Abstract
OBJECTIVE
The associations of prenatal exposures to maternal prepregnancy overweight and gestational diabetes mellitus (GDM) with offspring overweight are controversial. Research estimating risk for offspring overweight due to these exposures, separately and concomitantly, is limited.
RESEARCH DESIGN AND METHODS
Prevalence of overweight and abdominal obesity at age 16 years and odds ratios (ORs) for prenatal exposures to maternal prepregnancy overweight and GDM were estimated in participants of the prospective longitudinal Northern Finland Birth Cohort of 1986 (N = 4,168).
RESULTS
The prevalence and estimates of risk for overweight and abdominal obesity were highest in those exposed to both maternal prepregnancy overweight and GDM (overweight prevalence 40% [OR 4.05], abdominal obesity prevalence 25.7% [3.82]). Even in offspring of mothers with a normal oral glucose tolerance test during pregnancy, maternal prepregnancy overweight is associated with increased risk for these outcomes (overweight prevalence 27.9% [2.56], abdominal obesity prevalence 19.5% [2.60]). In offspring of women with prepregnancy normal weight, the prevalence or risks of the outcomes were not increased by prenatal exposure to GDM. These estimates of risk were adjusted for parental prepregnancy smoking, paternal overweight, and offspring sex and size at birth.
CONCLUSIONS
Maternal prepregnancy overweight is an independent risk factor for offspring overweight and abdominal obesity at age 16 years. The risks are highest in offspring with concomitant prenatal exposure to maternal prepregnancy overweight and GDM, whereas the risks associated with GDM are only small.
By 1961, Pedersen (1) had already described the phenotype of the infant with prenatal exposure to maternal diabetes as “Most conspicuous…, the round cherub's cheeks, buried eyes, and short neck.” He suggested that maternal hyperglycemia leads to fetal hyperinsulinemia and increased growth, a hypothesis that is still the basis of research on maternal-fetal metabolism. Increasing evidence suggests that prenatal exposure to a hyperglycemic environment can alter growth trajectories and homeostatic regulatory mechanisms, thus causing lifelong changes that result in an increased risk of overweight and obesity (2–5). However, in several studies the association of prenatal exposure to gestational diabetes mellitus (GDM) with overweight later in life has been attenuated when controlling for maternal weight (6–10).
We have previously observed adolescent offspring of mothers with GDM to have a higher BMI than offspring of mothers with no risk factors for GDM (11). In the present study, we estimated the risks of overweight and abdominal obesity at age 16 years associated with prenatal exposures to maternal prepregnancy overweight and GDM, separately and concomitantly, based on the prospective Northern Finland Birth Cohort of 1986 (NFBC 1986).
RESEARCH DESIGN AND METHODS
Maternal health care, screening and diagnosing GDM during the study period
In Finland, cost-free health care is offered to all pregnant women at maternity welfare clinics (MWCs). Practically all pregnant women attend these clinics. During the study period, GDM screening and diagnosis in the MWCs was based on assessment of risk factors, in accordance with national guidelines. The women were considered to be at risk for GDM if one or more of the following risk factors were present: age over 40 years, BMI ≥25 kg/m2, prior GDM, previous delivery of a macrosomic infant (birth weight >4,500 g), glucosuria, and suspected fetal macrosomia in the current pregnancy. These women underwent glucose tolerance testing, performed after an overnight fast, conducted by administering a 2-h, 75-g oral glucose tolerance test (OGTT). The upper ranges of normal capillary blood glucose concentrations in 1985–1986 were 5.5, 11.0, and 8.0 mmol/l at fasting and at 1 h and 2 h after the glucose load, respectively. Importantly, diagnosis of GDM was set after one abnormal value in the OGTT, according to prevailing national guidelines.
The women with diagnosed GDM received dietary advice and monitored their blood glucose values at home, reporting them weekly to the delivery diabetes nurse at the delivery hospital. If fasting plasma glucose concentrations repeatedly exceeded 5.3 mmol/l or 2-h postprandial concentrations exceeded 6.7 mmol/l, guargum or insulin therapy was initiated.
