Abstract
Neuronal αβ heteromeric and α7 homomeric nicotinic acetylcholine receptors (nAChRs) were compared in 4- and 27-month rabbits selected for learning proficiency. Sixty 4- and 60 27-month rabbits received the α7 nAChR agonist (MEM-3389), galantamine, or vehicle during training in trace eyeblink classical conditioning. Brain tissue from the best and worst young and older learners was analyzed with radioligand binding. Vehicle-treated 4- and 27-month good learners had higher αβ heteromeric nAChR binding in hippocampus and temporal–parietal cortex than poor learners, and this result was replicated in both age groups of rabbits treated with galantamine. Results indicate that anatomically more numerous nAChRs or functional activation of a greater number of nAChRs may characterize animals demonstrating optimal learning. During normal aging the expression of high-affinity binding sites declines. Age-related changes in the expression of hippocampal αβ heteromeric nAChRs may account for some of the documented age-related impairment in learning. However, individual differences in αβ heteromeric nAChRs also exist early in life, as better learning in 4-month rabbits was associated with significantly higher binding.
Keywords: Radioligand binding, Hippocampus, Temporal–parietal cortex, Trace eyeblink classical conditioning, Age differences
1. Introduction
Individual differences in learning and memory have been reported for several mammalian species and a variety of behavioral paradigms (e.g., Matzel and Gandhi, 2000; Wehner et al., 2001). During normal aging this individual variability increases, with some older organisms showing preserved learning and memory and others showing impairment (Kempermann and Gage, 2002; Lee et al., 2005; Olton et al., 1991; Van der Zee et al., 1997). Associated with preserved learning and memory in normal older organisms has been electrophysiological functioning comparable to young adult levels (McEchron et al., 2001; Schoenbaum et al., 2006). For example, excitability of CA1 neurons studied 24 h after training in hippocampus-dependent trace eyeblink conditioning differentiated good and poor learning in aged rabbits. Comparisons were made between young rabbits and aged rabbits that reached a behavioral criterion of 60% conditioned responses (CRs) called “learning-intact,” rabbits trained for 30 days that never demonstrated more than 30% CRs per session, called “failed to learn,” and naïve aging rabbits (McEchron et al., 2001). In general, aged rabbits required significantly more training trials to reach learning criterion than did young rabbits. However, hippocampal CA1 neurons from aged learning intact animals had significantly reduced post-burst afterhyperpolarizations and reduced spike frequency adaptation comparable to young rabbits. Hippocampal CA1 neurons from control groups of naïve and aging rabbits that failed to learn had significantly elevated post-burst afterhyperpolarizations and increased spike frequency adaptation. The data suggest that postsynaptic excitability of CA1 neurons is correlated with learning the hippocampus-dependent trace eyeblink conditioning task in both young and aged rabbits.
Among the mechanisms that support hippocampal electrophysiological function in adult organisms are neurotransmitter and receptor systems (Zhang et al., 2007). Extensive evidence indicates that nicotinic acetylcholine receptors (nAChRs) act as neuromodulators in communicative processes in the brain (Lindstrom, 1996) and that nAChRs are involved in cognitive and memory functions (Changeux et al., 1998; Dani and Bertrand, 2007). nAChRs in the central nervous system are composed of five subunits arranged around a ligand-gated excitatory ion channel. The most abundant nAChR subtypes are those that participate in high-affinity agonist binding associated with α4 and β2 subunits, and those sensitive to blockade by α-bungarotoxin and containing α7 subunits. In normal aging, it is the high-affinity agonist binding nAChR subunits that show the greatest deficits in human (e.g., Giacobini, 1992) and rodent brain (Araujo et al., 1990; Zhang et al., 1990). The loss in these sites is coincident with changes of expression of subunit proteins. Data in humans, rabbits, and rodents show consistency in the age-related loss of expression of αβ heteromeric nAChRs (Birtsch et al., 1997; Gahring et al., 2005; Li et al., in press). The pattern of age-related changes in neurons containing α7 homomeric nAChRs demonstrates age-related loss in a few brain sites but stability in most regions (Court et al., 1997; Falk et al., 2003; Gahring et al., 2005; Nordberg and Winblad, 1986).
We focused on the most abundant nAChR subtypes, those that participate in high-affinity agonist binding associated with α4 and β2 subunits and those containing α7 subunits. We used radioligand binding of tissue from hippocampus and temporal–parietal cortex to determine whether higher binding levels accompanied superior learning. Trace eyeblink classical conditioning was used to assess learning. This is a relatively difficult paradigm for rabbits in which a conditioned stimulus (CS) such as a tone is presented and then turned off before the unconditioned stimulus (US; corneal airpuff) is turned on. A learned or CR occurs when the rabbit produces an eyeblink before the onset of the US. We used trace procedures with sufficiently long trace intervals (400–500 ms) to insure that the task would be hippocampus-dependent for rabbits (Moyer et al., 1990; Rose et al., 2007).
One question of interest was whether cognition-enhancing drugs would improve learning in the difficult trace procedure in young and older rabbits. We used two drugs with different mechanisms of action as cognition enhancers. Galantamine is among the most effective cognition enhancers tested in the eyeblink classical conditioning model. A dose of 3.0 mg/kg galantamine ameliorates learning impairment in older rabbits in both the trace (Simon et al., 2004; Weible et al., 2004) and delay eyeblink classical conditioning paradigms (Woodruff-Pak et al., 2001, 2003). Galantamine has mechanisms of action that include both mild acetyl-cholinesterase inhibition and allosteric potentiating effects at nAChRs (Popa et al., 2006). AR-R-17779 (nowcalled MEM-3389) is an agonist shown to be selective to α7 homomeric nAChRs in frog oocytes (Papke et al., 2004). We anticipated that MEM-3389 would have efficacy in this model as a partial α7 agonist did improve eyeblink conditioning in 25-month rabbits (Woodruff-Pak et al., 1994). A dose–response study using the 750 ms delay eyeblink classical conditioning paradigm demonstrated that 1.0 mg/kg MEM-3389 significantly improved learning over vehicle (Li et al., in press).
A second question of interest was whether individual differences in nAChR binding levels would be associated with variation in learning rate in young and older organisms. Hippocampal and temporal–parietal cortical tissue from the best (“good learners;” n = 36) and worst (“poor learners;” n = 36) vehicle- and drug-treated performers was analyzed with radioligand binding ([3H]Epibatidine, [3H]Methyllycaconitine), and results from good and poor learners were compared.
A third question was whether cognition-enhancing drugs would increase nAChR binding levels in good learners above the levels in vehicle-treated good learners. A related issue was whether there would be differences in nAChR binding levels between drug-treated poor learners and vehicle-treated poor learners. We addressed these questions by comparing receptor binding in hippocampus and temporal–parietal cortex of young and older vehicle-treated good and poor learning rabbits to binding at these sites in young and older good and poor learning rabbits treated with MEM-3389 or galantamine.
2. Methods
2.1. Study population
A total of 120 female New Zealand white specific pathogen free (SPF) rabbits were tested. Sixty rabbits were retired breeders of a mean age of 27.4 months (S.D. = 2.5) and a mean weight of 4.1 kg (S.D. = 0.4) and 60 rabbits were young adults of a mean age of 4.0 months (S.D. = 0.0) and a mean weight of 2.8 kg (S.D. = 0.3). All rabbits were purchased from Covance (Denver, PA). They were individually housed in stainless steel cages in temperature and humidity-controlled rooms in an Association for Assessment and Accreditation of Laboratory Animal Care International-(AAALAC-) approved animal facility. They had ad lib access to food and water. The light/dark cycle was 12/12-h. The Institutional Animal Care and Use Committee (IACUC) at Temple University approved research procedures used in this study. This research was carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health.
2.2. Behavioral testing
Over the course of 7 days prior to training, the rabbits were gradually familiarized and adapted to Plexiglas restrainers for 30 min per day. Familiarization training took place in rabbits’ individual cages during the first 5 days. At the end of each familiarization session rabbits were rewarded with a treat formulated for rabbits (Kaytee Yogurt Dips). The last 2 days of familiarization took place outside the individual cages, and rabbits were fully restrained. On the seventh day, a local ophthalmic anesthetic (proparacaine hydrochloride) was applied to the left eye so that a 6–0 nylon suture loop could be placed in the temporal margin of the nictitating membrane (NM).
Rabbits were trained for 10 daily sessions in a 600 or 750 ms trace eyeblink classical conditioning paradigm with a 400 or 500 ms trace interval, respectively. There were 10 rabbits per age/drug/trace paradigm group. Thirty minutes prior to each of the training sessions, rabbits were injected subcutaneously with 1.0 ml/kg of one of three substances: sterile saline vehicle, an acetylcholinesterase inhibitor and allosteric nicotinic modulator (3.0 mg/kg galantamine), or a selective α7 nAChR agonist (1.0 mg/kg MEM-3389, formerly called AR-R-17779).
