Abstract
OBJECTIVE
Reduction of bone marrow–derived circulating progenitor cells has been proposed as a novel mechanism of cardiovascular disease in type 2 diabetes. The present study was designed to describe the extent and potential mechanisms of progenitor cell reduction during the natural history of type 2 diabetes.
RESEARCH DESIGN AND METHODS
We identified 425 individuals, divided into seven categories according to carbohydrate metabolism status (normal glucose tolerance [NGT], impaired fasting glucose, impaired glucose tolerance [IGT], and newly diagnosed type 2 diabetes) and diabetes duration (0–9, 10–19, and ≥20 years). These categories were examined as ideally describing the natural history of type 2 diabetes development and progression. We measured CD34+ and CD34+KDR+ progenitor cells by flow cytometry. We also evaluated progenitor cells in 20 coupled bone marrow and peripheral blood samples and examined progenitor cell apoptosis in 34 subjects.
RESULTS
In comparison to NGT, CD34+ cells were significantly reduced in IGT and had a first nadir in newly diagnosed type 2 diabetes and a second nadir after 20 years of diabetes. Statistical adjustment for possible confounders confirmed that CD34+ cell counts are deeply reduced at time of diagnosis, that they partially recover during the subsequent 0–19 years, and that they dip again after ≥20 years. A similar, but less consistent, trend was detected for CD34+KDR+ cells. Peripheral blood CD34+ cells were directly correlated with bone marrow CD34+ cells and inversely correlated with CD34+ cell apoptosis.
CONCLUSIONS
Circulating progenitor cell reduction marks the clinical onset of type 2 diabetes. Both defective mobilization and increased apoptosis may account for this phenomenon. While a partial recovery occurs during subsequent years, bone marrow reserve seems exhausted in the long term.
Type 2 diabetes is characterized by a two- to fourfold increased risk of cardiovascular disease (CVD) (1). This is generally attributed to the adverse effects of hyperglycemia and oxidative stress on vascular biology (2). However, type 2 diabetes is also associated with a constellation of additional risk factors, such as obesity, dyslipidemia, and hypertension, which concur to promote CVD. It has been also shown that patients with pre-diabetic conditions, such as impaired fasting glucose (IFG) and impaired glucose tolerance (IGT), are themselves at increased risk of CVD (3). This suggests that abnormalities in carbohydrate metabolism form a continuum that progressively worsens cardiovascular health, but the mechanisms are not clearly understood.
A relatively novel paradigm of CVD pathogenesis is the loss of normal endothelial turnover caused by a reduction of circulating endothelial progenitor cells (EPCs) (4). EPCs are mainly derived from bone marrow and are involved in the homeostasis of healthy and damaged endothelium, as well as in physiologic and compensatory angiogenesis (5). Given their paramount role in the cardiovascular system, reduction of EPCs is believed to promote CVD development or progression (4).
EPCs are reduced in the presence of risk factors for and of established CVD and predict future cardiovascular events (4,6–8). Both type 1 and type 2 diabetes are associated with a significant reduction of circulating EPCs (9,10), which parallels the severity of cardiovascular complications (11). Experimental studies suggest that the mechanisms linking hyperglycemia to progenitor cell reduction include defective mobilization from the bone marrow and reduced survival (9).
We have also shown that type 2 diabetes and IGT are associated with a significant reduction of total circulating CD34+ cells (10,12). These cells form a more generic, immature population of bone marrow–derived progenitors that include EPCs and nonendothelial progenitor cells potentially involved in cardiovascular homeostasis, such smooth muscle and cardiomyocyte progenitor cells (13). This may have important implications, as CD34+ cell count is strongly correlated with all cardiovascular parameters and risk estimates (6) and predicts cardiovascular outcomes in patients with metabolic disorders (14). However, it is not clear to what extent progenitor cell defects in type 2 diabetic patients are related to diabetes, per se, or to the associated cardiovascular risk factors. Moreover, in order prevent or treat diabetes complications, an increasing number of drugs are prescribed to these patients that may influence progenitor cells, thus masquerading the relationship between diabetes and cell count.
The aims of this study were 1) to define cross-sectionally the time course of progenitor cell alterations during the natural history of type 2 diabetes, 2) to clear out possible confounders of this relationship, and 3) to identify potential mechanisms of progenitor cell reduction.
