Abstract
Background
Fetal alcohol disorders are preventable, but self-reported alcohol consumption can be misleading and impede effective treatment. Biomarkers represent an alternative method for assessing alcohol use, and this study evaluated the relationship between blood phosphatidylethanol (PEth) and alcohol use in a sample of reproductive age women.
Methods
Alcohol use was estimated by validated self-report methods in 80 non-pregnant women ages 18 to 35. PEth was measured by a contracted laboratory using a liquid chromatography-tandem mass spectrometry assay. Regression methods appropriate for the distribution of PEth were used to define its relationship to alcohol consumption during the prior 2 weeks and explore the effects of drinking patterns on this association. Receiver operating characteristic analysis was used to estimate the sensitivity of PEth for various drinking levels at 95% specific cutoffs.
Results
PEth had a positive linear association with grams of alcohol consumed (p<0.001), and was detectable in 93% of subjects consuming an average of 2 or more drinks per day. The relationship between total alcohol consumption and PEth may be stronger in women with recent heavy drinking days. The relationship between drinking and PEth varied considerably between individuals, and sensitivity for a certain amount of drinking was low at a highly specific cutoff concentration.
Conclusions
PEth is a highly sensitive indicator of moderate and heavy alcohol consumption in reproductive age women and may complement the use of self-report alcohol screens when additional objective markers of alcohol use are desirable. However, choosing a highly valid cutoff concentration for PEth to differentiate various levels of alcohol consumption may not be feasible.
Keywords: phosphatidylethanol, alcohol drinking, women, biomarker
INTRODUCTION
Fetal alcohol spectrum disorders (FASD) are preventable birth defects that occur in approximately 1 in 1,000 live births in the US (May and Gossage, 2001). Fetal alcohol syndrome is a severe form of FASD related to chronic heavy drinking during pregnancy, and is characterized by facial dysmorphology, diminished growth, and mental retardation. Other alcohol-related birth defects can also occur, including heart, kidney, and auditory deficits. Alcohol-related neurodevelopmental disorder can be a subtler condition that may also be associated with heavy and perhaps even moderate drinking during pregnancy, and is expressed as learning deficits and behavioral problems that may only become apparent as the child matures (Floyd et al., 2005, Bertrand, 2004).
Detection of alcohol use must precede preventive interventions for FASD in women who drink while trying to conceive or during pregnancy, and several self-report screens have been validated for detecting potentially harmful alcohol use in pregnant and reproductive-age women (Bailey and Sokol, 2008). While the use of a validated self-report screen for drinking is recommended and effective, these can be misleading in some circumstances, either due to an error in communication or administration, or by intentional under-reporting of alcohol use (Del Boca and Darkes, 2003). As a result, laboratory measures of alcohol exposure may be a helpful adjunct to self-report screens (Bearer, 2001), and may help clarify the controversial role of light to moderate drinking during pregnancy as a cause of FASD (Henderson et al., 2007), an association that may be partly due to inaccurate alcohol assessments. Unfortunately, traditional alcohol biomarkers such as GGT, aminotransferases, and erythrocyte mean corpuscular volume do not have the requisite sensitivity and specificity for confidently predicting alcohol exposure, and thus have limited utility for clinical and research applications (Conigrave et al., 2003). This is particularly true in younger people, in whom the sensitivity of such markers is particularly low. However, minor, non-oxidative metabolites of ethanol may provide high sensitivity and specificity for detecting alcohol use (Wurst et al., 2005). For example, urine and hair ethyl glucuronide have been evaluated as alcohol consumption biomarkers during pregnancy (Wurst et al., 2008). In urine, this marker has clinical potential, but has a short half-life, with sensitivity dropping sharply after approximately 24 hours of abstinence (Wojcik and Hawthorne, 2007). Hair ethyl glucuronide appears to identify cases of otherwise undetected drinking (Wurst et al, 2008). However, since detectable hair ethyl glucuronide persists for several months, positive results may not be specific for drinking during pregnancy, and reductions in concentration may not occur rapidly enough for optimal assessment of drinking reduction during pregnancy. Blood phosphatidylethanol (PEth) is another product of ethanol elimination with high potential for clinical utility (Hartmann et al., 2007). PEth is synthesized from phosphatidylcholine and ethanol via a phospholipase D catalyzed reaction, and can be measured in red blood cells using one of several analytical techniques (Gustavsson, 1995, Gunnarsson et al., 1998). Like urinary ethyl glucuronide, PEth has the advantage of high specificity as a product of ethanol elimination, but has a longer half-life of approximately 4 days (Varga et al., 2000). In addition, since it does not reflect alcohol toxicity, only exposure, it may in theory have greater sensitivity in young people relative to traditional markers. To evaluate the potential clinical and research utility of this biomarker for drinking detection in young women and ultimately FASD prevention, we undertook this initial observational study to characterize the relationship of PEth to alcohol consumption in non-pregnant women of reproductive age.
