Abstract
Mice, in which the genetics can be manipulated and the life span is relatively short, enable evaluation of the effects of specific gene expression on cochlear degeneration over time. Antioxidant enzymes such as Cu/Zn superoxide dismutase (SOD1) protect cells from toxic, reactive oxygen species and may be involved in age-related degeneration. The effects of SOD1 deletion and over-expression on the cochlea were examined in Sod1-null mice, Sod1 transgenic mice and in age- and genetics-matched controls. Auditory brainstem responses (ABR) were measured and cochleae were histologically examined. The absence of SOD1 resulted in hearing loss at an earlier age than in wildtype or heterozygous mice. The cochleae of the null mice had severe spiral ganglion cell degeneration at 7–9 months of age. The stria vascularis in the aged, null mice was thinner than in the heterozygous or wildtype mice. Over-expression of SOD1 did not protect against hearing loss except at 24 months of age. In conclusion, SOD1 seems important for survival of cochlear neurons and the stria vascularis, however even half the amount is sufficient and an over abundance does not provide much protection from age-related hearing loss.
Keywords: Presbycusis, Aging, Cochlea, Mice, Hearing, Mitochondria
1. Introduction
Presbycusis or age-related hearing loss is, in part, a consequence of cochlear degenerative changes. Mice, in which the genome can be manipulated and the life span is relatively short, enable evaluation of the effects of specific gene expression on cochlear degeneration over time and may, therefore, provide insights into the mechanisms underlying degeneration. Antioxidant enzymes are thought to protect cells from the damaging effects of oxygen over time (Ames, 2004). Copper/zinc superoxide dismutase (SOD1), is a cytosolic enzyme that converts the superoxide radical to hydrogen peroxide and O2 and in so doing provides protection to cells from the damaging effects of the superoxide and hydroxyl radicals. The hydrogen peroxide is subsequently converted to water and oxygen (Scheffler, 1999). The generation of the superoxide radical, , is related to the cellular energy production and oxygen consumption of mitochondria. In addition to SOD1 several other antioxidant enzymes are involved in protection from reactive oxygen species including glutathione peroxidase and manganese superoxide dismutase (SOD2) that is present within mitochondria. Genes encoding these enzymes are, therefore, of interest as potential contributors to aging and presbycusis.
SOD1 is prevalent in cochlear tissues. It is expressed in the spiral ligament, stria vascularis, spiral limbus, cells of the organ of Corti (Pierson and Gray, 1982; Rarey and Yao, 1996; Yao and Rarey, 1996) and spiral ganglion cells (Staecker et al., 2001). All these cells contain numerous mitochondria (Spoendlin, 1981; Spicer and Schulte, 2002; Nakazawa et al., 1995) and are, therefore, vulnerable to oxidative damage from respiration.
In order to examine the effects of eliminating the free-radical scavenging activity of SOD1, Sod1 null mice were originally created on a CD-1/129S background (Reaume et al., 1996). At 13 months of age these animals have hair cell and spiral ganglion cell degeneration and hearing loss (McFadden et al., 1999a,b, 2001). This result is likely due to the absence of the protective function of SOD1 (Sha et al., 2001; Zhang et al., 2002), however, the background strain, CD-1, is outbred and unknown genetic modifiers may also affect the phenotype. The phenotypic variability reported by McFadden et al. is consistent with this interpretation. We, therefore, used a newly created SOD1 null mouse on a B6 background (Matzuk et al., 1998) to evaluate the effect of the deletion on cochlear structure and function with age. This strain has the advantage that the effects of SOD1 deletion can be determined on a more genetically defined, inbred strain.
Additional evidence for a protective role of SOD1 is suggested by the slightly larger hearing loss resulting from acoustic trauma in young Sod1 null mice than wildtype mice (Ohlemiller et al., 1999). Constitutive over-expression of SOD1, however, did not have any protective effect against acute acoustic trauma in 2-month-old transgenic mice or on age-related hearing loss up to 7 months of age (Coling et al., 2003). An acute traumatic experience is quite different than aging or the maintenance of cellular homeostasis over time, and age-related effects on hearing loss in B6 mice (the background strain of the SOD1 over-expressing transgenic mice) occur beyond 7 months of age, as measured by ABR threshold elevations (Zheng et al., 1999). We, therefore, examined SOD1 over-expressing mice at much older ages (up to 24 months) to determine whether an over-abundance of SOD1 would reduce the characteristic hearing loss and loss of spiral ganglion neurons in B6 mice related to the ahl gene (Johnson et al., 1997, 2000).
2. Materials and methods
2.1. Mice
The effect of SOD1 deficiency on auditory brainstem response (ABR) threshold and cochlear structure was examined in male and female Sod1 null mice from strain B6;129S7-Sod1tm1Leb/J, formerly B6;129S Sod1tm1Leb/tm1Leb (Matzuk et al., 1998). Wild type (+/+, n = 42) and heterozygous mice (+/−, n = 44) were tested at 7–9, 12, 15 and 18 months of age (20–45 g). Homozygous null mutants (−/−, n = 31) were tested at 7–9, 12 and 15 months of age. These mice when obtained from Dr. Russell Lebovitz in 1998 were approximately 50% B6 and 50% 129S7. Since then they were backcrossed twice more to B6, so were approximately 87.5% B6 and 12.5% 129S7. We compared the ABR results to those obtained previously for B6 mice (Keithley et al., 2004).
The effects of over-expression of SOD1 on age-related hearing loss were examined in B6-TgN(SOD1)3Cje mice that are hemizygous for a human SOD1 transgene on an otherwise B6 background (Epstein et al., 1987). These transgenic mice are viable, fertile and express about three times the normal level of CuZn-SOD in the brain (Przedborski et al., 1992) and cochlea (Coling et al., 2003). Animals were examined at 12, 15, 18 and 24 months of age (n = 18).