RESEARCH DESIGN AND METHODS
An expanded description of materials and methods is given in the online appendix (available at http://care.diabetesjournals.org/cgi/content/full/dc09-1999/DC1).
We identified 425 subjects for whom carbohydrate metabolism state and cardiovascular parameters were known. They were divided into 205 subjects with normal glucose tolerance (NGT), 40 with IFG, 43 IGT, 32 with new-onset type 2 diabetes, 64 with ≤10 years long-lasting diabetes, 20 with 10–19 years long-lasting diabetes, and 22 with ≥20 years long-lasting diabetes. CD34+ and CD34+KDR+ cell count was performed in all subjects. Vascular endothelial growth factor (VEGF) concentrations were determined in a subgroup of 98 patients. Progenitor cell apoptosis was assessed in 34 patients, and bone marrow samples were available for 20 patients. More details on patients' descriptions and characterizations can be found in the online appendix.
Assessment of progenitor cell count and apoptosis
Peripheral blood and bone marrow progenitor cells were counted by flow cytometry using antibodies against CD34 and KDR, as previously described in detail by our group (15). CD34+ cells were considered generic circulating progenitors, while CD34+KDR+ cells were considered EPCs. Apoptosis of CD34+ cells was evaluated by costaining with Annexin V. For more details on flow cytometry see the online appendix.
Statistical analyses
An expanded statistical section can be found in the online appendix. Data are expressed as means ± SD, unless otherwise specified. Progenitor cell count is always expressed as cell count per 106 events. To derive an estimate of progenitor cell variation that was independent of possible confounders, we used nonstandardized coefficients from multiple linear regression analyses, in which each category of patients was entered as a dichotomous variable (0 or 1) compared with NGT. To adjust data, we first used a full model (model 1) including all variables listed in Table 1 (except for total cholesterol, due to collinearity). Given the large uncertainty of such a estimate, to select a limited number of unrelated highly significant variables, we applied a stepwise regression approach and repeated the analyses controlling only for this parsimonious set of variables (model 2). SPSS version 13.0 was used, and statistical significance was accepted at P < 0.05.
Table 1.
Clinical characteristics of the study sample
| NGT | IFG | IGT | New-onset diabetes | Diabetes duration <10 years | Diabetes duration 10–20 years | Diabetes duration ≥20 years | |
|---|---|---|---|---|---|---|---|
| n | 205 | 40 | 43 | 32 | 64 | 20 | 22 |
| Age (years) | 46.6 ± 12.3 | 55.0 ± 10.9* | 53.0 ± 8.5* | 57.0 ± 0.7* | 65.1 ± 11.3* | 68.6 ± 10.5* | 70.4 ± 8.6* |
| Male sex | 98 (47.8) | 28 (70.0)* | 23 (53.5) | 21 (65.6) | 40 (62.5)* | 9 (45.0) | 17 (77.3)* |
| Plasma glucose (mg/dl) | 87.5 ± 9.0 | 113.9 ± 6.5* | 101.4 ± 14.3* | 142.8 ± 41.0* | 186.8 ± 67.3* | 205.8 ± 82.6* | 234.3 ± 95.9* |