METHODS
Subjects
Eighty generally healthy women between the ages of 18 and 35 were recruited from a university-affiliated women's health center and by advertisement. Other than age, the main inclusion criterion was self-reported consumption of any amount of alcohol on at least 2 days per week. This requirement was used to eliminate an excessive number of negative PEth assays due to inclusion of alcohol abstainers, since ethanol is a prerequisite for PEth synthesis. We anticipated that this strategy would provide a number of relatively light drinkers with low or undetectable PEth that would serve as a control group in estimating the utility of PEth for detecting drinking. The main exclusion criterion was current pregnancy, as it was imperative to minimize the potential for under-reporting consumption in this study, which utilized self-reported alcohol consumption as the reference standard for measuring alcohol use. Subjects received $25 to compensate them for their time and effort following data collection. The protocol was approved by the university Institutional Review Board.
Estimation of alcohol use
A daily drinking estimate for the preceding 30 days was obtained using timeline followback methodology (Sobell and Sobell, 1992). This validated technique uses a calendar, the subject's usual drinking patterns, and memory cues such as highlighting important dates or occasions to elicit estimated daily alcohol consumption. Subjects reported the type of beverages and amount consumed. These estimates were converted into grams of ethanol based on the alcohol content of the specific beverages and volume ingested. Given the estimated half-life of 4 days (Varga et al., 2000), we used the grams of ethanol consumed in the past-2-weeks as the main predictor of PEth, roughly corresponding to 3 half-lives. Since the relationship between PEth and total consumption might logically be influenced by the blood ethanol concentration that is attained, the drinking frequency, and the time elapsed between drinking and PEth measurement, we also quantified heavy drinking days (defined as 4 or more drinks in one day), number of drinking days (defined as a day during which any amount of alcohol was consumed), days since last heavy drinking day, and days since last drink.
Measurement of PEth
Ten ml of blood was drawn into a tube containing 100 mg sodium fluoride and 20 mg potassium oxalate. Samples were shipped fresh or following a maximum of 2 weeks storage at −20° C to a contracted laboratory (US Drug Testing Laboratories, Des Plaines, IL) which was masked to the status of the study subjects. PEth concentration has been shown to be stable in blood samples under similar conditions (Aradottir and Olsson, 2005). The assay employed by the laboratory was an adaptation of previously published methods (Tolonen et al., 2005, Gunnarsson et al., 1998). Phosphatidylpropanol was added to 1 mL of sample as an internal standard, and a liquid-liquid extraction for lipids was then completed. The LC-MS-MS assay (Waters Quattro Ultima coupled to a Waters 2790 Separations Module) in multiple reaction monitoring mode detected a product ion of the isomers 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylethanol and 1-oleoyl-2-palmitoyl-sn-glycero-3-phosphatidylethanol, which include approximately 40% of total PEth (Gunnarsson et al., 1998). The within-batch coefficient of variation for the assay was 3.1%, the between-batch coefficient of variation 6.3%, and the statistical limit of quantification 19.8 ng/mL. A test was considered positive at 20 ng/mL or higher (i.e., above the assay cutoff where PEth can reliably be detected over background noise).
