Abstract
OBJECTIVE
We have recently shown that a high-fat high-carbohydrate (HFHC) meal induces an increase in plasma concentrations of endotoxin (lipopolysaccharide [LPS]) and the expression of Toll-like receptor-4 (TLR-4) and suppresser of cytokine signaling-3 (SOCS3) in mononuclear cells (MNCs) in addition to oxidative stress and cellular inflammation. Saturated fat and carbohydrates, components of the HFHC meal, known to induce oxidative stress and inflammation, also induce an increase in LPS, TLR-4, and SOCS3.
RESEARCH DESIGN AND METHODS
Fasting normal subjects were given 300-calorie drinks of either glucose, saturated fat as cream, orange juice, or only water to ingest. Blood samples were obtained at 0, 1, 3, and 5 h for analysis.
RESULTS
Indexes of inflammation including nuclear factor-κB (NF-κB) binding, and the expression of SOCS3, tumor necrosis factor-α (TNF-α), and interleukin (IL)-1β in MNCs, increased significantly after glucose and cream intake, but TLR-4 expression and plasma LPS concentrations increased only after cream intake. The intake of orange juice or water did not induce any change in any of the indexes measured.
CONCLUSIONS
Although both glucose and cream induce NF-κB binding and an increase in the expression of SOCS3, TNF-α, and IL-1β in MNCs, only cream caused an increase in LPS concentration and TLR-4 expression. Equicaloric amounts of orange juice or water did not induce a change in any of these indexes. These changes are relevant to the pathogenesis of atherosclerosis and insulin resistance.
Our recent work has shown that a high-fat high-cholesterol (HFHC) meal induces oxidative and inflammatory stress in addition to inducing an increase in plasma endotoxin (lipopolysaccharide [LPS]) levels and the expression of Toll-like receptor (TLR)-4, the specific receptor for LPS (1). In contrast, a high-fiber and fruit meal does not induce any of these changes. These data are of great interest because the content of LPS in these meals is not significantly different, and, thus, it would appear that the inflammatory nature of the meal may lead to a partial breakdown of the intestinal barrier that normally protects the body from invasion of bacteria and the entry of LPS from the gut. The concept of this immunological barrier of the gut has developed rapidly over the past few years and is vital to the protection from bacterial toxins and immunological responses to the commensal and pathogenic intestinal bacteria.
In this context, we wanted to analyze which macronutrient was responsible for the induction of oxidative stress and inflammation, on the one hand, and the increase in LPS concentrations and the expression of TLR-4 and suppresser of cytokine signaling (SOCS)-3 on the other. To elucidate this, we investigated the effect of glucose, the most important carbohydrate, cream, a saturated fat, and orange juice, a carbohydrate-containing food product, which does not induce either oxidative stress or inflammation.
SOCS3 is a protein that has been shown to interfere with insulin and leptin signal transduction (2–5). Our recent work has shown that SOCS3 expression in the circulating mononuclear cells (MNCs) of the obese human is markedly increased when compared with that in normal subjects (6). In addition, our work demonstrated that SOCS3 expression in MNCs is inversely related to the tyrosine phosphorylation of the insulin receptor and directly related to BMI and insulin resistance (homeostasis model assessment of insulin resistance [HOMA-IR]), consistent with its role in the pathogenesis of insulin resistance. Leptin resistance in human obesity leads to the inability of leptin to cause satiety and weight loss, whereas insulin resistance makes the obese vulnerable to diabetes. Human obesity is also a state of chronic inflammation characterized by an increase in inflammatory mediators in plasma, in adipose tissue, and in circulating mononuclear cells (7,8). Because SOCS3 is induced in animal models by proinflammatory stimuli like the cytokines, TNF-α, IL-6, and IL-1β (3,4,9) and because macronutrient intake causes oxidative stress (10,11) and inflammation (12,13), it is possible that the intake of glucose and saturated fat (cream) induces an increase in the expression of SOCS3 as a part of macronutrient-induced inflammatory stress in parallel with increases in the activation of the proinflammatory transcription factor, nuclear factor-κB (NF-κB), and the expression of TNF-α, IL-6, and IL-1β.
