Abstract
Overactivity of the renin-angiotensin system (RAS) is involved in the pathogenesis of Hypertension and a hyper-functioning brain RAS has been highlighted in several genetic and experimental models. Until now, Angiotensin (Ang)-II was thought to be the main actor of this system and the ACE/Ang-II/AT1 receptor axis was the main target for antihypertensive therapies. A new member of the RAS, ACE2 (angiotensin converting enzyme type 2) has been identified in organs and tissues related to cardiovascular function (e.g. heart, kidney, vessels) and appears to be part of a counter-regulatory pathway buffering the excess of Ang-II. We recently identified the ACE2 protein in brain regions involved in the central regulation of blood pressure (BP) and showed that it regulates, and is regulated by, other components of the RAS. Here, we present evidence for brain ACE2’s involvement in the central regulation of BP, autonomic and cardiac function. We show that lack of ACE2 is deleterious for the central regulation of BP and that brain ACE2 gene therapy can restore baroreflex and autonomic functions and prevent the development of Hypertension. Additionally, and independently of Ang-II levels reduction, we will highlight some of the mechanisms responsible for the beneficial effects of central ACE2 in cardiovascular function.
Introduction
Hypertension affects about 73.6 million people in the United States from age 20 and over. However, only 25% of these patients have their blood pressure under control. One of the reasons for this poor record is that most of hypertension, about 90 to 95%, are from unknown cause, also called primary hypertension, or essential hypertension. The sympathetic nervous system exerts a fundamental role in the homeostatic control of blood pressure (Grassi & Mancia, 2004). Studies have shown that several hemodynamic changes in hypertension such as elevated cardiac output and heart rate, as well as alteration in vascular resistance, can be neurogenic (Julius & Majahalme, 2000). Indeed, neurogenic hypertension is associated with a rise in sympathetic outflow and often, an inhibition of parasympathetic drive, thus resulting in the increase of cardiac output and peripheral vascular resistance. In addition, plasma norepinephrine level, a marker of sympathetic activation, is elevated in essential hypertensive patients compared to age-matched normotensive controls (Goldstein, 1983).
The renin angiotensin system
The renin-angiotensin system (RAS) has been shown to play an important role in blood pressure regulation (Bader & Ganten, 2008). The classical view of the RAS (Paul et al., 2006), also called endocrine RAS, is the release of angiotensinogen from the liver, which is cleaved in the circulation by renin, the enzyme secreted from the juxtaglomerular apparatus of the kidney to form the decapeptide angiotensin (Ang)-I. Ang-I is then transformed into the octapeptide Ang-II by angiotensin converting enzyme (ACE), a membrane-bound metalloproteinase, which is predominantly expressed in high concentrations on the surface of endothelial cells in the pulmonary circulation. Ang-II, as the primary modulator in this system, is then acting on specific receptors; essentially type 1 receptors, leading to vasoconstriction. In recent years, the discovery of these RAS components in various tissues led to the concept of “local” or “tissue” RAS (Lavoie & Sigmund, 2003). This concept was based on the discovery of RAS components in “unlikely” places, such as the “kidney enzyme” renin being found in the brain, and where the endocrine actions of the system could not explain the findings, for example the beneficial effects of ACE inhibitors in patients with normal plasma Ang-II levels. It is now established that a local RAS is present in various tissues throughout the body, regulating local organ function and interacting with the endocrine as well as other tissues RAS.