All animals were bred and housed in the standard animal facility under normal mouse rearing conditions at The Jackson Laboratory (Bar Harbor, ME). Mice were genotyped by the Jackson Laboratory Genotyping Service, as described in the Jax Mice Genotyping Protocols (http://jaxmice.jax.org/pub-cgi/protocols/protocols.sh?objtype=prot_query_top&uclass=read). The stock number is 002972 for B6;129S7-Sod1tm1Leb/J; the stock number is 002629 for C57BL/6-Tg(SOD1)-3Cje/J. All experimental procedures that involved mice were approved by the Animal Care and Use Committee at The Jackson Laboratory.
2.2. Assessment of hearing
When mice reached the designated age, they were anesthetized with Avertin (tribromoethanol stabilized in tertiary amyl hydrate, 5 mg tribromoethanol/10 g body weight) and hearing was measured with a click stimulus and at 8, 16, and 32 kHz using tone pips (3 ms duration) in a closed acoustic system. Stimuli were presented to both ears simultaneously at decreasing intensities (5 dB steps). Recording electrodes (Model F-E2, Astro-Med, Inc.) were inserted subcutaneously at the vertex (active), ventrolateral to the left ear (ground) and ventrolateral to the right ear (reference). Stimulus evoked signals were recorded in an ABR recording system (Intelligent Hearing System, IHS, Miami. FL) (see Zheng et al., 1999 for details). The threshold response was recorded for each animal for each stimulus.
Following the ABR measurement, while still under deep anesthesia, the animals were perfused with saline followed by 4% paraformaldehyde. The animals were decapitated and the tissue was sent to the University of California, San Diego for tissue processing.
2.3. Histopathology
The cochleae were dissected and decalcified in 8% EDTA (pH 7.2) for one to two weeks and then cryosectioned (8 μm) parallel to the modiolus. Sections were collected on Superfrost Plus slides (Fisher Scientific, Inc.) and stained with osmium and cresyl violet.
Histological evaluation of each cochlea was performed by an individual who was unaware of the animal ’s age or genotype. Hair cells and pillar cells were evaluated in individual cochlear sections. A radial-section of the organ of Corti was considered normal if inner and outer hair cell nuclei could be identified. The stria vascularis was evaluated for pathological changes as described for aged rats (Keithley et al., 1992) and for thinning of the tissue. The thickness of the stria vascularis at a location in the apical turn, 10–15% from the apical end of the cochlear duct was measured in digital photomicrographs using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD). The measurement was made by using a cursor to draw a line from the margin of the stria to the junction of the basal cells with the spiral ligament half-way between the attachment of Reissner’s membrane and the spiral prominence. Three to five sections were measured for each cochlea. Spiral ganglion cells (types I and II were not differentiated) were counted in each cross-section of Rosenthal’s canal of individual sections. Cells were identified by the presence of a nucleus. The corresponding area of Rosenthal’s canal was measured on digital photomicrographs of each canal profile. The perimeter of the canal was traced with a calibrated cursor using Image-Pro Plus software. The computer then calculated the area within the outline. The density of cells was calculated by dividing the number of counted cells within each cross-sectional profile of Rosenthal’s canal by the area of that profile. No attempt was made to correct for “plit nuclei” so the absolute number of cells was not determined. Relative numbers among the genotypes were compared. Two to five cross-sections of each turn were evaluated for each cochlea. The canal was divided into three segments of unequal length representing the basal, middle and apical portions of the canal and referred to as turns (Keithley and Feldman, 1979; Dazert et al., 1996). The density of cells (number of cells/area) was determined for the apical, middle and basal region of each cochlea by averaging the individual section densities for each turn.
3. Results
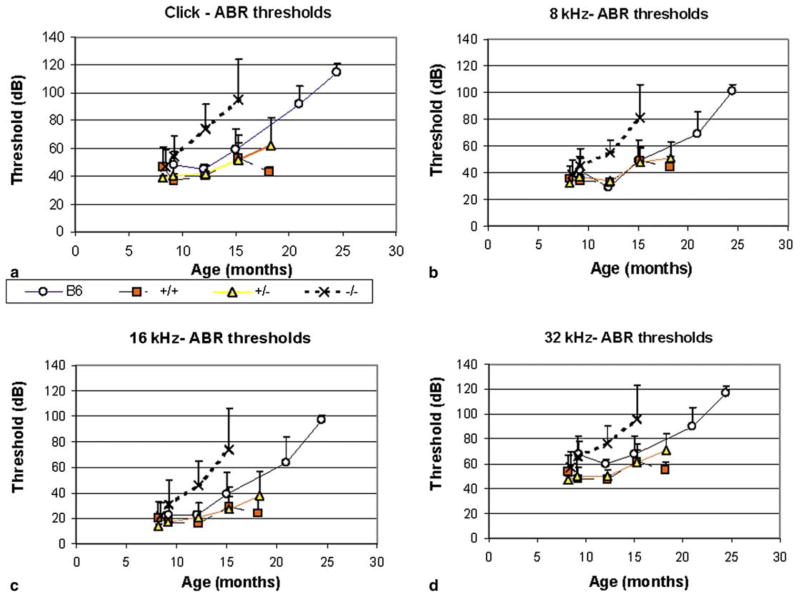
The mean ABR thresholds as a function of age for all stimuli for wildtype (+/+), heterozygotes (+/−) and Sod1 null (−/−) animals are shown in Fig. 1. For comparison, threshold data for the parental B6 strain, collected with the same equipment and previously published (Keithley et al., 2004) are also included. While all three genotypes have similar thresholds at 7–9 months, at 12 months the Sod1 null mice have significantly increased thresholds for all stimuli. Three months later, the thresholds were further elevated in the null mice relative to the other genotypes. (Two-way ANOVAs for 4 genotypes (B6, +/+, +/−, −/−) and 3 age groups [7–9, 12 and 15 months] with Tukey post hoc testing, [click: (3, 2, genotype * age = 6, 111) F = 23.1 for genotype and 22.87 for age, p < 0.001; 8 kHz: F = 19.15 for genotype and 33.15 for age, p < 0.001; 16 kHz: F = 20.92 for genotype and 25.76 for age, p < 0.001; 32 kHz: F = 19.83 for genotype and 15.72 for age, p < 0.001.) Although the background strain of B6 is well known for its age-related hearing loss that begins with the high frequencies (Willott, 1986), the thresholds at 32 kHz of the Sod1 null mice were about 20 dB greater than those of the B6 mice even at 12 months of age. The B6 mice had thresholds at 32 kHz about 10 dB greater than the wildtype controls and heterozygous mice at 12 months of age. In contrast to a previous report (McFadden et al., 1999b), the ABR thresholds of Sod1 heterozygotes were not different than those of the wildtype mice at any age or any frequency (two-way ANOVA for 2 genotypes, +/+, +/−, and 4 age groups, 7–9, 12, 15, 18 months, click: (df = 1, 3, 83, F = 0.43, p = 0.51).
