Abstract
OBJECTIVE
To evaluate the effects of two low-fat hypocaloric diets differing in the carbohydrate-to-protein ratio, with and without resistance exercise training (RT), on weight loss, body composition, and cardiovascular disease (CVD) risk outcomes in overweight/obese patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
A total of 83 men and women with type 2 diabetes (aged 56.1 ± 7.5 years, BMI 35.4 ± 4.6 kg/m2) were randomly assigned to an isocaloric, energy-restricted diet (female subjects 6 MJ/day, male subjects 7 MJ/day) of either standard carbohydrate (CON; carbohydrate:protein:fat 53:19:26) or high protein (HP; 43:33:22), with or without supervised RT (3 days/week) for 16 weeks. Body weight and composition, waist circumference (WC), and cardiometabolic risk markers were assessed.
RESULTS
Fifty-nine participants completed the study. There was a significant group effect (P ≤ 0.04) for body weight, fat mass, and WC with the greatest reductions occuring in HP+RT (weight [CON: −8.6 ± 4.6 kg, HP: −9.0 ± 4.8 kg, CON+RT: −10.5 ± 5.1 kg, HP+RT: −13.8 ± 6.0 kg], fat mass [CON: −6.4 ± 3.4 kg, HP: −6.7 ± 4.0 kg, CON+RT: −7.9 ± 3.7 kg, HP+RT: −11.1 ± 3.7 kg], and WC [CON: −8.2 ± 4.6 cm, HP: −8.9 ± 3.9 cm, CON+RT: −11.3 ± 4.6 cm, HP+RT: −13.7 ± 4.6 cm]). There was an overall reduction (P < 0.001) in fat-free mass (−2.0 ± 2.3 kg), blood pressure (−15/8 ± 10/6 mmHg), glucose (−2.1 ± 2.2 mmol/l), insulin (−4.7 ± 5.4 mU/l), A1C (−1.25 ± 0.94%), triglycerides (−0.47 ± 0.81 mmol/l), total cholesterol (−0.67 ± 0.69 mmol/l), and LDL cholesterol (−0.37 ± 0.53 mmol/l), with no difference between groups (P ≥ 0.17).
CONCLUSIONS
An energy-restricted HP diet combined with RT achieved greater weight loss and more favorable changes in body composition. All treatments had similar improvements in glycemic control and CVD risk markers.
Caloric restriction and physical activity are cornerstones of obesity and type 2 diabetes management (1). The substitution of some dietary carbohydrate for protein in a low-fat (<30% total energy) diet may improve body composition and cardiovascular disease (CVD) risk factors including insulin sensitivity, glycemic control, and blood lipids in overweight/obese populations, including patients with type 2 diabetes (2–5). However, these studies have almost invariably assessed modified macronutrient compositions without the inclusion of any exercise intervention.
Layman et al. (6) showed additive effects of a high-protein (HP) diet combined with exercise for improving body composition in healthy overweight/obese women with greater weight and fat loss following a lifestyle intervention of exercise plus an HP diet, compared with HP diet alone or a standard carbohydrate (CON) diet with or without exercise. A study by Meckling et al. (7) showed similar effects. In both these studies, the exercise programs used were primarily aerobic exercise based, whereas resistance exercise training (RT) may be more efficacious (8). During weight loss, RT can maintain and/or increase lean tissue and improve physical functioning (8), and its importance for improving glycemic control in patients with type 2 diabetes is well established (9). Recently, Kerksick et al. (10) showed greater improvements in body composition in obese women following a 14-week RT program when carbohydrate was substituted with protein. This suggests that an HP diet combined with RT may improve weight loss, body composition, and cardiometabolic risk factors. However, despite its importance, no studies have evaluated these effects in individuals with type 2 diabetes. This study compared the effects of an energy-restricted HP diet and an isocaloric “traditional” CON diet with and without RT on weight loss, body composition, CVD risk factors, and glycemic control in patients with type 2 diabetes. We hypothesized that HP+RT treatment would produce the greatest improvements on these outcomes.
RESEARCH DESIGN AND METHODS
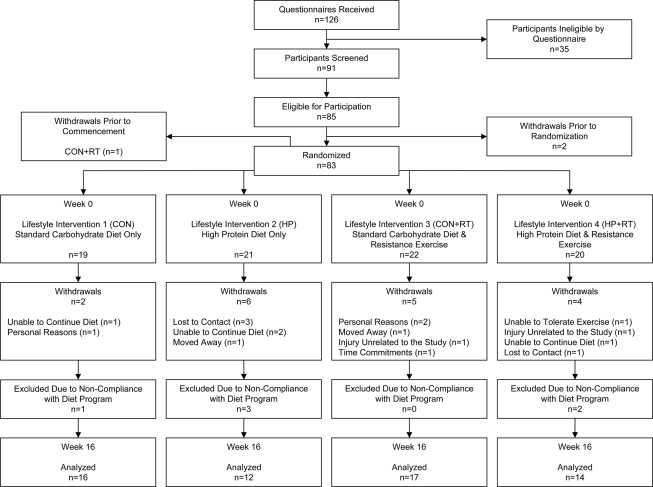
A total of 83 overweight/obese sedentary men and women (BMI 35.3 ± 4.5 kg/m2; aged 55.0 ± 8.4 years) with type 2 diabetes were recruited by public advertisement (Fig. 1). Participants were excluded if they had known proteinuria; had a malignancy; had a history of liver, kidney, cardiovascular, respiratory, or gastrointestinal disease; had uncontrolled hypertension; were pregnant or lactating; were a smoker; or were using insulin. Participants with any musculoskeletal injury or joint or peripheral vascular disease sufficient to impede exercise or who had participated in regular physical exercise (more than two 30-min sessions/week of moderate/vigorous aerobic exercise or one 30-min session/week of RT) during the 6 months prior to study were also excluded. Participants provided written informed consent. The study was approved by the human research ethics committees of the Commonwealth Scientific and Industrial Research Organisation (CSIRO) and the University of Adelaide.
