treatment significantly increased V3 current in keratinocytes in response to a low concentration, but not at high concentrations of V3 agonists. G) Co-immunoprecipitation of TRPV3 and EGFR in skin tissues from V3-YFP transgenic mice. Immunoprecipitates (IP) were formed with the indicated antibodies and visualized on Western blot (WB). TRPV3-YFP recognized by monoclonal anti-GFP; TRPV3-YFP band indicated by arrow. EGFR was IP’d by polyclonal anti-EGFR. H) TGF-α–induced tyrosine phosphorylation of TRPV3 in HEK293 cells. HEK293 cells were transiently transfected with the cDNAs of TRPV3-GFP and EGFR and treated with, or without, TGF-α (100 ng/ml) as shown. TRPV3-GFP was IP’d by a monoclonal anti-GFP and WB’d by a pan- phosphotyrosine antibody. EGFR was IP’d by polyclonal anti-EGFR and WB’d by a pan- phosphotyrosine antibody. See also Figure S4.
The results presented so far raise the possibility that TRPV3 and EGFR might be in a signaling complex. To test this hypothesis, co-immunoprecipitation (co-IP) experiments were first performed in a heterologous expression system. In cells transfected with TRPV3, either alone or together with EGFR, both endogenous (data not shown) and overexpressed EGFR (Fig. S4), were found to co-IP with TRPV3. Next, we confirmed this finding in keratinocytes (Fig. 4G) using skin tissues from TRPV3-YFP transgenic mice (Huang et al., 2008). These results suggest that EGFR can directly or indirectly associate with TRPV3 in both heterologous and native systems. Consistent with the close association of these molecules, we found that activation of EGFR with TGF-α resulted in tyrosine-phosphorylation of TRPV3 (Fig. 4H).
Altered keratinocyte differentiation in TRPV3-deficient mice
EGFR signaling is known to have at least two distinct functions in epidermis (Schneider et al., 2008). In the basal layer, TGF-α/EGFR signaling promotes keratinocyte proliferation (Schneider et al., 2008). The function of EGFR signaling in suprabasal cells is to promote late terminal differentiation (Ballaro et al., 2005; Dlugosz et al., 1994; Peus et al., 1997; Wakita and Takigawa, 1999). While proliferating keratinocytes in the basal layer express structural keratins K5/K14, differentiating keratinocytes express the structural keratins K1/K10 (Byrne et al., 2003). As keratinocytes move closer to the skin surface, expression of K1/K10 declines and loricrin expression increases (Byrne et al., 2003), as they undergo cornification. Consistent with previous studies (Wakita and Takigawa, 1999), we observed that EGF significantly reduced the expression of the early differentiation marker, K1, in suspended keratinocytes (Fig. S5). We reasoned that a reduced rate of autonomous EGFR-dependent proliferation or terminal differentiation would accelerate keratinocyte early differentiation in V3 KO cells. Compared to WT skin sections, V3 KO epidermis exhibited a > 2-fold increase in the thickness of the K1-positive layer (Fig. 5A), while cell sizes and densities were normal (Fig. S5). Similar results were reported in transgenic mice with defective TGF-α/EGFR signaling, or keratinocytes cultured in the presence of EGFR inhibitors (Ballaro et al., 2005; Peus et al., 1997). No significant change was observed in the K14 layer of V3 KO epidermis (Fig. 5B). Loricrin expression was relatively less elevated in V3 KO epidermis (Fig. 5C). Consistent with the increased early differentiation in V3 KO epidermis, thickness of K10- (interaction partner of K1) layer also increased in V3 KO animals (Fig. 5D). Finally, V3 cKO animals exhibited similar alterations in keratinocyte differentiation (Fig. 5E).
Figure 5. Genetic inactivation of TRPV3 results in increased expression of early epidermal differentiation markers in skin.