The conditioning apparatus consisted of eight separate sound-attenuating chambers, permitting up to eight rabbits to be trained simultaneously. A speaker mounted to the wall of each chamber delivered a pure tone that was used as the CS. The headpiece, affixed behind the rabbit’s ears and under its muzzle, held a plastic tube to deliver 3 psi corneal-directed air puff unconditioned stimulus (US) and a minitorque potentiometer (San Diego Instruments, San Diego, CA) to measure the rabbit’s NM/eyeblink response. Elastic eyelid retractors kept the rabbit’s eye open. The potentiometer was secured to the NM via a lever and the nylon suture loop. Analog output from the potentiometer was digitized, stored and analyzed using a PC (Chen and Steinmetz, 1998). This system also controlled the timing and presentation of the stimuli. For all experiments, the inter-trial interval was randomized and ranged between 20 and 30 s. A single session lasted approximately 45 min and consisted of 90 paired CS–US trials.
The eyeblink classical conditioning paradigms were 600 and 750 ms trace. For the 600 ms trace paradigm, a 1 kHz, 85 dB SPL tone CS sounded for 200 ms followed by a 400 ms blank period ended by a 100-ms corneal air puff US commenced 600 ms after the onset of the CS. For the 750 ms trace paradigm, a 1 kHz, 85 dB SPL tone CS sounded for 250 ms followed by a 500 ms blank period ended by a 100-ms corneal air puff US that onset 750 ms after CS onset. The rabbits received 10 training sessions (5 days per week for 2 weeks).
Changes in the position of the NM detected by the potentiometer were processed and stored in 3-ms bins by the computer. The program recorded a response when the NM moved a minimum of 0.5 mm. A CR was recorded if the response occurred between 25 and 600 ms or 25 and 750 ms after the onset of the CS in the 600 and 750 ms trace paradigms, respectively. An unconditioned response (UR) alone was recorded if the response took place more than 600 or 750 ms after the onset of the CS in the 600 and 750 ms trace paradigms, respectively. A trial was eliminated if NM activity crossed the response threshold within 100 ms prior to the onset of the CS. The dependent measure, trials to learning criterion was assessed as the number of trials presented before a rabbit produces 8 CRs in 9 consecutive trials in a session of 40% CRs or more.
2.3. Drugs and drug administration
The drug initially called AR-R-17779 was synthesized and supplied by Memory Pharmaceuticals as MEM-3389. Dose–response testing with 4- and 27-month rabbits in our laboratory indicated that a dose of 1.0 mg/kg improved conditioning significantly in both age groups in the 750 ms delay eyeblink conditioning paradigm. Galantamine hydrobromide was supplied by Janssen Pharmaceutica, N.V. and Ortho-McNeil Neurologics. A previous dose–response study in our laboratory identified of 3.0 mg/kg as optimal for improving eyeblink conditioning in rabbits (Woodruff-Pak and Santos, 2000). Drugs were dissolved in sterile saline and administered subcutaneously (sc) at 1 ml/kg 30 min before behavioral testing began. There were a total of 10 injections before the 10 training sessions, and an 11th injection was administered 30 min before euthanasia so that receptor binding assays would be carried out on tissue at drug levels comparable to training
2.4. Nicotinic acetylcholine receptor binding assays
Three days after behavioral testing, each rabbit was injected with the drug it had received for the previous 2 weeks: 3.0 mg/kg galantamine, 1.0 mg/kg MEM-3389, or 1.0 ml/kg sterile saline. Thirty minutes after injection, the rabbit was euthanized with an overdose of pentobarbital and decapitated. The brain was rapidly removed, dissected, and stored at −80 °C. For binding assays for this study, brain tissues from hippocampus and temporal–parietal cortex were analyzed. Behavioral data, including trials to learning criterion and percentage of CRs over the 10 training sessions, were used to select the top and bottom 3 performers from each of the age, drug treatment, and trace paradigm groups of 10 rabbits (n = 72 rabbits total) for behavioral and radioligand binding analyses. A comparison of mean trials to learning criterion for the 36 good learners (518 trials, S.D. = 285) and the 36 poor learners (999 trials, S.D. = 173) was statistically significant (t[70] = 8.65; p < 0.0001). Tissue was pooled from the 600 and 750 trace groups for the top 3 and bottom 3 performers of each age and drug treatment condition to provide sufficient tissue for receptor binding studies. Thus, each membrane assay included hippocampal or temporal–parietal cortical tissue from 6 rabbit brains.
2.5. Membrane preparation for radioligand binding assays
Membrane preparations were performed according to Davies et al. (1999). Ice cold (10%, w:v) sucrose buffer (0.32M sucrose, 1mM EDTA, 0.1mM phenylmethyl sulfonyl fluoride (PMSF), 0.01% (w:v) sodium azide, pH 7.4) was added to weighed crude tissue. The tissue was homogenized in the sucrose buffer using a glass–Teflon homogenizer (10 strokes at 600 rpm). The tissue homogenate was centrifuged at 20,000 × g for 30 min at 4 °C. The pellet was washed twice by resuspension in 10% (w:v) original weight phosphate buffer (50mM phosphate, 1mM EDTA, 0.1mM PMSF, 0.01% (w:v) sodium azide, pH 7.4). The tissue was homogenized with a glass–Teflon homogenizer and centrifuged again at 20,000 × g for 30 min at 4 °C. The final pellet was resuspended in phosphate buffer to a concentration of ~6 ml/g original weight and homogenized with a glass–Teflon homogenizer. Protein content was determined using Pierce BCA Protein Assay Kit (Pierce Chemical Company, Rockford, IL). The prepared membrane was stored in 1.0 ml aliquots at −80 °C.
During membrane preparation prior to ligand binding assay, the brain samples were homogenized, centrifuged and resuspended three times with large volumes of buffer, which should be sufficient for removing the drugs administered to rabbits during training. We confirmed that there is a negligible amount of cognition-enhancing drug remaining because the Kd value of the radioligand in each group after drug treatment was not significantly changed from the vehicle-treated control.
2.6. Radioligand binding assay
The binding of [3H]-labeled ligand to membranes of rabbit hippocampus and temporal–parietal cortex was investigated using [3H]Epibatidine ([3H]Epi) and [3H]Methyllycaconitine ([3H]MLA). The assay was performed according to Davies et al. (1999) and Wickramaratna et al. (2004). For [3H]Epi, membranes were diluted in phosphate buffer to give a protein content of 0.5 mg in a final assay volume of 0.5 ml. Total binding of [3H]Epi was determined using six concentrations from 0.03 to 1.0 nM. Radioligand concentrations were prepared by serial dilution. Non-specific binding of [3H]Epi was defined by the addition of 100 µM nicotine. For [3H]MLA, membranes were diluted in phosphate buffer supplemented with 0.1% (w:v) BSA to a protein content of 0.5 mg in a final assay volume of 0.5 ml. Total binding of [3H]MLA was determined using six concentrations from 0.3 to 10 nM. Non-specific binding of [3H]MLA was obtained by the addition of 300µM nicotine or 100µM MLA (similar non-specific binding).
Total binding assay volumes were 0.5 ml, consisting of 0.3 ml of diluted membrane, 0.1 ml phosphate buffer for total binding or 0.1 ml of cold ligand for non-specific binding, and 0.1 ml of radioligand. Samples were run in duplicates for both total binding and non-specific binding. After 90 min incubation at 0 °C, bound and free radioligands were separated by filtration with GF/B filters that had been soaked in a solution of 0.2% polyethylenimine, 0.1 mg BSA/ml, and 50mM Tris for 60 min. Filters were washed three times with ice cold 1 × PBS. Radioactivity on filters was determined by liquid scintillation counting.
Specific binding was determined by the difference between non-specific binding and total binding. Maximum binding (Bmax) and dissociation constant (Kd), expressed in femtomole per milligram protein (fmol/mg protein) and nanomolar (nM), respectively, were determined by non-linear regression fitting to a single-site ligand binding model found in the software Prism 3.0 (GraphPad Software, Inc., San Diego, CA).
2.7. Statistical analyses
Statistical Package for Social Sciences (SPSS) version 14 was used to carry out univariate and repeated measures analysis of variance (ANOVA) of conditioning data. There were three independent repeats for each receptor binding assay. Age and drug comparisons of Bmax and Kd were made with one-way ANOVA for each brain site for [3H]Epi and [3H]MLA using Prism 3.0 (GraphPad Software, Inc., San Diego, CA).
3. Results
Data were analyzed progressively. In Section 3.1 on “The effects of age and drug on trace eyeblink conditioning” the aim was to determine whether there were significant differences between the 600 and 750 ms trace eyeblink classical conditioning paradigms and to examine age and drug effects. The total sample size for these analyses was 120 rabbits. In the absence of differences between the 600 and 750 ms trace paradigms, we were able to collapse eyeblink conditioning data from these two trace intervals. Collapsing the trace interval doubled the number of good and poor learners in each age and drug treatment group. The main analyses presented in Section 3.2 on “Effects of age, drug, and learning proficiency” involve three variables: age, drug, and learning ability. Comparisons were made between 4- and 27-month rabbits treated with vehicle, 3.0 mg/kg galantamine, or 1.0 mg/kg MEM-3389 that were selected as the best and worst learners in their age and drug treatment group. The total sample size for these analyses was 72 rabbits, as radioligand binding was not carried out on the brains of intermediate learning rabbits. Acquisition of trace eyeblink conditioning and expression of αβ heteromeric and α7 nAChRs in hippocampus and temporal–parietal cortex were the dependent measures.
