Abstract
C57BL/6 (B6) mice experience hearing loss and cochlear degeneration beginning about mid-life, whereas CAST/Ei (CAST) mice retain normal hearing until old age. A locus contributing to the hearing loss of B6 mice, named age-related hearing loss (ahl), was mapped to Chromosome 10. A homozygous, congenic strain of mice (B6.CAST-+ahl), generated by crossing B6 (ahl/ahl) and CAST (+ahl/+ahl) mice has the same genomic material as the B6 mice except in the region of the ahl locus, which is derived from CAST. In this study, we have determined the extent of the CAST-derived region of Chromosome 10 in the congenic strain and have examined mice of all three strains for hearing loss and cochlear morphology between 9 and 25 months of age. Results for B6 mice were similar to those described previously. CAST mice showed no detectable hearing loss even at 24 months of age; however, they had a small amount of ganglion cell degeneration. B6.CAST-+ahl mice were protected from early onset hearing loss and basal turn degeneration, but older animals did show some hearing loss and ganglion cell degeneration. We conclude that loci in addition to ahl contribute to the differences in hearing loss between B6 and CAST mice. These results illustrate the complex inheritance of age-related hearing loss in mice and may have implications for the study of human presbycusis.
Keywords: ahl, Presbycusis, Aging, Cochlea, Mice, Hearing
1. Introduction
The C57BL/6 inbred strain of mice, hereafter referred to as B6, was found to have age-related hearing loss (Mikaelian, 1979; Henry and Chole, 1980) and has been used in many studies to evaluate cochlear structure and function (Willott, 1986; Willott et al., 1988; Willott, 1991; Hultcrantz and Li, 1993; White et al., 2000; McFadden et al., 2001; Bartolome et al., 2001). Cochleae of these mice are characterized by early degeneration of outer hair cells and spiral ganglion cells and ultimately by the degeneration of the entire organ of Corti and most of the spiral ganglion cells in the basal turn. The phenotype is similar to that seen in some humans with presbycusis in which the basal turn of the cochlea is degenerated (Schuknecht and Gacek, 1993), and it was assumed that by studying cochlear pathology insight would be gained about the mechanisms of age-related hearing loss. Recently Hequembourg and Liberman (2001) provided evidence that the cellular origin of the cochlear degeneration might be the early degeneration of the type IV fibrocytes in the spiral ligament. The loss of these cells might lead to insu/cient potassium recycling to the endolymph and subsequent degeneration of the organ of Corti; however, it is not known whether the observed degree of fibrocyte degeneration is extensive enough to cause this secondary pathology.
In a genetic approach to understanding presbycusis, a locus associated with the B6 hearing loss phenotype was mapped to Chromosome 10 and given the name ‘age-related hearing loss’, symbol ahl (Erway et al., 1993; Johnson et al., 1997, 2000). The modifier of deaf waddler gene (mdfw), which modifies the hearing loss phenotype in mice with mutations of the Atp2b2 gene (a member of the plasma membrane Ca2+ pump family of genes) may be allelic with ahl (Zheng and Johnson, 2001). The ahl locus also has been identified as a contributor to susceptibility to acoustic trauma (Davis et al., 2001; Jimenez et al., 2001). Recent evidence indicates that inbred strain-specific alleles of the cadherin 23 gene (Cdh23) are responsible for the hearing loss effects attributed to the ahl/mdfw locus (Noben-Trauth et al., 2003).
Quite unlike the B6 animals, CAST/Ei mice (henceforth CAST), which are inbred from wild-derived Mus musculus castaneus, still have good hearing at 15 months of age (Zheng et al., 1999). It was by crossing these mice with B6 mice that the ahl locus was mapped and its effect evaluated up to 18 month of age (Johnson et al., 1997). It is assumed that CAST mice are homozygous for a dominant, wild-type allele at the ahl locus (+ahl) that confers resistance to hearing loss.
B6.CAST-+ahl is an inbred strain of congenic mice that is identical to the B6 strain except for a small region of Chromosome 10 containing the ahl locus from the CAST strain (Johnson et al., 1997). This congenic strain was still under development at the time of the 1997 publication in which it was originally described. Since then it has been backcrossed twice more to B6 mice and then inbred for many additional generations. The 1997 paper reported results for N7 or N8 generation backcross mice that were either homozygous for B6 alleles or heterozygous for B6 and CAST alleles at the ahl locus. The current study reports on the completed B6.CAST-+Ahl congenic strain after 10 back-cross generations (N10) and extensive inbreeding so that all loci are now homozygous. We compare auditory brainstem response (ABR) thresholds and cochlear pathology of mice from this congenic strain with those of mice from the parental B6 and CAST inbred strains sampled at different ages. These comparisons provide a means to evaluate the isolated genetic effect of the ahl locus on hearing loss in these mice.