Fig. 1.
Mean (standard deviation) auditory brainstem response thresholds for (a) clicks and tone pip stimuli at (b) 8, (c) 16 and (d) 32 kHz. The thresholds for Sod1 null mice (−/−) are greater than for the B6 (Keithley et al., 2004), wildtype (+/+), and heterozygous (+/−) mice at 12 and 15 months of age (three-way ANOVA, p < 0.001). Between 5 and 20 animals were evaluated at each time point for each genotype.
Histological examination of cochlear sections from the wildtype and heterozygous mice at 7–9 months of age revealed a normal organ of Corti (hair cell nuclei present in individual sections) in 34 of 36 cochleas and normal appearing stria vascularis in 35 of the 36 cochleas. Measurements of strial thickness in the apical turn indicated a mean (standard deviation) thickness of 18.9 (3.8) μm in the wildtype and 18.3 (3.7) μm in the heterozygous mice. The organ of Corti in the Sod1 null mice at 7–9 months of age appeared normal in 12 of 14 mice (Fig. 2). The stria vascularis appeared normal and was 18.7 (3.6) μm thick in the apical turn which was no different than the other genotypes (Fig. 3). The ganglion cell density in all turns, however, was reduced relative to the wildtype and heterozygous animals (Figs. 4 and 5; three-way ANOVA, age, cochlear turn, genotype (2, 2, 2, 541, F = 14.15 for age, 76.13 for cochlear turn, and 139.04 for genotype, p < 0.001 for each factor).
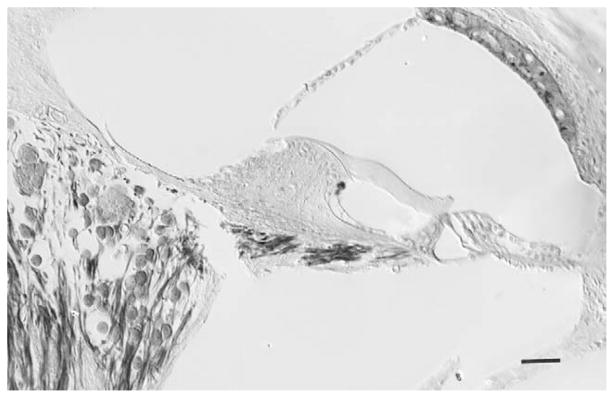
Fig. 2.
Photomicrograph (Nomarski DIC optics) of a radial section of the organ of Corti in the apical turn of a Sod1-null mouse at 9.5 months of age. The organ of Corti contains three outer hair cell nuclei and one inner hair cell and the spiral ganglion shows signs of cellular degeneration. This section is from a cochlea that was embedded in araldite with the addition of a plasticizer so that 15 μm sections could be cut with a glass knife (Keithley and Feldman, 1979). The tissue was osmicated prior to embedding and the section was not stained. This cochlea was not used for data collection reported in the results section. The break in the osseous spiral lamina is a preparation artifact. Scale bar, 50 μm.
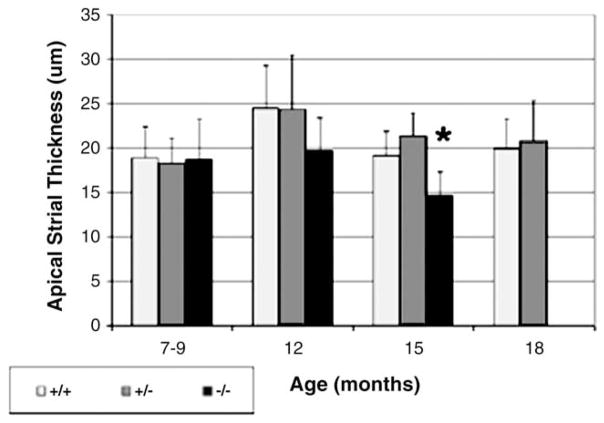
Fig. 3.
Mean (standard deviation) thickness of the stria vascularis in the apical turn for each genotype in each age group (*, p < 0.001, three-way ANOVA, see text).
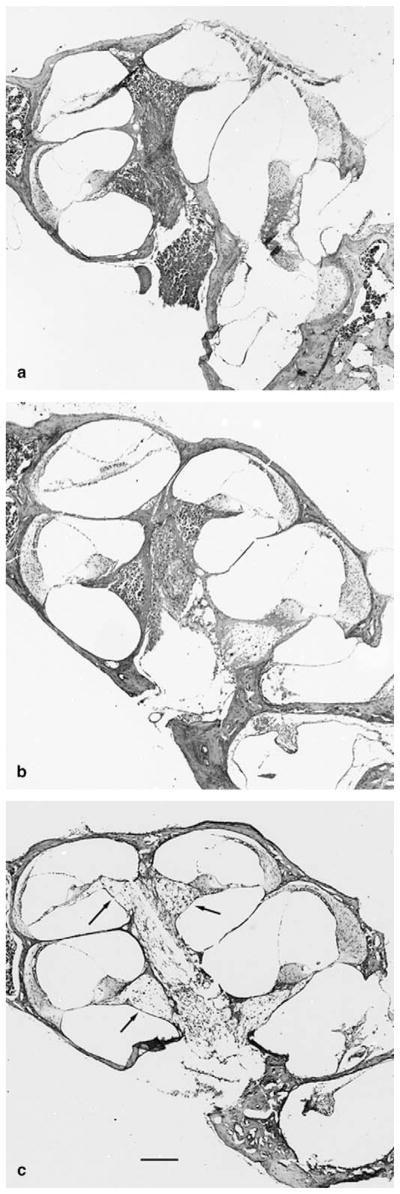
Fig. 4.