The brain RAS
Components of the RAS have been identified in all brain nuclei involved in the central regulation of blood pressure, including the subfornical organ (SFO), paraventricular nucleus (PVN), rostral ventrolateral medulla (RVLM), area posterma (AP), nucleus of the tractus solitarius (NTS), and others (Davisson, 2003). In addition to Ang II, other angiotensin peptides have also been identified in the brain, such as Ang-(1–7) (Schiavone et al., 1988), Ang IV (Faure et al., 2008; Yang et al., 2008) and recently, Ang-(1–12)(Nagata et al., 2006). Ang-(1–7) has opposite properties compared to Ang-II. For example, it stimulates nitric oxide (NO) release, improves baroreflex function and promotes vasodilatation (Sakima et al., 2005; Sampaio et al., 2007) while Ang-II impairs these mechanisms. Most of the previously mentioned nuclei are inside the blood brain barrier and therefore protected from systemic neuromediators. However, some of them, called circumventricular organs (CVO), such as the SFO and AP, are lacking a blood brain barrier and as a result, constitute “opened windows” to the brain for small peptides, like Ang-II (Johnson & Thunhorst, 1997). Indeed, in addition to locally generated Ang-II in the brain, blood borne Ang-II can reach the brain via the CVO and interact with angiotensin receptors located in these areas to exert central effects in addition to its peripheral effects (Lazartigues et al., 2007) (Xia et al., 2009).
Role of ACE2 in the brain
Almost a decade ago, a new member of the RAS was discovered and, as the first homolog of ACE, was named ACE2. Unlike ACE, ACE2 is a mono-carboxypeptidase and shares 42% homology with ACE (Tipnis et al., 2000). While ACE generates Ang-II from the degradation of Ang-I, ACE2 is able to cleave Ang-II and produce the vasodilating peptide Ang-(1–7) (Vickers et al., 2002). Studies have shown that peripheral ACE2 is able to reduce cardiac hypertrophy and prevent the development of hypertension in various animal models (Diez-Freire et al., 2006; Rentzsch et al., 2007). In the central nervous system (CNS), Yamazato et al. showed that ACE2 over-expression in the RVLM could reduce the elevated blood pressure in spontaneously hypertensive (SH) rats (Yamazato et al., 2007).
Focusing on one of the CVO, we observed that brain-targeted ACE2 over-expression in the SFO reduces the acute Ang-II-mediated pressor and drinking responses (Feng et al., 2008). Beyond the obvious reduction of Ang-II levels due to ACE2 overexpression, we noticed that these responses were also associated with the down-regulation of AT1 receptor expression both at the mRNA and protein levels. These data suggest that adenovirus-mediated ACE2 expression definitely plays a regulatory role in counter balancing the Ang-II effects, but also may regulate AT1 receptor expression. However, due to the short term expression and the low efficiency of the virus vectors, these acute studies could not address the long-term effects of ACE2 expression and dissect these mechanisms in detail.
To further investigate the role of ACE2 in the CNS, we generated a new transgenic mouse model (syn-hACE2), in which the expression of the human ACE2 gene is driven by a synapsin promoter, allowing its expression to every neuron in the brain. Syn-hACE2 transgenic mice exhibit normal baseline cardiac hemodynamic parameters, with blood pressure and heart rate in the same range as their control non-transgenic littermates (NT). Similarly, these transgenic mice have unaltered spontaneous baroreflex sensitivity (SBRS) and autonomic function in baseline. A more important question related to the phenotype of these mice in the face of a hypertensive challenge. To test the role of central ACE2 in the development of high blood pressure, we used the Ang-II ‘slow pressor dose’ model (600 ng/kg.min) which has been shown to result in neurogenic hypertension (Zimmerman et al., 2002). Interestingly, our data show that ACE2 over-expression in the brain blunted the development of low dose Ang-II-induced neurogenic hypertension in syn-hACE2 transgenic mice, as well as the associated increase in water intake. In parallel to these reductions in blood pressure and water intake, baroreflex sensitivity and parasympathetic tone were preserved from the inhibitory effects of Ang-II infusion, while paradoxically, sympathetic outflow did not appear to be significantly reduced. Most importantly, co-infusion of Ang-II with the Ang-(1–7) antagonist, D-ala7-Ang-(1–7) totally reversed the ACE2 blood pressure-lowering effects, suggesting that Ang-(1–7) plays a pivotal role in the prevention of hypertension in this model.