A–E) Immunofluorescence analyses of frozen skin sections from P4 pups. A, A′) Compared to WT mice, the immunofluorescence of keratin protein 1 (K1; a keratinocyte structural protein and a marker for the differentiating spinous and granular layers) was elevated in V3 KO skin sections. Integrin α6 antibody labeled the basement membrane, the boundary between epidermis and dermis. DAPI is a nuclear marker. The K1-positive layer was 2-fold thicker in V3 KO epidermis (quantified in the A′ panel). B, B′) Normal immunofluorescence of keratin protein 14 (K14; a keratinocyte structural protein and a marker for the proliferating basal layer). C, C′) Slightly but significantly elevated loricrin (a marker for the differentiating granular layer) immunofluorescence in V3 KO epidermis. D, D′) Elevated keratin protein 10 (K10; a keratin protein associated with K1 immunofluorescence in V3 KO epidermis. E, E′) Elevated K1 immunofluorescence in V3 cKO epidermis. See also Figure S5.
Ca2+ is an important regulator of keratinocyte differentiation both in vitro and in vivo (Yuspa et al., 1989). We next examined Ca2+-dependent keratinocyte differentiation in vitro using a well-established Ca2+ switch protocol. In these experiments, epidermal basal cells were selectively cultured in 0.05 mM Ca2+ medium and terminal differentiation was induced by raising [Ca2+]o to 0.2–1.4 mM (Yuspa et al., 1989). Compared to WT cells, more loricrin was expressed in cultured V3 KO keratinocytes after induction of differentiation (Fig. S5). Collectively, these results suggested a role of TRPV3 in keratinocyte differentiation both in vitro and in vivo.
Defective epidermal barrier function and TGase activity in TRPV3-deficient mice
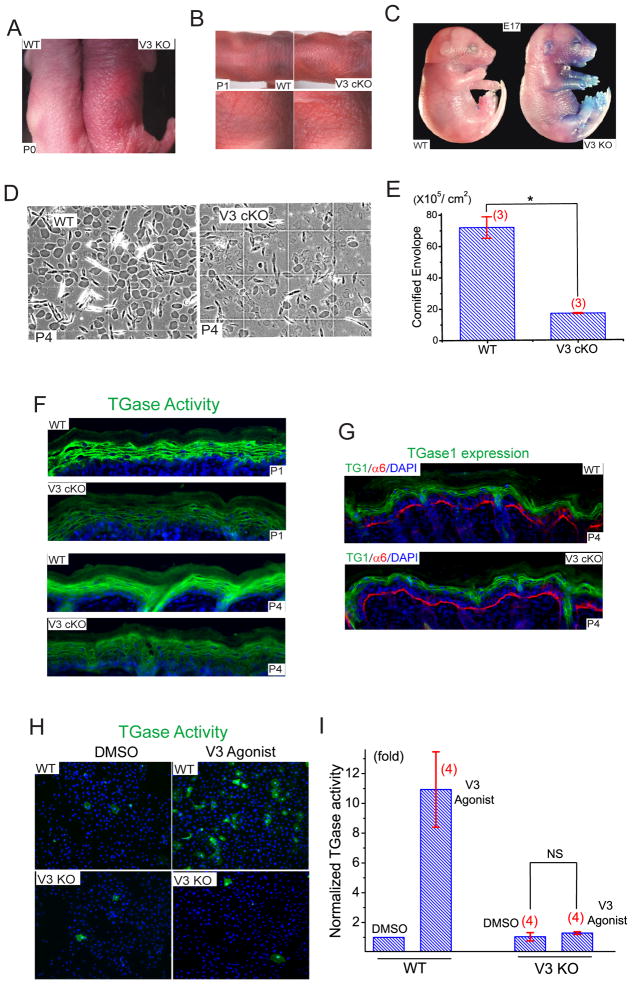
In addition to hair abnormalities, the skin of newborn V3 KO and cKO mice was red in color (erythroderma), dry, and scaly (Fig. 6A & B), resembling the skin phenotype of mice with defective barrier formation (Koch et al., 2000; Sevilla et al., 2007). Before hair penetration (P0-P3), the skin of V3 KO mice was rougher and shinier than WT. To measure skin barrier integrity, we used toluidine blue exclusion. In newborn (P0) V3 WT, KO, and E17 WT mice, dye was almost completely excluded, indicating normal maturation of the skin barrier. In E17 V3 KO embryos, however, dye permeability was significant, particularly in ventral areas (Fig. 6C). EGF is known to increase the thickness of stratum corneum (Ponec et al., 1997), and consistent with a role for TRPV3 in barrier formation, the stratum corneum layer of V3 KO (Fig. S2) and cKO (Fig. 2C, 2D) mice, was significantly thinner and more compact than WT littermates. We next examined the morphology of the mature cornified envelope (CE). Mature CE in WT skin was symmetrical and smooth while immature CE was irregular and fragile. The density of mature CE in V3 KO (Fig. S6) and cKO mice (Fig. 6D, 6E) was only 15–18 % of that in WT mice.