Study population and data collection
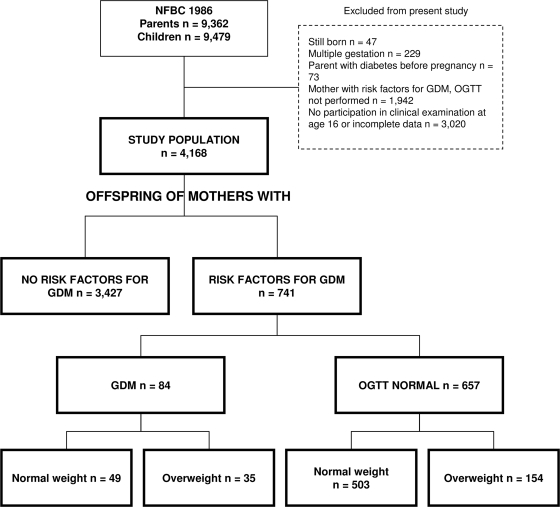
We used data based on participants of the NFBC 1986 (n = 9,362 mothers and fathers and 9,479 offspring) who were recruited and longitudinally assessed as described previously (11,12). Briefly, births to women with an expected delivery date between 1 July 1985 and 30 June 1986 in the two northernmost provinces of Finland were eligible. Data on parents and children were acquired prospectively, starting in the 12th gestational week, and were collected antenatally, at birth, and at the ages of 7 and 16 years. The latest follow-up, occurring in 2001–2002 at offspring age 16 years, consisted of questionnaires for parents and children (participation rate 80%) and a clinical examination of the children (participation rate 74%) for those participants who were alive and traceable. In the present study, we excluded children born from multiple gestations (n = 229), of parents with diabetes diagnosed before pregnancy (n = 73), and of mothers with risk factors for GDM but without an OGTT performed in pregnancy (n = 1,942) and those who had not participated in the clinical follow-up examination or had incomplete data on the outcome variables (n = 3,020). The study population included 4,168 adolescents, 2,092 males and 2,076 females (Fig. 1). Adolescents and parents received written and oral information and gave their written informed consent. The Ethics Committee of Northern Osthrobothnia Hospital District approved the study.
Figure 1.
Flow chart of the NFBC 1986 study population.
Trained nurses helped mothers fill in two questionnaires at MWCs. These questionnaires covered the early (data since 12th–16th gestational week) and late pregnancy (after 24 weeks of gestation including the perinatal period). A third questionnaire was filled in at the hospital by the attending midwives, who also recorded gestational age, weight, and length at birth. The course of pregnancy and delivery, including complications and diseases, were further confirmed from MWC and hospital patient records, as was the neonatal outcome. In 2000–2001 the parents and children filled in detailed postal questionnaires. At age 16 years, the adolescents attended a clinical examination performed by trained nurses. Measurements taken included, among others, height, weight, and waist circumference at the level midway between the lowest rib margin and the iliac crest.
Data on maternal prepregnancy overweight, susceptibility to GDM, and OGTT results in pregnancy were used to create an exposure variable as follows: Maternal prepregnancy BMIs were calculated and classified as normal weight/overweight with a cut off at 25 kg/m2. The mothers were classified according to their predisposition to GDM and OGTT results in pregnancy (no risk factors for GDM, risk factor/s for GDM but diagnostic OGTT normal, and GDM). Thereafter, a five-class variable was created: prenatal exposure to overweight and GDM (1), GDM only (2), overweight and maternal risk factors for GDM (3), maternal risk factors for GDM only (4), and no risk factors for GDM (5) (Fig. 1).
Possible confounding and intervening variables were treated as follows: Sex, socioeconomic status, prenatal exposure to maternal and paternal smoking, and paternal prepregnancy overweight were considered confounding factors. Offspring size at birth was considered an intervening factor. Duration of maternal education was used as a measure for socioeconomic status and classified as low/high with a cut off at 9 years. Prepregnancy smoking was classified as smoker and nonsmoker for both parents. Fathers' BMIs in 1985–1986 were calculated and classified as for mothers in prepregnancy. Offspring size at birth was classified as appropriate for gestational age (AGA) and small (SGA) and large (LGA) for gestational age according to ±2 SDs of the sex– and gestational age–specific cohort distributions.