Recent work has shown that high-fat diet–induced insulin resistance is TLR-4–dependent such that TLR-4 deletion protects mice from NF-κB–mediated inflammation and the development of insulin resistance (14,15). In addition, it has also been shown that the plasma concentration of LPS is significantly increased in type 2 diabetic patients and that its concentration is significantly related to plasma insulin concentration and HOMA-IR, an index of insulin resistance, showing a link between LPS concentration and insulin resistance (16). However, there are no data available on the effect of specific macronutrients on TLR expression or on inflammatory processes triggered by TLR-dependent mechanisms.
On the basis of the above, we hypothesized that the intake of glucose and cream induces an increase in the expression of SOCS3 and TLR-4 and the plasma concentration of LPS as a part of postprandial inflammation induced by an HFHC meal (1). We hypothesized further that because orange juice does not induce oxidative stress or inflammation, its intake will not induce either an increase in the expression of SOCS-3 or TLR-4 or that of plasma LPS concentrations. We also examined the expression of other SOCS family members, SOCS1 and SOCS7, in these experiments for comparison with the effect on SOCS3 and because they are also thought to contribute to insulin resistance in experimental animal models.
RESEARCH DESIGN AND METHODS
Four groups (12 each) of healthy normal-weight (BMI of 21.5–24.4 kg/m2, aged 25–47 years) subjects ingested either 75 g (= 300 calories) of glucose (Glucola drink; Fisher Scientific, Pittsburgh, PA), 33 g (= 300 calories) of cream (gourmet heavy whipping cream; Land O'Lakes, Arden Hills, MN), an equicaloric amount of orange juice, or 300 ml of water after an overnight fast. The content of the dairy cream used includes 70% saturated fat, 28% unsaturated fat, <2% protein, and no carbohydrates. All subjects were given 10 min to finish their drinks. Blood samples were collected before and at 1, 3, and 5 h after glucose, cream, or water intake. The Human Research Committee of the State University of New York at Buffalo approved this protocol. Informed consent was obtained from all subjects.
In our previous work, we had used orange juice obtained from a local supermarket and had used portions of the juice from ½- or 1-gallon packages for multiple experiments. To minimize any potential for instability of orange juice constituents, we used packages of recently produced “Not from Concentrate” Florida Orange Juice (provided by the Florida Department of Citrus). Each package, once opened, was discarded after a single experiment.
MNC isolation and NF-κB DNA binding activity
Fasting blood samples were collected in sodium EDTA as an anticoagulant. MNCs isolated and NF-κB DNA binding activity was measured as described previously (1).
Total RNA isolation and real-time RT-PCR
Total RNA isolation and RT-PCR for SOCS3 and TLR inflammatory cytokines was performed as described previously (1,8). The specificity and the size of the PCR products were tested by adding a melt curve at the end of the amplifications and by running it on a 2% agarose gel. All values were normalized to expression of three housekeeping genes (β-actin, cyclophilin, and ubiquitin).
Western blotting
MNC total cell lysates were prepared, and electrophoresis and immunoblotting were performed as described before (12). Polyclonal or monoclonal antibodies against TLR-2 (Imgenex, San Diego, CA), SOCS3 and TLR-4 (Abcam, Cambridge, MA), and actin (Santa Cruz Biotechnology, Santa Cruz, CA) were used, and all values were corrected for loading to actin levels.
Measurement of plasma glucose, insulin, and free fatty acid concentrations
Insulin, glucose, and free fatty acids (FFAs) were measured as described previously (1).
Plasma endotoxin and lipopolysaccharide-binding protein concentrations
Plasma endotoxin concentration was measured by a commercially available kit (Cambrex limulus amebocyte lysate kit; Lonza, Walkersville, MD) as described previously (1). Lipopolysaccharide-binding protein (LBP) was measured using an immunoassay kit from Cell Sciences (Canton, MA).
Determination of LPS content in food challenges
Dilutions comparable to the amounts ingested from cream, glucose, and orange juice were prepared in plasma or endotoxin-free water. Plasma was used as a diluent to maintain a testing medium similar to that in the postchallenge LPS measurements. LPS concentrations were then measured as described using a limulus amebocyte lysate assay, and endotoxin was calculated (n = 3 each).