Figure 6. Defective barrier formation and Ddiminished TGase activity in the skin of TRPV3-deficient mice.
A) Compared to newborn (P0) WT mice (on the left), V3 KO skin was dry, reddened, and scaly. B) Similar dry and scaly skin was also seen in neonatal (P1) cKO mice. C) Toluidine blue dye exclusion assay of embryonic day 17 (E17) embryos. Staining indicates dye permeability and defective or immature barrier function. The upper and lower halves of the pictures were taken separately, but shown in combination for the purpose of illustration. D, E) Compared to WT littermate pups (P4), the cornified cell envelopes (CE) of skins of V3 cKO pups were significantly less mature. F) Compared to WT littermates, reduced TGase activity was detected in the frozen skin sections of neonatal (P1; upper two panels) V3 cKO mice. TGase activity was detected using an immunofluorescence-coupled in situ enzymatic assay. Positive staining was restricted to the granular layer of epidermis. Reduced TGase activity in the P4 (lower two panels) skin of V3 cKO. G) Expression levels of TGase1 were comparable for both WT and V3 cKO mice. H) Short (40 min) application of V3 agonist cocktail (50 μM 2-APB + 200 μM Carvacrol) dramatically increased TGase activity in primary cultured keratinocytes from WT, but not V3 KO mice. I) V3 agonist cocktail induced an ~11-fold increase of TGase activity in WT keratinocytes. See also Figure S6.
Multiple mechanisms might lead to defective CE formation. TGases are a family of enzymes that cross-link proteins essential for CE formation (Lorand and Graham, 2003). Among them, TGase1 and TGase3, are expressed in the epidermis and are regulated by intracellular Ca2+ (Lorand and Graham, 2003). One possibility is that the activity of transglutaminases (TGases) was reduced in V3 KO mice. EGF or TGF-α is known to dramatically increase the activity of TGases and CE formation in cultured keratinocytes in suspension, which presumably mimics the conditions of suprabasal keratinocytes (Wakita and Takigawa, 1999). We found that the activity of TGases (Koch et al., 2000; Raghunath et al., 1998), was significantly lower in both newborn (P1) and P4 V3 cKO epidermis (Fig. 6F), but not in age-matched V1 and V4 KO epidermis (Fig. S6). The expression level of TGase1, however, was comparable for WT and V3 cKO (Fig. 6G). When TGase activity was measured in cultured keratinocytes, TGase activity was abnormally low in both WT and V3 KO keratinocytes (Fig. 6H, 6I). Application of V3 agonist cocktail dramatically increased TGase activity (>10 fold) in WT, but not V3 KO cells. Application of V1 and V4 agonists resulted in either no change, or a slight increase, in TGase activity in WT keratinocytes (Fig. S6). These results suggest that activation of TRPV3 increased intracellular [Ca2+], TGase activity, and subsequent CE formation in the epidermis.
Discussion
Intracellular Ca2+ regulates both the production and release of EGFR ligands (Denning et al., 2000; Dlugosz et al., 1994). Since TGF-α production/release is an auto-induction process (Coffey et al., 1987), the reduced production of TGF-α might result from reduced EGFR activity. The positive feedback loop (Fig. 7) in which TGF-α/EGFR activation potentiates TRPV3-mediated Ca2+ entry, which in turn potentiates TGF-α/EGFR signaling, may provide an explanation for its unique property of auto-induction (Coffey et al., 1987). Ca2+-induced differentiation dramatically increases TGF-α production, suggesting a non-proliferative role of the TGF-α/EGFR signaling axis (Denning et al., 2000). On the other hand, EGF/TGF-α itself is known to promote late terminal differentiation both in vitro and in vivo, by dramatically increasing TGase activity and CE formation while suppressing the expression of K10 (Dlugosz et al., 1994; Wakita and Takigawa, 1999). Thus, for suprabasal cells, the function of EGFR signaling is to promote late terminal differentiation (Wakita and Takigawa, 1999). The primary defect in V3 KO mice is late terminal differentiation, but not proliferation. Thus, the feed-forward mechanism described above may contribute to the process of late terminal differentiation.