Statistical methods
Descriptive statistics were calculated for PEth and alcohol consumption. Because PEth concentration was censored below the limit of quantification (i.e., 20 ng/mL), the linearity of the relationship between alcohol consumption and PEth was estimated using tobit regression. This method simultaneously accounts for the probability of dependent variables being quantifiable, and the relationship between dependent and independent variables in the quantifiable range of the dependent variable (Austin et al., 2000). For the tobit regression, PEth concentration served as the dependent variable, and grams of ethanol consumed over the 2 weeks preceding PEth measurement served as the main independent variable. Secondarily, we explored for interactions of total alcohol consumption with the number of heavy drinking days, number of drinking days, number of days since last heavy drinking day, and number of days since last drink. Finally, since classification of drinking status is a potentially important clinical application, a simple logistic regression was employed to predict each subject's probability of being in a certain drinking category (i.e., averaging more than 1 drink per day or more than 2 drinks per day in the past 2 weeks) based on their blood PEth concentration. A receiver operating characteristic curve (ROC) was generated by plotting sensitivity against 1-specificity for every observed PEth value, and the area under the curve (AUC) was estimated for each of these drinking categories. The AUC quantifies the probability that a woman in a higher drinking category would have a higher PEth concentration relative to a woman in a lower drinking category (e.g., a woman drinking more than 2 drinks per day compared to a woman drinking less). In addition to the AUC, because high specificity is paramount in clinical settings, we also report the sensitivity at a 95% specific PEth cutoff concentration for each drinking category.
RESULTS
Eighty subjects were recruited over a 4 month period. The median age was 26 (interquartile range 23 to 30), 89 % were non-Hispanic white, 7 % were non-Hispanic black, and 4 % identified themselves with other ethnic groups. The median grams of ethanol in the 14 days preceding blood sampling was 322 (interquartile range 211 to 534; range 17 to 1,378). This median translates to 23 standard drinks or an average of 1.6 drinks per day, with a standard drink roughly equivalent to 12 ounces of beer, 5 ounces of wine, or 1.5 ounces of an 80-proof beverage. The median PEth concentration was 45 ng/mL (interquartile range 0 to 84, range 0 to 565). Twenty-nine percent (n=23) of the subjects did not have quantifiable PEth, and this was related to the amount of alcohol consumed. For example, PEth was quantified in 8 of 15 (53%) subjects drinking up to one drink per day, in 23 of 37 (62%) drinking >1 to 2 drinks per day, and 26 of 28 (93%) averaging more than 2 drinks per day.
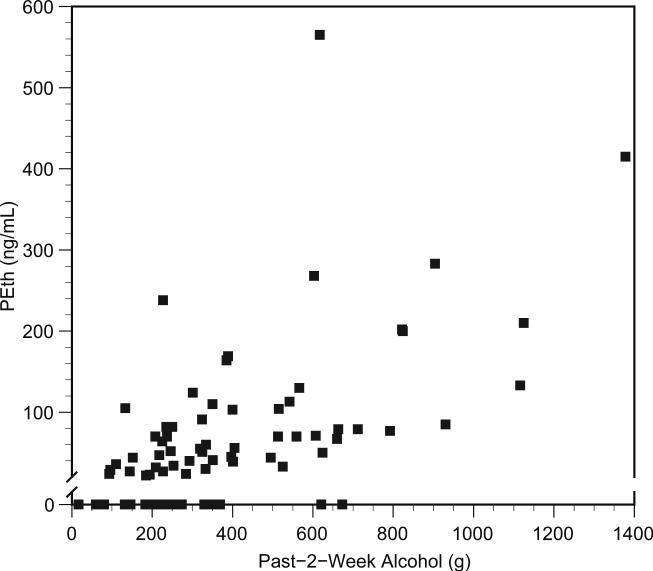
A scatter plot of the results is shown in the figure. The tobit regression demonstrated a significant linear relationship between PEth and total consumption in the past 2 weeks (regression coefficient = 0.26, p<0.001). In clinically relevant terms, this would roughly translate into an average elevation in PEth concentration of 50 ng/mL for an increase of one drink per day during the 2 weeks preceding PEth measurement. The relationship of total consumption to PEth concentration in our subjects did not depend on the number of heavy drinking days (p-value for interaction=0.467), number of days during which any alcohol was consumed (p>0.500), or days since last drink (p=0.255). However, the p-value for interaction with days since last