Abstract
OBJECTIVE
To compare the mortality of patients with an acute Charcot foot with a matched population with uninfected neuropathic foot ulcers (NFUs).
RESEARCH DESIGN AND METHODS
Data were extracted from a specialist departmental database, supplemented by hospital records. The findings were compared with the results of earlier populations with Charcot foot and uninfected NFUs managed from 1980. Finally, the results of all patients with acute Charcot foot and all control subjects managed between 1980 and 2007 were compared with normative mortality data for the U.K. population.
RESULTS
A total of 70 patients presented with an acute Charcot foot (mean age 57.4 ± 12.0 years; 48 male [68.6%]) between 2001 and 2007; there were 66 matched control subjects. By 1 October 2008, 13 (eight male; 18.6%) patients with a Charcot foot had died, after a median of 2.1 years (interquartile range 1.1–3.3). Twenty-two (20 male; 33.3%) control subjects had also died after a median of 1.3 years (0.6–2.5). There was no difference in survival between the two groups (log-rank P > 0.05). Median survival of all 117 patients with acute Charcot foot managed between 1980 and 2007 was 7.88 years (4.0–15.4) and was not significantly different from the control NFU patients (8.43 years [3.4–15.8]). When compared with normative U.K. population data, life expectancy in the two groups was reduced by 14.4 and 13.9 years, respectively.
CONCLUSIONS
These data confirm that the mortality in patients presenting to our unit with either an acute Charcot foot and an uninfected neuropathic ulcer was unexpectedly high.
Three studies of the long-term follow-up of acute Charcot neuropathic osteoarthropathy have been published. Two of them describe a very low mortality with, respectively, no deaths in 55 patients followed for a mean 92.6 weeks (1) and two deaths in 115 patients followed for 4 years (2). These findings conflict with those of our own earlier study (3), in which the outcome in 47 patients was compared with a matched population with uninfected neuropathic foot ulcers (NFUs) (44.7% of patients with Charcot's disease died after a mean 3.7 years mean follow-up, which was not significantly different from a mortality of 34.0% after 3.1 years in the control group). The reason for the much higher mortality in our earlier series is not clear but could reflect population selection. The aim of the present study was to attempt to examine the clinical outcome in a more recent series of patients managed at the same unit. If the high mortality of both the Charcot patients and the NFU control subjects was not confirmed, it might be explained by unreliability of our earlier observations, or it could reflect more comprehensive strategies to reduce cardiovascular risk in recent years (4). If, on the other hand, the mortality was shown to be equally poor in the new population, it should prompt a search for possible explanations.
RESEARCH DESIGN AND METHODS
Data on patients with diabetes referred to the specialist multidisciplinary outpatient foot care service at the city hospital campus of Nottingham University Hospitals National Health Service Trust have been recorded since 1982. The number of referrals to the service has increased progressively, as has the completeness of the dataset. The latest version of the database dates from 2000, holds details on ∼1,800 patients, and has been approved by the Caldicott Guardian of the Trust for the purposes of audit. Demographic and clinical details are recorded at the time of presentation of each new episode.
Each diabetic foot lesion is classified, according to the size (area and depth), sepsis, arteriopathy, and denervation [S(AD)SAD] system (5), in which cases of acute Charcot foot are specifically identified. The diagnosis of an acute Charcot foot was based on the presence of unexplained subacute inflammation with fracture or dislocation visible on plain X-ray. In more recent years, magnetic resonance imaging (MRI) was used to define the presence of marrow edema if there were no changes apparent on plain X-ray. In those with an associated ulcer, the diagnosis of osteomyelitis was excluded using conventional clinical, biochemical, and radiological tools. Outcome was determined on 1 October 2008 using the specialist database, the hospital database that is automatically updated with the date of death when it occurs and the hardcopy case records when necessary. Renal dysfunction was determined retrospectively by examination of case records and defined for the purposes of this study as an estimated glomerular filtration rate (Modification of Diet in Renal Disease formula) <30 ml/min or as a serum creatinine concentration exceeding 130 μmol/l on more than one occasion.