Figure 7. A working model for the role of TRPV3 in keratinocyte cell biology.
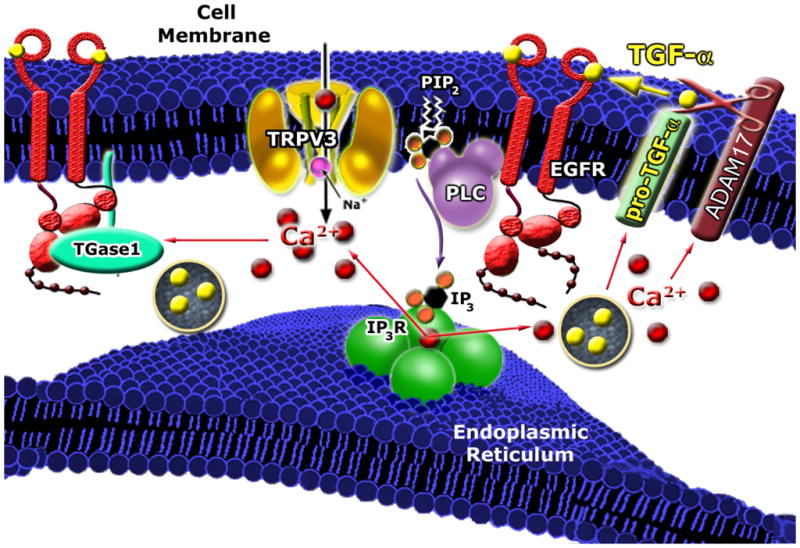
Activation of TRPV3 in vivo (potentially by an endogenous mechanism such as temperature or other unidentified cellular cues) may lead to an increase of Ca2+-dependent production/shedding/release of TGF-α or other EGFR ligands and an elevation of TGase (TGase 1 and TGase 3) activity. TGF-α in turn activates EGFR that physically associates with TRPV3 to form a signaling complex, which subsequently sensitizes TRPV3’s responses to the putative endogenous activation mechanism(s). Thus, a positive-feedback loop is formed between TRPV3 and TGF-α/EGFR. The combined function(s) of the TRPV3/ADAM17/EGFR/TGase complex may lead to terminal differentiation of suprabasal keratinocytes. Impairment of TRPV3/EGFR signaling leads to a “wavy hair” phenotype. TRPV3/ADAM17/EGFR/TGases signaling is required for skin barrier formation; reduced activity leads to a “dry skin” phenotype. Dysregulation of the TRPV3/ADAM17/EGFR/TGase signaling axis might also lead to other skin diseases.
It is still not clear how TRPV3 is activated to trigger or promote late terminal differentiation. Temperatures in the range of 31–39 °C activate TRPV3 in heterologous expression systems (Ramsey et al., 2006). Thus temperature may be the primary activator for keratinocyte TRPV3. Consistent with this notion, temperature is known to affect the barrier function and modulate the effect of EGF/TGF-α on keratinocyte differentiation (Denda et al., 2007; Ponec et al., 1997). At skin temperatures in vivo (~32 °C), TRPV3 is constitutively but weakly active. Thus release of TGF-α may increase the activity of weakly constitutively active TRPV3.