heavy drinking day was 0.190, which we considered marginal given the sample size of 80. As a result we dichotomized at the median of 5 days and ran the analyses in each of the two resulting groups. In the 41 subjects in whom the last heavy drinking day was greater than or equal to 5 days, the regression coefficient for past 2 week total consumption was 0.11 (p=0.227), and 19 subjects (46%) did not have quantifiable PEth concentrations. In the 39 subjects reporting a heavy drinking day in the prior 1 to 4 days, the regression coefficient was 0.24 (p<0.001), and 4 subjects (10%) did not have quantifiable PEth concentrations.
Plot of blood phosphatidylethanol (PEth) vs. total reported alcohol consumption in the prior 2 weeks. The PEth axis is interrupted at the limit of quantification (i.e., 20 ng/mL).
The results from the ROC analyses are included in the table. The areas under the curves indicate that a woman within a particular drinking category would usually have a higher PEth value compared to a woman drinking less alcohol (e.g., women consuming greater than 1 drink per day would have higher PEth 77% of the time relative to women drinking less than 1 drink per day). Overall, due to between-subject variability in the relationship of PEth concentration to the grams of alcohol ingested, it was not possible to define a highly sensitive cutoff for differentiating various drinking categories while maintaining very high specificity (e.g., 95% of women drinking less than 1 drink per day would have PEth < 45 ng/mL, but only 61% of women drinking more than 1 drink per day would have PEth ≥ 45 ng/mL). However, it should be noted that in many cases there were small differences in alcohol consumption between groups (e.g., a person consuming less than one drink daily may have consumed approximately 190 grams of ethanol, whereas a person consuming more than one drink daily may have consumed only slightly more than that).
Table.
Results of ROC Analyses
| Case Definition | Median grams ethanol in cases (range) [interquartile range] | Median grams ethanol in non-cases* (range) [interquartile range] | PEth concentration at 95% specificity | Sensitivity at 95% specificity | Area under the ROC curve (95% CI) |
|---|---|---|---|---|---|
| > 1 Drink per Day (n=64) | 355 (205-1,378) [250-603] | 134 (17-196) [93-152] | 45 ng/mL | 61 % | 0.77 (0.66 to 0.88) |
| >2 Drinks per Day (n=28) | 619 (397-1,378) [520-807] | 231 (17-389) [184-310] | 127 ng/mL | 32% | 0.80 (0.70 to 0.90) |
“Non-cases” included all subjects drinking below the case threshold (e.g., non-cases for the heaviest drinking category included all subjects averaging less than or equal to 2 drinks per day). “Grams ethanol” refers to the 2 weeks preceding blood collection.
DISCUSSION
This study evaluated the relationship of alcohol drinking to PEth in women of reproductive age. Results demonstrated that PEth above the limit of quantification was highly sensitive for drinking averaging at least 2 drinks per day during the weeks preceding measurement, and concentrations above 127 ng/mL were highly specific for drinking in excess of 2 drinks per day. In general however, there was substantial variation observed between individuals in PEth concentration given similar self-report of recent drinking. This demonstrates that highly specific cutoff concentrations for heavy drinking are likely to have low sensitivity. Conversely, highly sensitive cutoff concentrations for screening would result in substantial misclassification of moderate drinkers as heavier drinkers. Such misclassification may be less of an issue in obstetrical applications as ideally no patients would have detectable PEth during pregnancy or while trying to conceive.