Figure 1.
Participant flow.
In a parallel study, participants were blocked matched for age, sex, and weight, then randomized to one of four lifestyle interventions: an energy-restricted standard carbohydrate, low-protein, low-fat diet alone (CON) or with RT (CON+RT); or an isocaloric higher-protein, moderate-carbohydrate, low-fat diet alone (HP) or with RT (HP+RT) for 16 weeks. At baseline (week 0) and week 16, participants attended the CSIRO research clinic after an overnight fast for assessment. At the clinic testing visits, height (week 0 only), body weight, blood pressure, waist circumference (WC), and body composition were measured before a venous blood sample was drawn and muscle strength was assessed. Prior to the clinical assessments, a 24-h urine sample was collected.
Medications at baseline and changes throughout the study were documented. Lipid-lowering and antihypertensive medications were encouraged to remain constant throughout the intervention. Participants were asked not to modify their lifestyle patterns other than necessary during the intervention to comply with the study protocol.
Diet interventions
The diets were designed to be isocaloric with moderate energy restriction (∼6,000 kJ/day for women, ∼7,000 kJ/day for men). The planned macronutrient profile of the CON diet was 53% of total energy as carbohydrate, 19% (∼0.7 g · kg−1 · day−1) protein, and 26% fat. The target profile for the HP diet was 43% carbohydrate, 33% (∼1.2 g · kg−1 · day−1) protein, and 22% fat. To facilitate dietary compliance, detailed dietary advice, meal planning, and recipe information were provided at baseline and every 2 weeks by a qualified dietitian. Key foods representative of each diet's macronutrient profile (∼50% total energy) were supplied every 2 weeks. Diets were structured to include specific food quantities/weights to ensure correct macronutrient and energy profile were achieved. These foods were listed in a quantitative food record completed daily by participants. Participants were asked to weigh and measure their food using scales provided. Dietary composition was assessed by a qualified dietitian based on the analysis of 7 consecutive days from the semiquantitative food record of each 2-week period using a computerized database (Foodworks Professional Edition, version 4, 1998; Xyris Software, Highgate Hill, Australia). Participants who did not complete the food records were excluded as noncompliers.
Exercise intervention
The CON+RT and HP+RT group participants followed a progressive RT program. Eight separate exercises (leg press, knee extension, chest press, shoulder press, lat pull down, seated row, triceps press, and sit-ups) were performed using weight-stacked machines (Maxim Health Fitness, Adelaide, Australia), except sit-ups, on 3 nonconsecutive days/week. The weight loading was set at 70–85% one repetition maximum (1RM, protocol described below), determined for each exercise at Week 0. This allowed ∼8–12 reps to volitional fatigue with two sets per exercise and 1–2 min rest between sets to be performed. Once participants could successfully perform two sets of >12 repetitions, the weight load was increased to maintain the training load. Resistance exercise training sessions lasted ∼45 min and were conducted at the CSIRO gymnasium under professional supervision. Participants completed a training diary, and if a scheduled session was missed, they were encouraged to attend a make-up session as soon as possible.
Outcomes, body weight, composition, blood pressure, and muscle strength
Body mass was measured using calibrated electronic digital scales (Mercury; AMZ 14, Tokyo, Japan), and body composition was measured using dual-energy X-ray absorptiometry (DXA; Lunar Prodigy; General Electric, Madison, WI) to assess total body fat mass and total body fat-free mass (FFM). WC was measured on a horizontal plane 2 cm proximal to the uppermost lateral border of the right iliac crest. Seated blood pressure was measured using an automated sphygmomanometer (DYNAMAP 8100; Criticon, Tampa, FL).
Muscle strength (1RM) was assessed pre- and postintervention for chest press and lat pull down according to standard guidelines (11). 1RM of the other five weight-stacked exercises were also assessed in the participants in the exercise groups at baseline only to set initial training loads. To determine 1RM, following a low-intensity warm-up, participants performed four to five trials (separated by a 2-min resting interval) using varying moderate-heavy weights to determine the highest weight that could be lifted with only one repetition through the full range of motion with correct technique.
Biochemical analyses
Serum lipids and insulin, plasma glucose, C-reactive protein (CRP), and creatinine were measured using standard methods (12). A1C, measured using high-performance liquid chromatography, and 24-h urinary urea, creatinine, and albumin were assessed at a commercial laboratory (IMVS, Adelaide, Australia). Creatinine clearance was calculated as (urine creatinine [μmol/l] × urine volume [ml])/(plasma creatinine [μmol/l] × minutes) and corrected for body surface area (13).
Statistical analysis
Statistical analyses were performed using SPSS for Windows (version 17.0; SPSS, Chicago, IL). Prior to hypothesis testing, data were examined for normality. Non–normally distributed variables (insulin, CRP, and urinary albumin) were logarithmically transformed before analysis. Differences in baseline characteristics were compared by one-way ANOVA for continuous variables and χ2 tests for categorical variables. This investigation represents an efficacy trial to determine the physiological/metabolic effects of the treatments. Primary analysis was conducted on participants who completed the study per protocol. Secondary intention-to-treat (ITT) analysis was also conducted for the primary outcome measures (body weight and composition, cardiometabolic and glycemic control), including participants who completed the study irrespective to protocol adherence. Changes over time in the groups were assessed using repeated-measures ANOVA. The effects of the treatments on changes were assessed using one-way ANOVA, with group (CON, HP, CON+RT, and HP+RT) as a between-subject factor. Where there was a significant main effect, post hoc tests with Dunnett adjustment for multiple comparisons were performed to determine differences between group means compared with HP+RT; based on a prior hypothesis, the HP+RT group would achieve greater changes compared with the other treatments. Age and sex were included as covariates. No significant effect of sex or age was observed for any of the outcomes. To compare the magnitude of change between the two diets (CON and CON+RT versus HP and HP+RT) and exercise versus nonexercise (CON and HP versus CON+RT and HP+RT), planned contrasts were used in the statistical model. Pearson correlation coefficients were used to determine the relationships between variables. Statistical significance was set at P < 0.05. Data are means ± SD.