| A1C (%) | 5.1 ± 0.37 | 6.0 ± 0.42* | 5.6 ± 0.59 | 7.1 ± 0.29* | 8.2 ± 2.5* | 9.6 ± 2.2* | 9.3 ± 1.9* |
| BMI (kg/m2) | 24.7 ± 4.4 | 27.1 ± 3.9* | 27.5 ± 4.1* | 27.6 ± 4.5* | 28.2 ± 4.8* | 29.8 ± 6.4* | 27.8 ± 4.7* |
| Smoking habit | 49 (23.9) | 3 (7.5)* | 7 (16.3) | 7 (21.8) | 12 (18.7) | 3 (15.0) | 8 (36.4) |
| Systolic blood pressure (mmHg) | 122.9 ± 13.0 | 125.6 ± 17.2 | 129.0 ± 12.0* | 141.2 ± 17.2* | 147.1 ± 21.8* | 150.5 ± 21.1* | 150.2 ± 22.3* |
| Diastolic blood pressure (mmHg) | 81.6 ± 9.7 | 82.6 ± 12.1 | 83.6 ± 12.1 | 87.1 ± 12.3* | 87.3 ± 10.8* | 83.3 ± 11.0 | 81.4 ± 10.7 |
| Total cholesterol (mg/dl) | 200.1 ± 40.8 | 202.1 ± 32.9 | 210.9 ± 34.0* | 190.6 ± 37.2 | 179.7 ± 44.9* | 200.2 ± 27.7 | 187.0 ± 38.6 |
| HDL cholesterol (mg/dl) | 56.1 ± 15.7 | 53.4 ± 19.1 | 53.9 ± 15.1 | 43.2 ± 14.2* | 43.6 ± 12.7* | 50.6 ± 14.9 | 48.1 ± 17.8 |
| LDL cholesterol (mg/dl) | 125.4 ± 37.1 | 126.7 ± 32.6 | 128.1 ± 29.7 | 110.7 ± 32.5* | 105.6 ± 40.1* | 123.4 ± 26.1 | 109.4 ± 36.0* |
| Triglycerides (mg/dl) | 97.2 ± 54.5 | 110.2 ± 67.8 | 144.3 ± 66.5* | 183.3 ± 127.4* | 152.4 ± 78.2* | 130.7 ± 82.7 | 147.7 ± 74.7* |
| Diabetic retinopathy | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 11 (17.2)* | 9 (45.0)* | 7 (31.8)* |
| Chronic renal failure | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 8 (12.5)* | 7 (35)* | 5 (22.7)* |
| CVD | 14 (6.8) | 2 (5.0) | 2 (4.7) | 6 (18.8) | 39 (60.9)* | 15 (75.0)* | 21 (95.5)* |
| Medications | |||||||
| Statin | 2 ± 0.9 | 0 ± 0.0 | 1 ± 2.2 | 3 ± 9.4 | 20 ± 31.3* | 8 ± 40.0* | 8 ± 36.4* |
| ACE inhibitors/angiotensin receptor blockers | 8 ± 3.9 | 1 ± 2.5 | 1 ± 2.2 | 4 ± 12.5 | 32 ± 50.0* | 12 ± 60.0* | 17 ± 77.3* |
| Other antihypertensive | 17 ± 8.3 | 4 ± 10.0 | 4 ± 9.3 | 3 ± 9.4 | 33 ± 51.6* | 12 ± 60.0* | 16 ± 72.7* |
| Aspirin | 0 ± 0.00 | 0 ± 0.0 | 1 ± 2.2 | 2 ± 6.3 | 13 ± 20.3* | 9 ± 45.0* | 9 ± 41.0* |
| Insulin | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 19 ± 29.7* | 8 ± 40.0* | 14 ± 63.6* |
| Oral hypoglycemic agents | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 15 ± 23.4* | 11 ± 55.0* | 5 ± 22.0.7* |
Data are means ± SD or n (%). Patients were divided into seven categories according to carbohydrate metabolism and duration of diabetes, as appropriate.
*Significantly different versus NGT in least significant difference post-ANOVA test.
RESULTS
Study population
Detailed clinical characteristics of the study patients are reported in Table 1. Patients were divided into seven categories according to their degree of glucose tolerance or the duration of diabetes, as appropriate. Patients' distribution in these groups was not uniform, because about one-half was classified as having NGT. Patients with both IFG and IGT were classified as IGT. As expected, moving from NGT to worsening categories of carbohydrate abnormality was associated with a progressively worsening cardiovascular profile, while cardiovascular parameters in diabetic patients were likely influenced by medications. The prevalence of CVD increased markedly with longer diabetes duration.