The outcomes considered were 1) overweight including obesity and 2) abdominal obesity of the offspring at age 16 years. Overweight including obesity was defined according to the International Obesity Task Force age- and sex-specific criteria (13). The ratio of waist to height is a measure of central fatness that has emerged as a significant predictor of cardiovascular disease in children and adolescents (14). In the present study, abdominal obesity was defined as waist-to-height ratio >0.5.
Statistical methods
Statistical analyses were performed using SPSS version 15.0 (SPSS, Chicago, Illinois). The distributions of variables for clinical characteristics were skewed and therefore logarithmically transformed. These data are presented as geometric means and 95% confidence intervals (CIs). ANOVA was used for comparisons of variables between groups. Categorical data are presented as percentages. Pearson's χ2 test was used to evaluate differences between groups for categorized variables.
Logistic regression analysis was used to evaluate the independent associations of prenatal exposures with outcome variables. To create the adjusted regression models, prenatal exposure and confounding/intervening variables with a statistically significant odds ratio (OR) in the unadjusted analyses were entered simultaneously. All two-way interactions between predictors were tested for and found to be nonsignificant (data not shown).
In data attrition analysis, there were no statistically significant differences between the study population and the overall cohort in maternal age, child birth weight, or birth length. The mothers and fathers in the study population had a lower BMI at the initiation of the study compared with the overall cohort (geometric means of mothers' BMI 21.3 vs. 22.1 kg/m2, P < 0.001; fathers' BMI 23.8 vs. 23.9 kg/m2, P = 0.005).
RESULTS
Prenatal and birth data
The prevalence of GDM was 2.0%. Insulin therapy was initiated in 9.5% of the mothers with GDM. Mothers with GDM were more often overweight before pregnancy than mothers with a normal OGTT (41.7 vs. 23.6%, respectively). The maternal, paternal, and newborn characteristics, assorted according to maternal glucose metabolism in pregnancy and overweight, are shown in Table 1.
Table 1.
Characteristics of mothers, fathers, and children in the NFBC 1986 assorted according to maternal prepregnancy weight and glucose metabolism in pregnancy
| Maternal glucose metabolism and weight |
P* | |||||
|---|---|---|---|---|---|---|
| GDM |
OGTT normal |
Control | ||||
| Overweight | Normal weight | Overweight | Normal weight | |||
| n† | 30–35 | 42–49 | 136–154 | 439–503 | 2,837–3,427 | |
| Mothers | ||||||
| Age (years) | 34.8 (32.8–36.9) | 27.4 (25.8–29.1) | 29.4 (28.5–30.3) | 27.2 (26.8–27.7) | 26.9 (26.7–27.0) | <0.001 |