Statistical analysis
Statistical analysis was performed using SigmaStat software (Systat Software, San Jose, CA). Data on all of the figures are means ± SEM. Percent changes were calculated from baselines and are presented as means ± SEM. Statistical analysis of changes from baselines was performed using Holm-Sidak one-way repeated-measures ANOVA (RMANOVA). The Dunnett two-factor RMANOVA method was used for all multiple comparisons between different groups.
RESULTS
Plasma glucose, insulin, and lipid concentrations
There was no significant difference in fasting plasma glucose, insulin, or FFAs between the groups. There was a significant increase in glucose (from 80 ± 2 to 110 ± 9 mg/dl, P < 0.01) at 1 h after glucose intake, whereas there was no significant change in glucose concentrations after orange juice, cream, or water. Insulin concentrations increased significantly by 10- and 6.6-fold (P < 0.001) after glucose and orange juice intake, respectively, but did not change significantly after cream or water intake. Lipid concentrations did not change after glucose, orange juice, or water intake, whereas the intake of 33 g (300 calories) cream caused a significant increase in the plasma concentration of FFAs (from 0.32 ± 0.09 to 0.60 ± 0.14 mmol/l), triglycerides (from 103 ± 59 to 171 ± 60 mg/dl), and VLDL cholesterol (from 19.5 ± 13 to 32.3 ± 15 mg/dl at 3 h; P < 0.01 for all). There was no significant change in the concentration of total cholesterol, LDL cholesterol, and HDL cholesterol after cream.
Effect of glucose, orange juice, and cream intake on SOCS3 expression
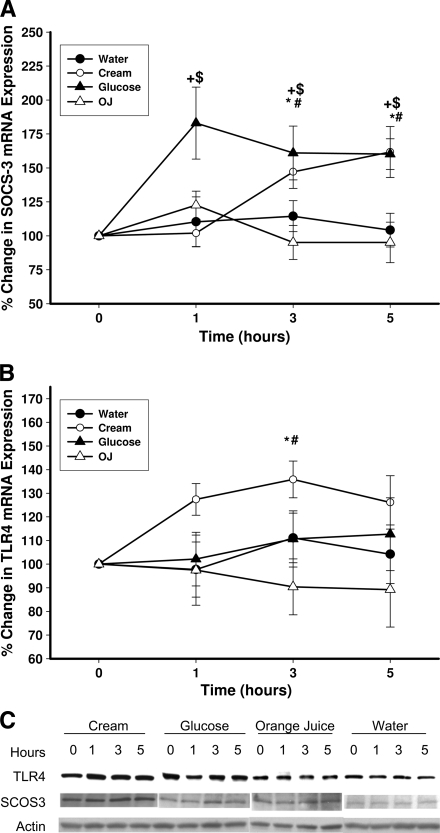
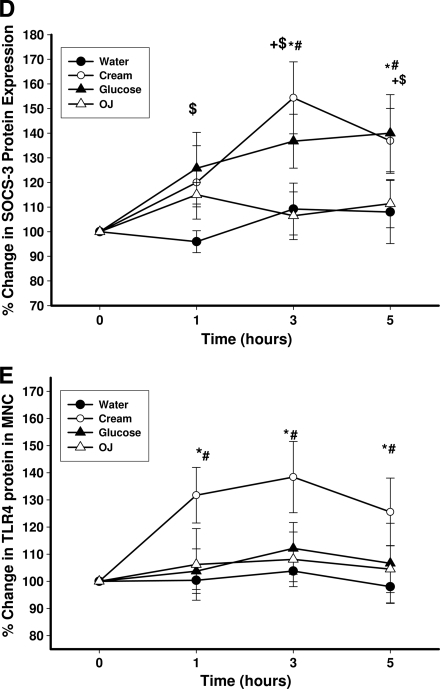
The intake of glucose caused a significant increase in mRNA expression of SOCS3 in MNCs (by 77 ± 22, 58 ± 16, and 54 ± 17% over the baseline at 1, 3, and 5 h after glucose intake, respectively (P < 0.02) (Fig. 1A). There was a significant increase in the mRNA expression of SOCS3 after cream intake by 45 ± 14 and 56 ± 15% over the baseline at 3 and 5 h, respectively (P = 0.014) (Fig. 1A), whereas there was no significant change in SOCS3 mRNA after orange juice or water intake. SOCS1 and SOCS7 mRNA expression did not change significantly after any challenge (data not shown). SOCS3 protein in MNCs also increased significantly after the intake of glucose and cream by 45 ± 16 and 53 ± 18% over the baseline, respectively, but not after orange juice or water (P < 0.05) (Fig. 1C and D).