Prior PEth validation studies in clinical populations have used an alternative and less sensitive PEth assay (Hansson et al., 1997, Hartmann et al., 2007). Such studies demonstrated nearly perfect specificity and very high sensitivity in differentiating known heavy drinkers (e.g., entering alcohol detoxification programs) from known abstainers and social drinkers. High sensitivity for heavy drinking has also been demonstrated in uncontrolled studies (Aradottir et al., 2006, Wurst et al., 2004). Our results with the MS assay reported here demonstrate quantifiable PEth in many social drinkers and in most women who exceed an average of 2 drinks per day. This is consistent with a prior study in patients with liver disease and hypertension, demonstrating quantifiable PEth in some light to moderate drinkers, even down to an average of less than one drink per day in some cases (Stewart, in press). While there is some variability in the extraction of lipids from the blood samples (Hansson et al., 1997), reasons for the individual variability in the relationship between PEth concentration and the amount of alcohol consumed have not been well-characterized, but our results suggested that some of the variability may be related to the recency of relatively heavy drinking. This seems logical as higher blood alcohol concentrations might in theory result in enhanced synthesis of PEth in red blood cells. For clinical application, any quantifiable PEth concentration by mass spectrometry indicates some amount of drinking during the prior days to weeks, and concentrations greater than 127 ng/mL appear highly specific for averaging greater than 2 drinks daily. However, lower levels do not rule out heavier drinking, due to the observed variability between subjects in the relationship between alcohol consumption and PEth concentration, and because PEth may be decreasing due to recent drinking reduction or abstinence. These estimates should be considered preliminary, since they are based on a study sample of 80 women in whom we can not definitively exclude erroneous self-report.
Strengths of this study included the detailed assessment of alcohol use and its association with a novel alcohol biomarker in a population of particular importance, and inclusion of a full range of recent alcohol exposures. This inclusion of a broad range of recent drinking activity explains why there was no cutoff concentration for PEth that was both highly sensitive and highly specific for a certain level of consumption. The main limitation was reliance on self-reported drinking as a reference standard, which provides a reasonable estimate but is certainly not error free. Any non-random misclassification based on this standard would bias the estimated sensitivity and specificity of PEth for specific drinking categories. The study also evaluated non-pregnant women, and it is the prospect of pregnancy that makes reproductive age women of particular relevance for validating alcohol consumption biomarkers. A similar study during pregnancy would be ideal, but ensuring accurate self-report and recruitment of women with a range of alcohol involvement during pregnancy would be difficult. An additional limitation was the sample size, which was not large enough for reliably estimating the effects of drinking patterns (e.g., binging vs. regular moderate drinking) on the relationship between PEth and total consumption. Controlled drinking experiments over a period of several weeks could address these issues but would be expensive to conduct. Alternatively, additional epidemiologic studies such as this one may provide additional confidence if results were similar. Finally, the PEth assay evaluated in this study is not routine, is currently only available in specialized laboratories, and would be expensive for clinical use. However, a monoclonal antibody to PEth has been developed, and less expensive immunoassays that can be utilized in most clinical laboratories may be on the horizon (Nissinen et al., 2008).
Identification of alcohol use in pregnant women and women who are trying to conceive is an important goal in the prevention of FASD, and validated self-report instruments are available for this purpose. This study suggests that PEth measured by mass spectrometry can be used as a very sensitive indicator of drinking in excess of approximately 2 drinks per day in circumstances where there is a desire to obtain additional objective indicators of alcohol use, with diminished sensitivity for lower levels of drinking. PEth may also be useful in clinical research protocols for this same purpose. Future studies would be useful for identifying sources of variability in PEth concentration among women drinking at similar levels, verify a similar relationship between PEth and drinking during pregnancy, and to confirm the relationship between the amount of alcohol consumed and PEth concentration.
Acknowledgements
Supported in part by NIAAA awards K23AA014188 and P50AA010761.