| Height (m) | 1.62 (1.60–1.64) | 1.63 (1.61–1.65) | 1.63 (1.62–1.64) | 1.64 (1.63–1.64) | 1.63 (1.63–1.63) | NS |
| Weight (kg) | 75.9 (72.9–79.1) | 56.4 (54.5–58.4) | 76.8 (75.1–78.5) | 57.1 (56.5–57.6) | 56.0 (55.8–56.2) | <0.001 |
| Prepregnancy BMI (kg/m2) | 29.0 (28.1–29.9) | 21.2 (20.7–21.8) | 28.9 (28.4–29.5) | 21.3 (21.1–21.5) | 21.0 (20.9–21.1) | <0.001 |
| % Nulliparous | 9.1 | 41.3 | 24.0 | 37.2 | 36.6 | <0.001 |
| % Smoker | 11.4 | 14.6 | 14.6 | 14.3 | 18.3 | NS |
| % Education <9 years | 20.0 | 2.2 | 9.3 | 5.1 | 4.4 | <0.001 |
| Fathers | ||||||
| Height (m) | 1.77 (1.74–1.79) | 1.78 (1.76–1.80) | 1.76 (1.75–1.78) | 1.77 (1.76–1.78) | 1.77 (1.77–1.77) | NS |
| Weight (kg) | 83.0 (77.8–88.5) | 76.4 (73.0–80.0) | 75.3 (73.5–77.0) | 74.6 (73.8–75.5) | 73.9 (73.6–74.2) | <0.001 |
| BMI (kg/m2) | 26.7 (25.3–28.1) | 24.3 (23.4–25.1) | 24.2 (23.8–24.6) | 23.8 (23.6–24.1) | 23.7 (23.6–23.8) | <0.001 |
| % Overweight | 56.7 | 38.1 | 36.0 | 32.0 | 28.3 | 0.001 |
| % Smoker | 31.3 | 42.9 | 38.4 | 35.8 | 37.8 | NS |
| Children | ||||||
| Newborn | ||||||
| Gestational age (weeks) | 38.5 (37.8–39.1) | 39.0 (38.6–39.5) | 39.4 (39.1–39.6) | 39.5 (39.4–39.7) | 39.5 (39.4–39.5) | 0.001 |
| Weight (kg) | 3.70 (3.49–3.92) | 3.67 (3.53–3.82) | 3.78 (3.68–3.88) | 3.69 (3.64–3.74) | 3.48 (3.46–3.50) | <0.001 |
| % Male | 60.0 | 55.1 | 55.8 | 52.1 | 49.5 | NS |
| % SGA | 0 | 0 | 1.9 | 0.6 | 2.2 | NS |
| % LGA | 8.6 | 2.0 | 10.4 | 6.2 | 0.4 | <0.001 |
| Age 16 years | ||||||
| Height (m) | 1.72 (1.69–1.74) | 1.71 (1.69–1.74) | 1.71 (1.69–1.72) | 1.70 (1.69–1.71) | 1.69 (1.69–1.69) | 0.001 |
| Weight (kg) | 66.7 (61.9–72.0) | 61.6 (58.6–64.8) | 65.2 (63.1–67.4) | 59.8 (58.9–60.8) | 58.9 (58.6–59.2) | <0.001 |
| BMI (kg/m2) | 22.7 (21.3–24.1) | 21.0 (20.5–21.5) | 22.4 (21.8–23.1) | 20.7 (20.5–21.0) | 20.7 (20.6–20.8) | <0.001 |
| Waist (cm) | 77.4 (73.1–82.0) | 74.6 (72.1–77.3) | 77.4 (75.7–79.2) | 72.9 (72.3–73.6) | 72.6 (72.4–72.9) | <0.001 |
Data are geometric means (95% CI) for continuous variables and percentage for categorical variables.
*P value for difference between groups from ANOVA for continuous variables and χ2 test for categorical variables.
†n varies due to incomplete data, most often missing paternal BMI. NS, not significant.
Outcome variables
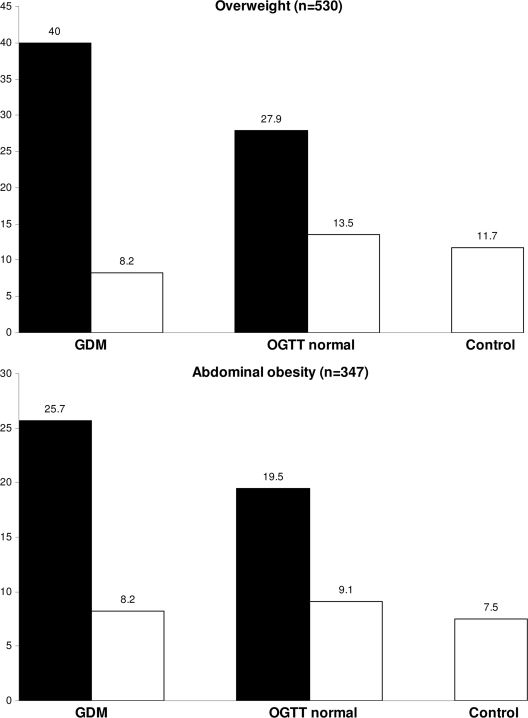
At age 16 years, 12.7% of the offspring in the whole cohort were overweight, and 8.3% had abdominal obesity. In offspring of mothers with prepregnancy normal weight, the prevalences of these outcome variables were similar irrespective of maternal glucose metabolism in pregnancy. In offspring of mothers with prepregnancy overweight, the prevalences of the outcome variables were increased, especially when prenatal exposure to GDM was also present (Fig. 2).