Figure 1.
Change in SOCS-3 (A, C, and D) and TLR-4 (B, C, and E) mRNA and protein expression in MNCs from normal subjects after a 300-calorie drink of cream (○), glucose (▴), orange juice (OJ, ▵), or water (●). Data are means ± SEM. * and +, P < 0.05 with RMANOVA comparing changes in relation to baseline after cream and glucose challenges; # and $, P < 0.05 with two-way RMANOVA for comparisons of cream and glucose changes, respectively, to water (n = 12).
Effect of glucose, orange juice, and cream intake on TLR-4 expression
The intake of glucose did not result in any change in TLR-4 expression, whereas cream intake induced a significant increase in TLR-4 mRNA expression by 37 ± 11% over the baseline (P < 0.05) (Fig. 1B). Orange juice or water did not induce any change in TLR-4. Similarly, there was a significant increase in TLR-4 protein levels in MNCs after cream intake by 38 ± 18% over the baseline but not after glucose, orange juice, or water intake (P < 0.05) (Fig. 1C and E). There was no significant change in TLR-2 mRNA or protein expression after any of the macronutrient challenges (data not shown).
Effect of glucose, orange juice, and cream intake on proinflammatory cytokine expression
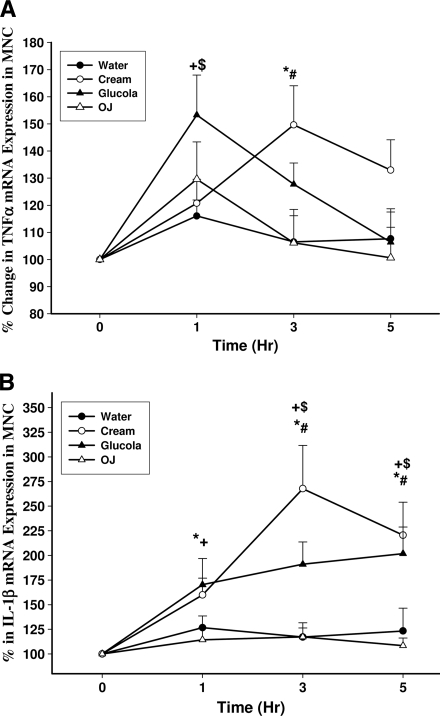
TNF-α mRNA expression in MNCs increased significantly by 53 ± 16% at 1 h and 51 ± 10% at 3 h over the baseline after glucose and cream intake, respectively (P < 0.05) (Fig. 2A). There was also a significant increase in IL-1β mRNA expression in MNCs after glucose and cream intake by 96 ± 25% at 5 h and 168 ± 32% at 3 h over the baseline, respectively (P < 0.01) (Fig. 2B). On the other hand, IL-6 expression did not alter significantly after glucose or cream intake (data not shown). There was no increase in the expression of any of these cytokines after orange juice or water.
Figure 2.
Change in TNF-α (A) and IL-1β (B) mRNA expression in MNCs from normal subjects after a 300-calorie drink of cream (○), glucose (▴), orange juice (OJ, ▵), or water (●). Data are means ± SEM. * and +, P < 0.05 with RMANOVA comparing changes in relation to baseline after cream and glucose challenges; # and $, P < 0.05 with two-way RMANOVA for comparisons of cream and glucose changes, respectively, to water (n = 12).