3.1. Effects of age and drug on trace eyeblink conditioning
Behavioral data were collected in trace classical conditioning paradigms at two different CS–US intervals (600 and 750 ms) that included two different trace intervals (400 and 500 ms, respectively). Of the 60 rabbits tested in the 600 ms trace paradigm, 30 were 4 months of age and 30 were a mean age of 27 months. Groups of 10 young and 10 older rabbits were treated with vehicle, 3.0 mg/kg galantamine, or 1.0 mg/kg of MEM-3389. The 30 young and 30 older rabbits tested in the 750 ms trace paradigm were dosed in the same manner. Performance of the 60 rabbits tested in the 600 ms trace paradigm was compared to the performance of the 60 rabbits tested in the 750 ms trace paradigm. A multivariate analysis of variance (MANOVA) was used with the dependent measures of mean total percentage of CRs and trials to a learning criterion of 8 CRs in 9 consecutive trials (in a session of 40% CRs or more). The effect of paradigm was not significant for either mean total percentage of CRs, F(1, 118) = 2.88; p = 0.093, or trials to criterion, F(1, 118) = 2.53; p = 0.115. Data were collapsed over trace paradigm for further analyses of age and drug effects on eyeblink classical conditioning so that the trace conditioning data would match the individual rabbit tissue pooled for radioligand binding assays.
Results indicated large age differences in acquisition, and cognition-enhancing drugs facilitated acquisition of trace eyeblink conditioning in 4- but not 27-month rabbits. A 2 (age; 4- and 27-month) by 3 (drug; vehicle, 3.0 mg/kg galantamine, 1.0 mg/kg MEM-3389) by 10 (training sessions) repeated measures analysis of variance (ANOVA)was carried out in the entire sample of 120 rabbits using the dependent measure, percentage of CRs. The main effects of age, F(1, 114) = 20.45; p < 0.001, drug, F(2, 114) = 4.67; p = 0.011, and training sessions, F(9, 1026) = 58.84; p < 0.001, were statistically significant. (Fig. 1A and B). The interaction between training sessions and age, F(9, 1026) = 2.96; p = 0.002, was the only significant interaction effect. Post hoc analyses of the significant effect of drug using the Tukey B test indicated that percentage of CRs was significantly higher in both galantamine- and MEM-3389 treated rabbits than in vehicle-treated rabbits (p < 0.05).
Fig. 1.
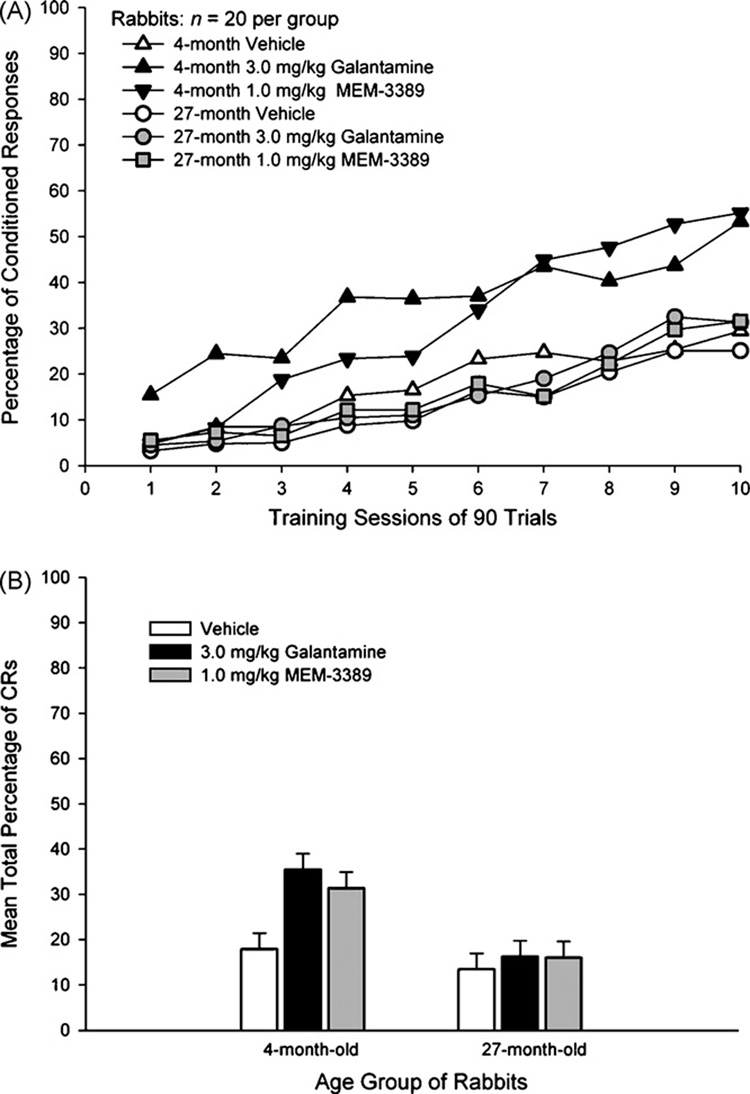
(A) Percentage of CRs in 10 90-trial training sessions of combined 600 and 750 ms trace eyeblink classical conditioning in 120 4- and 27-month rabbits treated with 3.0 mg/kg galantamine, 1.0 mg/kg MEM-3389, or vehicle. The effects of age, drug, and training session were statistically significant. (B) Data from A above averaged over the 10 training sessions to show the significant age and drug effects.
Using the dependent measure trials to learning criterion, a 2 (age) by 3 (drug) ANOVA was carried out. With trials to learning criterion, the higher the score, the worse the learning. Rabbits that learned more slowly had higher trials to learning criterion scores. The main effects of age, F(1, 114) = 20.96; p < 0.001, and drug, F(2, 114) = 4.07; p = 0.020, were significant (Fig. 2). The age by drug interaction effect was also significant, F(2, 114) = 4.52; p = 0.013. Trials to learning criterion were significantly lower in galantamine- and MEM-3389-treated 4-month rabbits than in vehicle-treated 4-month rabbits, but the difference in trials to criterion between drug-and vehicle-treated 27-month rabbits was not statistically significant.
Fig. 2.
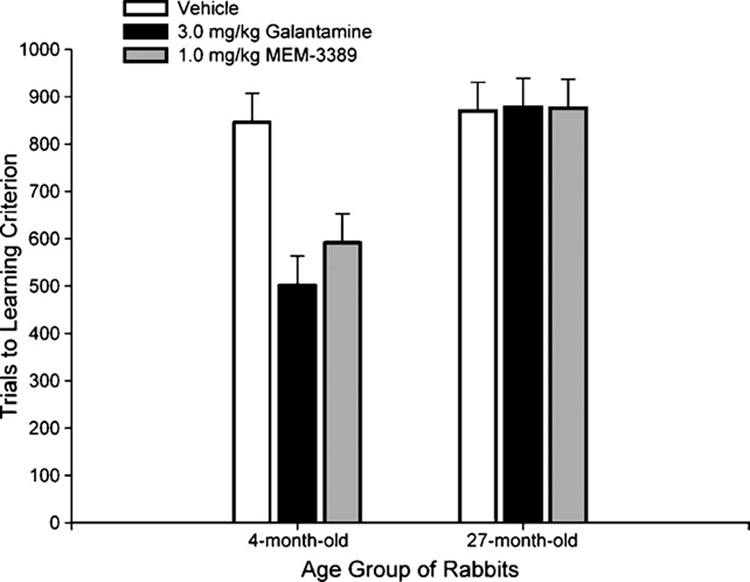
Trials to a learning criterion of 8 CRs in 9 consecutive trials (in a session with a minimum of 40% CRs) in 120 rabbits tested in the 600 or 750 ms trace eyeblink classical conditioning paradigm treated with 3.0 mg/kg galantamine, 1.0 mg/kg MEM-3389, or vehicle. Rabbits in the 27-month age group took significantly more trials to attain learning criterion than 4-month rabbits, and galantamine and MEM-3389 significantly reduced trials to criterion over vehicle in 4-month but not in 27-month rabbits.
3.2. Effects of age, drug, and learning proficiency
From each of the age, drug treatment, and trace paradigm groups of 10 rabbits, the top 3 performers were classified as good learners, and the bottom 3 performers were classified as poor learners. Analyses were carried out to compare eyeblink conditioning in the 4- and 27-month rabbits selected to be the best or worst learners. A 2 (age) by 3 (drug) by 2 (good versus poor learner) ANOVA using the mean total (over 10 training sessions) percentage of CRs as the dependent measure revealed statistically significant main effects for age, F(1, 60) = 25.93; p < 0.001, drug, F(2, 60) = 5.47; p = 0.007, and learner, F(1, 60) = 151.63; p < 0.001 (Fig. 3). The interaction effect between age and drug was also significant, F(2, 60) = 4.02; p = 0.023, but none of the other interaction effects attained statistical significance. Four-month rabbits learned significantly better than 27-month rabbits, and regardless of age, good learners acquired significantly more CRs than poor learners. Post hoc analysis of the significant drug effect using the Tukey B test indicated that treatment with galantamine and MEM-3389 resulted in better learning than treatment with vehicle, and the two drugs were equally effective. The significant interaction effect between age and drug occurred because the cognition-enhancing drugs were more effective in 4- than in 27-month rabbits.