We provide evidence that TRPV3 and TGF-α/EGFR are in the same signaling complex regulating epidermal homeostasis. While loss-of-function in TGF-α/EGFR leads to “wavy hair” (Luetteke et al., 1994; Luetteke et al., 1993; Mann et al., 1993; Schneider et al., 2008; Sibilia and Wagner, 1995; Threadgill et al., 1995), elevated TGF-α/EGFR activities cause a “hairless” phenotype (Ferby et al., 2006; Schneider et al., 2008; Wang et al., 2006). Interestingly, while our V3 KO mice exhibit “wavy hair”, mice carrying a gain-of-function mutation in TRPV3 are hairless (Asakawa et al., 2006). It may prove informative to generate transgenic mice with TRPV3 loss-of-function and concurrent TGF-α/EGFR gain-of-function, or with TRPV3 gain-of-function and concurrent TGF-α/EGFR loss-of-function.
The EGFR is the prototype of the Receptor Tyrosine Kinase (RTK) family. EGFR signaling is necessary for proper development and tissue homeostasis while its dysregulation rapidly results in defects in cellular proliferation and differentiation. The consequences of its malfunction are abnormal hair follicle morphogenesis, impaired would healing, and tumorigenesis (Schneider et al., 2008). We have identified another key element in this important signaling pathway, the TRPV3 channel. Our studies not only provide the first in vivo evidence in mammals for the close interaction of RTK and TRP channels, but also suggest that TRPV3 can be a novel target for hair growth and removal agents, and as well as in treatment of skin cancers or other dermatological diseases.
Experimental Procedures
Conditional and global disruption of Trpv3 in mice
Mouse Trpv3 was disrupted either globally or in a keratinocytes-specific manner (see Extended Experimental Procedures in the Supplementary Information).
Real-Time Semi-Quantitative PCR
After a small piece of back skin was dissolved in TRIzol® (Invitrogen, Carlsbad, CA), mRNA was purified using RNeasy columns (Qiagen Inc., Valencia, CA). First-strand cDNA was synthesized using Superscript III RT (Invitrogen) and utilized for Semi-Quantitative PCR based on intron-spanning primers. A Bio-Rad iQ iCycler was used to measure the expression level of transcripts. The primer sequences were provided in Extended Experimental Procedures in the Supplementary Information.
Preparation and culture of mouse keratinocytes
Mice (P0-P2) were sacrificed and soaked in 10% povidone-iodine for 5 min. After rinsing in 70% ethanol multiple times, the skin was removed and placed in a Petri dish containing PBS solution with 0.25% trypsin (Invitrogen) for incubating at 4 °C overnight. Epidermis was then separated from the subcutaneous tissues. Vortexing dissociated cells and keratinocytes were first plated in a high [Ca2+]o (1.4 mM) minimal essential medium (MEM; GIBCO), which was replaced with a low [Ca2+]o (0.05 mM; differentiation-restricted) medium after 6 h. The keratinocytes were then cultured in MEM containing 8% Chelex-treated (Bio-Rad) FBS with the final [Ca2+] adjusted to 0.05mM. Suspension cultures were on polyhydroxyethylmethacrylate (poly-HEMA)-coated plates as described previously (Wakita and Takigawa, 1999).
NHEK cell culture
Normal human epidermal keratinocytes were obtained from Invitrogen and cultured in EpiLife Medium supplemented with Human Keratinocyte Growth Supplement (Invitrogen).
Immunoblotting and Immunoprecipitation
Back skin lysates for the immunodetection of EGFR and P-EGFR were prepared as follows: a small piece of back skin was lysed on ice for 30 min using 1 ml of lysis buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 1 mM NaF, 1 mM Na3VO4, 1 mM PMSF, 0.25% sodium deoxycholate, and 1X protease inhibitor cocktail). Immuoprecipitation and immuoblotting were then performed using skin lysates as described in Extended Experimental Procedures in the Supplementary Information..
Histology and Immunostaining
Tissues were fixed for 3 h with 4% paraformaldehyde (PFA) at 4°C, embedded in OCT or paraffin. Sections (~ 4 μm) of paraffin embedded tissues were stained with hematoxylin and eosin (H&E). Immunohistochemistry was performed on cryostat sections (~10 μm) using antibody dilutions described in Extended Experimental Procedures in the Supplementary Information.
Dye Exclusion Assays
Toluidine-blue staining of mouse embryos and newborn pups was performed as described in the Supplementary Information.