2. Materials and methods
2.1. Mice
Three strains of mice from The Jackson Laboratory (Bar Harbor, ME, USA) were compared for their age-related hearing loss and cochlear degeneration pattern. The first group (n = 32) consisted of mice of the common B6 inbred strain, which are homozygous for the recessive Chromosome 10 ahl susceptibility allele (ahl/ahl). The second group (n = 19) consisted of mice of the CAST strain, which are homozygous for the resistance allele (+ahl/+ahl). The third group (n = 33) consisted of inbred congenic mice, B6.CAST-+ahl, which also are homozygous for the resistance allele (+ahl/+ahl), but on an otherwise B6 genetic background. The animals were housed in the standard animal facility under normal mouse rearing conditions at The Jackson Laboratory. All experimental procedures involving the mice were approved by the Animal Care and Use Committee at The Jackson Laboratory.
2.2. Assessment of hearing
When mice reached the designated age, they were anesthetized with Avertin (tribromoethanol stabilized in tertiary amyl hydrate), and hearing was measured with a click stimulus and at 8, 16, and 32 kHz using tone pips (3 ms duration) in a closed acoustic system. Stimuli were presented to both ears simultaneously at decreasing intensities (5 dB steps) in an ABR recording system (Intelligent Hearing System, Miami, FL, USA) (see Zheng et al., 1999 for details). The threshold response was recorded for each animal for each stimulus.
2.3. Tissue preparation
Following the ABR measurement, while still under deep anesthesia, the animals were perfused with saline followed by 4% paraformaldehyde. The animals were decapitated and the tissue was sent to the University of California, San Diego, CA, USA, for tissue processing. The cochleae were dissected and decalcified in 8% EDTA (pH 7.2) for 1–2 weeks and then cryosectioned (10 μm) parallel to the modiolus from dorsal to ventral. Sections were collected on superfrost plus slides (Fisher Scientific, Inc.) and stored at −20°C until they were stained with osmium and cresyl violet.
2.4. Histopathology
Histological evaluation of each cochlea was performed by an individual who was unaware of the animal’s age or genotype. Because the B6 mouse is characterized by a loss of the entire organ of Corti in the basal turn (Henry and Chole, 1980; Willott, 1991), each cochlea was scored for presence or absence of any cells of the organ of Corti. Comparisons were then made among the three strains to determine whether one strain was more likely to have total degeneration than another. It was not possible to identify hair cell loss in the sections. The stria vascularis was evaluated for a loss of cells as indicated by a thinning or distortion of the normal structure. Spiral ganglion cells (types I and II) in Rosenthal’s canal were counted in cochlear sections. Two to five cross-sections of each turn were evaluated for each cochlea. The mouse cochlea has approximately 1 and 3/4 to 2 turns. The canal therefore, was divided into three segments of unequal length, basal, middle and apical (Keithley and Feldman, 1979; Dazert et al., 1996). The basal turn including the hook is represented in the first half turn, the middle is three quarters of a turn and the apical portion comprises the final half turn. The density of cells was calculated by dividing the number of cells present within each cross-sectional profile of Rosenthal’s canal by the area of that profile as measured with the computer program Image-Pro Plus (Media Cybernetics, Silver Spring, MD, USA). Digital photomicrographs of each profile were collected and the perimeter of the canal traced, by hand, with a calibrated cursor. The area within the outlined canal was calculated with a computer. The density of cells (number of cells/area) was determined for the apical, middle and basal turns of each cochlea by averaging the individual densities for each turn. An analysis of variance (ANOVA) was used to test the following hypotheses: (1) there is a loss of cells over time within each strain for each cochlear turn and,(2) there is a difference among the strains in the amount of ganglion cell loss in the basal cochlear turn.
3. Results
3.1. Genetic definition of the B6.CAST-+ahl congenic region
The B6.CAST-+ahl congenic strain was still under development when it was originally described (Johnson et al., 1997). To better define the extent of the CAST/Ei-derived region in the now completed congenic strain, we typed 15 genetic markers along the length of Chromosome 0 (Table 1). The genetic map positions for these markers as assigned by the Mouse Genome Database (MGD) indicate that the congenic region extends for a genetic distance of about 9 cM. The DNA sequence positions in megabases (Mb) for these markers as assigned by Ensembl (http://www.ensembl.org) from the public sequence and by the Celera Discovery System (http://cds.celera.com) indicate that the congenic region consists of about 27 Mb. Cdh23, the gene most likely responsible for the ahl phenotype (Noben-Trauth et al., 2003), lies within this congenic interval.