This protective role of ACE2 was also tested in a genetic model of hypertension, the R+A+ mouse developed by Dr. Curt D. Sigmund at The University of Iowa. These mice overexpress both human renin and angiotensinogen genes throughout their body and are chronically hypertensive (Merrill et al., 1996). Breeding these mice with the syn-hACE2 model allowed us to generate a triple-transgenic (SARA) mouse with chronic elevation of Ang-II in the brain and the periphery and overexpression of ACE2 in the CNS. Interestingly, the enhanced water intake was prevented and autonomic function improved in the SARA mice. Moreover, hypertension was signifiantly reduced confirming the potential of ACE2 in the buffering of an overactive RAS (Xia et al., 2009).
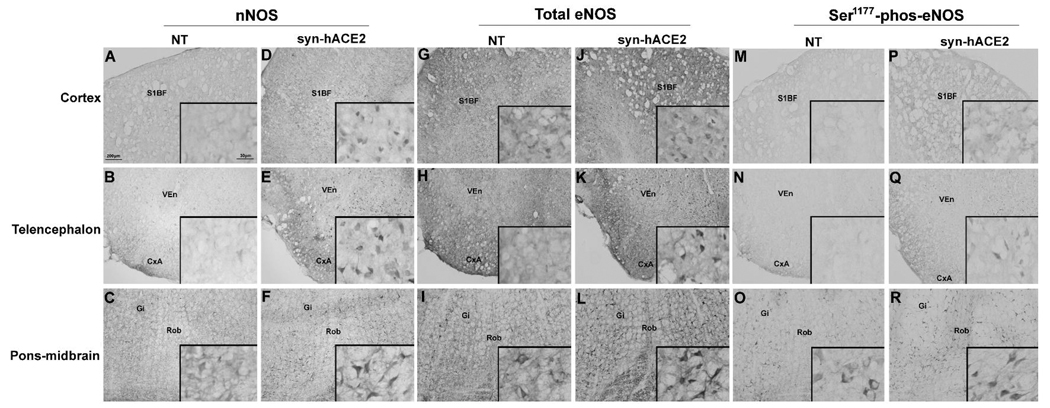
To gain insight on the signaling pathways and molecular mechanisms leading to ACE2-mediated reduction of hypertension development, we focused on the regulation of nitric oxide synthase (NOS) expression. Indeed, as a direct product of NOS, nitric oxide (NO) release has been shown to reduce sympathetic activity in the central nervous system (Sakai et al., 2005) and its release is known to be enhanced following activation of the Ang-(1–7) receptor (Sampaio et al., 2007). We hypothesized that ACE2 overexpression would lead to an increase in Ang-(1–7)-mediated NO release and therefore modulate the blood pressure and cardiovascular function. Using the syn-hACE2 transgenic mouse model, we examined the expression of the neuronal NOS (nNOS), endothelial NOS (eNOS) as well as the phosphorylated form of this protein (Ser1177-phosphorylated-eNOS). We observed that the expression of nNOS, eNOS and Ser1177-phosphorylated-eNOS were up-regulated in syn-hACE2 mice throughout the brain, including in cardiovascular regions as well as non-cardiovascular regions, such as cortex, telencephalon and pons-midbrain (Figure 1). These data suggest that ACE2 over-expression up-regulates constitutive NOS isoforms expression and phosphorylation, which may have participated to the blunting of hypertension through an enhanced release of NO in the CNS.
Figure 1. NOS expression in the brain.
Immunohistochemistry for nNOS, eNOS and Ser1177-phosphorylated-eNOS was visualized by DAB. Baseline levels of nNOS, eNOS and Ser1177-phosphorylated-eNOS were all significantly elevated throughout the brain in syn-hACE2 mice in cortex (D, J, P), telencephalon (E, K, Q) and pons-midbrain (F, L, R) compared to controls in cortex (A, G, M), telencephalon (B, H, N) and pons-midbrain (C, I, O). Representative microphotographs were taken using 10× magnification and inserts are 63× magnification. Abbreviations: NT, non-transgenic; syn-hACE2, human ACE2 transgenic mice; nNOS, neuronal nitric oxide synthase; eNOS, endothelial nitric oxide synthase; S1BF, the primary somatosensory cortex-barrel field; VEn, ventral endopiriform nucleus; CxA, cortex-amygdala transition zone ; GI, gigantocellular reticular nucleus; Rob, raphe obscurus nucleus.