In vivo and in vitro Transglutaminase Activity Assay
Detection of TGase activity in skin sections (in vivo) and cultured keratinocytes (in vitro) used the amine donor substrate monodansylcadaverine (Molecular Probes) as described in Extended Experimental Procedures.
Analysis of Cornified Envelopes
A piece of back skin (P4) was isolated and treated as described previously (Koch et al., 2000). Briefly, CEs were prepared by boiling skin for 30–60 min in a buffer consisting of 20 mM Tris-HCl, pH 7.5, 5 mM EDTA, 10 mM DTT, and 2% SDS. After centrifugation (5,000 g), CEs were washed twice at room temperature with a buffer consisting of 20 mM Tris-HCl, pH 7.5, 5 mM EDTA, 10 mM DTT, and 0.2% SDS. The density of CE was manually determined using a hemacytometer.
TGF-α ELISA
The medium of near confluent NHEK keratinocytes was harvested for TGF-α measurements using an ELISA kit for human TGF-α (Calbiochem).
Electrophysiology
Whole-cell recordings were performed in primary keratinocytes. Details of recording conditions are described in Extended Experimental Procedures.
Ca2+ imaging
Mouse and NHEK primary keratinocytes were loaded with 5 μM Fura-2 AM in culture medium at 37°C for 60 min. Cells were then washed in modified Tyrode’s solution for 10–30 min. Fluorescence at different excitation wavelengths was recorded on an EasyRatioPro system (Photon Technology International, Birmingham, NJ). Fura-2 ratios (F340/F380) recorded changes in [Ca2+]i upon stimulation. Ionomycin (1 μM) was added at the conclusion of all experiments to induce a maximal response for comparison.
Data analysis
Data are presented as the mean ± SEM. Statistical comparisons were made using analysis of variance (ANOVA). A P value < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work is supported by HHMI (to N.C.A & D.E.C), startup funds to H.X. from the Department of MCDB and Biological Science Scholar Program, the University of Michigan, and NIH RO1 grants (NS062792 to H.X and AR045973 to A.A.D). We thank Dr. Leonidas Tsiokas for the EGFR construct, Dr. Nan Hatch and Dr. Dave Ornitz for the FGFR2 constructs, and Dr. Makato Suzuki for TRPV4 KO mice. We are grateful to S. Hein, X. Wang (Rockefeller University), X. Wang, E. Mills, R. Hume, J. Kuwada, M. Akaaboune, and C. Collins for assistance, and L. Yue and D. Ren for comments on an earlier version of the manuscript. We appreciate the encouragement and helpful comments from other members of the Xu and Clapham laboratories.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asakawa M, Yoshioka T, Matsutani T, Hikita I, Suzuki M, Oshima I, Tsukahara K, Arimura A, Horikawa T, Hirasawa T, et al. Association of a mutation in TRPV3 with defective hair growth in rodents. J Invest Dermatol. 2006;126:2664–2672. doi: 10.1038/sj.jid.5700468. [DOI] [PubMed] [Google Scholar]
- Ballaro C, Ceccarelli S, Tiveron C, Tatangelo L, Salvatore AM, Segatto O, Alema S. Targeted expression of RALT in mouse skin inhibits epidermal growth factor receptor signalling and generates a Waved-like phenotype. EMBO reports. 2005;6:755–761. doi: 10.1038/sj.embor.7400458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne C, Hardman M, Nield K. Covering the limb--formation of the integument. Journal of anatomy. 2003;202:113–123. doi: 10.1046/j.1469-7580.2003.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J Biol Chem. 2004;279:21569–21575. doi: 10.1074/jbc.M401872200. [DOI] [PubMed] [Google Scholar]
- Coffey RJ, Jr, Derynck R, Wilcox JN, Bringman TS, Goustin AS, Moses HL, Pittelkow MR. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. Nature. 1987;328:817–820. doi: 10.1038/328817a0. [DOI] [PubMed] [Google Scholar]
- Coulombe PA, Kopan R, Fuchs E. Expression of keratin K14 in the epidermis and hair follicle: insights into complex programs of differentiation. J Cell Biol. 1989;109:2295–2312. doi: 10.1083/jcb.109.5.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denda M, Sokabe T, Fukumi-Tominaga T, Tominaga M. Effects of skin surface temperature on epidermal permeability barrier homeostasis. J Invest Dermatol. 2007;127:654–659. doi: 10.1038/sj.jid.5700590. [DOI] [PubMed] [Google Scholar]