Fig. 3.
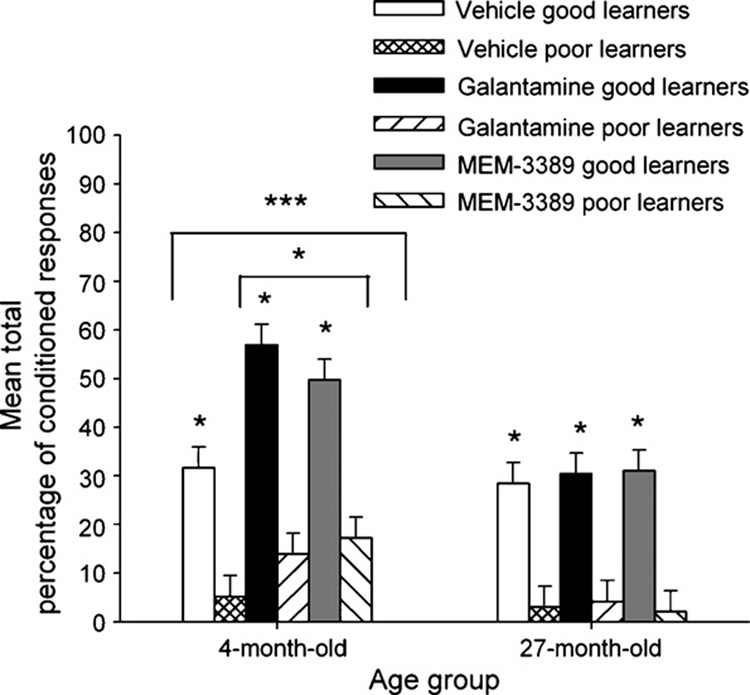
Mean total percentage of CRs in rabbits selected as the best (“good learner”) and worst (“poor learner”) performers in 600 or 750 ms trace eyeblink classical conditioning. A total of 18 4-month old and 18 27-month good learner rabbits were compared to 18 each of 4- and 27-month poor learner rabbits treated with vehicle, 3.0 mg/kg galantamine, or 1.0 mg/kg MEM-3389. There were highly significant differences between good and poor learners as well as significant age and drug effects. Single asterisks over each good learner group indicate statistically significant differences between good and poor learners. The bar with an asterisk over galantamine and MEM-3389 groups of young rabbits indicates statistically significant differences between vehicle- and drug-treated good learners. Asterisks over the bar over all young rabbits indicate significant differences between 4- and 27-month rabbits. *p < 0.05; ***p < 0.001.
Associated with the dramatic difference in learning between good and poor learners were statistically significant differences in αβ heteromeric nAChR expression. Comparisons of the vehicle-treated rabbits indicated that both 4- and 27-month good learners expressed higher levels of αβ heteromeric nAChRs than poor learners in hippocampus (Fig. 4A) and in temporal–parietal cortex (Fig. 4B). There were significantly higher Bmax values for [3H]Epibatidine ([3H]Epi) binding of αβ heteromeric nAChRs in the hippocampus of good than of poor learners, F(3, 11) = 34.46; p = 0.0004. Post hoc analyses using Tukey’s Multiple Comparison Test indicated that αβ heteromeric nAChR expression in hippocampus was greater in 4- and 27-month good learners (p = 0.001 and 0.001, respectively). There were significantly higher Bmax values for [3H]Epi binding of αβ heteromeric nAChRs in temporal–parietal cortex in good than in poor learners, F(3, 11) = 6.958; p = 0.022. Post hoc analyses indicated that αβ heteromeric nAChR expression in temporal–parietal cortex was greater in 4- and 27-month good learners (p = 0.05 and 0.01, respectively).
Fig. 4.
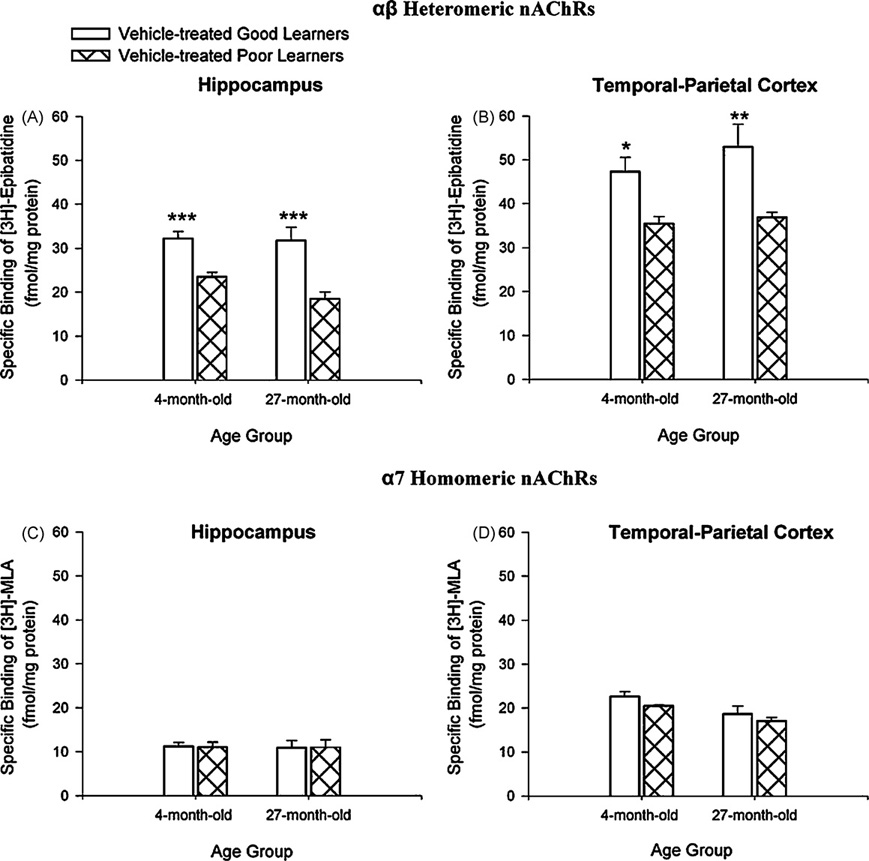
(A) In vehicle-treated rabbits, there was significantly higher expression of αβ heteromeric nicotinic acetylcholine receptors (nAChRs) in hippocampus in 4- and 27-month good learners. (B) Four- and 27-month good learner rabbits also had significantly higher expression of αβ heteromeric nAChRs in temporal–parietal cortex. Expression of α7 homomeric nAChRs was not different between vehicle-treated good and poor learners in (C) hippocampus or (D) temporal–parietal cortex. *p < 0.05; **p < 0.01; ***p < 0.001.
Comparisons of the α7 homomeric nAChRs binding in vehicle-treated rabbits indicated that 4- and 27-month good learners did not express higher levels than poor learners in hippocampus (Fig. 4C) or in temporal–parietal cortex (Fig. 4D). In both 4- and 27-month vehicle-treated rabbits, Bmax values for [3H]Methyllycaconitine ([3H]MLA) binding of α7 homomeric nAChRs in hippocampus and temporal–parietal cortex were not different between vehicle-treated good and poor learners.
Regardless of age, vehicle-treated good learner rabbits had higher levels of αβ heteromeric nAChRs in the hippocampus than vehicle-treated poor learner rabbits. Vehicle-treated good learner rabbits in both age groups also had higher levels of αβ heteromeric nAChRs in temporal–parietal cortex that did poor learner rabbits. Hippocampal and temporal–parietal α7 homomeric nAChRs were not different in vehicle-treated 4- or 27-month good and poor learners.
Administration of the cognition-enhancing drugs galantamine and MEM-3389 was associated with significant enhancement of learning in 4-month rabbits (Fig. 1 and Fig 2). Good learner rabbits treated with these drugs had significantly higher expression of αβ heteromeric nAChRs in most cases in comparison to poor learner rabbits (Fig. 5A and B). In hippocampus, there was significantly greater upregulation of αβ heteromeric nAChRs in good than in poor learners (F(7, 23) = 60.86; p < 0.0001). Post hoc analyses indicated that αβ heteromeric nAChR expression in hippocampus was greater in 4- and 27-month good learners treated with galantamine (p = 0.001 and 0.001, respectively) and 4-month rabbits treated with MEM-3389 (p < 0.01) (Fig. 5A). In temporal–parietal cortex, therewas significantly greater upregulation of αβ heteromeric nAChRs in good than in poor learners (F(7, 23) = 10.93; p < 0.0001). Post hoc analyses indicated that αβ heteromeric nAChR expression in temporal–parietal cortex was greater in 27-month good learners treated with galantamine and MEM-3389 (p < 0.05 and 0.05, respectively) (Fig. 5B). For 4-month good learner rabbits treated with galantamine or MEM-3389, the highest αβ heteromeric nAChR expression was in hippocampus, whereas for 27-month good learning rabbits treated with galantamine or MEM-3389, there was higher expression in both hippocampus and temporal–parietal cortex.
Fig. 5.