Effect of glucose, orange juice, and cream intake on NF-κB DNA binding
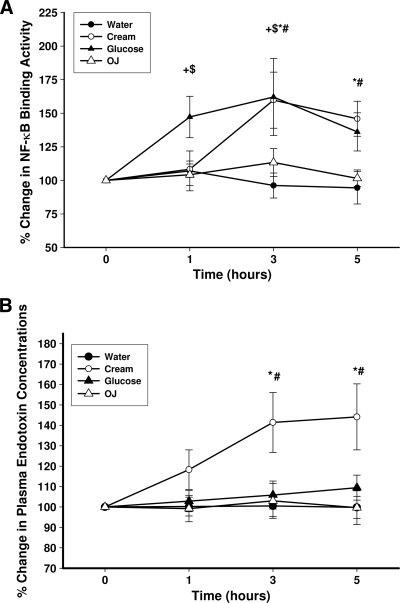
DNA binding by NF-κB increased significantly by 53 ± 17 and 54 ± 18% over the baseline (P < 0.05) (Fig. 3A) at 3 h after glucose and cream intake, respectively. There was no significant change in NF-κB binding activity after orange juice or water intake.
Figure 3.
Change in NFκB binding activity in MNC (A) and plasma endotoxin concentrations (B) in normal subjects after a 300-calorie drink of cream (○), glucose (▴), orange juice (OJ, ▵), or water (●). Data are means ± SEM. * and +, P < 0.05 with RMANOVA comparing changes in relation to baseline after cream and glucose challenges; # and $, P < 0.05 with two-way RMANOVA for comparisons of cream and glucose changes, respectively, to water (n = 12).
Effect of glucose, orange juice, and cream intake on plasma LPS and LBP concentrations
Plasma endotoxin concentrations increased significantly after the intake of cream from 0.29 ± 0.03 to 0.41 ± 0.07 endotoxin units (EU)/ml at 3 h (45 ± 17% over the baseline, P < 0.05) (Fig. 3B) but not after glucose, orange juice, or water intake. The concentration of LPS in plasma after the intake of cream continued to be significantly higher than basal levels up to 5 h. Endotoxin contents of cream and orange juice drinks were 104 ± 24 and 85 ± 21 EU/ml, respectively. This was equivalent to a total endotoxin load of 10,400 EU for the cream taken and 55,250 EU for the orange juice drink. Glucola drink and water did not contain measurable endotoxin. Considering that the amount of endotoxin ingested is extremely small (<5 μg) in comparison to a total estimated amount of 1 g in the gut, it is unlikely that the LPS content in drinks contributes significantly to the increase in plasma endotoxin concentrations. The LBP concentration increased significantly after the intake of glucose by 16% over the baseline at 5 h (from 9.4 ± 0.8 to 11 ± 1.2 μg/ml, P < 0.0.5) but not after cream or orange juice.
CONCLUSIONS
Our data show clearly for the first time that the intake of either 33 g cream or 75 g glucose resulted in a significant increase in the expression of SOCS3 mRNA in the circulating MNCs. With cream, this increase was observed at 1 h and continued until at least 5 h, at which time the experiment ended. Glucose caused an increase in SOCS3, which peaked at 1 h (83% over baseline) and was still elevated at 5 h. In parallel with the induction of SOCS3, there occurred an increase in NF-κB binding, consistent with an acute inflammatory response in MNCs. On the other hand, whereas the intake of cream resulted in an increase in both plasma LPS concentration and TLR-4 expression, glucose had no effect on either TLR-4 expression or plasma LPS concentration. Orange juice did not induce either SOCS3 or TLRs nor did it induce an inflammatory response.