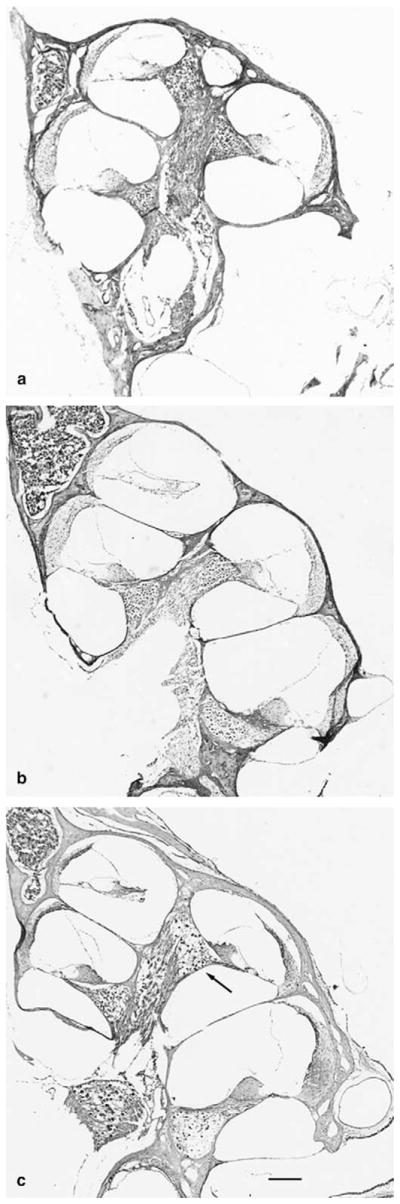
Photomicrographs of the cochlea stained with cresyl violet from (a) wildtype (+/+) mouse, (b) heterozygous (+/−) mouse and, (c) Sod1-null (−/−) mouse at 7–9 months of age. Arrow, spiral ganglion with reduced number of cells. Scale bar for all micrographs, 150 μm.
Fig. 5.
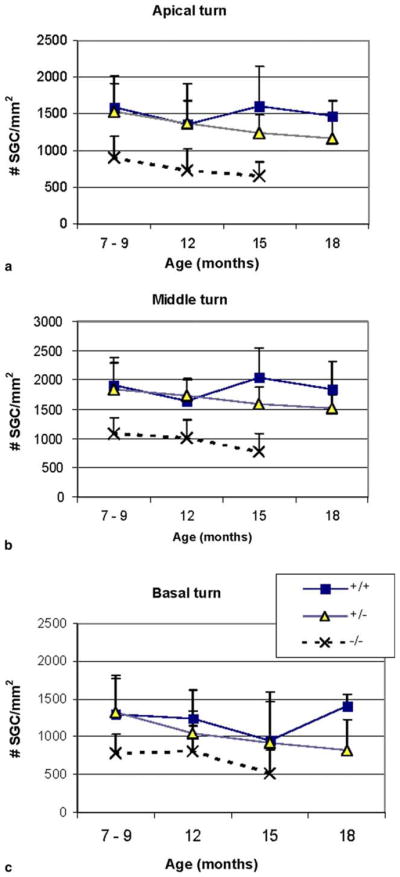
Mean (standard deviation) spiral ganglion cell density as a function of age in the (a) apical (b) middle and (c) basal turns of wildtype (+/+), heterozygous (+/−), and Sod1-null (−/−) mice cochleas. The Sod1-null mice have fewer cells at all ages in all cochlear turns (ANOVA, p < 0.001).
At 15 and 18 months the basal turn of the cochleae from most mice, independent of genotype, had degenerative changes within the organ of Corti including the loss of inner and outer hair cell nuclei and the collapse of the pillar cells. This age-related degeneration is consistent with the B6 genotype background of all these mice. The portion of the basilar membrane within the basal turn of the cochlea represents frequencies higher than 32 kHz (Ehret, 1983; Koay et al., 2002; Francis et al., 2004), so the loss of cells in this region is not reflected in the ABR thresholds that were measured. The organ of Corti more apical to the basal turn showed degenerative changes in all genotypes and the cryostat sections preclude any comparisons among genotypes.
The stria vascularis in the wildtype and heterozygous mice did not show degenerative changes even at 18 months of age (Fig. 3). The stria vascularis in the Sod1 null mice, on the other hand, was significantly reduced in thickness at 15 months of age (Figs. 3 and 6) (Tukey post hoc testing following ANOVA [three-way ANOVA for 3 genotypes, +/+, +/−, −/− and 3 age groups, 7–9, 12 and 15 months, df = 2, 2, 186, F = 15.2, p < 0.001 for genotype, F = 22.3, p < 0.001 for age and F = 5.6, p < 0.001 for interactions].
Fig. 6.