Observed and adjusted CD34+ cell counts in the natural course of type 2 diabetes
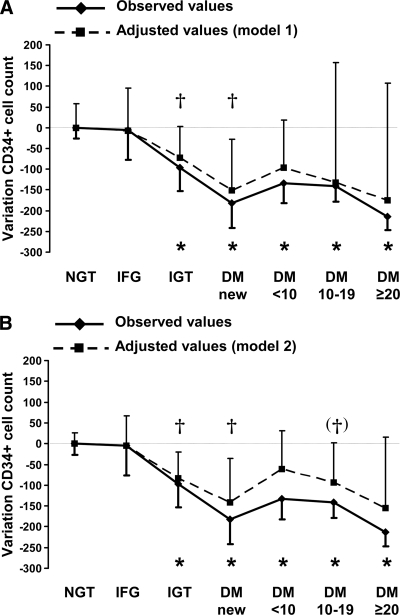
We examined CD34+ cell counts in subjects grouped according to carbohydrate metabolism and diabetes duration. Circulating CD34+ cells were significantly reduced in subjects with IGT (−21.9%; P = 0.016 after Bonferroni correction) and further reduced in new-onset type 2 diabetes in comparison with NGT (−40.8%; P < 0.001). Diabetic patients with 0–19 years of disease duration still had significantly lower circulating CD34+ cells than those with NGT, but on average cell counts were not different from that of new-onset diabetic patients. Patients with ≥20 years diabetes duration had the lowest mean cell count, which, however, was not significantly different from that of new-onset diabetes and other disease durations (−47.9%; P < 0.001 vs. NGT; P = 0.43 vs. new onset). These data indicate that observed levels of circulating CD34+ cells have a first nadir very early in newly diagnosed type 2 diabetic patients and that a second nadir occurs at least 20 years later (supplemental Table 1) (Fig. 1, continuous line).
Figure 1.
Observed and adjusted variation of circulating CD34+ cells in patients grouped according to carbohydrate metabolism or diabetes (DM) duration, as appropriate. The mean value of patients with NGT was taken to represent the zero point. Bars indicate 95% CIs of means (observed values) and estimates (adjusted values). *Observed values significantly different versus NGT. †Adjusted values significantly different versus NGT. Model 1 (A) and model 2 (B) refers to the strategy used to control for confounders (see statistical analyses).
We then reasoned that the observed pattern of CD34+ cell variation across categories of carbohydrate metabolism and diabetes duration could be influenced by many confounders, such as age, concomitant risk factors, CVD, microangiopathy, and medications, which have been previously shown to affect circulating progenitors (16). Therefore, we ran multiple linear regression analyses in order to correct for possible confounders the absolute CD34+ cell variation in each category (supplemental Table 1). To this end, we used two models (see statistical analyses): model 1 included all variables listed in Table 1 and was therefore highly redundant; model 2 included, at each step, only variables selected by a stepwise multiple regression procedure (IFG: none; IGT: age; newly diagnosed diabetes: plasma glucose; 0–9 years: age and A1C; 10–19 years: age; ≥20 years: age and A1C). Using both models, the trend of adjusted values was quite similar to that of observed values (supplemental Table 1) (Fig. 1A and B, dashed line). The gap between the two curves is to be considered attributable to confounders. Bars in the figure indicate 95% CIs of the mean (solid lines) or 95% CI of the estimate (dashed lines). In model 1, the uncertainty of the estimate was very large due to the high number of variables that were controlled for. In model 2, which was purportedly built to reduce uncertainty without missing highly significant covariates, bars were clearly narrower. The trend of adjusted values confirms a first nadir of CD34+ cell count at diagnosis of type 2 diabetes (−33.8%, P = 0.01 in model 1; −31.7%, P = 0.01 in model 2) and a second nadir after ≥20 years of disease duration but was marginally significant (−39.7% in model 1; −34.9% in model 2).
Observed and adjusted CD34+KDR+ cell counts in the natural course of type 2 diabetes
When we used the same statistical approach to analyze the time course of CD34+KDR+ variation in categories of patients describing the natural history of type 2 diabetes, we found a similar trend as for CD34+ cells, but results were less consistent. A trend toward CD34+ KDR+ nadir in newly diagnosed type 2 diabetes and a subsequent reduction in longer-standing type 2 diabetes was detected (Fig. 1) (supplemental Table 2). The gap between observed and adjusted data was larger and more inconsistent then for CD34+ cells.