REFERENCES
- ARADOTTIR S, ASANOVSKA G, GJERSS S, HANSSON P, ALLING C. Phosphatidylethanol (PEth) concentrations in blood are correlated to reported alcohol intake in alcohol-dependent patients. Alcohol & Alcoholism. 2006;41:431–7. doi: 10.1093/alcalc/agl027. [DOI] [PubMed] [Google Scholar]
- ARADOTTIR S, OLSSON BL. Methodological modifications on quantification of phosphatidylethanol in blood from humans abusing alcohol, using high-performance liquid chromatography and evaporative light scattering detection. BMC Biochemistry. 2005;6:18. doi: 10.1186/1471-2091-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AUSTIN PC, ESCOBAR M, KOPEC JA. The use of the Tobit model for analyzing measures of health status. Qual Life Res. 2000;9:901–10. doi: 10.1023/a:1008938326604. [DOI] [PubMed] [Google Scholar]
- BAILEY BA, SOKOL RJ. Pregnancy and alcohol use: evidence and recommendations for prenatal care. Clin Obstet Gynecol. 2008;51:436–44. doi: 10.1097/GRF.0b013e31816fea3d. [DOI] [PubMed] [Google Scholar]
- BEARER CF. Markers to detect drinking during pregnancy. Alcohol Res Health. 2001;25:210–8. [PMC free article] [PubMed] [Google Scholar]
- BERTRAND JF,RL, WEBER MK, O'CONNOR M, RILEY EP, JOHNSON KA, et al. National Task Force on FAS/FAE. Fetal Alcohol Syndrome: guidelines for referral and diagnosis., Centers for Disease Control and Prevention. 2004.
- CONIGRAVE KM, DAVIES P, HABER P, WHITFIELD JB. Traditional markers of excessive alcohol use. Addiction. 2003;98(Suppl 2):31–43. doi: 10.1046/j.1359-6357.2003.00581.x. [DOI] [PubMed] [Google Scholar]
- DEL BOCA FK, DARKES J. The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction. 2003;98(Suppl 2):1–12. doi: 10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- FLOYD RL, O'CONNOR MJ, SOKOL RJ, BERTRAND J, CORDERO JF. Recognition and prevention of fetal alcohol syndrome. Obstet Gynecol. 2005;106:1059–64. doi: 10.1097/01.AOG.0000181822.91205.6f. [DOI] [PubMed] [Google Scholar]
- GUNNARSSON T, KARLSSON A, HANSSON P, JOHNSON G, ALLING C, ODHAM G. Determination of phosphatidylethanol in blood from alcoholic males using high-performance liquid chromatography and evaporative light scattering or electrospray mass spectrometric detection. Journal of Chromatography B, Biomedical Sciences & Applications. 1998;705:243–9. doi: 10.1016/s0378-4347(97)00541-0. [DOI] [PubMed] [Google Scholar]
- GUSTAVSSON L. Phosphatidylethanol formation: specific effects of ethanol mediated via phospholipase D. Alcohol & Alcoholism. 1995;30:391–406. [PubMed] [Google Scholar]
- HANSSON P, CARON M, JOHNSON G, GUSTAVSSON L, ALLING C. Blood phosphatidylethanol as a marker of alcohol abuse: levels in alcoholic males during withdrawal. Alcoholism: Clinical & Experimental Research. 1997;21:108–10. [PubMed] [Google Scholar]
- HARTMANN S, ARADOTTIR S, GRAF M, WIESBECK G, LESCH O, RAMSKOGLER K, WOLFERSDORF M, ALLING C, WURST FM. Phosphatidylethanol as a sensitive and specific biomarker: comparison with gamma-glutamyl transpeptidase, mean corpuscular volume and carbohydrate-deficient transferrin. Addiction Biology. 2007;12:81–4. doi: 10.1111/j.1369-1600.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- HENDERSON J, GRAY R, BROCKLEHURST P. Systematic review of effects of low-moderate prenatal alcohol exposure on pregnancy outcome. Bjog. 2007;114:243–52. doi: 10.1111/j.1471-0528.2006.01163.x. [DOI] [PubMed] [Google Scholar]