Figure 2.
Prevalence (%) of overweight and abdominal obesity in offspring of the NFBC 1986 at age 16 years. Data assorted according to maternal glucose metabolism in pregnancy and prepregnancy BMI. □, Offspring of normal weight mothers; ■, offspring of overweight mothers. (Note difference in scale on y-axis.)
Estimates of risk
In unadjusted analyses, the risks for the outcomes were greatest in offspring with prenatal exposures to both GDM and maternal overweight (overweight OR 4.05, abdominal obesity 3.82). Prenatal exposure to maternal overweight associated with increased risk of overweight (OR 2.56) and abdominal obesity (2.60), even in offspring of mothers with a normal OGTT during pregnancy. In offspring of normal weight women, prenatal exposure to maternal GDM was not associated with increased risks of the outcome measures. These associations remained even after adjustment for confounding/intervening factors (Table 2).
Table 2.
Estimates of risk for overweight and abdominal obesity in offspring at age 16 years for prenatal exposures and possible confounding/intervening factors
| Risk factors | Overweight |
Abdominal obesity |
||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted OR | Adjusted OR† | Unadjusted OR | Adjusted OR† | |||||
| Maternal | ||||||||
| Glucose metabolism in pregnancy and prepregnancy weight | ||||||||
| Control | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | ||||
| OGTT normal | ||||||||
| Normal weight | 1.18 | 0.90, 1.56 | 1.13 | 0.83, 1.54 | 1.23 | 0.89, 1.72 | 1.16 | 0.80, 1.67 |
| Overweight | 2.92** | 2.03, 4.22 | 2.56** | 1.69, 3.88 | 2.97** | 1.95, 4.51 | 2.60** | 1.62, 4.17 |
| GDM | ||||||||
| Normal weight | 0.67 | 0.24, 1.89 | 0.73 | 0.26, 2.08 | 1.09 | 0.39, 3.06 | 1.22 | 0.43, 3.48 |
| Overweight | 5.03** | 2.54, 9.97 | 4.05** | 1.90, 8.62 | 4.25** | 1.97, 9.16 | 3.82* | 1.66, 8.82 |
| Smoking | ||||||||
| No | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | ||||
| Yes | 1.39* | 1.11, 1.74 | 1.31* | 1.00, 1.71 | 1.54* | 1.18, 2.00 | 1.42* | 1.04, 1.93 |
| Education | ||||||||
| High | 1 (ref.) | 1 (ref.) | ||||||
| Low | 1.41 | 0.93, 2.12 | NA | 1.41 | 0.86, 2.30 | NA | ||
| Paternal | ||||||||
| Overweight | ||||||||
| No | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | ||||
| Yes | 2.19** | 1.79, 2.68 | 2.14** | 1.74, 2.63 | 2.11** | 1.66, 2.68 | 2.02** | 1.58, 2.59 |
| Smoking | ||||||||
| No | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | ||||
| Yes | 1.26* | 1.04, 1.53 | 1.20 | 0.97, 1.49 | 1.41* | 1.12, 1.78 | 1.36* | 1.05, 1.76 |
| Offspring sex | ||||||||
| Female | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||||
| Male | 1.37* | 1.14, 1.64 | 1.32* | 1.07, 1.62 | 0.94 | 0.75, 1.17 | NA | |
| Birth size | ||||||||
| AGA | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||||
| SGA | 0.65 | 0.30, 1.43 | NA | 1.06 | 0.48, 2.31 | NA | ||
| LGA | 1.99* | 1.09, 3.63 | 1.28 | 0.62, 2.61 | 2.11* | 1.06, 4.18 | 1.39 | 0.62, 3.09 |
*P < 0.05,
**P < 0.001,
†adjusted for factors with a statistically significant OR in the unadjusted analyses.