It would thus appear that the oxidative stress– and inflammation-inducing actions of the HFHC meal are due to the combination of saturated fat and the carbohydrate (glucose) at least. On the other hand, only cream induced an increase in plasma LPS concentration and an increase in the expression of TLR-4, the receptor for LPS. Thus, saturated fats may have a more profound role in the pathogenesis of postprandial inflammation, as they may also perpetuate inflammation through the increases in LPS and TLR-4. By implication, saturated fats also appear to increase the permeability of intestinal epithelium and contribute to the breakdown of the intestinal barrier. Both carbohydrate and saturated fat induced an increase in the expression of SOCS3, the mediator of interference in insulin signal transduction. In contrast, orange juice does not induce any of the changes in oxidative and inflammatory stress or an increase in either LPS or TLR-4 or SOCS3. By implication, it may not affect the intestinal barrier either, because there is no increase in plasma LPS concentration after orange juice, despite it containing concentrations of endotoxin similar to those of cream. It is possible that the observed effect of orange juice is attributable to its flavonoids, naringenin and hesperidin, because they exert reactive oxygen species–suppressive (17) and anti-inflammatory effects. These potent effects have been demonstrated in experimental animal models in relation to endotoxin-induced inflammation and in cells in vitro (18,19). In this context, it is important to state that our recent data demonstrate that the consumption of orange juice with an HFHC meal prevents not only oxidative stress and inflammation but also the increase in LPS concentrations and the increase in the expression of TLR-4 and SOCS3 observed after ingestion of the HFHC meal alone (20).
It is also relevant that LBP increased after glucose intake but not after cream. Thus, the increase in LBP after an HFHC meal observed by us was probably due to the carbohydrate component of the meal. LBP did not alter after the intake of orange juice or water either.
SOCS3 has been shown previously to be induced by TNF-α, IL-6, and IL-1β and is thus a product of inflammation. Therefore, it is of interest that mRNAs for TNF-α and IL-1β were induced by glucose and cream intake in parallel with the increase in NF-κB binding. However, it was surprising that IL-6 was not induced by either glucose or cream. SOCS3, as its name suggests, was initially discovered as a molecule that interferes with cytokine signaling. It has since been shown to also interfere with both insulin and leptin signal transduction (4,21).
The induction of SOCS3 by cream and glucose intake in combination with the fact that its expression is increased in obese individuals suggests the possibility that chronic excessive fat and carbohydrate intake may result in a chronic increase in SOCS3 expression. It is also of interest that the intake of such macronutrients may result in resistance to leptin, one of the major signals that promotes satiety and thus potentially reduces food intake. Similarly, it is intriguing that the intake of a macronutrient should cause the induction of a molecule (SOCS3) that would interfere with the signal transduction of insulin, a hormone, which causes the assimilation of nutrients after a meal, including the distribution and storage of fat, carbohydrates, and proteins. Because the intake of a modest amount of proinflammatory macronutrients led to the induction of SOCS3, which induces concomitant insulin and leptin resistance, our observation raises the issue about the search for foods that are not likely to induce SOCS3 or inflammation.
Although SOCS1 and SOCS7 genes are also induced by inflammatory cytokines and have been reported to modulate insulin signaling in vitro and in experimental animal models (2,22), there are no data about their relevance to human insulin resistance. Indeed, their expression did not change after glucose and cream intake.
SOCS3 may interfere with insulin signal transduction at various levels. First, it can bind to the β-subunit of the insulin receptor (IR-β), reduce tyrosine phosphorylation of IR-β, and prevent the docking of insulin receptor substrate (IRS)-1 to the receptor (23). Second, it may bind to IRS-1, and by so doing, it may facilitate its ubiquitination and proteasomal degradation (2). By binding to IRS-1, it may also prevent the binding of IRS-1 to the p85 subunit of phosphatidylinositol 3-kinase and thus prevent insulin signaling. We have recently shown that SOCS3 expression is increased in obese individuals and is inversely related to the tyrosine phosphorylation of the IR-β and that it is directly related to insulin resistance (HOMA-IR), BMI, and other indexes of inflammation (6). Our current observations are consistent with its potential role as a mediator of insulin resistance in the obese.
SOCS3 also interferes with leptin signal transduction by reducing the phosphorylation of the leptin receptor and Janus kinase (24) attached to the leptin receptor. This results in a reduction of phosphorylation of signal transducer and activator of transcription (STAT) and thus the dimerization of STAT. This in turn prevents the nuclear translocation of STAT. Thus, the necessary gene transcription in response to the leptin signal cannot occur (5). The increase in SOCS3 expression and the presence of leptin resistance in the obese and their potential reversal with macronutrient restriction have important implications.