Table 1.
The extent of the congenic interval in the B6.CAST-+Ahl inbred strain, as determined from the genotypes of Chr 10 markers
| Genetic marker | Genotype | MGD position (cM) | Ensembl position (Mb) | Celera position (Mb) |
|---|---|---|---|---|
| D10Mit80 | BB | 4 | 11.4 | 8.5 |
| D10Mit169 | BB | 11 | 19.6 | 16.7 |
| D10Mit4 | BB | 19 | 25.7 | 23.9 |
| D10Mit251 | BB | 21 | 27.7 | 25.8 |
| D10Mit52 | BB | 23 | 33.5 | 31.6 |
| D10Mit156 | BB | 23 | 36.2 | 34.3 |
| D10Mit53 | BB | 25.5 | 39.9 | 37.9 |
| D10Mit108 | CC | 25.5 | 42.6 | 40.6 |
| D10Mit138 | CC | 30 | 53.7 | 51.5 |
| D10Mit59 | CC | 30 | 56.8 | 54.6 |
| Cdh23a | CC | 30.3 | 59.9–60.3 | 58.4–58.8 |
| D10Mit130 | CC | 31.5 | 66.1 | 64.5 |
| D10Mit113 | BB | 34 | 67.2 | 65.5 |
| D10Mit31 | BB | 36 | 68 | 66.3 |
| D10Mit21 | BB | 43 | 80.3 | 78.4 |
| D10Mit134 | BB | 59 | 104 | 103.5 |
C57BL/6J-derived alleles are designated ‘BB’ and CAST/Ei-derived alleles ‘CC’.
Cdh23 is the gene most likely responsible for the ahl phenotype (Noben-Trauth et al., 2003).
3.2. ABR thresholds
The CAST animals (+ahl/+ahl) retained good hearing until the oldest age studied, 24 months (Table 2). The variation in ABR thresholds among CAST mice was very small. There were no statistically significant differences among the animals in the different age groups for any of the presented stimuli, although a slight threshold elevation (about 10 dB for the 32 kHz stimulus) was observed at the oldest age tested.
Table 2.
Average ABR thresholds of C57BL/6J, B6.CAST-+ahl, and CAST/Ei mice tested at different ages for responses to click, 8, 16, and 32 kHz stimuli
| Inbred strain | Age (months) | Number tested | Click | Mean ABR thresholds (dB SPL) |
||
|---|---|---|---|---|---|---|
| 8 kHz | 16 kHz | 32 kHz | ||||
| C57BL/6J | 1–2 | 13 | 41 (8) | 28 (4) | 16 (4) | 41 (9) |
| C57BL/6J | 9 | 7 | 48 (10) | 42 (9) | 23 (10) | 68 (10) |
| C57BL/6J | 12 | 4 | 45 (4) | 29 (5) | 23 (3) | 60 (4) |
| C57BL/6J | 15 | 7 | 59 (15) | 49 (15) | 39 (17) | 68 (14) |
| C57BL/6J | 18 | 6 | 77 (10) | 58 (5) | 53 (16) | 83 (5) |
| C57BL/6J | 21 | 5 | 92 (13) | 69 (17) | 64 (20) | 90 (15) |
| C57BL/6J | 24 | 9 | 115 (6) | 101 (5) | 97 (4) | 117 (5) |
| B6.CAST-+ahl | 12 | 4 | 48 (6) | 39 (8) | 24 (6) | 46 (6) |
| B6.CAST-+ahl | 15 | 6 | 53 (4) | 51 (5) | 23 (3) | 54 (4) |
| B6.CAST-+ahl | 18 | 5 | 68 (8) | 48 (4) | 34 (4) | 67 (4) |
| B6.CAST-+ahl | 21 | 4 | 81 (14) | 59 (25) | 43 (20) | 76 (11) |
| B6.CAST-+ahl | 24 | 8 | 98 (17) | 79 (15) | 68 (16) | 98 (15) |
| CAST/Ei | 4 | 5 | 30 (0) | 19 (4) | 10 (0) | 32 (3) |
| CAST/Ei | 12 | 3 | 35 (0) | 33 (10) | 8 (3) | 43 (6) |
| CAST/Ei | 15 | 4 | 35 (0) | 36 (3) | 14 (3) | 48 (6) |
| CAST/Ei | 18 | 4 | 35 (0) | 39 (3) | 11 (3) | 45 (4) |
| CAST/Ei | 21 | 4 | 38 (3) | 38 (3) | 11 (3) | 46 (3) |
| CAST/Ei | 24 | 4 | 45 (14) | 44 (9) | 18 (5) | 58 (12) |
Standard deviations are given in parentheses to show the degree of threshold variation that was observed among mice within each test group for each presented stimulus.