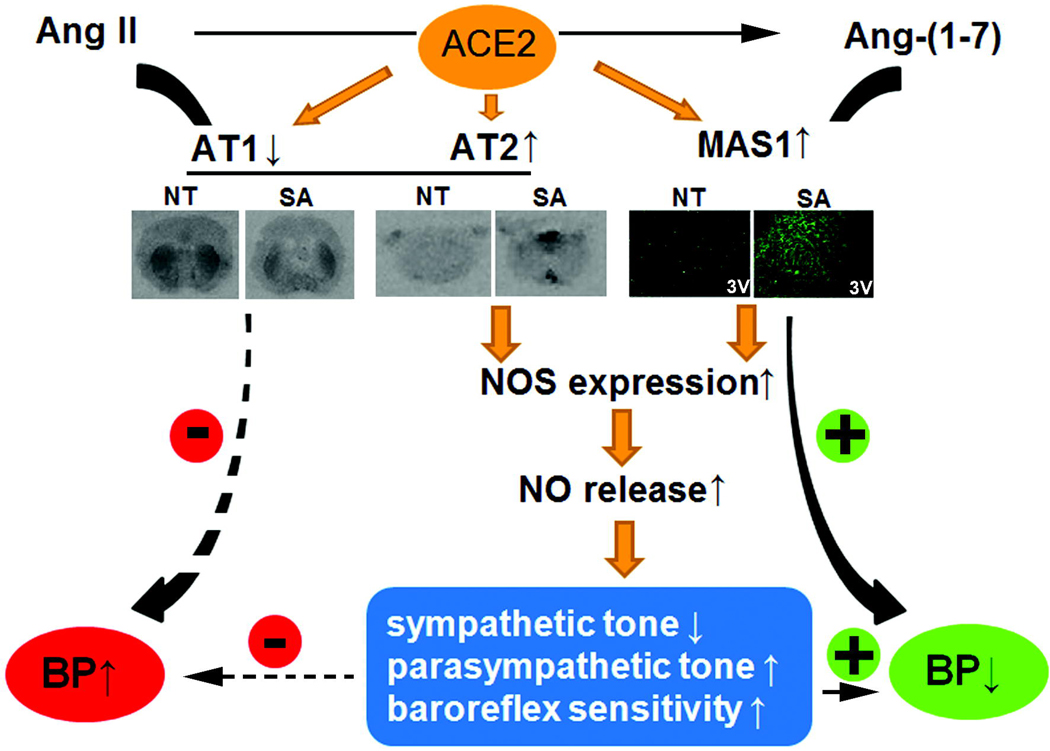
As previously mentioned above, we found that adenovirus-mediated ACE2 over-expression was able to down-regulate AT1 receptors in the SFO. Since Mas and AT2 receptors have been reported to mediate NOS activation and NO release (Sosa-Canache et al., 2000; Xu et al., 2008), we further investigated whether ACE2 over-expression would modify Mas and AT2 receptor expression in the CNS. Using immuno-histochemistry, we observed that ACE2 was able to modulate the expression of these receptors and eventually increase the AT2/AT1 and Mas/AT1 ratios in the SFO (Table 1). While Ang-II infusion resulted in a dramatic reduction of these ratios in control and transgenic mice, likely resulting from the up-regulation of AT1 receptors, syn-hACE2 were less affected and therefore protected against the deleterious effects of the vasopressor peptide. However, blockade of the Ang-(1–7) receptor reversed this protective effect, suggesting that Ang-(1–7) plays a critical role in the modulation of not only Mas but also AT1 and/or AT2 receptors (Table 1). More work is definitely necessary to dissect the precise mechanisms of these heterologous regulations. Parallel to the immuno-histochemistry experiments, receptor autoradiography was also performed for AT1 and AT2 subtypes, as well as immuno-fluorescence for Mas expression. As summarized in Figure 2, we confirmed that ACE2 over-expression resulted in down-regulation of the AT1 and up-regulation of both Mas and AT2 receptor subtypes in various brain regions of the syn-hACE2 mouse model.