It is of interest that although the intake of cream induced an increase in the expression of TLR-4 and in plasma LPS concentrations, glucose had no effect. Because TLR-4 is the specific receptor for LPS, the concomitant increase in both is a recipe for an amplified inflammatory signal. It would be of interest to examine the effects of repeated intake of cream as reflected in these indexes and the overall magnitude of the inflammatory process. Previously, Dasu et al. (25) reported that high glucose concentrations upregulated TLR-4 expression and its downstream signaling in a monocytic cell line. However, the excursions of glucose concentrations in normal and even obese subjects do not achieve those concentrations postprandially. Thus, the observations of Dasu et al. are relevant to diabetic individuals. Our investigation deals with postprandial changes in normal subjects. As far as the effects of cream intake are concerned, the amount taken is modest, and the increase in triglycerides is consistent with that in previous studies. It should, therefore, be noted that although the amounts of cream and glucose taken induced similar increases in reactive oxygen species generation and NF-κB binding, glucose was not able to induce an increase in the expression of TLR-4.
In summary, the intake of a modest amount of glucose or cream results in a significant induction of SOCS3 mRNA and protein in parallel with the induction of an inflammatory response characterized by an increase in NF-κB binding in MNCs and the induction of two of the cytokines, TNF-α and IL-1β, which are known to induce SOCS3 in experimental animals. In addition, the intake of cream but not of glucose also induces an increase in the expression of TLR-4 mRNA and protein while also inducing an increase in plasma LPS concentrations. Both SOCS3 and TLR-4 are putative mediators of insulin resistance. In contrast, orange juice intake does not induce oxidative stress, inflammation, SOCS3, TLR-4, or an increase in plasma LPS concentrations.
Acknowledgments
The study was supported in part by a grant from the State of Florida, Department of Citrus. P.D. was also supported by the National Institutes of Health (R01-DK-069805 and R01-DK-075877) and the American Diabetes Association (708CR13). The data were obtained from our laboratory and were under our control, and the interpretations and conclusions are those of the investigators. Furthermore, the Principal Investigator (P.D.) takes the full responsibility for them.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Ghanim H, Abuaysheh S, Sia CL, Korzeniewski K, Chaudhuri A, Fernandez-Real JM, Dandona P: Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells following a high-fat high-carbohydrate meal: implications for insulin resistance. Diabetes Care 2009; 32: 2281– 2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rui L, Yuan M, Frantz D, Shoelson S, White MF: SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem 2002; 277: 42394– 42398 [DOI] [PubMed] [Google Scholar]
- 3.Senn JJ, Klover PJ, Nowak IA, Zimmers TA, Koniaris LG, Furlanetto RW, Mooney RA: Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem 2003; 278: 13740– 13746 [DOI] [PubMed] [Google Scholar]
- 4.Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, Hilton DJ, Hotamisligil GS, Van Obberghen E: SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J Biol Chem 2001; 276: 47944– 47949 [DOI] [PubMed] [Google Scholar]
- 5.Bjørbaek C, El-Haschimi K, Frantz JD, Flier JS: The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem 1999; 274: 30059– 30065 [DOI] [PubMed] [Google Scholar]
- 6.Ghanim H, Aljada A, Daoud N, Deopurkar R, Chaudhuri A, Dandona P: Role of inflammatory mediators in the suppression of insulin receptor phosphorylation in circulating mononuclear cells of obese subjects. Diabetologia 2007; 50: 278– 285 [DOI] [PubMed] [Google Scholar]
- 7.Bistrian BR, Khaodhiar L: Chronic systemic inflammation in overweight and obese adults. JAMA 2000; 283: 2235; author reply 2236 [PubMed] [Google Scholar]