The B6 mice (ahl/ahl), on the other hand, exhibited elevated ABR thresholds by 15–16 months of age (Table 2). The number of animals with hearing loss and the magnitude of the loss increased with age. The thresholds in this group had a great deal of variation (as high as 50 dB) among animals tested at the same age. By 24 months, however, all B6 mice were deaf.
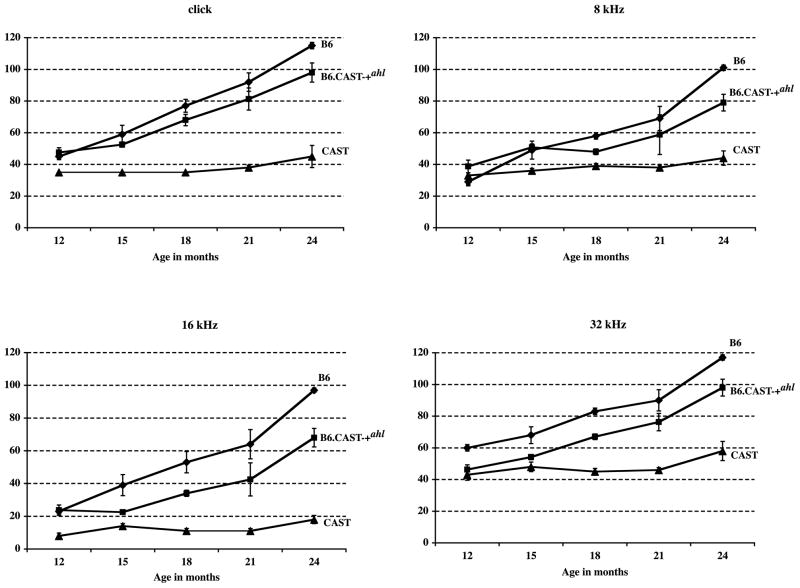
The congenic B6.CAST-+ahl mice (+ahl/+ahl) also had hearing loss with age, but less than that of B6 mice (Table 2). At 15 months of age and older, the average 16 kHz ABR thresholds of B6.CAST-+ahl mice were about 20 dB below those of equivalently aged B6 mice. ABR threshold variation among mice within this group was especially high at the 21 and 24 month test ages, whereas variation among B6 mice was highest between 15 and 21 months. A comparison of the hearing loss progression among the three strains is illustrated in Fig. 1. The degree of hearing loss in the B6.CAST-+ahl mice was delayed by approximately 3–6 months compared with B6 mice. For example, the average thresholds of B6 mice at 18 months of age were about the same as B6.CAST-+ahl mice at 21–24 months of age.
Fig. 1.
Comparison of hearing loss progression in B6, CAST, and B6.CAST-+ahl mice. Means (with standard error bars) of ABR thresholds (dB SPL) for each of the three strains for click, 8, 16, and 32 kHz stimuli. The numbers of mice tested for each strain at each age are given in Table 2.