Table 1.
AT2/AT1, Mas1/AT1 receptors ratio in subfornical organ.
| AT2/AT1 | MAS1/AT1 | |
|---|---|---|
| Subfornical organ | ||
| NT+saline | 1.0 ±0.14 | 1.0 ± 0.17 |
| SA+saline | 1.89 ± 0.04* | 1.86 ± 0.22* |
| NT+Ang II | 0.09 ± 0.06† | 0.89± 0.14 |
| SA+Ang II | 0.53 ± 0.08† | 1.07 ± 0.20 |
| SA+Ang II+D-ala7-Ang-(1–7) | 0.29 ± 0.08§ | 0.57 ± 0.12 |
Data represent the relative receptor density normalized to NT+saline. Values are expressed as mean ±SEM.
P<0.05 vs. NT;
P<0.05 vs. saline
P<0.05 vs. SA+Ang II.
Figure 2. ACE2 and blood pressure regulation.
ACE2 over expression in the central nervous system regulates AT1, AT2 and Mas receptors’ expression and activates nitric oxide synthase signaling pathway, leading to the modulation of baroreflex sensitivity, sympathetic and parasympathetic tone and eventually resulting in the decrease of neurogenic hypertension. (+) indicates a reinforcement and (−) indicates an inhibition of the pathways. Abbreviations: NT, non-transgenic; SA, syn-hACE2 transgenic mice.
In summary, using adenovirus and mouse genetic models, we have shown that over-expression of ACE2 would not only promote the conversion of the vasoconstrictor Ang-II into the vasodilator Ang-(1–7), but also modulate the expression of their various receptors to the detriment of hypertension. Moreover, increased expression of constitutive NOS isoforms and phosphorylation of eNOS were associated with the receptor modulation and may have promoted enhanced NO release also contributing to the improvement of baroreflex and autonomic functions; and ultimately to the buffering of hypertension in this model (Figure 2).
Conclusion
ACE2, as an important member of the RAS, ACE2 has been reported to participate in the regulation of BP and cardiovascular function in the brain and at the periphery. In this short review of our recent work, we provide evidence that modulation of ACE2 expression in the CNS may play an important role in protecting against the development of neurogenic hypertension through regulation of baroreflex and autonomic function. The molecular mechanisms by which this protective effect occurs seem to include the regulation of angiotensin receptors expression. ACE2 appears to be able to adjust the AT2/AT1 and Mas/AT1 ratios in a way opposing the development of hypertension. We further showed that NO signaling pathways are also affected by ACE2 over-expression in the CNS and might participate in the overall reduction of the neurogenic hypertension in syn-hACE2 mice. In conclusion, ACE2 plays a regulatory role in the central regulation of BP and cardiovascular function and could become an attractive target for the treatment of hypertension and other cardiovascular diseases resulting from an overactive RAS.
Acknowledgements
The authors would like to thank Felicia M. Rabey and Lenice Kappes Becker for excellent technical assistance. Transgenic mice were generated at the University of Iowa Transgenic Animal Facility directed by Dr. Curt D. Sigmund and supported in part by grants from the NIH and from the Roy J. and Lucille A. Carver College of Medicine. We wish to thank Norma Sinclair, Patricia Yarolem and Joanne Schwarting for their technical expertise in generating transgenic mice. This work was supported, in part, by an American Heart Association Postdoctoral Fellowship to Dr. Yumei Feng, an American Physiological Society Postdoctoral Fellowship to Dr. Huijing Xia and NIH grants NS052479, RR018766 and HL093178 to Dr. Eric Lazartigues.