- Denning MF, Dlugosz AA, Cheng C, Dempsey PJ, Coffey RJ, Jr, Threadgill DW, Magnuson T, Yuspa SH. Cross-talk between epidermal growth factor receptor and protein kinase C during calcium-induced differentiation of keratinocytes. Experimental dermatology. 2000;9:192–199. doi: 10.1034/j.1600-0625.2000.009003192.x. [DOI] [PubMed] [Google Scholar]
- Dlugosz AA, Cheng C, Denning MF, Dempsey PJ, Coffey RJ, Jr, Yuspa SH. Keratinocyte growth factor receptor ligands induce transforming growth factor alpha expression and activate the epidermal growth factor receptor signaling pathway in cultured epidermal keratinocytes. Cell Growth Differ. 1994;5:1283–1292. [PubMed] [Google Scholar]
- Du X, Tabeta K, Hoebe K, Liu H, Mann N, Mudd S, Crozat K, Sovath S, Gong X, Beutler B. Velvet, a dominant Egfr mutation that causes wavy hair and defective eyelid development in mice. Genetics. 2004;166:331–340. doi: 10.1534/genetics.166.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferby I, Reschke M, Kudlacek O, Knyazev P, Pante G, Amann K, Sommergruber W, Kraut N, Ullrich A, Fassler R, et al. Mig6 is a negative regulator of EGF receptor-mediated skin morphogenesis and tumor formation. Nat Med. 2006;12:568–573. doi: 10.1038/nm1401. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet. 2002;3:199–209. doi: 10.1038/nrg758. [DOI] [PubMed] [Google Scholar]
- Horiuchi K, Le Gall S, Schulte M, Yamaguchi T, Reiss K, Murphy G, Toyama Y, Hartmann D, Saftig P, Blobel CP. Substrate selectivity of epidermal growth factor-receptor ligand sheddases and their regulation by phorbol esters and calcium influx. Molecular biology of the cell. 2007;18:176–188. doi: 10.1091/mbc.E06-01-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HZ, Gu Q, Wang C, Colton CK, Tang J, Kinoshita-Kawada M, Lee LY, Wood JD, Zhu MX. 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem. 2004;279:35741–35748. doi: 10.1074/jbc.M404164200. [DOI] [PubMed] [Google Scholar]
- Huang SM, Lee H, Chung MK, Park U, Yu YY, Bradshaw HB, Coulombe PA, Walker JM, Caterina MJ. Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J Neurosci. 2008;28:13727–13737. doi: 10.1523/JNEUROSCI.5741-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch PJ, de Viragh PA, Scharer E, Bundman D, Longley MA, Bickenbach J, Kawachi Y, Suga Y, Zhou Z, Huber M, et al. Lessons from loricrin-deficient mice: compensatory mechanisms maintaining skin barrier function in the absence of a major cornified envelope protein. J Cell Biol. 2000;151:389–400. doi: 10.1083/jcb.151.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HS, Xu XZ, Montell C. Activation of a TRPC3-dependent cation current through the neurotrophin BDNF. Neuron. 1999;24:261–273. doi: 10.1016/s0896-6273(00)80838-7. [DOI] [PubMed] [Google Scholar]
- Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nature reviews. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- Luetteke NC, Phillips HK, Qiu TH, Copeland NG, Earp HS, Jenkins NA, Lee DC. The mouse waved-2 phenotype results from a point mutation in the EGF receptor tyrosine kinase. Genes & development. 1994;8:399–413. doi: 10.1101/gad.8.4.399. [DOI] [PubMed] [Google Scholar]
- Luetteke NC, Qiu TH, Peiffer RL, Oliver P, Smithies O, Lee DC. TGF alpha deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell. 1993;73:263–278. doi: 10.1016/0092-8674(93)90228-i. [DOI] [PubMed] [Google Scholar]
- Mann GB, Fowler KJ, Gabriel A, Nice EC, Williams RL, Dunn AR. Mice with a null mutation of the TGF alpha gene have abnormal skin architecture, wavy hair, and curly whiskers and often develop corneal inflammation. Cell. 1993;73:249–261. doi: 10.1016/0092-8674(93)90227-h. [DOI] [PubMed] [Google Scholar]