3.3. Histological results
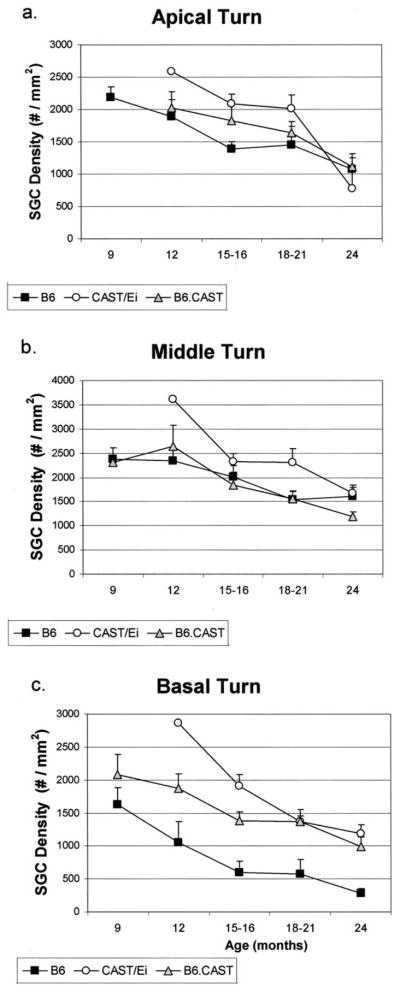
Consistent with their excellent hearing, the CAST animals had excellent morphology of the organ of Corti even at the oldest ages (Fig. 2a). None of the animals showed total degeneration of the organ of Corti in any turn. The spiral ganglion cell density, however, decreased over the life span of the animal with a significant loss of cells in the apical, middle and basal turns (one-way ANOVA, P < 0.001 for each turn, apical turn F(3, 27) = 17.19; middle turn F(3, 40) = 10.95; basal turn F(3, 28) = 8.47) (Fig. 3a–c). The stria vascularis showed little or no age-related degenerative changes, except in two animals (18 and 24 months) in which it was degenerated in the apical turn.
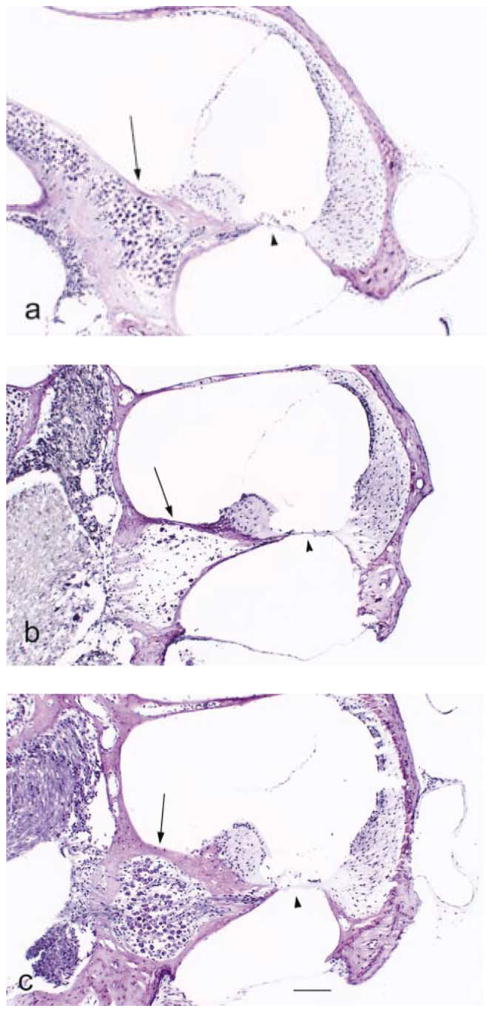
Fig. 2.
Photomicrographs of the cochlear basal turn from mice of each strain. Arrow, spiral ganglion cells, arrowhead, organ of Corti. (a) B6, 24 months old. There is no organ of Corti and the spiral ganglion is severely degenerated. (b) CAST, 21 months old. The organ of Corti and spiral ganglion look normal. (c) B6.CAST-+ahl, 18 months old. The organ of Corti and spiral ganglion look normal. Scale bar for all three photographs, 100 μm.
Fig. 3.
Mean (S.E.M.) spiral ganglion cell density in the basal, middle and apical regions of Rosenthal’s canal for each of the three mouse strains as a function of age. All strains show a decrease in ganglion cell density in each turn with age (ANOVA, P < 0.001). In the basal turn, the B6 mice have severe spiral ganglion cell degeneration. Comparison among the strains show that the B6.CAST-+ahl and CAST mice have significantly more cells in the basal turn than do the B6 mice even at the oldest ages (ANOVA, P < 0.001). Sixty-seven cochleae were used in this analysis: 9 months, B6, n = 7 cochleae, CAST, n = 0, B6.CAST-+ahl, n = 3; 12 months, B6, n = 4, CAST, n = 1, B6.CAST-+ahl, n = 5; 15–16 months, B6, n = 6, CAST, n = 7, B6.CAST-+ahl, n = 6; 18–21 months, B6, n = 4, CAST, n = 6, B6.CAST-+ahl, n = 9; 24 months B6, n = 7, CAST, n = 4, B6.CAST-+ahl, n = 7.