Potential mechanisms of progenitor cell reduction
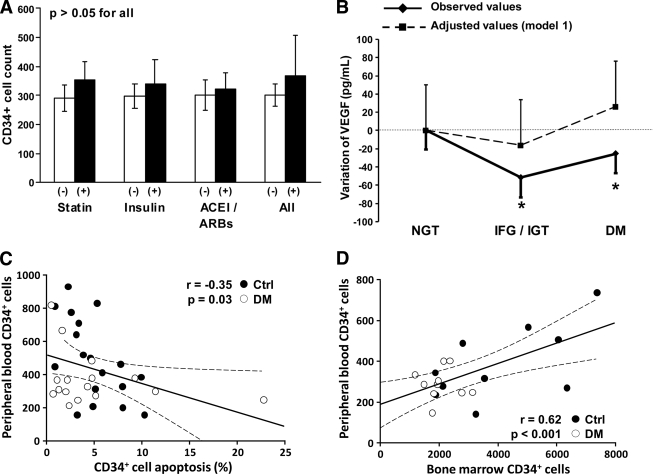
Pharmacologic effects, VEGF stimulation, cell apoptosis, and bone marrow mobilization were assessed as potential explanations for CD34+ progenitor cell reduction. When we examined whether the increase and stabilization of CD34+ cell count in patients with 0–19 years diabetes duration was related to pharmacological interventions established after a diagnosis of diabetes, which might influence progenitor cell biology (such as insulin, statins, and ACE inhibitors/angiotensin receptor blockers) (4), we found that none of these treatments or their combination was associated with significant changes in CD34+ cells (Fig. 2A).
Figure 2.
Potential mechanisms of CD34+ progenitor cell reduction. A: Circulating CD34+ cell counts in pooled patients with 0–19 years of disease duration, divided according to the use of drugs known to affect peripheral blood progenitor cells: statins, ACE inhibitors, angiotensin receptor blockers (ARBs), insulin, and all these medications together. B: Variation of plasma VEGF concentrations in pre-diabetic (IFG/IGT) and diabetic (DM) versus subjects with NGT. *Significant versus observed NGT values. C: A significant negative correlation was found between peripheral blood CD34+ cell count and the apoptotic rate of CD34+ cells. D: A significant direct correlation was found between peripheral blood and bone marrow CD34+ cell counts. ●, nondiabetic subjects; ○, diabetic patients; Ctrl, control.
Observed plasma VEGF concentrations were reduced in pre-diabetic and diabetic patients as compared with patients with NGT, but statistical significance was lost after adjusting for confounders in model 1 (Fig. 2B). We found no significant correlation between plasma VEGF concentrations and CD34+ (r = −0.12; P = 0.25) or CD34+KDR+ cells (r = −0.02; P = 0.84).
In a subset of 34 subjects, we found a significant inverse correlation between peripheral blood CD34+ cell count and the percentage of CD34+ cell apoptosis (Fig. 2C). In this small substudy, a trend toward lower CD34+ cell count and in diabetes versus NGT was confirmed (361 ± 36 vs. 497 ± 60; P = 0.07), but the apoptotic rate was not different (4.9 ± 1.3% vs. 5.0 ± 0.7%; P = 0.92).
In a sample of 10 nondiabetic and 10 diabetic subjects CD34+ cells were measured in both peripheral blood and bone marrow. We found a direct correlation between cell counts in the two compartments (Fig. 2D). CD34+ cells were lower in diabetic than in nondiabetic patients in both peripheral blood (290 ± 24 vs. 388 ± 57; P = 0.13) and bone marrow (2,061 ± 183 vs. 4,026 ± 640; P = 0.008).