In unadjusted analyses of the confounding/intervening variables, prenatal exposures to paternal overweight, maternal and paternal smoking, male sex, and being born LGA were associated with increased risks of both outcome measures. In adjusted analyses, the associations remained for prenatal exposures to maternal smoking and paternal overweight for both outcome measures (Table 2). The estimates of risk did not differ between sexes, except for the association of prenatal exposure to smoking with the outcome measures, which was found only in same-sex parent-child pairs (data not shown).
CONCLUSIONS
Our study presents estimates of risk of overweight and abdominal obesity for prenatal exposures to maternal prepregnancy overweight and GDM separately. Maternal overweight emerged as an essential risk factor for both outcomes. The risks associated with concomitant exposure to maternal prepregnancy overweight and GDM were high. In offspring of normal-weight women, no statistically significant risks for overweight and abdominal obesity were associated with prenatal exposure to GDM.
Previous studies assessing overweight after prenatal exposure to GDM have not always controlled for maternal overweight. The studies that have controlled for maternal overweight are conflicting. Four studies have found an independent association between prenatal exposure to maternal GDM and offspring overweight (14–17). The results from the Pima Indian Study (14) may not be generalized to other populations, and two other studies were retrospective and lacked a control group of mothers with normal glucose tolerance (16,17). Four retrospective studies have found that prenatal exposure to GDM was not independently associated with offspring obesity (6–9); one lacked a control group of mothers with normal glucose tolerance (8), and one was questionnaire-based (9). A recent study by Catalano et al. (10) suggests that maternal prepregnancy overweight is the strongest predictor of childhood obesity, independent of maternal glucose metabolism during pregnancy or offspring birth weight. Our results support this; in addition, we found that prenatal exposure to prepregnancy overweight combined with GDM conveys an even higher risk for offspring overweight than prenatal exposure to maternal prepregnancy overweight alone.
It has been suggested that the theory of fuel-mediated teratogenesis might be expanded to include fetal overnutrition due to maternal obesity in pregnancy (18). To test the fetal overnutrition hypothesis, the effect of maternal and paternal prepregnancy BMI on later offspring BMI should be compared (19). Some previous studies have found support for the hypothesis (19,20), while others have challenged it (21–23). In the present study, a greater fraction of the risk for overweight and abdominal obesity at age 16 years was attributable to maternal than paternal overweight during the fetal period of the offspring. Thus, our results support the fetal overnutrition hypothesis. However, this must be treated with caution, as maternal prepregnancy overweight may influence offspring overweight not only via the intrauterine milieu, but also via genetic and/or postnatal environment and lifestyle factors, which are beyond the scope of the present study.
A recent meta-analysis has estimated the OR of exposure to maternal smoking for obesity between the ages of 3 and 33 years to be ∼1.50 (24), but to our knowledge there are no previous studies that have assessed the effects of prenatal exposure to GDM, parental overweight, and smoking simultaneously. In addition to confirming the association of maternal smoking with offspring overweight, even when adjusting for several factors, we observed that prenatal exposure to paternal smoking was associated with increased risk of abdominal obesity in offspring. The association of intrauterine exposure to smoking with the outcome measures was stronger in same-sex parent-child pairs; we speculate this may be due to an additional lifestyle effect, assuming that smoking may associate with an obesity-prone lifestyle adopted from the same-sex parent.
The screening and diagnosis of GDM is a subject of debate (2). In the present study, the screening for GDM was risk-factor based; the cut offs for the OGTT results in pregnancy differed from those recommended by the American Diabetes Association (25), and the diagnosis of GDM was made after one abnormal value in the 75-g OGTT. Thus, some women with no risk factors for GDM but with the disease may have gone undetected, and women with relatively mild disturbances in glucose metabolism are included in the GDM group. However, as we observed that prenatal exposure to GDM increased the risks for the outcome measures in offspring of overweight mothers quite strikingly, it seems that even mild disturbances in maternal glucose metabolism are a risk factor for offspring overweight and abdominal obesity.