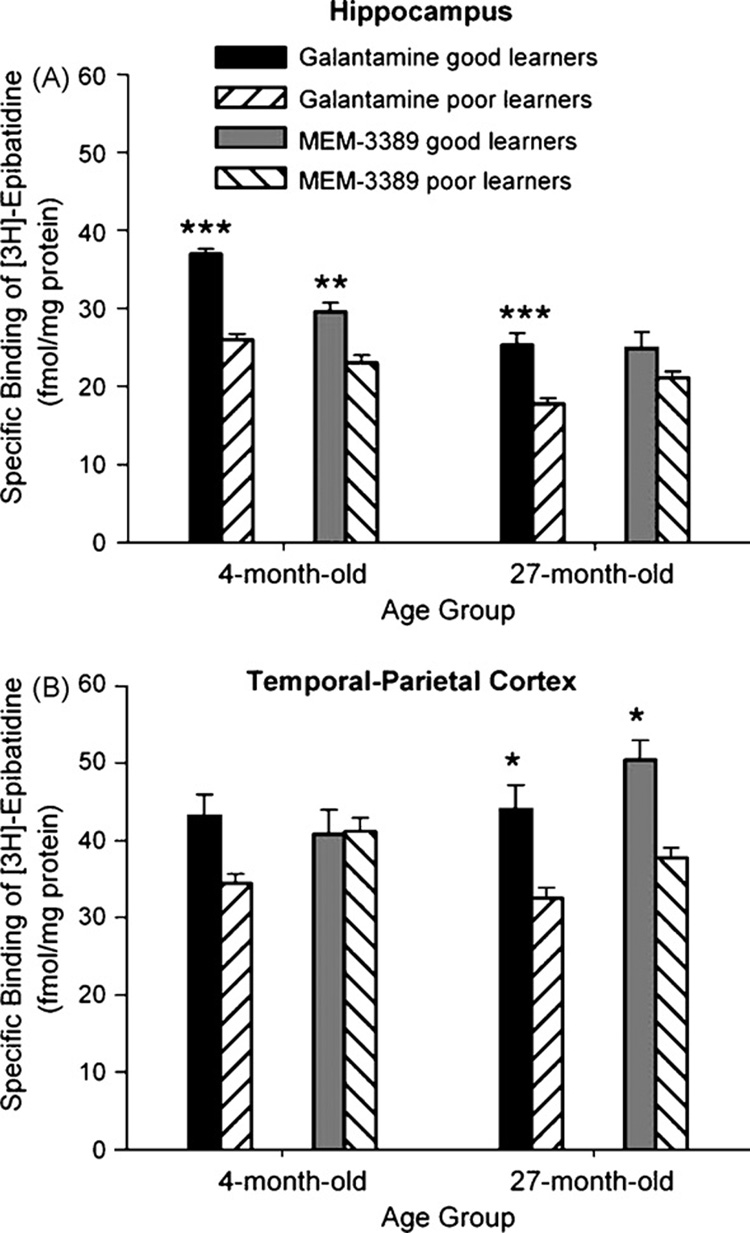
(A) In rabbits treated with galantamine or MEM-3389, there was significantly higher expression of αβ heteromeric nicotinic acetylcholine receptors (nAChRs) in hippocampus in 4- and 27-month good learners. (B) In temporal–parietal cortex, 27-month good learner rabbits treated with galantamine or MEM-3389 had significantly higher expression of αβ heteromeric nAChRs. *p < 0.05; **p < 0.01; ***p < 0.001.
There were few differences between α7 homomeric nAChRs in good and poor learners treated with MEM-3389 or galantamine just as reported for good and poor learners treated with vehicle (shown in Fig. 4). In most groups, learning proficiency was not associated with levels of α7 homomeric nAChRs. The only exception was in the hippocampus in which there was significantly greater expression of α7 homomeric nAChRs in good than in poor learners, F(7, 23) = 4.846; p = 0.0059. Post hoc analyses indicated that α7 homomeric nAChR expression in hippocampus was significantly greater in 27-month good learners treated with MEM-3389 (p < 0.05) (Fig. 6A). In temporal–parietal cortex, overall expression α7 homomeric nAChRs in good versus poor learners was not different, F(7, 23) = 2.339; p = 0.0834 (Fig. 6B).
Fig. 6.
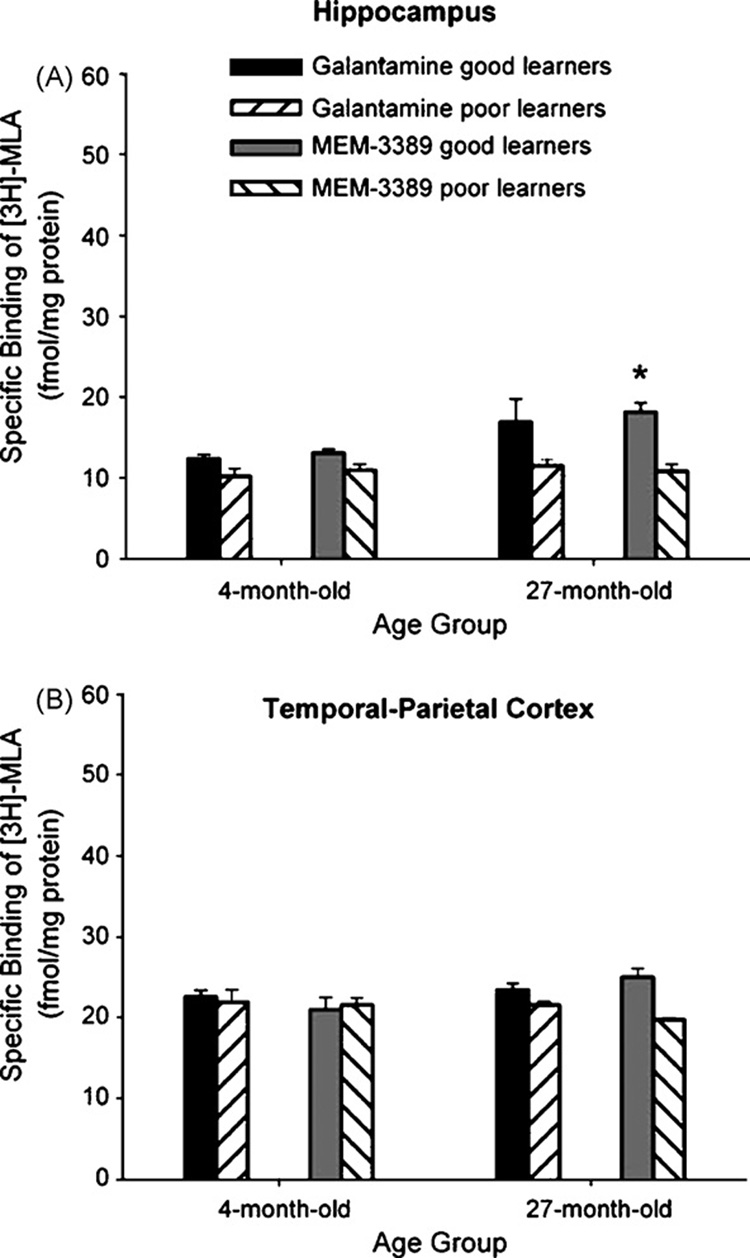
(A) In 27-month good learner rabbits treated with MEM-3389, there was significantly higher expression of α7 homomeric nicotinic acetylcholine receptors (nAChRs) in hippocampus. (B) There were no significant differences in temporal–parietal cortex between good and poor learners in α7 homomeric nAChRs in 4- or 27-month rabbits. *p < 0.05.
Previous analyses examined receptor binding in vehicle-and drug-treated animals separately. Here we compared nAChR expression in drug-treated rabbits to vehicle-treated rabbits. Bmax values for [3H]Epi binding of αβ heteromeric nAChRs and for [3H]MLA binding of α7 homomeric nAChRs in hippocampus and temporal–parietal cortex were compared between 4- and 27-month vehicle- and drug-treated rabbits. In hippocampus, there was significantly greater upregulation of αβ heteromeric nAChRs, F(11, 35) = 40.04; p < 0.0001. Post hoc analyses indicated that αβ heteromeric nAChR expression in hippocampus was greater in young galantamine-treated good learners than in vehicle treated good learners (p = 0.05) (Fig. 7A). However, in the case of older good learners, vehicle-treated good learner rabbits had higher αβ heteromeric nAChR expression in the hippocampus than older good learners treated with galantamine (p < 0.01) or MEM-3389 (p < 0.01) (Fig. 7A). There was significantly greater upregulation of α7 homomeric nAChRs in hippocampus, F(11, 35) = 3.621; p = 0.0049. Post hoc analyses indicated that α7 homomeric nAChR expression in hippocampus was greater in 27-month MEM-3389-treated good learners than in vehicle-treated good learners (p < 0.05) (Fig. 4C). In temporal–parietal cortex, increased expression of αβ heteromeric or α7 nAChRs in 4- or 27-month good learners was not different from increased nAChR expression in vehicle-treated rabbits (Fig. 7B and D).
Fig. 7.