References
- Bader M, Ganten D. Update on tissue renin–angiotensin systems. J Mol Med. 2008;86:615–621. doi: 10.1007/s00109-008-0336-0. [DOI] [PubMed] [Google Scholar]
- Davisson RL. Physiological genomic analysis of the brain renin-angiotensin system. Am J Physiol - Regul Integr Comp Physiol. 2003;285:R498–R511. doi: 10.1152/ajpregu.00190.2003. [DOI] [PubMed] [Google Scholar]
- Diez-Freire C, Vazquez J, Correa de Adjounian MF, Ferrari MFR, Yuan L, Silver X, Torres R, Raizada MK. ACE2 gene transfer attenuates hypertension-linked pathophysiological changes in the SHR. Physiol Genomics. 2006;27:12–19. doi: 10.1152/physiolgenomics.00312.2005. [DOI] [PubMed] [Google Scholar]
- Faure Sa, Bureau Aa, Oudart Na, Javellaud Ja, Fournier Ab, Achard J-Ma. Protective effect of candesartan in experimental ischemic stroke in the rat mediated by AT2 and AT4 receptors. J Hypertens. 2008;26:2008–2015. doi: 10.1097/HJH.0b013e32830dd5ee. [DOI] [PubMed] [Google Scholar]
- Feng Y, Yue X, Xia H, Bindom SM, Hickman PJ, Filipeanu CM, Wu G, Lazartigues E. Angiotensin-Converting Enzyme 2 Overexpression in the Subfornical Organ Prevents the Angiotensin II-Mediated Pressor and Drinking Responses and Is Associated With Angiotensin II Type 1 Receptor Downregulation. Circ Res. 2008;102:729–736. doi: 10.1161/CIRCRESAHA.107.169110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS. Plasma catecholamines and essential hypertension. An analytical review. Hypertension. 1983;5:86–99. doi: 10.1161/01.hyp.5.1.86. [DOI] [PubMed] [Google Scholar]
- Grassi G, Mancia G. Neurogenic Hypertension: Is the Enigma of Its Origin Near the Solution? Hypertension. 2004;43:154–155. doi: 10.1161/01.HYP.0000109870.99110.7e. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Thunhorst RL. The neuroendocrinology of thirst and salt appetite: visceral sensory signals and mechanisms of central integration. Front Neuroendocrinol. 1997;18:292–353. doi: 10.1006/frne.1997.0153. [DOI] [PubMed] [Google Scholar]
- Julius S, Majahalme S. The changing face of sympathetic overactivity in hypertension. Ann Med. 2000;32:365–370. doi: 10.3109/07853890008995939. [DOI] [PubMed] [Google Scholar]
- Lavoie JL, Sigmund CD. Overview of the renin-angiotensin system-An endocrine and paracrine system. Endocrinology. 2003;144:2179–2183. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- Lazartigues E, Feng Y, Lavoie JL. The two fACEs of the tissue renin-angiotensin systems: implication in cardiovascular diseases. Curr Pharm Des. 2007;13:1231–1245. doi: 10.2174/138161207780618911. [DOI] [PubMed] [Google Scholar]
- Merrill DC, Thompson MW, Carney CL, Granwehr BP, Schlager G, Robillard Je, Sigmund CD. Chronic hypertension and altered baroreflex responses in transgenic mice containing the human renin and human angiotensinogen genes. J Clin Invest. 1996;97:1047–1055. doi: 10.1172/JCI118497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochemical and Biophysical Research Communications. 2006;350:1026–1031. doi: 10.1016/j.bbrc.2006.09.146. [DOI] [PubMed] [Google Scholar]