Maternal hyperglycemia, irrespective of its etiology, has been postulated to have similar long-term effects on the offspring (15). However, the genetic factors contributing to the predisposition to metabolic disturbances in offspring and the timing of prenatal exposure to hyperglycemia are not identical in offspring of mothers with type 1, type 2, and GDM. As the data comparing long-term consequences of prenatal exposures to different diabetes types in humans is limited, the results of this study are comparable only with studies on prenatal exposure to GDM.
To date, the NFBC 1986 is one of the most comprehensive, prospective, long-term follow-up cohort of offspring exposed to GDM in a general population. All participants were white Caucasian, born in the same area during the same time period, and similarly followed-up at the same age. The virtually 100% coverage of antenatal care in the MWCs enabled extensive, prospective data collection. The exceptionally high retention rate further adds to the value of this study. In addition to distinguishing between the effects of GDM and maternal overweight, the present study accounted for several confounding factors, even paternal variables. Despite the large number of participants in the NFBC 1986, the number in the stratified analyses did not allow stable risk estimates for very many predictors. Thus, to avoid overparameterization, we chose to concentrate on prenatal factors as determinants of later overweight and abdominal obesity. Even though the study groups were quite small, the differences observed were statistically significant and clinically plausible, and we therefore consider the results highly relevant.
In summary, we present novel, prospective data on the risks of overweight and abdominal obesity associated with prenatal exposures to maternal prepregnancy overweight and GDM. Prenatal exposure to maternal prepregnancy overweight was an independent risk factor for both outcomes. The risks of overweight and abdominal obesity associated with concomitant prenatal exposures to maternal prepregnancy overweight and GDM were alarmingly high. Given the well-known health risks related to overweight and abdominal obesity and the rising prevalence of both maternal prepregnancy overweight and GDM, the results of the present study warrant public health attention.
Acknowledgments
This study was conducted with the support of the Academy of Finland and the European Commission (Framework 5 award QLG1-CT-2000-01643). J.P. has received funding from the National Graduate School of Clinical Investigation, the Northern Ostrobothnia Hospital District, the Alma and K.A. Snellman Foundation, and the Oulu University Scholarship Foundation. The authors' work was independent of the funders. No potential conflicts of interest relevant to this article were reported.
We thank Markku Koiranen and Tuula Ylitalo for their support in data delivery and management.
Footnotes
References
- 1.Pedersen J, Osler M: Hyperglycemia as the cause of characteristic features of the foetus and newborn of diabetic mothers. Dan Med Bull 1961; 8: 78– 83 [PubMed] [Google Scholar]
- 2.Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, Hod M, Kitzmiller JL, Kjos SL, Oats JN, Pettitt DJ, Sacks DA, Zoupas C: Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007; 30( Suppl. 2): S251– S260 [DOI] [PubMed] [Google Scholar]
- 3.Dabelea D: The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care 2007; 30( Suppl. 2): S169– S174 [DOI] [PubMed] [Google Scholar]
- 4.Vohr BR, Boney CM: Gestational diabetes: the forerunner for the development of maternal and childhood obesity and metabolic syndrome? Matern Fetal Neonatal Med 2008; 21: 149– 157 [DOI] [PubMed] [Google Scholar]
- 5.Simeoni U, Barker DJ: Offspring of diabetic pregnancy: long-term outcomes. Semin Fetal Neonatal Med 2009; 14: 119– 124 [DOI] [PubMed] [Google Scholar]
- 6.Whitaker RC, Pepe MS, Seidel KD, Wright JA, Knopp RH: Gestational diabetes and the risk of offspring obesity. Pediatrics 1998; 101: E9. [DOI] [PubMed] [Google Scholar]
- 7.Vohr BR, McGarvey ST, Tucker R: Effects of maternal gestational diabetes on offspring adiposity at 4–7 years of age. Diabetes Care 1999; 22: 1284– 1291 [DOI] [PubMed] [Google Scholar]