CONCLUSIONS
The present study was designed to describe the trend of progenitor cell decline during the natural history of type 2 diabetes. Since decades would be needed to address this issue prospectively, we cross-sectionally examined patients at different stages of disease development and progression, providing an overview that simulates a longitudinal perspective. The interest in better defining the relationships between progenitor cells and diabetes stands in the notion that progenitor cells are involved in cardiovascular homeostasis, and their reduction independently predicts cardiovascular events (14). In the present study, unadjusted data indicate that the level of circulating CD34+ cells starts to decline very early in the natural course of type 2 diabetes, having a first nadir at the time of diagnosis. Surprisingly, patients with 0–19 years of diabetes did not show a further progressive decline in progenitor cell count, as it would be straightforwardly expected from a time-dependent cumulative damage model (17). Rather, in this category of patients, CD34+ cell count proved to be slightly increased or stabilized. Only after at least 20 years of disease duration, there was a second nadir of circulating CD34+ cells. This trend was likely influenced by many confounders. First, there was an almost linear increase in age across categories of patients, and aging is known to progressively restrict bone marrow reserve and reduce circulating progenitor cell levels (6,18). Additionally, with worsening carbohydrate metabolism, concomitant abnormalities became more prevalent, including overweight, dyslipidemia, and hypertension, all of which have been previously associated with progenitor cell decline (6,19). Moreover, high glucose at the time of blood sampling may have influenced progenitor cell count, per se, as known from in vivo and in vitro observations (10,20). The higher prevalence of CVD in patients with longer disease duration may have confounded the relationship between cell count and diabetes, because CVD patients usually have lower levels of circulating progenitor cells (6). Finally, patients were prescribed with different classes of drugs, such as statins, ACE inhibitors, and insulin, all of which have the potential to modulate progenitor cells (4,21,22). To remove these confounders, we used two multiple linear regression models, and the variation of progenitor cell count attributable only to patients' category was estimated from regression coefficients. Model 1 was purportedly redundant, as it included all possible confounding variables, but led to a high uncertainty in the estimate of cell count reduction. Conversely, model 2 was highly conservative, as it included only variables selected by a preliminary stepwise analysis, thus producing narrower CIs of the estimate. Both models revealed a trend of progenitor cell count that was quite similar to observed data (supplemental Table 1). The gap between the two curves in Fig. 1 is to be considered attributable to confounders and vary according to the model used. Both analyses indicate that observed CD34+ cell reduction in these patients is partly attributable to aging, concomitant risk factors, or CVD and not entirely to their carbohydrate metabolism status or diabetes duration. Most importantly, adjusted data confirm that CD34+ cells decrease from NGT to IGT with a first nadir in newly diagnosed patients. The partial recovery of progenitor cells during the subsequent 20 years has not a clear explanation. Antihyperglycemic treatment, either with lifestyle or pharmacological interventions, might have had beneficial effects on progenitor cells. Adjustment for treatment should minimize this bias, but we could not control for cumulative exposure to antihyperglycemic drugs. Moreover, physical exercise, which can stimulate progenitor cells and is usually encouraged soon after diabetes diagnosis, may account in part for the observed cell values. To rule out that the development of CVD-stimulated repeated bursts of progenitor cell mobilization due to subclinical episodes of ischemia, we excluded patients with acute CVD and controlled for chronic CVD in model 1.
Interestingly, variation of CD34+ KDR+ cells, which can be equated to EPCs (23), was somehow similar to CD34+ cell variation (supplemental Fig. 1). We found a trend toward early EPC reduction in newly diagnosed type 2 diabetes and another dip in longer-lasting disease. However, fluctuations in CD34+KDR+ levels were much more pronounced, and the gap between observed and adjusted values was larger and more inconsistent than for CD34+ cells. This might reflect that endothelial-primed progenitors are more susceptible to the changing patient phenotype and cumulative therapeutic intervention than generic progenitor cells.
This is the first study thoroughly describing trends of progenitor cells during development and progression of type 2 diabetes, but it has several limitations. Its cross-sectional nature does not allow to draw definite conclusions on progenitor cell evolution at the single-patient level. The limited number of patients in some of the groups may account for an apparently inconsistent trend, while CIs of adjusted values are wide because a number of confounders had to be controlled for.