RESULTS
Participants
Of 83 randomized participants, 18 withdrew and 6 participants were excluded for dietary noncompliance (failure to complete food records); 59 participants completed the study per protocol (Fig. 1). There was no difference between treatment groups, diets, or exercise versus nonexercise groups in the number of participants who withdrew (P ≥ 0.34) or who were excluded (P ≥ 0.17). At baseline, there were no significant differences in age, weight, BMI, A1C, and sex distribution between groups (P ≥ 0.12). These characteristics were also similar between participants who completed or did not complete the study (P ≥ 0.09), except for age in which completers were on average 5 years younger (completers: aged 50.8 ± 10.4 years, dropouts: aged 56.1 ± 7.4 years; P = 0.02).
Dietary composition and exercise compliance
Based on the food records, participants showed good compliance with the prescribed diets (Table 1). Total energy intake was similar across treatment groups, while macronutrient composition was different between the diet groups; the HP and HP+RT groups consumed significantly more protein and less carbohydrate and fat compared with the CON and CON+RT groups. There was a significant diet effect for the urinary urea–to–creatinine excretion ratio (P = 0.003), which decreased in the CON diet groups (CON: 30.8 ± 8.6 to 26.7 ± 3.8, CON+RT: 25.9 ± 5.1 to 24.2 ± 4.0) and increased in the HP diet groups (HP: 31.1 ± 9.8 to 33.6 ± 11.2, HP+RT: 28.3 ± 9.5 to 31.5 ± 7.7), indicating higher protein intakes in the HP diet groups.
Table 1.
Macronutrient composition of the treatment groups
| CON | HP | CON+RT | HP+RT | Treatment group* | Diet† | |
|---|---|---|---|---|---|---|
| n | 16 | 12 | 17 | 14 | ||
| Energy (kJ) | 6,278 ± 648 | 6,321 ± 763 | 6,199 ± 696 | 6,339 ± 649 | 0.944 | 0.62 |
| Carbohydrate (g) | 197.4 ± 16.3 | 176.3 ± 23.7 | 195.0 ± 21.5 | 170.0 ± 23.1 | 0.001 | <0.001 |
| Carbohydrate (% of energy) | 53.6 ± 2.6 | 47.4 ± 1.6 | 53.6 ± 3.9 | 45.5 ± 2.4 | <0.001 | <0.001 |
| Protein (g) | 68.4 ± 5.9 | 119.0 ± 7.8 | 68.0 ± 8.3 | 117.1 ± 6.7 | <0.001 | <0.001 |
| Protein (% of energy) | 18.6 ± 0.9 | 32.3 ± 2.8 | 18.7 ± 1.3 | 31.6 ± 2.2 | <0.001 | <0.001 |
| Fat (g) | 38.5 ± 7.7 | 30.5 ± 8.2 | 37.5 ± 9.6 | 33.7 ± 5.5 | 0.041 | <0.01 |
| Fat (% of energy) | 22.6 ± 3.0 | 17.7 ± 3.0 | 22.3 ± 4.5 | 19.6 ± 1.9 | 0.001 | <0.001 |
| Saturated fat (% of total fat) | 34.1 ± 5.5 | 33.9 ± 5.0 | 33.2 ± 2.8 | 34.3 ± 4.3 | 0.90 | 0.68 |
| Polyunsaturated fat (% of total fat) | 19.8 ± 4.5 | 22.3 ± 3.6 | 21.4 ± 4.5 | 21.0 ± 4.2 | 0.47 | 0.39 |
| Monounsaturated fat (% of total fat) | 46.1 ± 6.6 | 43.9 ± 4.1 | 45.5 ± 5.4 | 44.8 ± 5.1 | 0.74 | 0.32 |
| Diet fiber (g) | 31.1 ± 2.9 | 24.7 ± 4.0 | 30.5 ± 4.4 | 22.6 ± 4.1 | <0.001 | <0.001 |
Data are means ± SD. The treatment groups were a standard carbohydrate, low-protein, low-fat diet alone (CON) or with resistance exercise training (CON+RT), or an isocaloric higher-protein, low-fat diet alone (HP) or with resistance exercise training (HP+RT).
*Differences between groups (one-way ANOVA).
†Comparison of the difference between the diets (CON and CON+RT vs. HP and HP+RT) (planned contrast).
Compliance with RT was defined as the number of exercise sessions completed at the prescribed training loads per total number of prescribed sessions. All participants in the CON+RT and HP+RT groups achieved compliance with the prescription (defined as 75% of prescribed sessions), on average completing 93% (43.5 ± 4.0 of 47) of the prescribed sessions. There was a significant effect of exercise treatment for 1RM lat pull down and chest press (P < 0.001) such that increases occurred in the diet+RT groups, with no change in the diet-only groups (Table 2), indicating compliance with the prescribed RT.
Table 2.