By 12 months of age the organ of Corti in the B6 mice had totally degenerated in three of four cochleae (Fig. 2b) in agreement with previously published descriptions (Henry and Chole, 1980; Willott, 1991). Older animals all showed a loss of the organ of Corti in the basal turn with the exception of one mouse at 21 and another at 24 months that did retain an organ of Corti in the basal turn. Over all ages, only four of the 27 B6 mouse cochleae had an organ of Corti in the basal turn. There was also a decrease in the ganglion cell density relative to the 9-month-old animals for each turn over time, with the largest loss of cells occurring in the basal turn (ANOVA, P < 0.001 for each turn; apical turn F(4, 40) = 9.69; middle turn F(4, 84) = 11.59; basal turn F(4, 45) = 14.36) (Fig. 3a–c). The stria vascularis did not show age-related degeneration; except in the apical turns of cochleae from three mice and the basal turns of cochleae from three other mice.
The cochleae of the congenic B6.CAST-+ahl mice (Fig. 2c) resembled those of CAST mice. The organ of Corti in the basal turn was present in 26 of 30 animals that were evaluated. Two of the animals with degeneration were 24 months old. There was a decrease in spiral ganglion cell density with age for all cochlear turns (ANOVA, P < 0.001; apical turn F(4, 53) = 9.22; middle turn F(4, 85) = 20.18; basal turn F(4, 38) = 9.42). As in the two parental mouse strains, the stria vascularis showed little atrophy even in the oldest animals studied. Two animals, one 12 months and one 18 months of age, had some atrophic changes in the stria vascularis of the apical turn.
Comparison of the organ of Corti degeneration among the strains showed that the B6 cochleae are significantly different than the other two strains (chi-square, P < 0.001). Comparison of the spiral ganglion cell density in the basal turn only, where B6 mice have the greatest cell loss, showed that, at 12 months of age and older, there was no difference in spiral ganglion cell density between B6.CAST-+ahl mice and CAST mice (one-way ANOVA, P < 0.001, F(2, 112) = 14.42). Both these strains had significantly more ganglion cells than B6 mice in the basal turn. It seems, then, that the normal allele at the ahl locus (+ahl) in the congenic mice did prevent the well-known degeneration of the organ of Corti and spiral ganglion cells characteristic of B6 mice. The loss of ganglion cells with age in the middle and apical turns may not be related to this allele, however.
4. Discussion
These are exciting times in the study of presbycusis, as the components and interactions between the different contributors to this condition are being identified (Fischel-Ghodsian et al., 1997; Seidman et al., 2002; Fransen et al., 2003). Since researchers began using mice as experimental animals and creating inbred strains, many genetic loci that affect inner ear structure and function have been mapped. With the advent of molecular biological techniques for identifying the genes associated with the described phenotypes, it will ultimately be possible to define profiles of the genes that affect particular structures and functions of the inner ear.
The well-known, age-related hearing loss that occurs in B6 mice (Mikaelian, 1979; Henry and Chole, 1980; Willott, 1991) has been attributed, in part, to the ahl gene on Chromosome 10 (Erway et al., 1993; Johnson et al., 1997). In order to test the isolated role of the ahl gene in the age-related hearing loss and cochlear degeneration of B6 mice, a congenic strain was created (Johnson et al., 1997). These congenic mice have a small portion of Chromosome 10, including the normal +ahl allele, from CAST, with the remaining genome B6. We show that the congenic interval of the now completed B6.CAST-+ahl inbred strain extends for a genetic distance of about 9 cM and contains about 27 Mb of DNA derived from the CAST/Ei strain. Phenotypic differences between the B6 and B6.CAST-+ahl strains, therefore, can be attributed to this Chromosome 10 region. The congenic region includes Cdh23, the gene thought to be responsible for the hearing loss attributed to the ahl locus (Noben-Trauth et al., 2003).
The B6.CAST-+ahl mice exhibited a hearing loss similar to that of B6 mice, although progression was delayed by about 3–6 months, whereas CAST mice retained normal hearing until old age (Fig. 1). Thus, the +ahl allele from CAST delays progression but does not prevent eventual hearing loss in old B6 mice. The typical B6 degenerative pattern of the basal turn, on the other hand, was not seen in the congenic mice even at the oldest ages. These results indicate that the loss of the organ of Corti and the spiral ganglion cells in the basal turn of B6 mice is related to the ahl locus. The apical turn degeneration seems not to be determined by the ahl locus, however. It follows, then, that genes other than ahl must contribute to the maintenance of good auditory thresholds over the life span of CAST mice. In fact, a second locus contributing to the retention of hearing in CAST mice was recently identified on Chromosome 5 and named ahl2 (Johnson and Zheng, 2002). CAST mice are among the few animal models that show very little age-related hearing loss and are worthy of further investigation to identify genes that maintain hearing. Although we detected no statistically significant ABR threshold elevations, these mice do develop a small amount of age-related spiral ganglion cell degeneration.