Notwithstanding these limitations, we suggest that our data may have pathophysiological implications. Of remarkable interest is the progenitor cell nadir in newly diagnosed type 2 diabetic patients. To dig deeper into this pathologic observation, we explored some mechanisms of CD34+ progenitor cell reduction, such as the effects of drugs, the role of VEGF, bone marrow mobilization, and apoptotic cell death. We found that none of the known EPC-modulating treatments were cross-sectionally associated with different CD34+ cell counts. VEGF appeared to play minor or no role in the variation of progenitor cell counts from NGT to pre-diabetes and overt diabetes, and no correlation between VEGF and cell counts was found. Conversely, we found that the lower the count of CD34+ cells the higher their apoptotic rate, indicating that apoptosis occurring in the bloodstream might reduce progenitor cell level. However, there was no difference in the apoptotic rate between diabetic patients and those with NGT, and the percentage of CD34+ cell apoptosis was too low (usually ≤10%) to explain a 40% decrease in cell count, suggesting that other mechanisms are operating. In principle, any peripheral cause of progenitor cell reduction (e.g., apoptosis) should be normally compensated by bone marrow mobilization to restore the peripheral blood progenitor cell pool. Thus, in the presence of a steady-state reduction of circulating progenitors, a functional bone marrow defect can be postulated. By showing a close direct correlation between peripheral blood and bone marrow CD34+ cells and a low bone marrow cell content in diabetic versus nondiabetic patients, we support the hypothesis of a bone marrow defect in diabetes. This is in compliance with our previous demonstration of a defective postischemic bone marrow mobilization in experimental diabetes (24) and with recent findings showing that a specific form of bone marrow neuropathy accounts for reduced circulating progenitors in a rat type 2 diabetic model (25). Therefore, we hypothesize that worsening glucose metabolism in IGT and newly diagnosed diabetes progressively induces a sort of bone marrow “stunning,” leading to the early progenitor cell nadir. Subsequent bone marrow adaptation or reduced cell apoptosis might explain a partial recovery, despite the development of concomitant risk factors and CVD. Interestingly, after ≥20 years of disease, a deeper nadir takes place that is poorly influenced by confounding factors, possibly suggesting an exhaustion of bone marrow adaptation capacity and functional reserve.
At present, there are many drugs able to increase circulating progenitor cells, including statins, ACE inhibitors, and glitazones, which are commonly used in diabetic patients (4,21). Given that CD34+ progenitor cell reduction predicts cardiovascular events beyond classical risk factors (6,14), we may be persuaded to aggressively treat patients in the early phases after diagnosis of type 2 diabetes in order to preserve marrow function longer and to prevent CVD.
Supplementary Material
Acknowledgments
G.P.F. is supported by a young investigator grant from the European Association for the Study of Diabetes (EASD).
No potential conflicts of interest relevant to this article were reported.
Parts of this article were presented in abstract form at the 44nd EASD Annual Meeting, Rome, Italy, 7–11 September 2008.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Almdal T, Scharling H, Jensen J, Vestergaard H: The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med 2004; 164: 1422– 1426 [DOI] [PubMed] [Google Scholar]
- 2.Avogaro A, Fadini GP, Gallo A, Pagnin E, de Kreutzenberg S: Endothelial dysfunction in type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis 2006; 16( Suppl. 1): S39– S45 [DOI] [PubMed] [Google Scholar]
- 3.Hu F, Stampfer M, Haffner S, Solomon C, Willett W, Manson J: Elevated risk of cardiovascular disease prior to clinical diagnosis of type 2 diabetes. Diabetes Care 2002; 25: 1129– 1134 [DOI] [PubMed] [Google Scholar]
- 4.Fadini GP, Agostini C, Sartore S, Avogaro A: Endothelial progenitor cells in the natural history of atherosclerosis. Atherosclerosis 2007; 194: 46– 54 [DOI] [PubMed] [Google Scholar]
- 5.Urbich C, Dimmeler S: Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 2004; 95: 343– 353 [DOI] [PubMed] [Google Scholar]
- 6.Fadini GP, de Kreutzenberg SV, Coracina A, Baesso I, Agostini C, Tiengo A, Avogaro A: Circulating CD34+ cells, metabolic syndrome, and cardiovascular risk. Eur Heart J 2006; 27: 2247– 2255 [DOI] [PubMed] [Google Scholar]