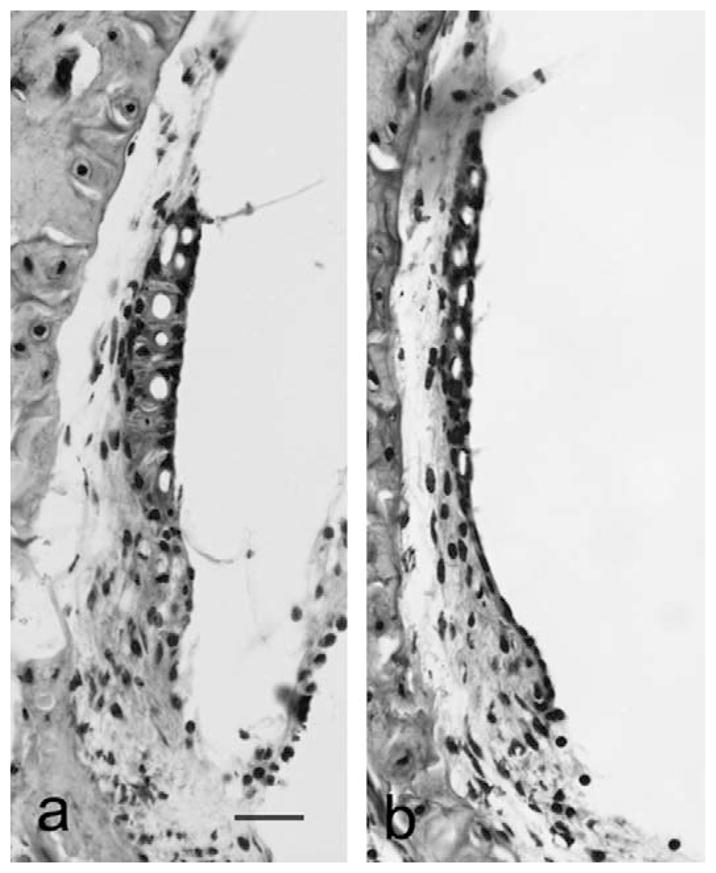
Photomicrographs of the stria vascularis from the apical turn (10–15% distance from the apical end) of (a) a wildtype, 15 month old mouse and (b) a Sod1-null, 15 month old mouse. Scale bar for both figures, 25 μm.
In the wildtype mice, the ganglion cell density did not decrease with age (Tukey post hoc test from above ganglion cell analysis, not significant). A decrease in cell density did occur, however, in the heterozygous mice by 15 months (Tukey post hoc test from the above analysis, p < 0.001). The Sod1 null mice, that had fewer ganglion cells than either the wildtype or heterozygous mice at 7–9 month of age, continued to experience ganglion cell degeneration over time in all turns (Tukey post hoc test from above analysis, p < 0.001) (Figs. 5 and 7). The large loss of neurons seen in all genotypes in the basal turn reflects the parental B6 strain.
Fig. 7.
Photomicrographs of the cochlea from 15–18 month old (a) wildtype (+/+) mouse, (b) heterozygous (+/−) mouse and, (c) Sod1-null (−/−) mouse. Arrows, spiral ganglion in the apical and middle turns with reduced number of cells. The basal turn has a loss of ganglion cells in all genotypes at this age. Scale bar for all micrographs, 150 μm.
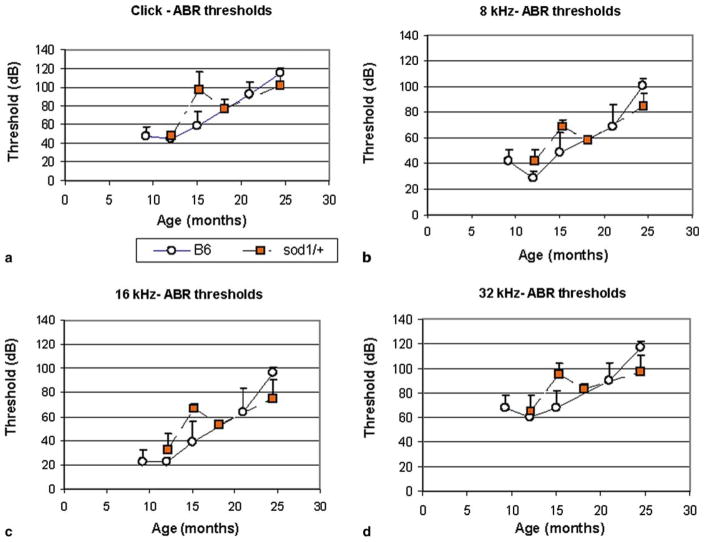
Over-expression of SOD1 did not delay the onset of hearing loss (Fig. 8) associated with the ahl gene in B6 mice. The thresholds of the hemizygous B6/TgN(SOD1)3Cje transgenic mice were no different than those of the parental B6 strain. It is possible, however, that a small amount of protection was provided at 24 months where the B6 animals (Keithley et al., 2004) had slightly higher ABR thresholds than the SOD1 transgenic mice (t-test, 32 kHz, p < 0.01). The cochleae of the SOD1 over-expressing mice appeared similar to those of B6 mice, with degeneration of hair cells and spiral ganglion cells in the basal turn in the aged mice.
Fig. 8.
Mean (standard deviation) ABR thresholds at each age for B6-TgN(SOD1)3Cje, transgenic mice that over-express SOD1. (a) Click stimuli, tone pip stimuli at (b) 8, (c) 16 and (d) 32 kHz. Thresholds for SOD1 transgenic mice (Sod1/+) are no different than those of B6 mice (Keithley et al., 2004) that were used as the background.
4. Discussion
The first transgenic mice in which the Sod1 gene was inactivated were generated by (Reaume et al., 1996; Cephalon, Inc., CD-1,129 SOD-1 deletion) to investigate the relationship between motor neuron degeneration and SOD1. The mice were created on a CD-1/129S background. These mice were used later by others to examine the effect of the absence of SOD1 on cochlear morphology with age. Because CD-1 mice are outbred, genetic variability is greater than in genetically homogeneous inbred strains, a feature emphasized by McFadden et al. (1999a,b) in their reports. Hair cell degeneration was highly variable among the mice that were evaluated. Losses were seen at 2 months of age and continued to be greater than in the wildtype animals up to 19 months of age leading to the conclusion that SOD1 is important for hair cell survival (McFadden et al., 1999a). At 13 months of age severe hearing loss was identified in both the heterozygous and null mice. The spiral ganglion cells in the basal turn of the null mice were also degenerated (McFadden et al., 1999b). Most of the heterozygous mice had a phenotype similar to the null mice, although there was a great deal of variability among the mice. The stria vascularis was not evaluated.