The original linkage cross that was used to map ahl (Johnson et al., 1997) was not large enough (38 progeny) to detect loci that make small contributions to age-related hearing loss in B6 mice. Use of the B6.CAST-+ahl congenic strain rather than B6 in a linkage cross with CAST would control for the large effect of ahl and improve chances for finding additional loci; however, a much larger cross than was used to initially map ahl would be needed to map these less in£uential loci.
We noted that, at certain ages, there were significant differences in hearing loss among genetically identical mice of the B6 and B6.CAST-+ahl inbred strains, which were reared in very similar environments. A similar variability of hearing loss among age-matched mice of the same inbred strain has been reported previously for B6 mice (Li and Borg, 1991; Prosen et al., 2003) and also for other strains with age-related hearing loss (Erway et al., 1993; Zheng et al., 1999). This within-strain, non-genetic variation must be caused by minor environmental differences or possibly by random stochastic factors. Over time, environmental insults (and perhaps negative stochastic events – for example, see Herndon et al., 2002) contribute to an accumulation of damage to the cochlea. Genetic factors like ahl presumably modulate the extent of this damage by altering resistance or repair mechanisms. It is only when the accumulated cochlear damage reaches a threshold level that we can measure a significant hearing loss.
In conclusion, our histopathology results show that the ahl gene is a major contributor to the loss of the organ of Corti and spiral ganglion cells in the basal turn of B6 mice. Our ABR threshold results show that progression of hearing loss in B6.CAST-+ahl mice is delayed relative to B6 mice but is not prevented. Additional genes, therefore, must contribute to the maintenance of normal auditory thresholds and ganglion cell survival characteristic of CAST mice, especially in old age.
Acknowledgments
The authors wish to thank Tim Truong, Kristin Meuller, Anna Vigran, Jenny Hong, Jennifer Lee, M.D., and Heping Yu for their technical contributions. This work was supported by NIH NIDCD DC RO-1 003395 (to E.M.K.), the Medical Research Service of the Department of Veterans Affairs, NIDCD contract DC 62108 (to K.R.J.) and TJL institutional shared services supported by National Cancer Institute grant CA34196.
Abbreviations
- ABR
auditory brainstem response
- ahl
age-related hearing loss
- ANOVA
analysis of variance
References
- Bartolome MV, Lopez LM, Gil-Loyzaga P. Galectine-1 expression in cochleae of C57BL/6 mice during aging. NeuroReport. 2001;12:3107–3110. doi: 10.1097/00001756-200110080-00025. [DOI] [PubMed] [Google Scholar]
- Davis RR, Newlander JK, Ling X, Cortopassi GA, Krieg EF, Erway LC. Genetic basis for susceptibility to noise-induced hearing loss in mice. Hear Res. 2001;155:82–90. doi: 10.1016/s0378-5955(01)00250-7. [DOI] [PubMed] [Google Scholar]
- Dazert S, Feldman ML, Keithley EM. Cochlear spiral ganglion cell degeneration in wild-caught mice as a function of age. Hear Res. 1996;100:101–106. doi: 10.1016/0378-5955(96)00100-1. [DOI] [PubMed] [Google Scholar]
- Erway LC, Willott JF, Archer JR, Harrison DE. Genetics of age-related hearing loss in mice: I. Inbred and F1 hybrid strains. Hear Res. 1993;65:125–132. doi: 10.1016/0378-5955(93)90207-h. [DOI] [PubMed] [Google Scholar]
- Fischel-Ghodsian N, Bykhovskaya Y, Taylor K, Kahen T, Cantor R, Ehrenman K, Smith R, Keithley EM. Temporal bone analysis of patients with presbycusis reveals high frequency of mitochondrial mutations. Hear Res. 1997;110:147–154. doi: 10.1016/s0378-5955(97)00077-4. [DOI] [PubMed] [Google Scholar]
- Fransen E, Lemkens N, Laer LV, Camp GV. Age-related hearing impairment (ARHI): environmental risk factors and genetic prospects. Exp Gerontol. 2003;38:353–359. doi: 10.1016/s0531-5565(03)00032-9. [DOI] [PubMed] [Google Scholar]