- 7.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G: Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 2005; 353: 999– 1007 [DOI] [PubMed] [Google Scholar]
- 8.Schmidt-Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, Dimmeler S, Zeiher AM: Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation 2005; 111: 2981– 2987 [DOI] [PubMed] [Google Scholar]
- 9.Fadini GP, Sartore S, Agostini C, Avogaro A: Significance of endothelial progenitor cells in subjects with diabetes. Diabetes Care 2007; 30: 1305– 1313 [DOI] [PubMed] [Google Scholar]
- 10.Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A: Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol 2005; 45: 1449– 1457 [DOI] [PubMed] [Google Scholar]
- 11.Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, Vigili de Kreutzenberg S, Tiengo A, Agostini C, Avogaro A: Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol 2006; 26: 2140– 2146 [DOI] [PubMed] [Google Scholar]
- 12.Fadini GP, Pucci L, Vanacore R, Baesso I, Penno G, Balbarini A, Di Stefano R, Miccoli R, de Kreutzenberg S, Coracina A, Tiengo A, Agostini C, Del Prato S, Avogaro A: Glucose tolerance is negatively associated with circulating progenitor cell levels. Diabetologia 2007; 50: 2156– 2163 [DOI] [PubMed] [Google Scholar]
- 13.Yeh E, Zhang S, Wu H, Korbling M, Willerson J, Estrov Z: Transdifferentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation 2003; 108: 2070– 2073 [DOI] [PubMed] [Google Scholar]
- 14.Fadini GP, de Kreutzenberg S, Agostini C, Boscaro E, Tiengo A, Dimmeler S, Avogaro A: Low CD34+ cell count and metabolic syndrome synergistically increase the risk of adverse outcomes. Atherosclerosis 2009; 207: 213– 219 [DOI] [PubMed] [Google Scholar]
- 15.Fadini GP, de Kreutzenberg S, Albiero M, Coracina A, Pagnin E, Baesso I, Cignarella A, Bolego C, Plebani M, Nardelli GB, Sartore S, Agostini C, Avogaro A: Gender differences in endothelial progenitor cells and cardiovascular risk profile: the role of female estrogens. Arterioscler Thromb Vasc Biol 2008; 28: 997– 1004 [DOI] [PubMed] [Google Scholar]
- 16.Egan CG, Lavery R, Caporali F, Fondelli C, Laghi-Pasini F, Dotta F, Sorrentino V: Generalised reduction of putative endothelial progenitors and CXCR4-positive peripheral blood cells in type 2 diabetes. Diabetologia 2008; 51: 1296– 1305 [DOI] [PubMed] [Google Scholar]
- 17.Brownlee M: Glycation products and the pathogenesis of diabetic complications. Diabetes Care 1992; 15: 1835– 1843 [DOI] [PubMed] [Google Scholar]
- 18.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C: Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol 2005; 45: 1441– 1448 [DOI] [PubMed] [Google Scholar]
- 19.Fadini GP, Agostini C, Boscaro E, Avogaro A: Mechanisms and significance of progenitor cell reduction in the metabolic syndrome. Metab Syndr Relat Disord 2009; 7: 5– 10 [DOI] [PubMed] [Google Scholar]
- 20.Seeger FH, Haendeler J, Walter DH, Rochwalsky U, Reinhold J, Urbich C, Rossig L, Corbaz A, Chvatchko Y, Zeiher AM, Dimmeler S: p38 mitogen-activated protein kinase downregulates endothelial progenitor cells. Circulation 2005; 111: 1184– 1191 [DOI] [PubMed] [Google Scholar]
- 21.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rutten H, Fichtlscherer S, Martin H, Zeiher AM: HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest 2001; 108: 391– 397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humpert PM, Djuric Z, Zeuge U, Oikonomou D, Seregin Y, Laine K, Eckstein V, Nawroth PP, Bierhaus A: Insulin stimulates the clonogenic potential of angiogenic endothelial progenitor cells by IGF-1 receptor dependent signalling. Mol Med 2008; 14: 301– 308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fadini GP, Baesso I, Albiero M, Sartore S, Agostini C, Avogaro A: Technical notes on endothelial progenitor cells: ways to escape from the knowledge plateau. Atherosclerosis 2008; 197: 496– 503 [DOI] [PubMed] [Google Scholar]
- 24.Fadini GP, Sartore S, Schiavon M, Albiero M, Baesso I, Cabrelle A, Agostini C, Avogaro A: Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia-reperfusion injury in rats. Diabetologia 2006; 49: 3075– 3084 [DOI] [PubMed] [Google Scholar]
- 25.Busik JV, Tikhonenko M, Bhatwadekar A, Opreanu M, Yakubova N, Caballero S, Player D, Nakagawa T, Afzal A, Kielczewski J, Sochacki A, Hasty S, Calzi SL, Kim S, Duclas SK, Segal MS, Guberski DL, Esselman WJ, Boulton ME, Grant MB: Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J Exp Med 2009; 206: 2897– 906 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.