- Miettinen PJ, Berger JE, Meneses J, Phung Y, Pedersen RA, Werb Z, Derynck R. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995;376:337–341. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- Montell C. The TRP superfamily of cation channels. Sci STKE 2005. 2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- Murillas R, Larcher F, Conti CJ, Santos M, Ullrich A, Jorcano JL. Expression of a dominant negative mutant of epidermal growth factor receptor in the epidermis of transgenic mice elicits striking alterations in hair follicle development and skin structure. The EMBO journal. 1995;14:5216–5223. doi: 10.1002/j.1460-2075.1995.tb00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiological reviews. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Pandiella A, Massague J. Multiple signals activate cleavage of the membrane transforming growth factor-alpha precursor. J Biol Chem. 1991;266:5769–5773. [PubMed] [Google Scholar]
- Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, et al. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- Peus D, Hamacher L, Pittelkow MR. EGF-receptor tyrosine kinase inhibition induces keratinocyte growth arrest and terminal differentiation. J Invest Dermatol. 1997;109:751–756. doi: 10.1111/1523-1747.ep12340759. [DOI] [PubMed] [Google Scholar]
- Ponec M, Gibbs S, Weerheim A, Kempenaar J, Mulder A, Mommaas AM. Epidermal growth factor and temperature regulate keratinocyte differentiation. Archives of dermatological research. 1997;289:317–326. doi: 10.1007/s004030050198. [DOI] [PubMed] [Google Scholar]
- Raghunath M, Hennies HC, Velten F, Wiebe V, Steinert PM, Reis A, Traupe H. A novel in situ method for the detection of deficient transglutaminase activity in the skin. Archives of dermatological research. 1998;290:621–627. doi: 10.1007/s004030050362. [DOI] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annual review of physiology. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Nelson KG, Snedeker S, Bossert NL, Walker MP, McLachlan J, DiAugustine RP. Expression of epidermal growth factor in suprabasal cells of stratified squamous epithelia: implications for a role in differentiation. Cell Growth Differ. 1994;5:527–535. [PubMed] [Google Scholar]
- Schneider MR, Werner S, Paus R, Wolf E. Beyond wavy hairs: the epidermal growth factor receptor and its ligands in skin biology and pathology. The American journal of pathology. 2008;173:14–24. doi: 10.2353/ajpath.2008.070942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilla LM, Nachat R, Groot KR, Klement JF, Uitto J, Djian P, Maatta A, Watt FM. Mice deficient in involucrin, envoplakin, and periplakin have a defective epidermal barrier. J Cell Biol. 2007;179:1599–1612. doi: 10.1083/jcb.200706187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibilia M, Wagner EF. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science. 1995;269:234–238. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC, et al. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- Wakita H, Takigawa M. Activation of epidermal growth factor receptor promotes late terminal differentiation of cell-matrix interaction-disrupted keratinocytes. J Biol Chem. 1999;274:37285–37291. doi: 10.1074/jbc.274.52.37285. [DOI] [PubMed] [Google Scholar]
- Wang X, Bolotin D, Chu DH, Polak L, Williams T, Fuchs E. AP-2alpha: a regulator of EGF receptor signaling and proliferation in skin epidermis. J Cell Biol. 2006;172:409–421. doi: 10.1083/jcb.200510002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zinkel S, Polonsky K, Fuchs E. Transgenic studies with a keratin promoter-driven growth hormone transgene: prospects for gene therapy. Proc Natl Acad Sci U S A. 1997;94:219–226. doi: 10.1073/pnas.94.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, et al. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci. 2006;9:628–635. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- Yuspa SH, Kilkenny AE, Steinert PM, Roop DR. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J Cell Biol. 1989;109:1207–1217. doi: 10.1083/jcb.109.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.