- 8.Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P: Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation 2004; 110: 1564– 1571 [DOI] [PubMed] [Google Scholar]
- 9.Emanuelli B, Glondu M, Filloux C, Peraldi P, Van Obberghen E: The potential role of SOCS-3 in the interleukin- 1β-induced desensitization of insulin signaling in pancreatic β-cells. Diabetes 2004; 53( Suppl. 3): S97– S103 [DOI] [PubMed] [Google Scholar]
- 10.Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P: Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab 2000; 85: 2970– 2973 [DOI] [PubMed] [Google Scholar]
- 11.Mohanty P, Ghanim H, Hamouda W, Aljada A, Garg R, Dandona P: Both lipid and protein intakes stimulate increased generation of reactive oxygen species by polymorphonuclear leukocytes and mononuclear cells. Am J Clin Nutr 2002; 75: 767– 772 [DOI] [PubMed] [Google Scholar]
- 12.Aljada A, Mohanty P, Ghanim H, Abdo T, Tripathy D, Chaudhuri A, Dandona P: Increase in intranuclear nuclear factor κB and decrease in inhibitor κB in mononuclear cells after a mixed meal: evidence for a proinflammatory effect. Am J Clin Nutr 2004; 79: 682– 690 [DOI] [PubMed] [Google Scholar]
- 13.Aljada A, Friedman J, Ghanim H, Mohanty P, Hofmeyer D, Chaudhuri A, Dandona P: Glucose ingestion induces an increase in intranuclear nuclear factor κB, a fall in cellular inhibitor κB, and an increase in tumor necrosis factor α messenger RNA by mononuclear cells in healthy human subjects. Metabolism 2006; 55: 1177– 1185 [DOI] [PubMed] [Google Scholar]
- 14.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS: TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006; 116: 3015– 3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araújo EP, Vassallo J, Curi R, Velloso LA, Saad MJ: Loss-of-function mutation in TLR4 prevents diet-induced obesity and insulin resistance. Diabetes 2007; 56: 1986– 1998 [DOI] [PubMed] [Google Scholar]
- 16.Creely SJ, McTernan PG, Kusminski CM, Fisher M, Da Silva NF, Khanolkar M, Evans M, Harte AL, Kumar S: Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab 2007; 292: E740– E747 [DOI] [PubMed] [Google Scholar]
- 17.Ghanim H, Mohanty P, Pathak R, Chaudhuri A, Sia CL, Dandona P: Orange juice or fructose intake does not induce oxidative and inflammatory response. Diabetes Care 2007; 30: 1406– 1411 [DOI] [PubMed] [Google Scholar]
- 18.Yeh CC, Kao SJ, Lin CC, Wang SD, Liu CJ, Kao ST: The immunomodulation of endotoxin-induced acute lung injury by hesperidin in vivo and in vitro. Life Sci 2007; 80: 1821– 1831 [DOI] [PubMed] [Google Scholar]
- 19.Bodet C, La VD, Epifano F, Grenier D: Naringenin has anti-inflammatory properties in macrophage and ex vivo human whole-blood models. J Periodontal Res 2008; 43: 400– 407 [DOI] [PubMed] [Google Scholar]
- 20.Ghanim H, Sia CL, Upadhyay M, Korzeniewski K, Viswanathan P, Abuaysheh S, Mohanty P, Dandona P: Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am J Clin Nutr 2010; 91: 940– 949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjørbaek C, Flier JS: Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med 2004; 10: 734– 738 [DOI] [PubMed] [Google Scholar]
- 22.Banks AS, Li J, McKeag L, Hribal ML, Kashiwada M, Accili D, Rothman PB: Deletion of SOCS7 leads to enhanced insulin action and enlarged islets of Langerhans. J Clin Invest 2005; 115: 2462– 2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emanuelli B, Peraldi P, Filloux C, Sawka-Verhelle D, Hilton D, Van Obberghen E: SOCS-3 is an insulin-induced negative regulator of insulin signaling. J Biol Chem 2000; 275: 15985– 15991 [DOI] [PubMed] [Google Scholar]
- 24.Gautier EL, Jakubzick C, Randolph GJ: Regulation of the migration and survival of monocyte subsets by chemokine receptors and its relevance to atherosclerosis. Arterioscler Thromb Vasc Biol 2009; 29: 1412– 1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I: High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes 2008; 57: 3090– 3098 [DOI] [PMC free article] [PubMed] [Google Scholar]