Subsequently a different Sod1 null mouse was generated (Matzuk et al., 1998) and is maintained on a pre-dominantly B6 background at The Jackson Laboratory, allowing evaluation of SOD1 activity relative to the expression of the ahl gene (Johnson et al., 1997). These mice are inbred so the effects of unknown modifiers are consistent in each mouse. They were chosen for the current analysis to minimize genetic variability. It is hypothesized that each of the metabolically active cochlear cell types with large numbers of mitochondria would be vulnerable to the absence of SOD1. It appears that the cochlear cell type first affected by the lack of SOD1 is the spiral ganglion cell. Degeneration of spiral ganglion cells in the Sod1 null mice had already occurred by 7–9 months, the earliest age at which the cochleas were examined. Degeneration continued to occur during the next 6 months. Although we were not able to do a quantitative assessment of the hair cells in surface preparations of the organ of Corti, most sections from 7–9 month-old mice contained intact hair cells, especially outer hair cells that are required for good auditory thresholds (Ryan and Dallos, 1975; Salvi et al., 2000). The thresholds were not elevated in the null mice at this age. The stria vascularis, a tissue with abundant mitochondria, appeared normal at 7–9 months of age, however by 15 months of age it was significantly thinner in the apical turn of Sod1-null mice. Mice heterozygous for SOD1 had some neuronal degeneration over time, while the wildtype mice did not show ganglion cell degeneration. This result implies that haploinsufficiency of SOD1 may begin to have an effect as these animals age. The ABR and strial measures in the heterozygous mice were not different than the wildtype mice. Since the ABR threshold reflects outer hair cell function that is dependent on normal strial function, it seems that even half as much of the enzyme is sufficient to maintain the health of these tissues.
The background strain, B6, of the Sod1 null mouse examined in this study is well known for its early onset hearing loss and degeneration of the organ of Corti and ganglion cells in the basal cochlear turn (Mikaelian, 1979; Henry and Chole, 1980; Willott, 1986). We therefore, included ABR threshold data for this strain that were measured in the same way as the thresholds reported here (Keithley et al., 2004). The hearing loss in the Sod1 null mice is significantly greater than in the B6 mice at 12 and 15 months of age. The magnitude of ganglion cell loss is also greater than in B6 mice especially above the basal turn (Keithley et al., 2004). Because the degeneration in the Sod1 null mice is greater than that of B6 mice, the absence of SOD1 must confer a greater susceptibility to age-related hearing loss than does the ahl gene (Johnson et al., 1997; Noben-Trauth et al., 2003) alone. Although there is evidence that Cdh23 underlies the ahl phenotype, its role in the development of age-related hearing loss is not clear. It may contribute to the morphogenesis and maintenance of stereocilia, making hair cells of mice with the Cdh23ahl variant more vulnerable to damage. It is likely, however, that the age-related effects of Cdh23ahl and Sod1 on hearing loss are independent because Cdh23, is not involved in any antioxidant pathway and over-expression of SOD1 in transgenic B6 mice does not reduce the age-related hearing loss attributable to Cdh23ahl.
In the cochlea, the cells of the stria vascularis (Sugar et al., 1972), the spiral ganglion cells (Ylikoski et al., 1978; Spoendlin, 1981) and the hair cells (Engstrom, 1967; Engstrom and Engstrom, 1972, 1977) all contain especially numerous mitochondria and therefore might be subject to damage by reactive oxygen species. Cochlear SOD1 activity was measurable in guinea pig cochlear fractions of both the spiral ligament/stria vascularis and the organ of Corti/limbus. The fraction containing the stria vascularis had a higher activity than the organ of Corti fraction, and catalase and glutathione peroxidase specific activities were greater than SOD1 in both of these fractions (Pierson and Gray, 1982). In a subsequent study, ELISAs of dissected rat cochlear tissues identified the stria vascularis/spiral ligament and cells of the organ of Corti as containing SOD1 (Yao and Rarey, 1996). Immunohistochemical localization of SOD1 (Rarey and Yao, 1996) again identified strong staining in the spiral ligament, limbus and organ of Corti cells, but only light staining of spiral ganglion cells. Most recently immunohistochemical assays have identified SOD1 in the spiral ganglion cells of mice as well as the previously identified locations (Staecker et al., 2001). Although the stria vascularis contains a large amount of SOD1, it has a greater catalase and glutathione peroxidase activity (Pierson and Gray, 1982; Staecker et al., 2001) that may protect it from greater degeneration over time. Perhaps in the absence of SOD1, SOD2 and these other enzymes are sufficient to protect the strial cells from superoxide damage. The spiral ganglion cells and the hair cells (McFadden et al., 1999a) appear to be more dependent on SOD1. The conclusion that different mitochondria-containing cells of the cochlea have different mechanisms for handling reactive oxygen species has implications for the application of therapies that use anti-oxidant drugs. The targeted cell type to be rescued should be considered.
SOD1 also may be involved in glutamate receptor-mediated excitotoxicity. Experiments with gene targeted and transgenic mice have shown a gene-dose effect of SOD1 on excitotoxic mechanisms (Schwartz et al., 1998). These authors demonstrated that 10-fold over expression of SOD1 in the striatum provided some protection against kainic acid induced excitotoxicity while 50% reduced expression resulted in increased amounts of neuronal cell death. It is possible that the spiral ganglion cells that are very sensitive to glutamate excitotoxicity (Pujol and Puel, 1999) are more vulnerable to excitation in the Sod1 null mice. This mechanism of ganglion cell degeneration could account for the early loss of these cells and is consistent with the finding of Ohlemiller et al. (1999) who demonstrated the Sod1 null mice were more vulnerable to acoustic trauma.