The cohort comprised all patients with newly diagnosed Charcot foot, presenting between January 2000 and October 2007. If a patient had bilateral disease, only the first episode was considered. Although the new population excluded any Charcot cases included in the earlier study of patients managed between 1980 and 2000 (3), there were four patients who had served as control subjects in the earlier series and who subsequently developed an acute Charcot foot and were included as index patients. The database was searched for control subjects who were matched by sex, diabetes type, age (±2 years), and diabetes duration at onset of the foot lesion (±2 years), who had no history of Charcot neuroparthropathy and who had palpable foot pulses and presented to the foot clinic with an uninfected NFU in the same period, and outcomes were compared. In addition, life expectancy was determined in the total series of patients with either a Charcot foot or NFU managed between 1980 and 2007 and compared with sex- and age-matched control subjects using the interim life tables of the U.K. Office for National Statistics.
Statistical analysis
Statistical analyses were performed using SPSS version 17.0 for Windows. The Student t test was used to assess differences in continuous variables when normally distributed, while the χ2 test was used for categorical variables. Kaplan-Meier survival curves were generated for the cohort, and the log-rank test was used to test equality of survivor functions between the various groups. Cox proportional hazards models were used to test whether there were significant differences in mortality risk.
RESULTS
2000–2007 cohort
A total of 70 eligible patients were identified who presented with an acute Charcot foot between 1 January 2000 and 31 October 2007. Forty-eight (68.6%) were male and 49 (70.0%) had type 2 diabetes. The mean (±SD) duration of diabetes until the onset of the Charcot foot was 16.1 ± 9.8 years, and mean age at the date of onset was 57.4 ± 12.0 years (Table 1). It was possible to identify control patients with uncomplicated neuropathic foot ulcers for 66 of 70 subjects. Forty-eight (72.2%) of the control patients were male and 17 (25.8%) patients had type 1 diabetes. Mean age was 58.1 ± 12.2 years, and mean duration of diabetes was 15.8 ± 9.6 years.
Table 1.
Baseline characteristic of patients for the current study, as well as for the total 1980–2000 cohort
| 2000–2007 |
2000–2007 |
1980–2007 |
1980–2007 |
|
|---|---|---|---|---|
| Charcot | Neuropathic ulcer | Charcot | Neuropathic ulcer | |
| n | 70 | 66 | 117 | 109 |
| n (%) male | 48 (68.6) | 48 (72.7) | 74 (63.2) | 73 (67.0) |
| n (%) type 2 diabetic | 49 (70.0) | 49 (74.2) | 78 (66.7) | 77 (70.6) |
| Mean age ± SD (years) | 57.4 ± 12.0 | 58.1 ± 12.2 | 58.1 ± 12.5 | 58.6 ± 12.5 |
| Mean diabetes duration ± SD (years) | 16.1 ± 9.8 | 15.8 ± 9.6 | 16.1 ± 10.4 | 15.5 ± 10.4 |
| Renal dysfunction (n) | 25* | 28 | 49† | 43‡ |
Renal dysfunction: serum creatinine concentration >130 μmol/l or estimated glomerular filtration rate <30 ml/min on two or more occasions.
*Missing data in two subjects.
†Missing data in four subjects.
‡Missing data in eight subjects.
Table 2.