- 8.Schaefer-Graf UM, Heuer R, Kilavuz O, Pandura A, Henrich W, Vetter K: Maternal obesity not maternal glucose values correlates best with high rates of fetal macrosomia in pregnancies complicated by gestational diabetes. J Perinat Med 2002; 30: 313– 321 [DOI] [PubMed] [Google Scholar]
- 9.Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA: Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics 2003; 111: e221– e226 [DOI] [PubMed] [Google Scholar]
- 10.Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, Amini SB: Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr 2009; 90: 1303– 1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vääräsmäki M, Pouta A, Elliot P, Tapanainen P, Sovio U, Ruokonen A, Hartikainen AL, McCarthy M, Järvelin MR: Adolescent manifestations of metabolic syndrome among children born to women with gestational diabetes in a general-population birth cohort. Am J Epidemiol 2009; 169: 1209– 1215 [DOI] [PubMed] [Google Scholar]
- 12.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH: Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000; 320: 1240– 1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarthy HD, Ashwell M: A study of central fatness using waist-to-height ratios in UK children and adolescents over two decades supports the simple message–‘keep your waist circumference to less than half your height’. Int J Obes (Lond) 2006; 30: 988– 992 [DOI] [PubMed] [Google Scholar]
- 14.Pettitt DJ, Knowler WC: Long-term effects of the intrauterine environment, birth weight, and breast-feeding in Pima Indians. Diabetes Care 1998; 21( Suppl. 2): B138– B141 [PubMed] [Google Scholar]
- 15.Metzger BE, Silverman BL, Freinkel N, Dooley SL, Ogata ES, Green OC: Amniotic fluid insulin concentration as a predictor of obesity. Arch Dis Child 1990; 65: 1050– 1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plagemann A, Harder T, Kohlhoff R, Rohde W, Dörner G: Overweight and obesity in infants of mothers with long-term insulin-dependent diabetes or gestational diabetes. Int J Obes Relat Metab Disord 1997; 21: 451– 456 [DOI] [PubMed] [Google Scholar]
- 17.Lee H, Jang HC, Park HK, Cho NH: Early manifestation of cardiovascular disease risk factors in offspring of mothers with previous history of gestational diabetes mellitus. Diabetes Res Clin Pract 2007; 78: 238– 245 [DOI] [PubMed] [Google Scholar]
- 18.Levin BE, Govek E: Gestational obesity accentuates obesity in obesity-prone progeny. Am J Physiol 1998; 275: R1374– R1379 [DOI] [PubMed] [Google Scholar]
- 19.Lawlor DA, Smith GD, O'Callaghan M, Alati R, Mamun AA, Williams GM, Najman JM: Epidemiologic evidence for the fetal overnutrition hypothesis: findings from the mater-university study of pregnancy and its outcomes. Am J Epidemiol 2007; 165: 418– 424 [DOI] [PubMed] [Google Scholar]
- 20.Parsons TJ, Power C, Manor O: Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: longitudinal study. BMJ 2001; 323: 1331– 1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davey Smith G, Steer C, Leary S, Ness A: Is there an intrauterine influence on obesity? Evidence from parent child associations in the Avon Longitudinal Study of Parents and Children (ALSPAC). Arch Dis Child 2007; 92: 876– 880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kivimäki M, Lawlor DA, Smith GD, Elovainio M, Jokela M, Keltikangas-Järvinen L, Viikari JS, Raitakari OT: Substantial intergenerational increases in body mass index are not explained by the fetal overnutrition hypothesis: the Cardiovascular Risk in Young Finns Study. Am J Clin Nutr 2007; 86: 1509– 1514 [DOI] [PubMed] [Google Scholar]
- 23.Lawlor DA, Timpson NJ, Harbord RM, Leary S, Ness A, McCarthy MI, Frayling TM, Hattersley AT, Smith GD: Exploring the developmental overnutrition hypothesis using parental-offspring associations and FTO as an instrumental variable. PLoS Med 208; 5: e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oken E, Levitan EB, Gillman MW: Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes (Lond) 2008; 32: 201– 210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2007; 30( Suppl. 1): S42– S47 [DOI] [PubMed] [Google Scholar]