Our results indicate that the effects of SOD1 on cochlear function are not strictly dose dependent. ABR thresholds of heterozygous mice are not different than wildtype mice demonstrating that reducing wildtype SOD1 activity by half does not increase vulnerability to hearing loss. Yet the absence of SOD1 leads to extensive cochlear degeneration as seen in Sod1 null mice. Over-expression of SOD1 above wildtype levels might reduce the amount of age-related hearing loss by providing extra protection from free radical damage to cochlear cells. Threshold data for the SOD1 over-expressing transgenic mice indicate however, that little or no protection was provided. The thresholds are not different than those of the background strain except at 24 months of age, close to the maximum life span of the animals. The ubiquitous over-expression of SOD1 does not extend life span in mice either (Huang et al., 2000). SOD1 over-expression also failed to protect mice from acoustic trauma (Coling et al., 2003), but did provide some protection against ototoxicity induced by the antibiotic drug, kanamycin (Sha et al., 2001; Kawamoto et al., 2004). In the context of these data, the observed up-regulation of SOD1 at 9 months of age in B6 mice (Staecker et al., 2001) must represent a futile cellular response to arrest the degeneration. On the other hand, the slight protection from hearing loss at 24 months might reflect protection from aging not related to the ahl gene. The mechanisms of induced degeneration in response to age, acoustic trauma and ototoxic drugs likely involve different cellular pathways.
In conclusion, SOD1 is an extremely important enzyme for cell survival in the cochlea. The spiral ganglion cells appear to be the first cells to be affected by its absence, but the hair cells and cells of the stria vascularis are also affected. ABR thresholds of heterozygous mice were not different from those of wildtype mice, however, there was some loss of ganglion cells by 15 months in the heterozygous mice. Over-expression of SOD1 does not provide protection against age-related hearing loss or neuronal degeneration that is associated with the ahl gene in the B6 mice, but may provide some protection against hearing loss in very old animals.
Acknowledgments
Support: NIH NIDCD, R01 DC03395 (E.M.K.), R01 DC005827 (K.R.J.), NSFC30440080 (Q.Y.Z.), Medical Research Service, Department of Veterans Affairs. We thank Drs. Peter Billings and Allen Ryan for interesting and helpful discussions. We thank Kristin Mueller and Tim Truong for their technical contributions.
References
- Ames BN. Mitochondrial decay, a major cause of aging, can be delayed. J Alzheimers Dis. 2004;6:117–121. doi: 10.3233/jad-2004-6202. [DOI] [PubMed] [Google Scholar]
- Coling DE, Yu KC, Somand D, Satar B, Bai U, Huang TT, Seidman MD, Epstein CJ, Mhatre AN, Lalwani AK. Effect of SOD1 overexpression on age- and noise-related hearing loss. Free Radic Biol Med. 2003;34:873–880. doi: 10.1016/s0891-5849(02)01439-9. [DOI] [PubMed] [Google Scholar]
- Dazert S, Feldman ML, Keithley EM. Cochlear spiral ganglion cell degeneration in wild-caught mice as a function of age. Hearing Res. 1996;100:101–106. doi: 10.1016/0378-5955(96)00100-1. [DOI] [PubMed] [Google Scholar]
- Ehret G. Peripheral anatomy and physiology. In: Willott JF, editor. The Auditory Psychobiology of the Mouse. Charles C. Thomas; Springfield, IL: 1983. pp. 169–200. [Google Scholar]
- Engstrom H. The ultrastructure of the sensory cells of the cochlea. J Laryngol Otol. 1967;81:687–715. [PubMed] [Google Scholar]
- Engstrom B, Engstrom H. Structural and physiological features of the organ of Corti. Audiology. 1972;11:6–28. doi: 10.3109/00206097209072578. [DOI] [PubMed] [Google Scholar]
- Engstrom H, Engstrom B. A survey of the cyto-architecture of the organ of Corti. Acta Otolaryngol. 1977;83:65–70. doi: 10.3109/00016487709128814. [DOI] [PubMed] [Google Scholar]
- Epstein CJ, Avraham KB, Lovett M, Smith S, Elroy-Stein O, Rotman G, Bry C, Groner Y. Transgenic mice with increased Cu/Zn-superoxide dismutase activity: animal model of dosage effects in Down syndrome. Proc Natl Acad Sci USA. 1987;84:8044–8048. doi: 10.1073/pnas.84.22.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis HW, Rivas A, Lehar M, Ryugo DK. Two types of afferent terminals innervate cochlear inner hair cells in C57BL/6J mice. Brain Res. 2004;1016:182–194. doi: 10.1016/j.brainres.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Henry KR, Chole RA. Genotypic differences in behavioral, physiological and anatomical expressions of age-related hearing loss in the laboratory mouse. Audiology. 1980;19:369–383. doi: 10.3109/00206098009070071. [DOI] [PubMed] [Google Scholar]
- Huang TT, Carlson EJ, Gillespie AM, Shi Y, Epstein CJ. Ubiquitous overexpression of CuZn superoxide dismutase does not extend life span in mice. J Gerontol A: Biol Sci Med Sci. 2000;55:B5–B9. doi: 10.1093/gerona/55.1.b5. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/6J mice. Hearing Res. 1997;114:83–92. doi: 10.1016/s0378-5955(97)00155-x. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY, Erway LC. A major gene affecting age-related hearing loss is common to at least ten inbred strains of mice. Genomics. 2000;70:171–180. doi: 10.1006/geno.2000.6377. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Sha SH, Minoda R, Izumikawa M, Kuriyama H, Schacht J, Raphael Y. Antioxidant gene therapy can protect hearing and hair cells from ototoxicity. Mol Ther. 2004;9:173–181. doi: 10.1016/j.ymthe.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Feldman ML. Spiral ganglion cell counts in an age-graded series of rat cochleas. J Comp Neurol. 1979;188:429–442. doi: 10.1002/cne.901880306. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Ryan AF, Feldman ML. Cochlear degeneration in aged rats of four strains. Hearing Res. 1992;59:171–178. doi: 10.1016/0378-5955(92)90113-2. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Canto C, Zheng QY, Fischel-Ghodsian N, Johnson KR. Age-related hearing loss and the ahl locus in mice. Hearing Res. 2004;188:21–28. doi: 10.1016/S0378-5955(03)00365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koay G, Heffner R, Heffner H. Behavioral audiograms of homozygous med(J) mutant mice with sodium channel deficiency and unaffected controls. Hearing Res. 2002;171:111–118. doi: 10.1016/s0378-5955(02)00492-6. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Dionne L, Guo Q, Kumar TR, Lebovitz RM. Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology. 1998;139:4008–4011. doi: 10.1210/endo.139.9.6289. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Reaume AG, Flood DG, Salvi RJ. Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol Aging. 1999a;20:1–8. doi: 10.1016/s0197-4580(99)00018-4. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Burkard RF, Jiang H, Reaume AG, Flood DG, Salvi RJ. Cu/Zn SOD deficiency potentiates hearing loss and cochlear pathology in aged 129, CD-1 mice. J Comp Neurol. 1999b;413:101–112. [PubMed] [Google Scholar]
- McFadden SL, Ding D, Salvi R. Anatomical, metabolic and genetic aspects of age-related hearing loss in mice. Audiology. 2001;40:313–321. [PubMed] [Google Scholar]
- Mikaelian DO. Development and degeneration of hearing in the C57/b16 mouse: relation of electrophysiologic responses from the round window and cochlear nucleus to cochlear anatomy and behavioral responses. Laryngoscope. 1979;89:1–15. doi: 10.1288/00005537-197901000-00001. [DOI] [PubMed] [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet. 2003;35:21–23. doi: 10.1038/ng1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Spicer SS, Schulte BA. Ultrastructural localization of Na, K-ATPase in the gerbil cochlea. J Histochem Cytochem. 1995;43:981–991. doi: 10.1177/43.10.7560888. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, McFadden SL, Ding DL, Flood DG, Reaume AG, Hoffman EK, Scott RW, Wright JS, Putcha GV, Salvi RJ. Targeted deletion of the cytosolic Cu/Zn-superoxide dismutase gene (Sod1) increases susceptibility to noise-induced hearing loss. Audiol Neurootol. 1999;4:237–246. doi: 10.1159/000013847. [DOI] [PubMed] [Google Scholar]
- Pierson MG, Gray BH. Superoxide dismutase activity in the cochlea. Hearing Res. 1982;6:141–151. doi: 10.1016/0378-5955(82)90050-8. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Kostic V, Carlson E, Epstein CJ, Cadet JL. Superoxide dismutase, catalase, and glutathione peroxidase activities in copper/zinc-superoxide dismutase transgenic mice. J Neurochem. 1992;58:1760–1767. doi: 10.1111/j.1471-4159.1992.tb10051.x. [DOI] [PubMed] [Google Scholar]
- Pujol R, Puel JL. Excitotoxicity, synaptic repair, and functional recovery in the mammalian cochlea: a review of recent findings. Ann NY Acad Sci. 1999;884:249–254. doi: 10.1111/j.1749-6632.1999.tb08646.x. [DOI] [PubMed] [Google Scholar]
- Rarey KE, Yao X. Localization of Cu/Zn-SOD and Mn-SOD in the rat cochlea. Acta Otolaryngol. 1996;116:833–835. doi: 10.3109/00016489609137935. [DOI] [PubMed] [Google Scholar]
- Reaume AG, Elliott JL, Hoffman EK, Kowall NW, Ferrante RJ, Siwek DF, Wilcox HM, Flood DG, Beal MF, Brown RH, Jr, Scott RW, Snider WD. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat Genet. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- Ryan AF, Dallos P. Absence of cochlear outer hair cells: Effect on behavioural auditory threshold. Nature. 1975;253:44–46. doi: 10.1038/253044a0. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Ding D, Wang J, Jiang HY. A review of the effects of selective inner hair cell lesions on distortion product otoacoustic emissions, cochlear function and auditory evoked potentials. Noise Health. 2000;2:9–26. [PubMed] [Google Scholar]
- Scheffler IE. Mitochondria. John Wiley & Sons, Inc; New York, NY: 1999. pp. 235–238. [Google Scholar]
- Schwartz PJ, Reaume A, Scott R, Coyle JT. Effects of over- and under-expression of Cu, Zn-superoxide dismutase on the toxicity of glutamate analogs in transgenic mouse striatum. Brain Res. 1998;789:32–39. doi: 10.1016/s0006-8993(97)01469-8. [DOI] [PubMed] [Google Scholar]
- Sha SH, Zajic G, Epstein CJ, Schacht J. Overexpression of copper/zinc-superoxide dismutase protects from kanamycin-induced hearing loss. Audiol Neurootol. 2001;6:117–123. doi: 10.1159/000046818. [DOI] [PubMed] [Google Scholar]
- Spicer SS, Schulte BA. Spiral ligament pathology in quiet-aged gerbils. Hearing Res. 2002;172:172–185. doi: 10.1016/s0378-5955(02)00581-6. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Differentiation of cochlear afferent neurons. Acta Otolaryngol. 1981;91:451–456. doi: 10.3109/00016488109138527. [DOI] [PubMed] [Google Scholar]
- Staecker H, Zheng QY, Van De Water TR. Oxidative stress in aging in the C57B16/J mouse cochlea. Acta Otolaryngol. 2001;121:666–672. doi: 10.1080/00016480152583593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugar JO, Engstrom H, Stahle J. Stria vascularis. Acta Otolaryngol, Suppl. 1972;301:61–77. doi: 10.3109/00016487209122690. [DOI] [PubMed] [Google Scholar]
- Willott JF. Effects of aging, hearing loss, and anatomical location on thresholds of inferior colliculus neurons in C57BL/6 and CBA mice. J Neurophysiol. 1986;56:391–408. doi: 10.1152/jn.1986.56.2.391. [DOI] [PubMed] [Google Scholar]
- Yao X, Rarey KE. Detection and regulation of Cu/Zn-SOD and Mn-SOD in rat cochlear tissues. Hearing Res. 1996;96:199–203. doi: 10.1016/0378-5955(96)00050-0. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Collan Y, Palva T. Ultrastructural features of spiral ganglion cells. Arch Oto-Rhino-Laryngol. 1978;104:84–88. doi: 10.1001/archotol.1978.00790020026007. [DOI] [PubMed] [Google Scholar]
- Zhang X, Han D, Ding D, Dai P, Yang W, Jiang S, Salvi RJ. Cochlear mitochondrial DNA3867bp deletion in aged mice. Chin Med J (Engl) 2002;115:1390–1393. [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hearing Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]