Mortality and median survival periods for patients presenting with an acute Charcot foot or with an uncomplicated neuropathic ulcer
| 2000–2007 |
1980–2007 |
|||
|---|---|---|---|---|
| Charcot | Neuropathic ulcer | Charcot | Neuropathic ulcer | |
| Median follow-up (interquartile range) to 1 October 2008 | 3.0 (1.9–4.5) | 4.4 (3.2–6.3) | 3.4 (2.0–6.8) | 5.5 (3.3–8.4) |
| Median follow-up (interquartile range) to death | 2.1 (1.1–3.3) | 1.3 (0.6–2.5) | 3.7 (1.5–6.8) | 2.7 (1.2–6.0) |
| Number of male deaths (% of all males) | 8 (16.7) | 20 (41.7) | 25 (33.8) | 38 (52.1) |
| Number of female deaths (% of all females) | 5 (22.7) | 20 (5.6) | 25 (58.1) | 12 (33.3) |
| Mean age (± SD) at death | 61.7 ± 11.5 | 65.1 ± 11.0 | 66.4 ± 11.6 | 66.5 ± 11.2 |
By 1 October 2008, 13 (eight male; 18.6%) patients with a Charcot foot had died at a mean age of 61.7 ± 11.5 years and after a median interval of 2.1 years (interquartile range 1.1–3.3). Twenty-two patients (20 male; 33.3%) in the control group had died by the same time at a mean age of 65.1 ± 11.0 years and after a median follow-up of 1.3 years (0.6–2.5). Data on renal function were missing in two Charcot patients, but impaired renal function was identified in 25 (35.7% of 70) and 28 (42.2% of 66) patients in the Charcot and the control groups, respectively. Of 13 patients with Charcot who died during follow-up, 61.5% had renal dysfunction compared with 63.6% of 22 who died in the control group. The patients who survived in the two groups were followed for a median period of 3.0 years (1.9–4.5) and 4.4 years (3.2–6.3), respectively. There was no significant difference in survival between the two groups, although significantly more male subjects died in the control group (P = 0.027).
When the results of the present populations of Charcot and NFU patients were compared with those studied between 1980 and 2001 and previously published (3), no difference was observed in survival for either the Charcot (log-rank 2.797, P = 0.094) or the NFU (log-rank 0.048, P = 0.827) groups. The data for all patients managed between 1980 and 2007 were therefore combined in order to compare them with U.K. normative data.
Total 1980–2007 populations
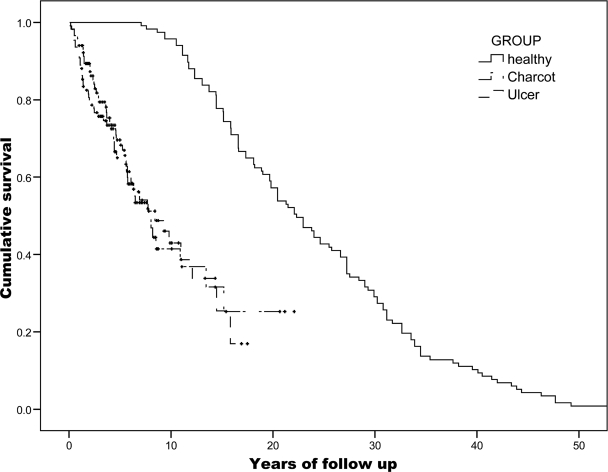
There was a total of 117 (67% type 2 diabetes; 63% male) patients with an acute Charcot foot (mean age 58.1 ± 12.5 years; mean disease duration 16.1 ± 10.4 years) and 109 (71% with type 2 diabetes; 71% male) with NFUs (mean age 58.6 ± 12.5 years; mean diabetes duration 15.5 ± 19.4 years) (Table 1). Forty-five (35%) patients (25 male) with Charcot disease died during the periods of follow-up compared with 50 (45.9%) NFU control subjects (38 male). The median survival of the patients who died was 3.7 years (interquartile range 1.5–5.8) for Charcot foot and 2.7 years (1.2–6.0) for NFUs, but this was not statistically significantly different (Mann-Whitney U; P = 0.15). The mortality rates for Charcot and NFU patients was 11 and 19%, respectively, at 1 year, 24 and 27% at 3 years, and 41 and 40% at 5 years. Patients with a Charcot foot died at a mean age of 66.4 ± 11.6 years, and this was similar to the NFU control subjects (66.5 ± 11.2 years) (Fig. 1). The mortality in both the Charcot and NFU control subjects was significantly higher in those patients with coexisting renal dysfunction (Charcot patients with and without renal dysfunction 53.1 and 26.6%, respectively, χ2 = 8.46, P = 0.015; NFU control subjects with and without renal dysfunction 62.8 and 27.6%, respectively, χ2 = 18.35, P < 0.001). The mortality of the men in the total NFU population was significantly higher than the women (38 vs. 12%; P = 0.05), but there was no equivalent sex difference in patients with acute Charcot (25 vs. 20%; P = 0.12). Logistic regression analysis of the whole cohort revealed that age (P < 0.001) and renal dysfunction (P < 0.001) were independent predictors of mortality, but that sex (P = 0.55) and the presence of Charcot (P = 0.31) were not.