Body weight, composition, muscle strength, cardiometabolic risk factors, and glycemic control before and after 16 weeks of either an energy-restricted standard carbohydrate, low-protein, low-fat diet alone (CON) or with resistance exercise training (CON+RT), or an isocaloric higher-protein, low-fat diet alone (HP) or with resistance exercise training (HP+RT)
| CON | HP | CON+RT | HP+RT |
P |
||||
|---|---|---|---|---|---|---|---|---|
| Time effect* | Group effect† | Diet comparison‡ | Exercise comparison§ | |||||
| n | 16 | 12 | 17 | 14 | ||||
| Body weight (kg) | ||||||||
| Week 0 | 97.0 ± 10.6 | 102.7 ± 15.4 | 105.0 ± 15.3 | 107.6 ± 15.5 | <0.001 | 0.04 | 0.18 | 0.02 |
| Week 16 | 88.4 ± 11.2 | 93.7 ± 13.8 | 94.5 ± 15.4 | 93.8 ± 13.5 | ||||
| Change | −8.6 ± 4.6‖ | −9.0 ± 4.8‖ | −10.5 ± 5.1 | −13.8 ± 6.0 | ||||
| BMI (kg/m2) | ||||||||
| Week 0 | 34.8 ± 4.9 | 35.6 ± 3.8 | 34.9 ± 4.9 | 36.6 ± 5.0 | <0.001 | 0.06 | 0.17 | 0.03 |
| Week 16 | 31.7 ± 5.1 | 32.5 ± 3.1 | 31.4 ± 4.3 | 31.9 ± 4.3 | ||||
| Change | −3.1 ± 1.6 | −3.2 ± 1.7 | −3.5 ± 1.7 | −4.7 ± 2.1 | ||||
| Total body fat mass (kg) | ||||||||
| Week 0 | 38.5 ± 8.0 | 40.4 ± 8.4 | 40.4 ± 10.0 | 42.9 ± 11.6 | <0.001 | 0.006 | 0.06 | <0.01 |
| Week 16 | 32.1 ± 9.5 | 33.2 ± 6.9 | 32.3 ± 10.7 | 31.5 ± 11.6 | ||||
| Change | −6.5 ± 3.7¶ | −7.1 ± 4.0‖ | −8.1 ± 3.8 | −11.4 ± 3.9 | ||||
| WC (cm) | ||||||||
| Week 0 | 111.3 ± 10.7 | 114.3 ± 6.8 | 113.7 ± 8.5 | 116.2 ± 12.7 | <0.001 | 0.006 | 0.19 | 0.48 |
| Week 16 | 103.2 ± 12.8 | 105.4 ± 6.7 | 102.4 ± 9.6 | 102.5 ± 11.8 | ||||
| Change | −8.2 ± 4.6¶ | −8.9 ± 3.9‖ | −11.3 ± 4.6 | −13.7 ± 4.6 | ||||
| Total FFM (kg) | ||||||||
| Week 0 | 58.5 ± 10.7 | 62.3 ± 13.0 | 64.6 ± 12.4 | 64.7 ± 11.5 | <0.001 | 0.91 | 0.80 | 0.51 |
| Week 16 | 56.3 ± 10.6 | 60.4 ± 13.2 | 62.2 ± 12.0 | 62.3 ± 10.7 | ||||
| Change | −2.2 ± 1.9 | −1.9 ± 1.5 | −2.4 ± 2.5 | −2.4 ± 3.1 | ||||
| Single repetition bench press (kg) | ||||||||
| Week 0 | 60.0 ± 18.1 | 68.5 ± 27.4 | 67.1 ± 22.4 | 64.6 ± 25.5 | <0.01 | <0.001 | 0.78 | <0.001 |
| Week 16 | 58.1 ± 17.7 | 66.0 ± 25.1 | 76.2 ± 23.6 | 75.5 ± 28.9 | ||||
| Change | −1.9 ± 4.8# | −2.5 ± 8.0** | 9.1 ± 8.5 | 10.9 ± 8.2 | ||||
| Single repetition lat pull down (kg) | ||||||||
| Week 0 | 49.8 ± 15.1 | 56.7 ± 15.4 | 55.0 ± 14.8 | 55.7 ± 18.7 | <0.001 | <0.001 | 0.51 | <0.001 |
| Week 16 | 50.4 ± 14.9 | 57.2 ± 15.9 | 66.2 ± 17.4 | 65.0 ± 20.7 | ||||
| Change | 0.6 ± 4.9# | 0.6 ± 3.7¶ | 11.2 ± 6.0 | 9.3 ± 4.9 | ||||
| Systolic blood pressure (mmHg) | ||||||||
| Week 0 | 137 ± 12 | 141 ± 11 | 137 ± 10 | 138 ± 9 | <0.001 | 0.90 | 0.86 | 0.943 |
| Week 16 | 124 ± 11 | 125 ± 11 | 122 ± 9 | 124 ± 9 | ||||
| Change | −13 ± 11 | −16 ± 13 | −16 ± 7 | −14 ± 9 | ||||
| Diastolic blood pressure (mmHg) | ||||||||
| Week 0 | 79 ± 9 | 83 ± 9 | 81 ± 8 | 79 ± 8 | <0.001 | 0.49 | 0.56 | 0.346 |
| Week 16 | 72 ± 6 | 74 ± 9 | 74 ± 6 | 72 ± 8 | ||||
| Change | −7 ± 6 | −10 ± 6 | −8 ± 5 | −7 ± 6 | ||||
| Plasma glucose (mmol/l) | ||||||||
| Week 0 | 9.2 ± 2.7 | 9.5 ± 2.9 | 8.7 ± 3.2 | 8.2 ± 2.1 | <0.001 | 0.90 | 0.79 | 0.483 |
| Week 16 | 7.1 ± 1.0 | 7.0 ± 1.0 | 6.8 ± 1.5 | 6.3 ± 1.0 | ||||
| Change | −2.2 ± 2.2 | −2.5 ± 2.7 | −1.9 ± 2.3 | −1.9 ± 1.6 | ||||
| Glycosylated hemoglobin (%) | ||||||||
| Week 0 | 7.6 ± 1.0 | 8.0 ± 1.8 | 7.3 ± 1.4 | 6.8 ± 1.0 | <0.001 | 0.21 | 0.16 | 0.179 |
| Week 16 | 6.4 ± 0.7 | 6.3 ± 0.9 | 6.2 ± 1.0 | 5.6 ± 0.6 | ||||