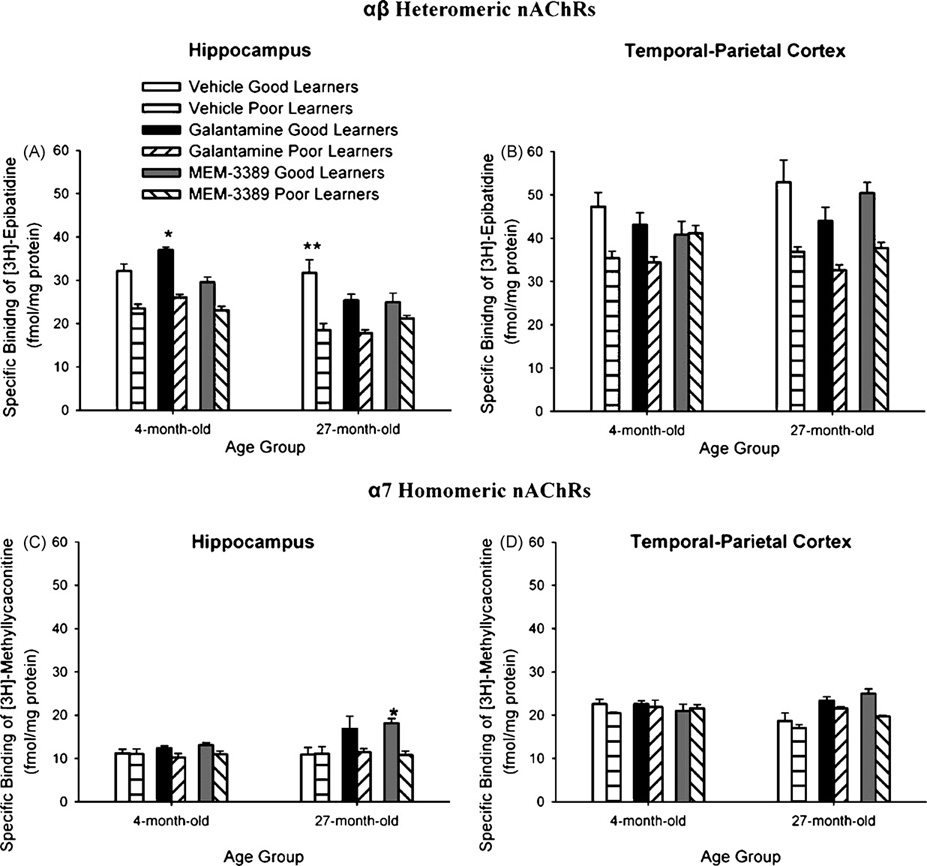
Comparison of levels of αβ heteromeric nicotinic acetylcholine receptors (nAChRs) in hippocampus (A) and temporal–parietal cortex (B) and of α7 homomeric nAChRs in hippocampus (C) and temporal–parietal cortex (D) in 4- and 27-month vehicle- and drug-treated good and poor learner rabbits. In 4-month good learner rabbits, there were significantly higher levels of αβ heteromeric nAChR expression in galantamine-treated than in vehicle-treated rabbits, and in 27-month good learner rabbits, there were significantly higher levels of αβ heteromeric nAChR expression in vehicle-treated rabbits (A). In 27-month good learner rabbits, there were significantly higher levels of α7 homomeric nAChR expression in MEM-3389-treated than in vehicle-treated rabbits (C). *p < 0.05; **p < 0.01.
4. Discussion
A total of 120 4- and 27-month rabbits were tested in the 600 and 750 ms trace eyeblink classical conditioning paradigms for ten sessions following injections of an α7 nAChR agonist (MEM-3389), a mild acetylcholinesterase inhibitor and allosteric modulator of nAChRs (galantamine), or vehicle. All groups of rabbits showed significant acquisition of CRs, but 4-month rabbits had superior acquisition of CRs over 27-month rabbits, and only 4-month rabbits had statistically significant facilitation of learning by galantamine and MEM-3389. As shown by previous investigations (Solomon and Groccia-Ellison, 1996; Thompson et al., 1996; Woodruff-Pak et al., 1987), there was variability in rate of acquisition. Some rabbits were very efficient learners, attaining learning criterion in relatively few sessions. Other rabbits learned slowly, and many never attained learning criterion. We classified rabbits as good or poor learners and investigated levels of nAChRs in hippocampus and temporal–parietal cortex of good and poor learning 4- and 27-month rabbit groups.
4.1. Variations in nAChRs and learning
The two major new results in this study both involve nAChRs and learning proficiency. First, there was significant variation in the expression of αβ heteromeric nAChRs in good and poor learners for both young and older rabbits that was associated with significant differences in learning in trace eyeblink classical conditioning. Finch and Kirkwood (2000) pointed out that neuron numbers vary widely among individuals, in part as a consequence of chance developmental variations. Here we demonstrated that αβ heteromeric nAChRs vary widely in rabbits, and this variation was associated with differences in learning. Individual variations in nAChR expression may also be related to outcomes of aging. The magnitude of nAChR loss required for functional impact may be greater in individuals endowed with higher nAChR expression in early life. The 4-month poor learners in this study would likely have become poor learners when tested at 27 months. However, the 4-month good learners may have still remained good learners at 27 months. Age-related deficits in nAChR expression in these good learner rabbits may have been postponed to a later point in the adult life span.
Liang et al. (2008) reported an association between good learning and nicotine induced selective-tuning of evoked potentials in auditory cortex. Good, intermediate, and poor learning rats were identified in an auditory-cued active avoidance task. Good learners exhibited nicotinic modulation of response magnitude to tone-evoked responses in auditory cortex, whereas intermediate and poor learners did not. Evoked responses in good learners were enhanced to the characteristic frequency and were suppressed to auditory stimuli at frequencies remote from the characteristic frequency. The investigators suggested that the data provided a neural basis for a nicotinic contribution to auditory-cognitive function. The characteristic stimulus had a more prominent response in auditory cortex of good learners, which could have been a consequence of higher expression of nAChRs as found in temporal–parietal cortex of good learning rabbits conditioned with a tone CS in the present study.
A second new finding is that treatment with cognition-enhancing drugs in good learners was associated with significantly increased expression of hippocampal nAChRs over vehicle treatment in good learners. However, the effective drug and the affected nAChRs were different for the two age groups of rabbits. In 4-month rabbits, galantamine-treated good learners had significantly increased expression of hippocampal αβ heteromeric nAChRs as opposed to vehicle-treated good learners. In 27-month rabbits, MEM-3389 treatment was associated with significantly increased expression of hippocampal α7 homomeric nAChRs in good learners over vehicle-treated good learners. This association with αβ heteromeric nAChRs was anticipated with galantamine, which is an allosteric modulator of nAChRs (Akk and Steinbach, 2005; Dajas-Bailador et al., 2003; Pereira et al., 1994). The association with α7 homomeric nAChRs was anticipated as well because MEM-3389 was demonstrated to be selective to α7 nAChRs in frog oocytes (Papke et al., 2004).
Comparing results in the present study using the 750 ms trace paradigm and cognition-enhancing drugs to previous results with 750 ms delay eyeblink conditioning and the same drugs (Li et al., in press), we add to the existing research literature documenting striking differences between the delay and trace paradigms. In the case of the 750 ms delay paradigm, doses of 3.0 mg/kg galantamine or 1.0 mg/kg MEM-3389 improved learning significantly over vehicle for 27-month as well as 4-month rabbits (Li et al., in press). In the 750 ms trace paradigm, only 4-month rabbits showed improved learning with doses of 3.0 mg/kg galantamine or 1.0 mg/kg MEM-3389. The difficult trace paradigm caused a floor effect for older rabbits as they failed to benefit from treatment with galantamine or MEM-3389. However, good learning 27-month rabbits had greater expression of nAChRs and significantly better performance in the trace paradigm than did poor learning rabbits. These results indicate that some groups of organisms (e.g., “good learners”) age more optimally than others.
4.2. Cognition-enhancement in delay and trace eyeblink conditioning
Two previous studies of trace eyeblink classical conditioning had used a dose of 3.0 mg/kg galantamine in rabbits, and there was significant learning improvement in both studies (Simon et al., 2004;Weible et al., 2004). One study (Simon et al., 2004) tested young rabbits, and results are consistent with the present study in that 3.0 mg/kg galantamine facilitated learning in 4-month rabbits. The other study (Weible et al., 2004) tested 2–3 month and 30–33 month rabbits, giving them 10 days of previous exposure to 3.0 mg/kg galantamine and then 20 training sessions in trace eyeblink conditioning. Older but not younger galantamine-treated rabbits attained learning criterion more rapidly. Older rabbits in that study benefited from additional administration of galantamine before training and from a longer training period (20 days in comparison to 10 days in the present study).We have twice demonstrated that 3.0 mg/kg galantamine facilitates learning in young rabbits in the less difficult 750 msdelay paradigm (Woodruff-Pak et al., 2001, 2003), and now we report, along with Simon et al. (2004) that 3.0 mg/kg galantamine facilitates learning in young rabbits in the 600 and 750 ms trace paradigms. It is difficult to show facilitation of learning in high-performing young rabbits, but galantamine is one of the few drugs we have tested that enables young rabbits to exceed their already close to optimal performance.