- Henry KR, Chole RA. Genotypic differences in behavioral, physiological and anatomical expressions of age-related hearing loss in the laboratory mouse. Audiology. 1980;19:369–383. doi: 10.3109/00206098009070071. [DOI] [PubMed] [Google Scholar]
- Hequembourg S, Liberman MC. Spiral ligament pathology: a major aspect of age-related cochlear degeneration in C57BL/6 mice. J Assoc Res Otolaryngol. 2001;2:118–129. doi: 10.1007/s101620010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors in£uence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- Hultcrantz M, Li HS. Inner ear morphology in CBA/Ca and C57BL/6J mice in relationship to noise, age and phenotype. Eur Arch Otorhinolaryngol. 1993;250:257–264. doi: 10.1007/BF00186222. [DOI] [PubMed] [Google Scholar]
- Jimenez AM, Stagner BB, Martin GK, Lonsbury-Martin BL. Susceptibility of DPOAEs to sound overexposure in inbred mice with AHL. J Assoc Res Otolaryngol. 2001;2:233–245. doi: 10.1007/s101620010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/6J mice. Hear Res. 1997;114:83–92. doi: 10.1016/s0378-5955(97)00155-x. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY, Erway LC. A major gene affecting age-related hearing loss is common to at least ten inbred strains of mice. Genomics. 2000;70:171–180. doi: 10.1006/geno.2000.6377. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY. ahl2, a second locus affecting age-related hearing loss in mice. Genomics. 2002;80:461–464. [PMC free article] [PubMed] [Google Scholar]
- Keithley EM, Feldman ML. Spiral ganglion cell counts in an age-graded series of rat cochleas. J Comp Neurol. 1979;188:429–442. doi: 10.1002/cne.901880306. [DOI] [PubMed] [Google Scholar]
- Li HS, Borg E. Age-related loss of auditory sensitivity in two mouse genotyes. Acta Otolaryngol. 1991;111:827–834. doi: 10.3109/00016489109138418. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Salvi R. Anatomical, metabolic and genetic aspects of age-related hearing loss in mice. Audiology. 2001;40:313–321. [PubMed] [Google Scholar]
- Mikaelian DO. Development and degeneration of hearing in the C57/b16 mouse: relation of electrophysiologic responses from the round window and cochlear nucleus to cochlear anatomy and behavioral responses. Laryngoscope. 1979;89:1–15. doi: 10.1288/00005537-197901000-00001. [DOI] [PubMed] [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet. 2003;35:21–23. doi: 10.1038/ng1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosen CA, Dore DJ, May BJ. The functional age of hearing loss in a mouse model of presbycusis. I Behavioral assessments. Hear Res. 2003;183:44–56. doi: 10.1016/s0378-5955(03)00211-9. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol. 1993;102:1–16. doi: 10.1177/00034894931020S101. [DOI] [PubMed] [Google Scholar]
- Seidman MD, Ahmad N, Bai U. Molecular mechanisms of age-related hearing loss. Ageing Res Rev. 2002;1:331–343. doi: 10.1016/s1568-1637(02)00004-1. [DOI] [PubMed] [Google Scholar]
- White JA, Burgess BJ, Hall RD, Nadol JB. Pattern of degeneration of the spiral ganglion cell and its processes in the C57BL/6J mouse. Hear Res. 2000;141:12–18. doi: 10.1016/s0378-5955(99)00204-x. [DOI] [PubMed] [Google Scholar]
- Willott JF. Effects of aging, hearing loss, and anatomical location on thresholds of inferior colliculus neurons in C57BL/6 and CBA mice. J Neurophysiol. 1986;56:391–408. doi: 10.1152/jn.1986.56.2.391. [DOI] [PubMed] [Google Scholar]
- Willott JF, Hunter KP, Coleman JR. Aging and presbycusis: effects on 2-deoxy-D-glucose uptake in the mouse auditory brain stem in quiet. Exp Neurol. 1988;99:615–621. doi: 10.1016/0014-4886(88)90178-1. [DOI] [PubMed] [Google Scholar]
- Willott JF. Aging and the Auditory System. Singular Publ; San Diego, CA: 1991. [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR. Hearing loss associated with the modifier of deaf waddler (mdfw) locus corresponds with age-related hearing loss in 12 inbred strains of mice. Hear Res. 2001;154:45–53. doi: 10.1016/s0378-5955(01)00215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]