Figure 1.
Kaplan-Meier survival curve cohort 1980–2007 versus an age- and sex-matched British population.
Kaplan-Meier survival analysis of both the Charcot and the NFU 1980–2007 cohorts were then compared with normative data for the U.K. population (Fig. 1). The greater mortality of both the Charcot and NFU groups compared was highly significant when compared with the general population. Median survival for the age- and sex-matched general population was 22.32 years (interquartile range 15.2–31.2) compared with median survival for Charcot patients and NFU patients of 7.88 years (4.0–15.47) and 8.43 years (3.4–15.8), respectively (P < 0.001 for comparison with general population).
CONCLUSIONS
These results confirm our earlier observations and indicate that mortality is high in a consecutive series of patients presenting with an acute Charcot foot to a single U.K. center between 2000 and 2007. Median survival for such a population with a mean age of 58 years is reduced from ∼22 to 8 years. This reduction in life expectancy is much less than that which can be attributed to diabetes alone, which has been recently estimated to be just 8 years for a patient aged 50 years, when compared with the nondiabetic population (6). This high mortality conflicts markedly with the findings in the two earlier published series of patients with a Charcot. The reason for this conflict is not clear but could be explained by population selection, and other studies are urgently needed. Nevertheless, our observations are similar to those recently reported by Sohn et al. (7), in which the 5-year mortality of 1,050 cases first diagnosed in 2003 was 28.3%, even though this recent study was based on data from Veterans Administration hospitals and therefore included an almost entirely male population whose age was also slightly older at 63.0 years.
When compared with the earlier cohort, there was no evidence of improved life expectancy despite the generalized introduction of strategies for cardiovascular risk reduction in recent years. The lack of improvement contrasts with the recent improvement in life expectancy in a less selected population with diabetic foot ulcers observed elsewhere in U.K. (4).
An observational study such as this has inherent weaknesses. Of these, the most important is the fact that the acute Charcot foot has no precise diagnostic markers, and diagnosis is based on clinical pattern recognition supported by various imaging techniques. Nevertheless, it is assumed that the patient populations reported by different groups in different centers are broadly similar. Despite these, the magnitude of the observed associations is strongly suggestive of a valid relationship.