In the present study, there was not a significant difference in performance between the 600 and 750 ms trace paradigms. It is well established that long time intervals between the CS and US (called the inter-stimulus interval, ISI) increase difficulty level and the number of training trials required to attain learning criterion (e.g., Schneiderman, 1966). The cerebellum is essential in all eyeblink classical conditioning paradigms (reviewed in Christian and Thompson, 2003) including the 750 ms trace conditioning paradigm (Woodruff-Pak et al., 1985). The trace paradigm is called “hippocampus dependent” because rabbits with bilateral lesions of the hippocampus do not acquire CRs when the trace period exceeds 300 ms (Moyer et al., 1990). Bilateral removal of the hippocampus does not impair delay eyeblink conditioning (Schmaltz and Theios, 1972), even in the long 750 ms delay paradigm in retired breeder rabbits (Woodruff-Pak et al., 1987).We compared acquisition 750 ms delay to acquisition in trace paradigms with a 300, 400, and 500 ms trace in 32-month rabbits (Rose et al., 2007). Learning was similarly impaired in the 600 and 750 ms trace conditioning paradigms, using a 400 and 500 ms trace intervals, respectively. In the present study we replicated the result that acquisition is similarly impaired with a 400 and 500 ms trace interval using a larger number of rabbits per group (10/group in the present study; 8/group in Rose et al., 2007) and with 4- as well as 27-month rabbits.
4.3. Age, nAChRs, and trace eyeblink conditioning
The hippocampus is essential in trace eyeblink conditioning, and input to hippocampus occurs via temporal lobe circuits. Although frontal cortex and cerebellum were also sites of interest because of their demonstrated involvement in trace eyeblink conditioning, our assays were limited to hippocampus and temporal–parietal cortex because frontal cortex in rabbit is small and tissue samples from the 6 rabbits pooled in each group were not sufficient to complete three independent assays. In the case of cerebellum, our previous work demonstrated that Bmax values of [3H]Epibatidine and [3H]Methyllycaconitine assays were too low to identify differences (Li et al., in press).
Expression of αβ heteromeric nAChRs in hippocampus was greater in 4- and 27-month good learner vehicle-treated rabbits andwas also greater in 4- and 27-month good learners treated with galantamine and 4-month rabbits treated with MEM-3389. Hippocampal differences in nAChR expression were limited to αβ heteromeric nAChRs as α7 homomeric nAChRs in hippocampus were not different between vehicle-or drug-treated 4- or 27-month good and poor learners. The α7 nAChR agonist MEM-3389 was associated with the only significant drug treatment effect on α7 homomeric nAChR expression in hippocampus, which was between 27-month drug and vehicle-treated good learners. The pattern of results was similar in hippocampus and temporal–parietal cortex, although drug effects were more evident in hippocampus.
Some portion of the age difference in learning in delay and trace eyeblink conditioning may result from age differences in αβ heteromeric nAChRs in the hippocampus. Mean Bmax values for hippocampal αβ heteromeric nAChR binding in a group of 10 27-month rabbits treated with vehicle and tested in the 750 ms delay eyeblink conditioning paradigm were significantly lower than hippocampal αβ heteromeric nAChR binding in 10 similarly treated 4-month rabbits (Li et al., in press). Results for αβ heteromeric nAChRs in rabbits are consistent with studies in a number of species. During normal aging, there is a diminution in the expression of high-affinity binding sites in the brain that has been reported in humans (e.g., Giacobini, 1992), monkeys (Wagster et al., 1990), and rodents (Araujo et al., 1990; Birtsch et al., 1997; Zhang et al., 1990). The fact that there was significantly better learning in 4- and well as 27-month rabbits having higher Bmax values for hippocampal αβ heteromeric nAChR binding indicates that individual differences in αβ heteromeric nAChRs exist in young adult animals.
Our data on nAChR binding are consistent with studies using different techniques and showing that tasks more difficult for older learners recruit more brain activity. For 27-month good learning rabbits treated with galantamine or MEM-3389, there was higher expression of αβ heteromeric nAChRs in both hippocampus and temporal–parietal cortex. In the case of young drug-treated good learners, only hippocampus was upregulated. Imaging studies have demonstrated that more difficult tasks recruit more brain regions (reviewed in Chein and Schneider, 2005), and that older adults show greater activations to the same task as young adults (Grady et al., 2008). Older drug-treated good learner rabbits also showed more significant increased expression of nAChRs when performing the same task as young rabbits. In the case of the difficult 750 ms trace eyeblink classical conditioning paradigm, this recruitment of greater nAChR expression was not sufficient to improve learning.
Acknowledgements
We thank Susan Seta, LaToya Roker, Renee Procopio and Steve Purcell for their assistance with rabbit familiarization training and behavioral testing. We gratefully acknowledge Janssen Pharmaceutica, N.V. and Ortho-McNeil Neurologics for supplying galantamine and Memory Pharmaceuticals for supplying MEM-3389, formerly identified as AR-R-17779. This research was supported by grants from the National Institute on Aging, R01 AG021925 and R01 AG023742 to DSW-P and grants from the National Institute on Drug Abuse, R01 DA17302 and P30 DA 13429 to L-YL-C.
Footnotes
Disclosure statement
The first author had contracts as a PI at Albert Einstein Healthcare Network with Janssen Pharmaceutica to test cognition-enhancing drugs, including galantamine, that were completed in 2005. The company reorganized into Janssen Pharmaceutica, N.V. and Ortho-McNeil Neurologics, which provided the galantamine for the present study that was initiated at Temple University in 2006. A contract with the first author as PI at Albert Einstein Healthcare Network with Memory Pharmaceuticals to test the calcium channel blocker, MEM-1003 was completed in 2003, and this company provided an α7 nicotinic agonist, MEM-3389 for the present study. No financial support from either company was provided to the first author or to Temple University for research described in this article.
The co-authors have no financial disclosures.
References
- Akk G, Steinbach JH. Galantamine activates muscle-type nicotinic acetylcholine receptors without binding to the acetylcholine-binding site. J. Neurosci. 2005;25:1001–1992. doi: 10.1523/JNEUROSCI.4985-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo DM, Lapchak PA, Meaney MJ, Collier B, Quirion R. Effects of aging on nicotinic and muscarinic autoreceptors function in the rat brain: relationship to presynaptic cholinergic markers and binding sites. J. Neurosci. 1990;10:3069–3078. doi: 10.1523/JNEUROSCI.10-09-03069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birtsch C, Wevers A, Traber J, Maelicke A, Bloch W, Schröder H. Expression of α4-1 and α5 nicotinic cholinoceptor mRNA in the aging rat cerebral cortex. Neurobiol. Aging. 1997;18:335–342. doi: 10.1016/s0197-4580(97)80316-8. [DOI] [PubMed] [Google Scholar]
- Changeux JP, Bertrand D, Corringer PJ, Dehaene S, Edelstein S, Lena C, Le Novere N, Marubio L, Picciotto M, Zoli M. Brain nicotinic receptors: structure and regulation, role in learning and reinforcement. Brain Res. Rev. 1998;26:198–216. doi: 10.1016/s0165-0173(97)00040-4. [DOI] [PubMed] [Google Scholar]
- Chein JM, Schneider W. Neuroimaging studies of practice-related change: fMRI and meta-analytic evidence of a domain-general control network for learning. Brain Res. Cogn. Brain Res. 2005;25:607–623. doi: 10.1016/j.cogbrainres.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Chen G, Steinmetz JE. A general-purpose computer system for behavioral conditioning and neural recording experiments. Behav. Res. Methods Instrum. Comput. 1998;30:384–391. [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn. Mem. 2003;11:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Court J, Lloyd S, Johnson M, Griffiths M, Birdsall NJ, Piggott MA, Okley AE, Ince PG, Perry EK, Perry RH. Nicotinic and muscarinic cholinergic receptor binding in the human hippocampal formation during development and aging. Dev. Brain Res. 1997;90:159–167. doi: 10.1016/s0165-3806(97)00052-7. [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador FA, Heimala K, Wonnacott S. The allosteric potentiation of nicotinic acetylcholine receptors by galantamine is transduced into cellular responses in neurons: Ca2+ signals and neurotransmitter release. Mol. Pharmacol. 2003;64:1217–1226. doi: 10.1124/mol.64.5.1217. [DOI] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Davies RL, Hardick DJ, Blagbrough IS, Potter B, Wolstenholme AJ, Wonnacott S. Characterisation of the binding of [3H]-methyllycaconitine: a new radioligand for labeling α7-type neuronal nicotinic acetylcholine receptors. Neuropharmacology. 1999;38:679–690. doi: 10.1016/s0028-3908(98)00221-4. [DOI] [PubMed] [Google Scholar]