- Paul M, Poyan Mehr A, Kreutz R. Physiology of Local Renin-Angiotensin Systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- Rentzsch B, Iliescu R, Todiras M, Popova E, Baltatu O, Santos R, Bader M. Transgenic ACE2 overexpression in vascular smooth muscle of SHR-SP rats reduces blood pressure and improves endothelial function. Hypertension. 2007;50:e89. doi: 10.1161/HYPERTENSIONAHA.108.114322. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hirooka Y, Shigematsu H, Kishi T, Ito K, Shimokawa H, Takeshita A, Sunagawa K. Overexpression of eNOS in brain stem reduces enhanced sympathetic drive in mice with myocardial infarction. Am J Physiol Heart Circ Physiol. 2005;289:H2159–H2166. doi: 10.1152/ajpheart.00408.2005. [DOI] [PubMed] [Google Scholar]
- Sakima A, Averill DB, Gallagher PE, Kasper SO, Tommasi EN, Ferrario CM, Diz DI. Impaired heart rate baroreflex in older rats: role of endogenous angiotensin-(1–7) at the nucleus tractus solitarii. Hypertension. 2005;46:333–340. doi: 10.1161/01.HYP.0000178157.70142.33. [DOI] [PubMed] [Google Scholar]
- Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1–7) Through Receptor Mas Mediates Endothelial Nitric Oxide Synthase Activation via Akt-Dependent Pathways. Hypertension. 2007;49:185–192. doi: 10.1161/01.HYP.0000251865.35728.2f. [DOI] [PubMed] [Google Scholar]
- Schiavone MT, Santos RAS, Brosnihan KB, Khosla MC, Ferrario CM. Release of Vasopressin from the Rat Hypothalamo-Neurohypophysial System by Angiotensin-(1–7) Heptapeptide. Proc Natl Acad Sci U S A. 1988;85:4095–4098. doi: 10.1073/pnas.85.11.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa-Canache B, Cierco M, Gutierrez CI, Israel A. Role of bradykinins and nitric oxide in the AT2 receptor-mediated hypotension. J Hum Hypertens. 2000;14 Suppl 1:S40–S46. doi: 10.1038/sj.jhh.1000986. [DOI] [PubMed] [Google Scholar]
- Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- Xia H, Feng Y, Obr TD, Hickman PJ, Lazartigues E. Angiotensin II Type 1 Receptor-Mediated Reduction of Angiotensin-Converting Enzyme 2 Activity in the Brain Impairs Baroreflex Function in Hypertensive Mice. Hypertension. 2009;53:210–216. doi: 10.1161/HYPERTENSIONAHA.108.123844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Costa-Goncalves AC, Todiras M, Rabelo LA, Sampaio WO, Moura MM, Sousa Santos S, Luft FC, Bader M, Gross V, Alenina N, Santos RAS. Endothelial Dysfunction and Elevated Blood Pressure in Mas Gene-Deleted Mice. Hypertension. 2008;51:574–580. doi: 10.1161/HYPERTENSIONAHA.107.102764. [DOI] [PubMed] [Google Scholar]
- Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK. Overexpression of Angiotensin-Converting Enzyme 2 in the Rostral Ventrolateral Medulla Causes Long-Term Decrease in Blood Pressure in the Spontaneously Hypertensive Rats. Hypertension. 2007;49:926–931. doi: 10.1161/01.HYP.0000259942.38108.20. [DOI] [PubMed] [Google Scholar]
- Yang Ra, Smolders Ia, De Bundel Da, Fouyn Ra, Halberg Mb, Demaegdt Hc, Vanderheyden Pc, Dupont AGd. Brain and peripheral angiotensin II type 1 receptors mediate renal vasoconstrictor and blood pressure responses to angiotensin IV in the rat. J Hypertens. 2008;26:998–1007. doi: 10.1097/HJH.0b013e3282f5ed58. [DOI] [PubMed] [Google Scholar]
- Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res. 2002;91:1038–1045. doi: 10.1161/01.res.0000043501.47934.fa. [DOI] [PubMed] [Google Scholar]