| Change | −1.1 ± 0.6 | −1.8 ± 1.6 | −1.1 ± 0.7 | −1.1 ± 0.7 | ||||
| Serum insulin (mU/l) | ||||||||
| Week 0 | 15.8 ± 10.0 | 12.4 ± 8.6 | 12.3 ± 4.8 | 15.2 ± 8.3 | <0.001 | 0.11 | 0.19 | 0.289 |
| Week 16 | 11.8 ± 10.2 | 9.0 ± 8.0 | 8.8 ± 3.4 | 7.2 ± 3.6 | ||||
| Change | −4.1 ± 4.2 | −3.5 ± 2.8 | −3.4 ± 4.0 | −7.9 ± 8.1 | ||||
| Triglycerides (mmol/l) | ||||||||
| Week 0 | 2.3 ± 1.3 | 2.0 ± 1.1 | 1.6 ± 0.5 | 1.8 ± 0.7 | <0.001 | 0.70 | 0.91 | 0.626 |
| Week 16 | 1.7 ± 1.5 | 1.6 ± 1.2 | 1.3 ± 0.5 | 1.2 ± 0.5 | ||||
| Change | −0.6 ± 1.1 | −0.4 ± 1.0 | −0.3 ± 0.5 | −0.5 ± 0.6 | ||||
| Total cholesterol (mmol/l) | ||||||||
| Week 0 | 4.8 ± 1.0 | 5.0 ± 1.1 | 4.3 ± 0.9 | 4.7 ± 0.9 | <0.001 | 0.89 | 0.54 | 0.628 |
| Week 16 | 4.1 ± 1.1 | 4.4 ± 1.4 | 3.5 ± 0.9 | 4.0 ± 0.8 | ||||
| Change | −0.7 ± 0.6 | −0.6 ± 0.8 | −0.8 ± 0.9 | −0.7 ± 0.6 | ||||
| HDL cholesterol (mmol/l) | ||||||||
| Week 0 | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.3 | 0.02 | 0.86 | 0.71 | 0.863 |
| Week 16 | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.2 | ||||
| Change | −0.0 ± 0.2 | −0.1 ± 0.2 | −0.1 ± 0.2 | −0.1 ± 0.2 | ||||
| LDL cholesterol (mmol/l) | ||||||||
| Week 0 | 2.7 ± 0.9 | 2.7 ± 0.9 | 2.4 ± 0.8 | 2.7 ± 0.6 | <0.001 | 0.37 | 0.27 | 0.208 |
| Week 16 | 2.4 ± 1.0 | 2.5 ± 1.2 | 1.9 ± 0.9 | 2.4 ± 0.6 | ||||
| Change | −0.3 ± 0.5 | −0.2 ± 0.6 | −0.5 ± 0.6 | −0.3 ± 0.4 | ||||
| CRP (mgl/l) | ||||||||
| Week 0 | 3.3 ± 2.4 | 4.5 ± 2.4 | 3.0 ± 2.6 | 4.0 ± 2.5 | <0.001 | 0.55 | >0.99 | 0.183 |
| Week 16 | 2.6 ± 1.9 | 3.5 ± 1.1 | 1.8 ± 1.1 | 3.0 ± 2.5 | ||||
| Change | −0.7 ± 1.5 | −0.9 ± 1.9 | −1.2 ± 1.9 | −1.0 ± 1.9 | ||||
| Creatinine clearance (ml/min per 1.73 m2) | ||||||||
| Week 0 | 119.7 ± 22.2 | 114.3 ± 46.9 | 116.6 ± 24.4 | 117.7 ± 24.9 | <0.001 | 0.68 | 0.55 | 0.46 |
| Week 16 | 113.1 ± 40.5 | 96.6 ± 32.1 | 108.7 ± 22.8 | 114.2 ± 15.8 | ||||
| Change | −6.6 ± 32.0 | −17.7 ± 37.6 | −7.9 ± 22.6 | −5.5 ± 16.4 | ||||
Data are means ± SD.
*Changes over time in the groups from weeks 0 to 16 (repeated-measures ANOVA).
†Treatment effect between groups for the change from weeks 0 to 16 (one-way ANOVA).
‡Comparison of the magnitude of change between diets (CON and CON+RT vs. HP and HP+RT) (planned contrast).
§Comparison of the magnitude of change between exercise and nonexercise treatments (CON and HP vs. CON+RT and HP+RT) (planned contrast).
‖P ≤ 0.05;
¶P < 0.01;
#P < 0.001 significantly different from HP+RT.
Body weight and composition
Overall, body weight was reduced (P < 0.001), with a significant group effect (P = 0.04), such that HP+RT achieved greatest weight loss (CON: −8.9%, HP: −8.7%, CON+RT: −10.0%, HP+RT: −12.7%) (Table 2). Post hoc analysis showed that the greater weight loss in HP+RT was statistically significant compared with CON and HP groups (P < 0.05), but compared with CON+RT statistical significance was not reached (P = 0.20). Overall, weight loss was not different between the two diets (P = 0.18); however, the exercise groups (HP+RT and CON+RT) lost more weight compared with the diet-only groups (CON and HP) (diet+RT: −12.0 ± 5.7 kg, diet only: −8.8 ± 4.6 kg; P = 0.02). ITT analysis showed a similar weight loss pattern, albeit the magnitude of effect was reduced (CON: −9.4 ± 6.5 kg, HP: −8.0 ± 4.8 kg, CON+RT: −10.5 ± 5.1 kg, HP+RT: −12.6 ± 6.5 kg; P = 0.16 group effect).