- MAY PA, GOSSAGE JP. Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res Health. 2001;25:159–67. [PMC free article] [PubMed] [Google Scholar]
- NISSINEN AE, MAKELA SM, VUORISTO JT, LIISANANTTI MK, HANNUKSELA ML, HORKKO S, SAVOLAINEN MJ. Immunological detection of in vitro formed phosphatidylethanol--an alcohol biomarker--with monoclonal antibodies. Alcoholism: Clinical & Experimental Research. 2008;32:921–8. doi: 10.1111/j.1530-0277.2008.00656.x. [DOI] [PubMed] [Google Scholar]
- SOBELL L, SOBELL M. Timeline follow-back: A technique for assessing self-reported ethanol comsumption. In: LITTEN RZ, ALLEN JP, editors. Measuring alcohol consumption: Psychological and biological methods. Humana Press; Totowa, NJ: 1992. [Google Scholar]
- STEWART SH, REUBEN A, BRZEZINSKI WA, KOCH DG, BASILE J, RANDALL PK, MILLER PM. Preliminary Evaluation of Phosphatidylethanol in Patients with Liver Disease and Hypertension. Alcohol & Alcoholism. doi: 10.1093/alcalc/agp039. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOLONEN A, LEHTO TM, HANNUKSELA ML, SAVOLAINEN MJ. A method for determination of phosphatidylethanol from high density lipoproteins by reversed-phase HPLC with TOF-MS detection. Anal Biochem. 2005;341:83–8. doi: 10.1016/j.ab.2005.03.001. [DOI] [PubMed] [Google Scholar]
- VARGA A, HANSSON P, JOHNSON G, ALLING C. Normalization rate and cellular localization of phosphatidylethanol in whole blood from chronic alcoholics. Clinica Chimica Acta. 2000;299:141–50. doi: 10.1016/s0009-8981(00)00291-6. [DOI] [PubMed] [Google Scholar]
- WOJCIK MH, HAWTHORNE JS. Sensitivity of commercial ethyl glucuronide (ETG) testing in screening for alcohol abstinence. Alcohol & Alcoholism. 2007;42:317–20. doi: 10.1093/alcalc/agm014. [DOI] [PubMed] [Google Scholar]
- WURST FM, ALEXSON S, WOLFERSDORF M, BECHTEL G, FORSTER S, ALLING C, ARADOTTIR S, JACHAU K, HUBER P, ALLEN JP, AUWARTER V, PRAGST F. Concentration of fatty acid ethyl esters in hair of alcoholics: comparison to other biological state markers and self reported-ethanol intake. Alcohol & Alcoholism. 2004;39:33–8. doi: 10.1093/alcalc/agh005. [DOI] [PubMed] [Google Scholar]
- WURST FM, ALLING C, ARADOTTIR S, PRAGST F, ALLEN JP, WEINMANN W, MARMILLOT P, GHOSH P, LAKSHMAN R, SKIPPER GE, NEUMANN T, SPIES C, JAVORS M, JOHNSON BA, AIT-DAOUD N, AKHTAR F, ROACHE JD, LITTEN R. Emerging biomarkers: new directions and clinical applications. Alcoholism: Clinical & Experimental Research. 2005;29:465–73. doi: 10.1097/01.alc.0000156082.08248.ab. [DOI] [PubMed] [Google Scholar]
- WURST FM, KELSO E, WEINMANN W, PRAGST F, YEGLES M, SUNDSTROM POROMAA I. Measurement of direct ethanol metabolites suggests higher rate of alcohol use among pregnant women than found with the AUDIT--a pilot study in a population-based sample of Swedish women. Am J Obstet Gynecol. 2008;198:407, e1–5. doi: 10.1016/j.ajog.2007.10.801. [DOI] [PubMed] [Google Scholar]