Our original study selected patients with uninfected neuropathic ulcers as control subjects in the belief that survival would be shown to be significantly worse in those with an acute Charcot foot. Patients with infection of either soft tissue or bone were excluded from the control subjects in case this could have had a confounding effect on mortality. In practice, we observed no difference between the two carefully matched groups, and mortality was equally high in those with neuropathic ulcers. Although this was a major surprise at the time, there is now increasing evidence to indicate that the mortality associated with neuropathic ulcers is high and approaches 50% at 5 years (8,9). Once again, we have confirmed our earlier observations and found no difference in survival between patients with a Charcot foot and a matched population with neuropathic ulcers, and this suggests that the poor survival of patients with an acute Charcot foot may be largely attributable to the distal symmetrical neuropathy with which the condition is universally associated. Distal symmetrical neuropathy has been shown to be independently associated with mortality in a number of studies (10–12), and it has recently been suggested that the process underlying the association is increased calcification and ossification of the arterial wall and the consequent increase in left ventricular strain (13). The association between neuropathy and early nephropathy is also clearly relevant. And while it was disappointing that we were unable to show any improvement in outlook despite the current more general use of intensive approaches to cardiovascular risk reduction for patients with diabetes (4), this may have occurred too recently to have had an impact on our data.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Armstrong DG, Todd WF, Lavery LA, Karkless LB, Bushman TR: The natural history of acute Charcot's arthropathy in a diabetic foot specialty clinic. Diabet Med 1997; 14: 357– 363 [DOI] [PubMed] [Google Scholar]
- 2.Fabrin J, Larsen K, Holstein P: Long-term follow-up in diabetic Charcot feet with spontaneous onset. Diabetes Care 2000; 23: 796– 800 [DOI] [PubMed] [Google Scholar]
- 3.Gazis A, Pound N, Macfarlane R, Treece K, Game FL, Jeffcoate WJ: Mortality in patients with diabetic neuropathic osteoartrhopathy (Charcot foot). Diabet Med 2004; 21: 1243– 1246 [DOI] [PubMed] [Google Scholar]
- 4.Young MJ, McCardle JE, Randall LE, Barclay JI: Improved survival of diabetic foot ulcer patients 1995–2008: possible impact of aggressive cardiovascular risk management. Diabetes Care 2008; 31: 2143– 2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Treece KA, Macfarlane RM, Pound N, Game FL, Jeffcoate WJ: Validation of a system of foot ulcer classification in diabetes mellitus. Diabet Med 2004; 21: 987– 991 [DOI] [PubMed] [Google Scholar]
- 6.Franco OH, Steyerberg EW, Hu FB, Mackenbach J, Nusselder W: Associations of diabetic mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med 2007; 167: 1145– 1151 [DOI] [PubMed] [Google Scholar]
- 7.Sohn M-W, Todd AL, Stuck RM, Frykberg RG, Budiman-Mak E: Mortality risk of Charcot arthropathy compared with that of diabetic foto ulcer and diabetes alone. Diabetes Care 2009; 32: 816– 821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moulik PK, Mtonga R, Gill GV: Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care 2003; 26: 494– 494 [DOI] [PubMed] [Google Scholar]
- 9.Robbins JM, Strauss G, Aron D, Long J, Kuba J, Kaplan Y: Mortality rates and diabetic foot ulcers: is it time to communicate mortality risk to patients with diabetic foot ulceration? J Am Podiatr Med Assoc 2008; 98: 489– 493 [DOI] [PubMed] [Google Scholar]
- 10.Coppini DV, Bowtell PA, Weng C, Young PJ, Sönksen PH: Showing neuropathy is related to increased mortality in diabetic patients: a survival analysis using an accelerated failure time model. J Clin Epidemiol 2000; 53: 519– 523 [DOI] [PubMed] [Google Scholar]
- 11.Forsblom CM, Sane T, Groop PH, Totterman KJ, Kallio M, Saloranta C, Groop L: Risk factors for mortality in type II (non-insulin-dependent) diabetes: evidence of a role for neuropathy and a protective effect of HLA-DR4. Diabetologia 1998; 41: 1253– 1262 [DOI] [PubMed] [Google Scholar]
- 12.Soedamah-Muthu SS, Chaturvedi N, Witte DR, Stevens LK, Porta M, Fuller JH: Relationship between risk factors and mortality in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study (PCS). Diabetes Care 2008; 31: 1360– 1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeffcoate W, Rasmussen LM, Hofbauer LC, Game FL: Medial arterial calcification in diabetes and its relationship to neuropathy. Diabetologia 2009; 52: 2478– 2488 [DOI] [PubMed] [Google Scholar]