Similarly, fat mass and WC reduced in all groups (P < 0.001) (Table 2), with a significant group effect (P ≤ 0.006) such that HP+RT had greatest reductions. Post hoc analysis showed that HP+RT had significantly greater reductions compared with CON and HP (P ≤ 0.02), but compared with CON+RT, statistical significance was not reached (fat mass: P = 0.06, WC: P = 0.32). Overall, compared with the diet-only groups, the exercising groups had greater reductions in fat mass (diet+RT: −9.6 ± 4.1 kg, diet only: −6.7 ± 3.8 kg; P < 0.01) and WC (diet+RT: −12.4 ± 4.7 cm, diet only: −8.5 ± 4.3 cm; P < 0.01). Overall, more fat mass loss occurred in participants consuming the HP diet (−9.4 ± 4.5 kg) compared with the CON diet (7.3 ± 3.8 kg; P = 0.06). There was an overall reduction in FFM (Table 2), with no effect of treatment group (P = 0.91), diet composition (P = 0.80), or exercise participation (P = 0.51). ITT analysis confirmed these results, with a significant group effect evident for fat mass and WC (P ≤ 0.02) but not for FFM (P = 0.75).
Cardiometabolic outcomes and glycemic control
Overall, blood pressure, lipids, glucose, A1C, and CRP were reduced (P ≤ 0.02) (Table 2) with no difference between treatment groups (P ≥ 0.37), diet composition (P ≥ 0.27), or exercise participation (P ≥ 0.21). Insulin concentrations also decreased (P < 0.001) with a differential (nonsignificant) effect evident between treatment groups (P = 0.11), such that HP+RT experienced approximately twofold greater reductions compared with the other groups. No differential insulin response between diet compositions (P = 0.19) or exercise participation (P = 0.29) were observed. Changes in insulin were significantly correlated with changes in weight (r = 0.35, P < 0.01) and fat mass (r = 0.36, P = 0.005). ITT analysis also showed no significant differences in the changes of these parameters between the groups.
Creatinine clearance and urinary albumin
Overall, creatinine clearance was reduced (P < 0.001) (Table 2), with no differential effect between treatments (P = 0.68), diet composition (P = 0.55), or exercise participation (P = 0.46). At week 0, 45 participants (CON diet: 27, HP diet: 18) had normoalbuminuria (urinary albumin excretion <20 μg/min), and values remained in the normoalbuminuria range at week 16, except in 1 participant in the CON group whose urinary albumin excretion increased (33.4 μg/min) to microalbuminuria classification (urinary albumin excretion 20–200 μg/min). At baseline, 14 participants (24%; CON diet: n = 6, HP diet: n = 8) had microalbuminuria; at week 16, 4 participants (CON: n = 2, HP: n = 2) remained in the microalbuminuria range and 10 participants decreased to normoalbuminuria classification (CON: n = 4, HP: n = 6).
Medication changes
A total of 33 participants were on hypoglycemic medication at baseline (CON: n = 11, HP: n = 7, CON+RT: n = 10, HP+RT: n = 5), and during the intervention, medication dose was reduced in 9 participants (CON: n = 2, HP: n = 1, CON+RT: n = 4, HP+RT: n = 2) and increased in 1 participant in the HP group. There were no significant differences between the treatment groups. At baseline, 36 and 29 participants were on antihypertensive medication (CON: n = 9, HP: n = 4, CON+RT: n = 14, HP+RT: n = 9) and lipid-lowering medication (CON: n = 9, HP: n = 5, CON+RT: n = 9, HP+RT: n = 6), respectively. Following the intervention, antihypertensives were reduced in six participants (CON+RT: n = 4, HP+RT: n = 2) and increased in 1 participant in the CON+RT group, and 1 participant in the HP+RT group ceased lipid-lowering medication.
CONCLUSIONS
This study showed substantial improvements in CVD risk factors and glycemic control following lifestyle intervention incorporating a structured, energy-restricted diet that occurred independent of macronutrient composition or participation in a RT program in sedentary obese patients with type 2 diabetes. The addition of RT increased weight and fat mass loss, which was further magnified by replacing some dietary carbohydrate with protein.
The HP+RT group exhibited at least a 3.3-kg greater weight and fat loss and 21% greater reduction in WC compared with the other treatment groups. Previous studies in healthy, overweight/obese individuals without type 2 diabetes show greater reductions in weight and fat mass of similar magnitude when exercise training is combined with a hypocaloric HP diet compared with an isocaloric CON diet (6,7). The CON diet was designed to reflect standard dietary recommendations (15–20% of total energy as protein) (1). The HP diet was designed to achieve protein intake of >25%, corresponding with typically prescribed HP diets; it provided an average protein intake of 1.12 g · kg−1 · day−1. Previous studies show that an HP diet compared with a CON diet is associated with greater reductions in fat mass (5,6). RT also reduces fat mass in obese individuals, independent of caloric restriction (14), suggesting that the separate interventional components of an HP diet and RT may have contributed to the greater weight and fat mass losses following the HP+RT treatment.
Although a significant main group effect was observed, post hoc analysis showed that greater weight, fat mass, and WC reductions in the HP+RT group only reached statistical significance compared with the CON and HP groups but not the CON+RT group (P ≥ 0.06), despite large absolute differences. Nevertheless, the additional weight and fat losses that occurred when RT was combined with an HP diet compared with a CON diet represent moderate to large effect sizes (0.67–0.85) and are considered clinically relevant and shown to produce important health outcomes (15). Furthermore, clinical and experimental evidence shows that WC is an independent predictor of CVD (16), and the 21% greater reductions following the HP+RT group would represent a significant health improvement. Power analysis estimates that a sample size of 24 subjects per group would have been needed for the observed 3.3-kg weight loss difference between the groups to have been statistically significant (80%, P = 0.05). Further larger studies are required to confirm these findings. In contrast, Layman et al. (6), who observed similar absolute weight and fat loss differences between treatment groups comparing CON and HP diets with and without exercise, achieved statistically significant diet and exercise effects with smaller sample sizes (n = 12 per group). However, this study only examined women, and sex differences in body composition responses to dieting have been previously observed (5). Although no sex effects were observed in our study, inclusion of men and women may have increased the data variability, subsequently lowering the statistical power.