- Falk L, Nordberg A, Seiger A, Kjaeldgaard A, Hellström-Lindahl E. Higher expression of α7 nicotinic acetylcholine receptors in human fetal compared to adult brain. Dev. Brain Res. 2003;142:151–160. doi: 10.1016/s0165-3806(03)00063-4. [DOI] [PubMed] [Google Scholar]
- Finch CE, Kirkwood TBL. Chance, Development, and Aging. New York: Oxford University Press; 2000. [Google Scholar]
- Gahring LC, Persiyanov K, Rogers SW. Mouse strain-specific changes in nicotinic receptor expression with age. Neurobiol. Aging. 2005;26:973–980. doi: 10.1016/j.neurobiolaging.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Giacobini E. Nicotine acetylcholine receptors in human cortex: aging and Alzheimer’s disease. In: Lippiello PM, Collins AC, Gray AC, Robinson JH, editors. Biology of Nicotine. New York: Raven; 1992. pp. 183–215. [Google Scholar]
- Grady CL, Yu H, Alain C. Age-related differences in brain activity underlying working memory for spatial and nonspatial auditory information. Cereb. Cortex. 2008;18:189–199. doi: 10.1093/cercor/bhm045. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. Genetic determinants of adult hippocampal neurogenesis correlate with acquisition, but not probe trial performance, in the water maze task. Eur. J. Neurosci. 2002;16:129–136. doi: 10.1046/j.1460-9568.2002.02042.x. [DOI] [PubMed] [Google Scholar]
- Lee HK, Min SS, Gallagher M, Kirkwood A. NMDA receptor-independent long-term depression correlates with successful aging in rats. Nat. Neurosci. 2005;8:1657–1659. doi: 10.1038/nn1586. [DOI] [PubMed] [Google Scholar]
- Li J-G, Lehr MA, Liu-Chen L-Y, Woodruff-Pak DS. Nicotinic acetylcholine receptors and modulation of learning in 4- and 27-month rabbits. Neuropsychopharmacol. doi: 10.1038/npp.2008.1. in press. [DOI] [PubMed] [Google Scholar]
- Liang K, Poytress BS, Weinberger NM, Metherate R. Nicotinic modulation of tone-evoked responses in auditory cortex reflects the strength of prior auditory learning. Neurobiol. Learn. Mem. 2008;90:138–146. doi: 10.1016/j.nlm.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J. Neuronal nicotinic acetylcholine receptors. Ion Channels. 1996;4:377–450. doi: 10.1007/978-1-4899-1775-1_10. [DOI] [PubMed] [Google Scholar]
- Matzel LD, Gandhi CC. The tractable contribution of synapses and their component molecules to individual differences in learning. Behav. Brain Res. 2000;110:53–66. doi: 10.1016/s0166-4328(99)00184-9. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Weible AP, Disterhoft JF. Aging and learning-specific changes in single-neuron activity in CA1 hippocampus during rabbit trace eyeblink conditioning. J. Neurophysiol. 2001;86:1839–1857. doi: 10.1152/jn.2001.86.4.1839. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav. Neurosci. 1990;104:243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Nordberg A, Winblad B. Brain nicotinic and muscarinic receptors in normal aging and dementia. In: Fisher A, Hanin I, Lachman C, editors. Alzheimer’s and Parkinson’s Diseases: Strategies for Research and Development. New York: Plenum; 1986. pp. 95–108. [Google Scholar]
- Olton DS, Markowska A, Breckler SJ, Wenk GL, Pang KC, Koliatsos V. Individual differences in aging: behavioral and neural analyses. Biomed. Environ. Sci. 1991;4:166–172. [PubMed] [Google Scholar]
- Papke RL, Porter Papke JK, Rose GM. Activity of alpha7-selective agonists at nicotinic and serotonin 5HT3 receptors expressed in Xenopus oocytes. Bioorg. Med. Chem. Lett. 2004;14:1849–1853. doi: 10.1016/j.bmcl.2003.09.104. [DOI] [PubMed] [Google Scholar]
- Pereira EF, Alkondon M, Reinhardt S, Maelicke A, Peng X, Lindstrom J, Whiting P, Albuquerque EX. Physostigmine and galanthamine: probes for a novel binding sie on the alpha 4 beta 2 subtype of neuronal nicotinic acetylcholine receptors stably expressed in fibroblast cells. J. Pharmacol. Exp. Ther. 1994;270:768–778. [PubMed] [Google Scholar]
- Popa RV, Pierra EF, Lopes C, Maelicke A, Albuquerque EX. The N-butylcarbamate derivative of galantamine acts as an allosteric potentiating ligand on alpha7 nicotinic receptors in hippocampal neurons: clinical implications for treatment in Alzheimer’s disease. J. Mol. Neurosci. 2006;30:227–232. doi: 10.1385/JMN:30:1:227. [DOI] [PubMed] [Google Scholar]
- Rose GM, Ong VS, Woodruff-Pak DS. Efficacy of MEM 1003, a novel calcium channel blocker, in delay and trace eyeblink conditioning in older rabbits. Neurobiol. Aging. 2007;28:766–773. doi: 10.1016/j.neurobiolaging.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Schmaltz LW, Theios J. Acquisition and extinction of a classically conditioned response in hippocampectomized rabbits (Oryctolagus cuniculus) J. Comp. Physiol. Psych. 1972;79:328–333. doi: 10.1037/h0032531. [DOI] [PubMed] [Google Scholar]
- Schneiderman N. Interstimulus interval function of the nictitating membrane response of the rabbit under delay versus trace conditioning. J. Comp. Physiol. Psych. 1966;62:397–402. [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding changes in orbitofrontal cortex in reversal-impaired aged rats. J. Neurophysiol. 2006;95:1509–1517. doi: 10.1152/jn.01052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon BB, Knuckley B, Powell DA. Galantamine facilitates acquisition of a trace-conditioned eyeblink response in healthy, young rabbits. Learn. Mem. 2004;11:116–122. doi: 10.1101/lm.66204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon PR, Groccia-Ellison ME. Classic conditioning in aged rabbits: delay, trace, and long-delay conditioning. Behav Neurosci. 1996;110:427–435. doi: 10.1037//0735-7044.110.3.427. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Moyer JR, Disterhoft JF. Trace eyeblink conditioning in rabbits demonstrates heterogeneity of learning ability both between and within age groups. Neurobiol. Aging. 1996;17:619–629. doi: 10.1016/0197-4580(96)00026-7. [DOI] [PubMed] [Google Scholar]
- Van der Zee EA, Kornforst-Collins MA, Maizels ET, Hunziker-Dunn M, Disterhoft JF. gamma Isoform-selective changes in PKC immunoreactivity after trace eyeblink conditioning in the rabbit hippocampus. Hippocampus. 1997;7:271–285. doi: 10.1002/(SICI)1098-1063(1997)7:3<271::AID-HIPO3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Wagster MV, Whitehouse PJ, Walker LC, Kellar KJ, Price DL. Laminar organization and age-related loss of cholinergic receptors in temporal neocortex of rhesus monkey. J. Neurosci. 1990;10:2879–2885. doi: 10.1523/JNEUROSCI.10-09-02879.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner JM, Radcliffe RA, Bowers BJ. Quantitative genetics and mouse behavior. Annu. Rev. Neurosci. 2001;24:845–867. doi: 10.1146/annurev.neuro.24.1.845. [DOI] [PubMed] [Google Scholar]
- Weible AP, Oh MM, Lee G, Disterhoft JF. Galantamine facilitates acquisition of hippocampus-dependent trace eyeblink conditioning in aged rabbits. Learn. Mem. 2004;11:108–115. doi: 10.1101/lm.69804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramaratna JC, Fry BG, Loiacono RE, Aguilar MI, Alewood PF, Hodgson WC. Isolation and characterization at cholinergic nicotinic receptors of a neurotoxin from the venom of the Acanthophis sp. Seram death adder. Biochem. Pharmacol. 2004;68:383–394. doi: 10.1016/j.bcp.2004.03.033. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Lavond DG, Thompson RF. Trace conditioning: abolished by cerebellar nuclear lesions but not lateral cerebellar cortex aspirations. Brain Res. 1985;348:249–260. doi: 10.1016/0006-8993(85)90443-3. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Lavond DG, Logan CG, Thompson RF. Classical conditioning in 3-, 30-, and 45-month rabbits: behavioral learning and hippocampal unit activity. Neurobiol. Aging. 1987;8:101–108. doi: 10.1016/0197-4580(87)90018-2. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Li Y-T, Kem WR. A nicotinic agonist (GTS-21), eyeblink classical conditioning, and nicotinic receptor binding in rabbit brain. Brain Res. 1994;645:309–317. doi: 10.1016/0006-8993(94)91665-9. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Santos IS. Nicotinic modulation in an animal model of a form of associative learning impaired in Alzheimer’s disease. Behav. Brain Res. 2000;113:11–19. doi: 10.1016/s0166-4328(00)00196-0. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Vogel RW, III, Wenk GL. Galantamine: effect on nicotinic receptor binding, acetylcholinesterase inhibition, and learning. PNAS (USA) 2001;98:2089–2094. doi: 10.1073/pnas.031584398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Vogel R, III, Wenk GL. Mecamylamine interactions with galantamine and donepezil: Effects on learning, acetyl-cholinesterase, and nicotinic acetylcholine receptors. Neuroscience. 2003;117:439–447. doi: 10.1016/s0306-4522(02)00872-2. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Watson ML, Gallagher M, Nicolle MM. Muscarinic receptor-mediated GTP-Eu binding in the hippocampus and prefrontal cortex is correlated with spatial memory impairment in aged rats. Neurobiol. Aging. 2007;28:619–626. doi: 10.1016/j.neurobiolaging.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wahlstrom G, Nordberg A. Influence of development and aging on nicotinic receptor subtypes in rodent brain. Int. J. Dev. Neurosci. 1990;8:715–721. doi: 10.1016/0736-5748(90)90065-a. [DOI] [PubMed] [Google Scholar]