Mechanisms underlying the greater weight and fat changes in HP+RT are not clear. Given that both diets were isocaloric and participants undertook the same RT program under controlled conditions, it suggests these changes reflect metabolic differences. The HP+RT group experienced approximately twofold greater reductions in fasting insulin compared with the other treatments, although, again, power was not sufficient to reveal significant differences. Nevertheless, there was a significant correlation between the changes in weight and fat mass and insulin levels. Insulin has fat-sparing effects and promotes adipose tissue accumulation (17). Hence, greater insulin reductions in the HP+RT group may explain the greater fat mass reductions; however, causation cannot be determined. Other evidence suggests that fat mass accumulation drives insulin resistance (17). Alternately, fat has more positive effects on energy balance compared with other macronutrients (18). Therefore, although unlikely, the possibility that lower fat intakes in the HP diet contributed greater fat reductions in the HP+exercise group cannot be dismissed.
In the exercising groups, high compliance with the RT program achieved substantial strength gains, which is associated with metabolic disease reductions (19). Nevertheless, FFM reduced similarly across treatment groups. Although previous studies have observed protective effects of RT (20) and HP diets (3) on FFM during caloric restriction, these effects have not been consistently shown (5), and perhaps even higher relative protein intakes are required. Compared with this study, experiments demonstrating FFM preservation following a hypocaloric HP diet with RT (6,21) administered higher relative protein intakes (1.12 vs. ≥1.4 g · kg−1 · day−1). A meta-analysis showed that the degree of FFM retention during weight loss increases with increasing quartiles of protein intake (2), suggesting that relative protein intakes may be an important weight loss program design consideration.
Substantial reductions in CVD risk markers and glycemic control occurred during the intervention with no observable differences between the groups. Previous studies report that RT (14) and HP diets (22) improve A1C independent of weight loss in type 2 diabetic patients. The lack of any difference in glycemic control between the treatment groups in the present study may therefore be due to the hypoglycemic effects of energy restriction (23), masking any potential effects of exercise or diet composition. Under milder energy restriction, RT has been shown to provide additional reductions in A1C (9). Longer-term studies are required to separate out these effects. Nevertheless, the overall A1C reductions observed is clinically relevant and associated with a 21% reduction in diabetes-related mortality (24).
HP diets having potentially adverse effects on renal function remains a concern (25). However, no differences in creatinine clearance or presence of microalbuminuria between the diets groups were observed. Other short-term, interventional studies also report no differences in renal function in obese individuals with type 2 diabetes following either an HP or low-protein diet (5,22). This suggests that a hypocaloric HP diet does not adversely affect renal function, at least over the short term, in subjects without overt renal impairment. Nevertheless, diabetes is associated with impaired renal function, and the longer-term effects in this patient population warrants further investigation.
Although not statistically significant, higher participant withdrawals/exclusions in the HP diet groups are noteworthy. The exact reason/s are not entirely clear, but this is not consistent with previous studies comparing HP versus CON diets that report similar dropouts with both dietary treatments and in some studies lower dropouts following the HP diet (5,7).
In conclusion, participation in RT produced greater weight and fat loss and increases in muscular strength compared with energy restriction alone. Additionally, replacement of some carbohydrate for protein further magnified these effects, resulting in greatest reductions in weight, fat mass, WC, and insulin. All treatments had similar improvements in glycemic control and CVD risk. A lifestyle modification program combining an energy-restricted HP diet and RT appears to be a preferred treatment strategy in overweight/obese individuals with type 2 diabetes. Further studies should evaluate the longer-term effects.
Acknowledgments
This work was supported with project grants from the National Heart Foundation of Australia, the Diabetes Australia Research Trust, and the Pork Cooperative Research Centre. George Weston Foods donated foods for this study. None of the funding agencies played a role in the conception, design or conduct of the study, collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript.
No potential conflicts of interest relevant to this article were reported.
We thank the volunteers who made the study possible through their participation. We gratefully acknowledge Anne McGuffin for coordinating this trial; Penelope Taylor and Heidi Sulda for assisting in delivering of the dietary intervention; Rosemary McArthur and Lindy Lawson for nursing expertise; and Candita Sullivan, Robb Muirhead, Cathryn Seccafien, Vanessa Russell, and Mark Mano for assisting with the biochemical assays. We thank Kylie Lange for assisting with the statistical analysis and David Jesudason for assisting with the medical supervision of the participants.
The authors' responsibilities were as follows: T.P.W. and G.D.B. were responsible for the conception and design of the study, coordinated the study, performed data analyses, interpreted the data, and coordinated and contributed to the writing of the manuscript. M.N. contributed to the conception and design of the study and data interpretation and the writing of the manuscript and designed the experimental diets. P.M.C. was responsible for the medical monitoring of the research participants and contributed to the data interpretation and writing of the manuscript. X.C. designed the experimental diets, coordinated the implementation of the dietary protocols, and contributed to the writing of the manuscript. J.B.K. assisted in the design of the experimental diets, contributed to the conception and design of the study, and contributed to the manuscript. All authors agreed on the final version of the manuscript.
Footnotes
Australian New Zealand clinical trials reg. ACTR no. 12608000206325, anzctr.org.au.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, Hoogwerf BJ, Lichtenstein AH, Mayer-Davis E, Mooradian AD, Wheeler ML: Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008; 31( Suppl. 1): S61– S78 [DOI] [PubMed] [Google Scholar]
- 2.Krieger JW, Sitren HS, Daniels MJ, Langkamp-Henken B: Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: a meta-regression 1. Am J Clin Nutr 2006; 83: 260– 274 [DOI] [PubMed] [Google Scholar]
- 3.Piatti PM, Monti F, Fermo I, Baruffaldi L, Nasser R, Santambrogio G, Librenti MC, Galli-Kienle M, Pontiroli AE, Pozza G: Hypocaloric high-protein diet improves glucose oxidation and spares lean body mass: comparison to hypocaloric high-carbohydrate diet. Metabolism 1994; 43: 1481– 1487 [DOI] [PubMed] [Google Scholar]
- 4.Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams T, Williams M, Gracely EJ, Stern L: A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med 2003; 348: 2074– 2081 [DOI] [PubMed] [Google Scholar]
- 5.Parker B, Noakes M, Luscombe N, Clifton P: Effect of a high-protein, high-monounsaturated fat weight loss diet on glycemic control and lipid levels in type 2 diabetes. Diabetes Care 2002; 25: 425– 430 [DOI] [PubMed] [Google Scholar]
- 6.Layman DK, Evans E, Baum JI, Seyler J, Erickson DJ, Boileau RA: Dietary protein and exercise have additive effects on body composition during weight loss in adult women. J Nutr 2005; 135: 1903– 1910 [DOI] [PubMed] [Google Scholar]
- 7.Meckling KA, Sherfey R: A randomized trial of a hypocaloric high-protein diet, with and without exercise, on weight loss, fitness, and markers of the metabolic syndrome in overweight and obese women. Appl Physiol Nutr Metab 2007; 32: 743– 752 [DOI] [PubMed] [Google Scholar]
- 8.Cauza E, Hanusch-Enserer U, Strasser B, Ludvik B, Metz-Schimmerl S, Pacini G, Wagner O, Georg P, Prager R, Kostner K, Dunky A, Haber P: The relative benefits of endurance and strength training on the metabolic factors and muscle function of people with type 2 diabetes mellitus. Arch Phys Med Rehabil 2005; 86: 1527– 1533 [DOI] [PubMed] [Google Scholar]
- 9.Dunstan DW, Daly RM, Owen N, Jolley D, De Courten M, Shaw J, Zimmet P: High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care 2002; 25: 1729– 1736 [DOI] [PubMed] [Google Scholar]
- 10.Kerksick C, Thomas A, Campbell B, Taylor L, Wilborn C, Marcello B, Roberts M, Pfau E, Grimstvedt M, Opusunju J, Magrans-Courtney T, Rasmussen C, Wilson R, Kreider RB: Effects of a popular exercise and weight loss program on weight loss, body composition, energy expenditure and health in obese women. Nutr Metab (Lond) 2009; 6: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription 6th ed.Philadelphia, Lippincott Williams and Wilkins, 2000 [Google Scholar]
- 12.Tay J, Brinkworth GD, Noakes M, Keogh J, Clifton PM: Metabolic effects of weight loss on a very-low-carbohydrate diet compared with an isocaloric high-carbohydrate diet in abdominally obese subjects. J Am Coll Cardiol 2008; 51: 59– 67 [DOI] [PubMed] [Google Scholar]
- 13.Du Bois D, Du Bois EF: A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989; 5: 303– 311, discussion 312–313 [PubMed] [Google Scholar]
- 14.Braith RW, Stewart KJ: Resistance exercise training: its role in the prevention of cardiovascular disease. Circulation 2006; 113: 2642– 2650 [DOI] [PubMed] [Google Scholar]
- 15.Tong PC, Lee ZS, Sea MM, Chow CC, Ko GT, Chan WB, So WY, Ma RC, Ozaki R, Woo J, Cockram CS, Chan JC: The effect of orlistat-induced weight loss, without concomitant hypocaloric diet, on cardiovascular risk factors and insulin sensitivity in young obese Chinese subjects with or without type 2 diabetes. Arch Intern Med 2002; 162: 2428– 2435 [DOI] [PubMed] [Google Scholar]
- 16.de Koning L, Merchant AT, Pogue J, Anand SS: Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J 2007; 28: 850– 856 [DOI] [PubMed] [Google Scholar]
- 17.Kahn BB, Flier JS: Obesity and insulin resistance. J Clin Invest 2000; 106: 473– 481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Astrup A: The role of dietary fat in the prevention and treatment of obesity: efficacy and safety of low-fat diets. Int J Obes Relat Metab Disord 2001; 25( Suppl. 1): S46– S50 [DOI] [PubMed] [Google Scholar]
- 19.Jurca R, Lamonte MJ, Church TS, Earnest CP, Fitzgerald SJ, Barlow CE, Jordan AN, Kampert JB, Blair SN: Associations of muscle strength and fitness with metabolic syndrome in men. Med Sci Sports Exerc 2004; 36: 1301– 1307 [DOI] [PubMed] [Google Scholar]
- 20.Janssen I, Fortier A, Hudson R, Ross R: Effects of an energy-restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care 2002; 25: 431– 438 [DOI] [PubMed] [Google Scholar]
- 21.Demling RH, DeSanti L: Effect of a hypocaloric diet, increased protein intake and resistance training on lean mass gains and fat mass loss in overweight police officers. Ann Nutr Metab 2000; 44: 21– 29 [DOI] [PubMed] [Google Scholar]
- 22.Gannon MC, Nuttall FQ, Saeed A, Jordan K, Hoover H: An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am J Clin Nutr 2003; 78: 734– 741 [DOI] [PubMed] [Google Scholar]
- 23.Lee M, Aronne LJ: Weight management for type 2 diabetes mellitus: global cardiovascular risk reduction. Am J Cardiol 2007; 99: 68B– 79B [DOI] [PubMed] [Google Scholar]
- 24.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR: Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405– 412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knight EL, Stampfer MJ, Hankinson SE, Spiegelman D, Curhan GC: The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med 2003; 138: 460– 467 [DOI] [PubMed] [Google Scholar]